Abstract
Objective To review systematically the evidence for an effect of long chain and shorter chain omega 3 fatty acids on total mortality, cardiovascular events, and cancer.
Data sources Electronic databases searched to February 2002; authors contacted and bibliographies of randomised controlled trials (RCTs) checked to locate studies.
Review methods Review of RCTs of omega 3 intake for 3 6 months in adults (with or without risk factors for cardiovascular disease) with data on a relevant outcome. Cohort studies that estimated omega 3 intake and related this to clinical outcome during at least 6 months were also included. Application of inclusion criteria, data extraction, and quality assessments were performed independently in duplicate.
Results Of 15 159 titles and abstracts assessed, 48 RCTs (36 913 participants) and 41 cohort studies were analysed. The trial results were inconsistent. The pooled estimate showed no strong evidence of reduced risk of total mortality (relative risk 0.87, 95% confidence interval 0.73 to 1.03) or combined cardiovascular events (0.95, 0.82 to 1.12) in participants taking additional omega 3 fats. The few studies at low risk of bias were more consistent, but they showed no effect of omega 3 on total mortality (0.98, 0.70 to 1.36) or cardiovascular events (1.09, 0.87 to 1.37). When data from the subgroup of studies of long chain omega 3 fats were analysed separately, total mortality (0.86, 0.70 to 1.04; 138 events) and cardiovascular events (0.93, 0.79 to 1.11) were not clearly reduced. Neither RCTs nor cohort studies suggested increased risk of cancer with a higher intake of omega 3 (trials: 1.07, 0.88 to 1.30; cohort studies: 1.02, 0.87 to 1.19), but clinically important harm could not be excluded.
Conclusion Long chain and shorter chain omega 3 fats do not have a clear effect on total mortality, combined cardiovascular events, or cancer.
Introduction
Consumption of long chain omega 3 fatty acids (eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA)) found in fatty fish and fish oils has been linked to the low incidence of coronary heart disease in the Inuit people of Greenland1; α linolenic acid (ALA), a shorter chain omega 3 found in some plant oils (and variably converted to eicosapentaenoic acid and docosahexaenoic acid) may also be protective.2 Omega 3 fats may protect against cardiovascular disease by lowering blood pressure and heart rate; reducing serum triglycerides, thrombotic tendency, inflammation, and arrhythmias; and improving endothelial function, insulin sensitivity, paraoxonase concentrations, and plaque stability.3-6
Toxic compounds, such as fat soluble methylmercury, dioxins, and polychlorinated biphenyls, are also found in oily fish and fish oils, but any harm from these compounds would be seen only after long term supplementation.7,8 Animal intervention studies and studies of adults after severe inadvertent exposure indicate that dioxins and polychlorinated biphenyls increase the risk of cancer.9,10 Methylmercury may increase the risk of myocardial infarction and cause neurological damage.11,12
Since a meta-analysis of the effect of omega 3 fats on cardiovascular morbidity and mortality in coronary heart disease suggested important benefits,13 a large intervention study has been published.14 Our meta-analysis included these new data, balanced protective effects with possible harm, assessed the effects of plant based omega 3 fats on health, included people without established cardiovascular disease, and highlighted important questions about the role of omega 3 fats on cardiovascular disease and mortality. We systematically reviewed the effects of long chain and short chain omega 3 fats (together and separately) on mortality, cardiovascular disease, cancer, and bleeding events and analysed all relevant randomised controlled trials (RCTs) and prospective cohort studies.
Methods
The study methods have been described in detail elsewhere.15
Search strategy and study selection
We searched the Cochrane Library, Medline, Embase, the National Research Register, and SIGLE (to February 2002); we checked the bibliographies of included studies and contacted the authors. Articles not in English were translated. We excluded trials if they were not randomised; they had no omega 3 arm; the participants were children or were critically ill; the duration was < 6 months; the intervention was multifactorial; or data on death, cardiovascular disease, or cancer were not available. We rejected cohort studies if they did not assess the intake of omega 3, follow-up was < 6 months, or the association between the intake of omega 3 and health was not investigated. Two reviewers assessed the inclusion of articles independently, and we contacted authors for more information on methodological quality, outcomes, and further studies.
Data extraction and quality assessment
Two reviewers independently extracted the data and assessed the quality of the studies. For RCTs we assessed the following quality characteristics: concealment of allocation to the study arms and the masking of participants, providers, and outcome assessors. We classed trials as having low risk of bias if allocation to the study arms was concealed and participants, providers, and outcome assessors were masked. Quality assessment of cohort studies was based on control group type, number lost to follow-up, baseline similarity, adjustment for dissimilarities, and masking.
Data synthesis
For RCTs we extracted the numbers of participants experiencing each outcome and total numbers randomised for each study arm, and combined by using relative risks in random effects meta-analysis.16 For cohort studies we used relative risk or odds ratio that had been adjusted for the most confounding factors, and we compared the most exposed quantile with the least exposed quantile. We used one analysis only for each cohort per outcome.
We used subgrouping of RCTs to explore the effects on mortality, cardiovascular events, and cancer of long chain versus short chain omega 3 fats and dietary advice versus supplementation. We used random effects meta-regression to analyse the effects of the dose of omega 3 and the duration of the trial. Sensitivity analyses assessed the robustness of RCT results to trial quality by restricting the analysis to studies with low risk of bias.
We used Cochran's test for heterogeneity to determine whether studies in a meta-analysis evaluated the same underlying sizes of effect. We used I2 to measure the degree of inconsistency among studies (the proportion of total observed variability due to genuine variation rather than random error within studies).17
Results
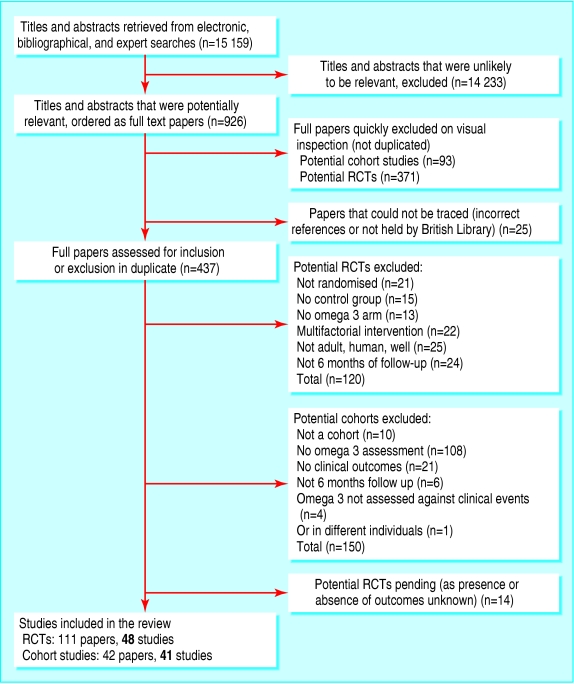
We screened 15 159 titles and collected 926 full text papers. Forty eight randomised controlled trials and 41 analyses of 26 cohort studies fulfilled all inclusion criteria (fig 1; for a complete set of references see bmj.com). Table 1 shows the main characteristics of the included studies.
Fig 1.
The selection process for randomised controlled trials (RCTs) and cohort studies on omega 3 fatty acid and health outcomes
Table 1.
Characteristics of trials and cohort studies on effects of omega 3 fats on mortality, cardiovascular disease (CVD), and cancer
| Criteria | Randomised controlled trials | Cohorts |
|---|---|---|
| Studies and participants | ||
| No of participants | 36 913 | 563 218, plus two where the size not described |
| No of studies | 48 | 47 published analyses from 26 |
| No of large studies | 8 (>500 participants) | 10 (>20 000 participants) |
| Follow-up | 0.5 to >∼6 years | 4 to 25 years |
| Risk of CVD | ||
| Have CVD | 21 | 2 |
| Moderate risk | 10 | 2 |
| Low risk | 17 | 22 |
| Sex | ||
| ≥70% men | 24 | 14 |
| 31% to 69% men | 17 | 4 |
| ≤30% men | 5 | 7 |
| Not stated | 2 | 1 |
| Main outcome* | ||
| CVD or mortality | 32 | 13 |
| Cancer | 0 | 10 |
| Other outcomes | 16 | 10 |
Several cohorts appear to have more than one main outcome.
Intervention or exposure
Dietary supplements were given in 44 trials (36 as capsules, six as oil, one each as liquid emulsion and enriched margarine), advice on eating oily fish in three, and advice on diet and food supplements in one. Supplements were long chain omega 3 fats (usually whole or concentrated fish oil; one small trial used refined eicosapentaenoic acid and one used refined docosahexaenoic acid), and five studies provided shorter chain omega 3 fats. Doses of long chain omega 3 fats (summing eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid) varied from 0.4 g to 7.0 g per day. Control groups received vegetable oils, other fats, “inert” or ill defined substances, different dietary advice, or nothing. The intervention lasted 6-11 months in 23 studies, 12-23 months in 16, 24-47 months in eight, and ≥ 48 months in one study.
Intake of omega 3 (varying combinations of eicosapentaenoic acid, docosahexaenoic acid, docosapentaenoic acid, along with α linolenic acid, supplemental fish oils, or dietary oily fish) was assessed by dietary and biochemical means in two cohorts, dietary means only in 18, and biochemical means only in 10. Groups with the lowest and highest intake of long chain omega 3 differed by 0.1-0.6 g omega 3 per day.
Methodological quality
Twenty five RCTs were rated as having a low risk of bias (table 2). Losses to follow-up were unclear in 16 cohort studies. In 15 cohort studies the outcome assessors were blinded to exposure, in two they were not blinded, and in nine blinding was unclear.
Table 2.
Quality assessment of randomised controlled trials of the effect of omega 3 fats on mortality, cardiovascular disease, and cancer
|
Study
|
Concealment of allocation
|
Masking
|
Dropouts for analysis of events (intervention, control)
|
|||
|---|---|---|---|---|---|---|
| Participants | Providers of care | Assessors of outcome | Summary risk of bias | |||
| Almallah 1998w1 | Done | Yes | Yes | Yes | ?/18, ?/18 | Low |
| Bairati 1992w2 | Done | Yes | Yes | Yes | 48/107, 38/98 | Low |
| Bellamy 1992*w3 | Unclear | Unclear | Unclear | Unclear | 3/60, 7/60 | Medium or high |
| Belluzzi 1996w4 | Done | Yes | Yes | Yes | 5/39, 2/39 | Low |
| Bemelmans 2002w5 | Done | Yes | Yes | Yes | ?/51, ?/52 | Low |
| Bonnema 1995w6 | Done | Yes | Yes | Yes | 0/14, 1/14 | Low |
| Borchgrevink 1966w7 | Done | Yes | Yes | Yes | ?/100, ?/100 | Low |
| Brox 2001w8 | Unclear | No | Yes | Yes | Seal oil 8/40, cod liver oil 2/40, 1/40 | Medium or high |
| Burr (DART) 1989w9 | Unclear | No | No | Yes | 0/1015, 0/1018 for mortality | Medium or high |
| Burr 2003w10 | Unclear | No | No | Yes | 0/1571, 0/1543 for mortality | Medium or high |
| Connor 1993w11 | Done | Yes | Yes | Yes | ?/8, ?/8 | Low |
| Dehmer 1998w12 | Not done | No | No | No | 3/46, 5/44 | Medium or high |
| Dry 1991w13 | Done | Yes | Yes | Yes | 0/6, 0/6 | Low |
| Eritsland 1996w14 | Done | No | No | Yes | 15/317, 14/293 | Medium or high |
| Franzen 1993w15 | Unclear | Yes | Unclear | Yes | 0/15, 0/15 | Medium or high |
| Geusens 1994*w16 | Unclear | Yes | Yes | Yes | Low dose 9/30, high dose 11/30, 10/30 | Medium or high |
| GISSI-P 1999*w17 | Done | No | Unclear | Yes | ?/5665, ?/5658 | Medium or high |
| Greenfield 1993w18 | Done | Yes | Yes | Yes | 3/16, 1/8 | Low |
| Hawthorne 1992w19 | Done | Unclear | Yes | Yes | 5/49, 6/47 | Medium or high |
| Johansen 1999*w20 | Unclear | Yes | Yes | Yes | 54/250, 58/250 | Medium or high |
| Katan 1997w21 | Done | No | Unclear | Yes | Low dose ?/15, med dose ?/15, high dose ?/14, ?/14 | Medium or high |
| Kaul 1992w22 | Unclear | No | No | Yes | ?/58, ?/49 | Medium or high |
| Lau 1993w23 | Done | Yes | Yes | Yes | 9/32, 16/32 | Low |
| Lau 1995w24 | Done | Yes | Yes | Yes | 0/25, 0/20 | Low |
| Leaf 1994w25 | Done | Yes | Yes | Yes | 69/275, 69/276 | Low |
| Loeschke 1996w26 | Done | Yes | Yes | Yes | 0/31, 0/33 | Low |
| Lorenz-Meyer 1996w27 | Done | Yes | Yes | Yes | ?/70, ?/65 | Low |
| Malaguarnera 1999*w28 | Unclear | No | Unclear | Unclear | ?/26, ?/26 | Medium or high |
| Maresta 2002w29 | Done | Yes | Yes | Yes | 44/169, 38/170 | Low |
| Mate-Jimenez 1991w30 | Done | No | Yes | Yes | 4/19, 6/19 | Medium or high |
| Milner 1989w31 | Done | No | Unclear | Yes | 0/100, 0/100 | Medium or high |
| Natvig 1968*w32 | Done | Yes | Yes | Yes | 2/6716, 2/6690 | Low |
| Nilsen 2001w33 | Done | Yes | Yes | Yes | ?/150, ?/150 | Low |
| Nye 1990*w34 | Unclear | Yes | Yes | Yes | 0/36, 0/37 | Medium or high |
| Reis 1991w35 | Done | Yes | Yes | Yes | 22/146, 10/72 | Low |
| Rossing 1996w36 | Done | Yes | Yes | Yes | 0/18, 0/18 | Low |
| Sacks (HARP) 1995*w37 | Unclear | Yes | Yes | Yes | 10/41, 11/39 | Medium or high |
| Sacks (TOHP 1) 1994*w38 | Done | Yes | Yes | Yes | 1/175, 1/175 | Low |
| Sarkkinen 1998w39 | Unclear | No | No | Yes | 0/41, 0/37 | Medium or high |
| Selvais 1995w40 | Done | Yes | Yes | Yes | 4/12, 2/12 | Low |
| Shimizu 1995w41 | Not done | No | No | Yes | ?/29, ?/16 | Medium or high |
| Singh 1997†w42 | Done | No | Yes | Yes | Fish oil 4/122, mustard oil 8/120, 6/118 | Medium or high |
| Sirtori 1998w43 | Done | Yes | Yes | Yes | 28/470, 39/465 | Low |
| Skoldstam 1992w44 | Done | No | No | No | 1/23, 2/23 | Medium or high |
| Terano 1999w45 | Done | No | No | Yes | 0/10, 0/10 | Medium or high |
| Thien 1993w46 | Done | Yes | Yes | Yes | 6/21, 6/16 | Low |
| Veale 1994w47 | Done | Yes | Yes | Yes | 4/19, 0/19 | Low |
| von Schacky 1999w48 | Done | Yes | Yes | Yes | ?/112, ?/111 | Low |
Allocation concealment was coded as done, unclear, or not done; blinding of participants, providers, and outcome assessors was coded as yes (where there was a clear and realistic attempt to blind—success of blinding was rarely checked in the studies), unclear, or no (in consultation with authors of trials where possible).
A trial was considered to be at low risk of bias if allocation concealment was “done” and blinding of participants, providers, and outcome assessors was coded “yes.” All other trials were considered at moderate or high risk of bias.
Did not respond to our request for further information on the quality of their studies or provide additional data on trial outcomes.
The BMJ has concerns about the validity of another paper written by this author.18
In the seven cohort studies that described omega 3 intake at baseline (five assessed long chain omega 3 fats only, one short chain omega 3 fats only, and one assessed both), the characteristics of participants with high and low intake of omega 3 fats differed. People who consumed most long chain omega 3 at baseline had an advantage with regard to lifestyle (smoking, diet, and exercise), interest in health, and social factors (education, living in town). Adjustment for these potential confounding factors may not have been adequate (table 3).
Table 3.
Adjustment carried out in 10 cohorts that assessed effects of omega 3 fats on cancer
| Factors* | No of cohorts adjusted† |
|---|---|
| Lifestyle factors | |
| Smoking | 2 |
| Alcohol intake | 2 |
| Physical activity | 1 |
| Dietary fibre intake | 1 |
| Saturated fat intake | 1 |
| Trans fat intake | 0 |
| Red meat intake | 2 |
| Fruit intake | 0 |
| Vegetable intake | 0 |
| Others‡: | |
| Body mass index | 4 |
| Total energy intake | 5 |
| Other dietary factors, height, parity, and menopausal status | |
| 1 each | Social factors |
| Place of residence | 2 |
| Education | 2 |
| Other‡: | |
| Ethnic origin | 1 |
| Socioeconomic status | 1 |
| Interest in health | |
| Vitamin E supplementation | 0 |
| Multivitamin supplementation | 0 |
| Others‡: | |
| Use of hormone replacement therapy | 1 |
| Family history of the disease studied | 2 |
| Precancerous symptoms | 1 |
Factors that differ consistently between people taking most and least fish based omega 3 fats; the association was consistent across all cohorts that assessed it and was seen in at least two cohorts.
The number of cohorts that adjusted for the factor in the most adjusted analysis, of the 10 cohorts used in the cancer outcomes analysis. Six cohorts also adjusted for age.
Unclear if consistently associated with omega 3 fats.
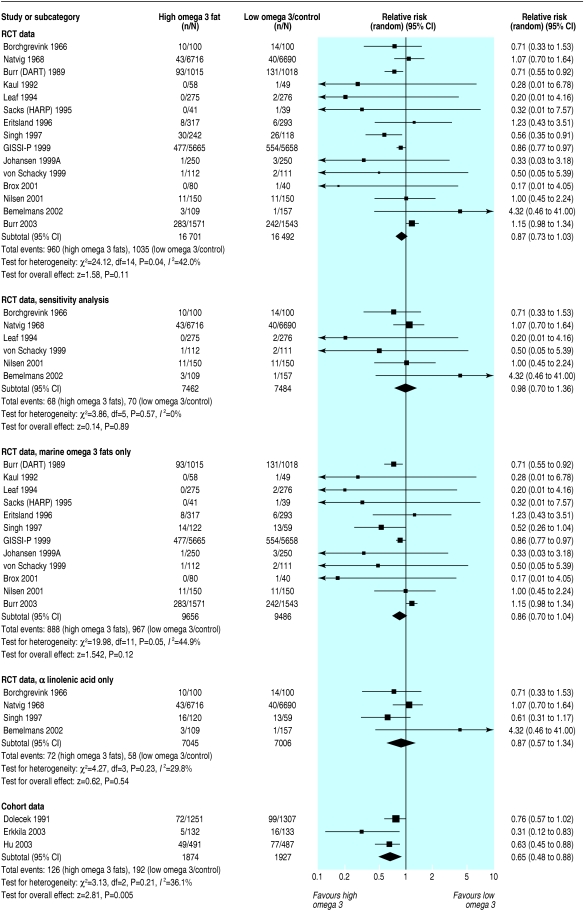
Total mortality
Deaths occurred in 15 RCTs (1995 deaths), and authors of 29 RCTs reported that no deaths occurred. Evidence that risk of death was reduced in participants randomised to omega 3 (relative risk 0.87, 95% confidence interval 0.73 to 1.03) was weak, and inconsistency was moderate (I2 = 42%) (fig 2). When analysis was restricted to studies at low risk of bias this effect was attenuated (0.98, 0.70 to 1.36; 138 deaths), and inconsistency between RCTs was low (I2 = 0%). This sensitivity analysis removed the RCT by Singh, whose studies have been questioned.18
Fig 2.
Effect of omega 3 fatty acids on mortality. For references see bmj.com
Results were similar for long chain versus short chain omega 3 (fig 2) and dietary advice versus supplements (data not shown). Meta-regression indicated that the risk of death increased as the length of the RCT increased (regression coefficient 0.008, 0.003 to 0.012). This is compatible with omega 3 fats having an early protective effect that later becomes harmful; however, the association was lost when we removed the large trial by Burr et al.14 Meta-regression did not suggest a relation between mortality and the dose of long chain omega 3. Cohort studies suggested that omega 3 protected against death (0.65, 0.48 to 0.88; I2 = 36%), but it was unclear whether adjustment for confounders was adequate.
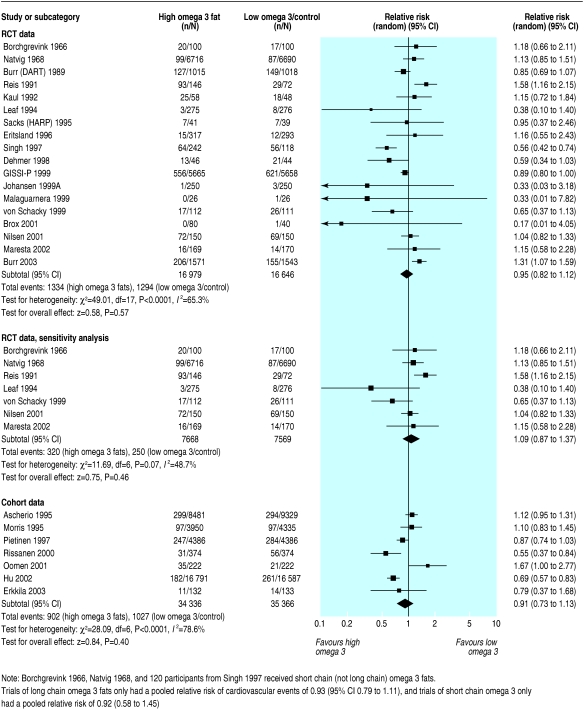
Combined cardiovascular events
Eighteen RCTs provided data on cardiovascular events in 2628 participants. The meta-analysis showed no definite effect of omega 3 fats on cardiovascular events, but confidence intervals were wide (0.95, 0.82 to 1.12) and inconsistency was high (I2 = 65%) (fig 3). Removing studies at moderate or high risk of bias reduced but did not remove inconsistency (1.09, 0.87 to 1.37; 570 events; I2 = 49%).
Fig 3.
Effect of omega 3 fatty acids on cardiovascular events. For references see bmj.com
Subgrouping by long chain versus short chain omega 3 or by advice to eat oily fish versus supplements did not generate robust effects of omega 3 fats on cardiovascular events. Cohort studies provided no strong evidence that omega 3 fats protect against cardiovascular events.
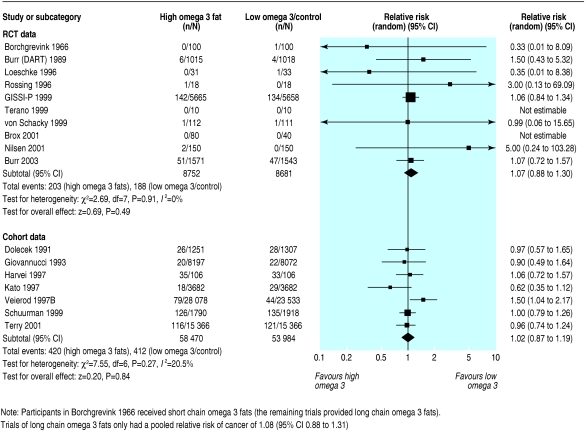
Cancer
Ten RCTs reported the incidence of cancer; 391 diagnoses of cancer or death from cancer occurred in 17 433 participants (two of the trials reported no cancers). We found no evidence that omega 3 fats had an effect on the incidence of cancer (1.07, 0.88 to 1.30) and there was no inconsistency (I2 = 0%) (fig 4). Five trials and seven events remained on sensitivity analysis.
Fig 4.
Effect of omega 3 fatty acids on the diagnosis of cancer or death from cancer. For references see bmj.com
Seven cohort studies provided data on cancer (832 events in the highest and lowest quantiles), and meta-analysis found no effect of high versus low intake of omega 3 (1.02, 0.87 to 1.19; I2 = 21%).
Outcomes related to bleeding
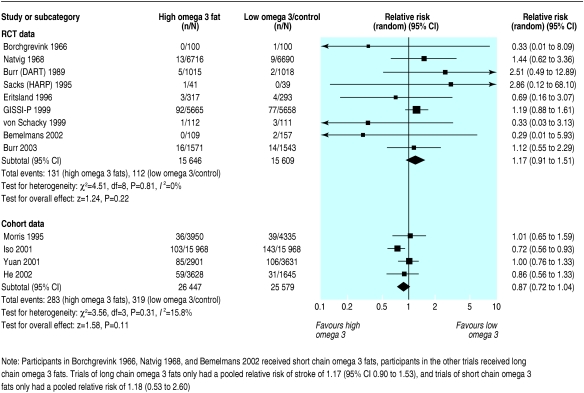
Nine RCTs reported at least one stroke (243 strokes in total), but little information was available specifically on haemorrhagic stroke. Omega 3 had no clear effect on the total numbers of strokes (1.17, 0.91 to 1.51; I2 = 0%), in sensitivity analysis (29 events), or in four cohort studies (0.87, 0.72 to 1.04) (fig 5).
Fig 5.
Effect of omega 3 fatty acids on stroke. For references see bmj.com
Discussion
Our meta-analysis of RCTs assessing the effects of increased omega 3 fats on total mortality found substantial variations between studies. Studies with stronger methodology had more consistent results, and the pooled relative risk of these studies was 0.98 (0.70 to 1.36; 138 events). We found no evidence from RCTs or cohort studies that omega 3 fats have an effect on combined cardiovascular events. Neither RCTs nor cohort studies showed significantly increased risks of cancer or stroke with higher intake of omega 3, but there were too few events to rule out important effects.
Strengths and weaknesses
The largest studies reviewed had greater potential for bias than some of the smaller ones. We hoped that pooling studies at low risk of bias might provide enough power to inform us of effects on health, but this was not the case (only 138 deaths and 570 cardiovascular events). Similarly, analysis of the effects of omega 3 on rarer outcomes such as stroke had insufficient power to detect clinically important effects. Unlike previous metaanalyses, we reviewed systematically the effects of omega 3 fats on mortality, cardiovascular disease, cancer, and bleeding events and analysed all relevant RCTs and prospective cohort studies. We also accounted for differences in study quality and examined the effects of long chain and short chain omega 3 fats in a wide group of participants; this provides high quality evidence to guide policy and practice.
Other studies
Our findings differ from those of a recent systematic review by Bucher et al,13 which reviewed trials assessing the effects of long chain omega 3 fats over at least six months in patients with coronary heart disease and found significant protection from mortality (0.8, 0.7 to 0.9) and sudden death (0.7, 0.6 to 0.9). Bucher et al analysed 11 RCTs (nine included here, two excluded as multifactorial interventions) with 15 806 participants and 1335 deaths but did not include the recent study by Burr et al with 3114 participants included in our study.14
A systematic review from the United States collated RCTs and cohort studies to assess the effects of marine omega 3 fats, but this study did not perform meta-analysis, reported the results of the largest studies only, and did not investigate reasons for conflicting results.19 A recent review from the United Kingdom of the benefits and risks of consumption of fish on cardiovascular disease mentioned only four large trials and did not pool the results.20
Our inclusion of studies with short chain omega 3 as the active intervention or with participants who had a lower risk of cardiovascular disease does not explain the differences between our results and those of Bucher et al. When we pooled studies in which participants were given only long chain omega 3 fats or were at high risk of cardiovascular disease the risk of death was not significantly reduced. However, when we removed the study by Burr et al from our meta-analysis, risk of death was similar to that reported by Bucher et al (0.83, 0.75 to 0.91). The study by Burr et al (included in our review as an unpublished study, since published) is the second largest study in terms of deaths reported (525 deaths; the GISSI-P study reports 1031 deaths).21
Why does the study by Burr et al contradict the other large studies by not suggesting a benefit of omega 3?14 The first possible explanation is that this RCT had the longest follow-up of all RCTs and the harmful effects of methylmercury could be cumulative. Time course analysis of the GISSI-P trial was consistent with the risk of death rising over the 42 months of the trial, but the increase in mortality was seen within the first year in the study by Burr et al.14,22 A second explanation is that the study by Burr et al was the only RCT that specifically enrolled men treated for angina. Post hoc subgroup analysis of the GISSI-P trial indicates that people with heart failure benefited most from omega 3 supplements (R Marchioli, personal communication, 2004). As heart failure is more common after myocardial infarction this may explain an attenuation of effect but would not explain the increase in risk suggested in the study by Burr et al. A third possibility is that omega 3 from oily fish has a different effect to fish oil supplements, but this was investigated by Burr et al and found not to explain the differences. It is therefore not clear why the results of Burr et al differ from the other large studies on fish based omega 3. It is possible that performance bias due to differential care in the intervention and control arms occurred in trials where the intervention was not masked, including GISSI-P and the Burr study,14,21,23 but dietary intake, other relevant risk factors, and pharmacotherapy appeared similar in both arms of these trials. It may be that the effect of omega 3 fats on cardiovascular disease is smaller than previously thought, or that its beneficial effect is limited to a specific group (such as patients after myocardial infarction or with heart failure) not represented in the study by Burr et al.14
Cohort studies or RCTs?
A recent meta-analysis assessed the effects of consumption of fish on stroke in cohort studies and found that people who ate white or oily fish at least once a week had a significantly reduced risk of stroke.24 We excluded cohort studies that assessed only total fish intake (as this is not clearly related to omega 3 intake). The web of lifestyle, interest in health, and social factors (health patterning) seen in the cohort studies included in our review provides an advantage to people taking most long chain omega 3 fats, and this makes adequate adjustment for confounding difficult, if not impossible. Thus, we must rely on high quality RCTs to provide non-confounded answers about the effects of omega 3 fats on health. Some effects of fish on health may be due to components other than omega 3—for example, selenium or vitamin D.
Interpretation
It is not clear whether long chain or short chain omega 3 fats (together or separately) reduce or increase total mortality, cardiovascular events, cancer, or strokes. Our findings do not rule out an important effect of omega 3 fats on total mortality, as robust trials at low risk of bias reported few deaths. There is no evidence that the source (dietary or supplemental) and dose of omega 3 fats affected the effectiveness of long chain omega 3 fats.
What is already known on this topic
A systematic review of randomised controlled trials in coronary heart disease showed reduced mortality in patients taking supplemental long chain omega 3 fats
What this study adds
This systematic review assessed the health effects of long chain and shorter chain omega 3 fats (together or separately) on total mortality, cardiovascular events, cancer, and strokes in a wide group of participants and found no evidence of a clear benefit of omega 3 fats on health
UK guidelines encourage the general public to eat more oily fish, and higher amounts are advised after myocardial infarction (supported by trials after myocardial infarction).20,25,26 This advice should continue at present but the evidence should be reviewed regularly. It is probably not appropriate to recommend a high intake of omega 3 fats for people who have angina but have not had a myocardial infarction.
Adjustment for lifestyle factors appeared to be inadequate in the cohort studies, so policy and lifestyle decisions should be based on data from RCTs. To understand the effects of omega 3 fats on health, we need more high quality RCTs (with adequate concealment of allocation and masking of participants and health providers) of long duration that also report the associated harms.
Supplementary Material
 A complete set of references is available on bmj.com
A complete set of references is available on bmj.com
Thanks to Theresa Moore and Margaret Burke from the Cochrane Heart Group, and to all of the authors of primary studies who helped us build up the data. This paper is based on a Cochrane review accepted for publication in The Cochrane Library (see www.TheCochraneLibrary.net for information).
Contributors: All authors commented critically on the manuscript and agreed the final version. NEC helped design the review, provided a clinical perspective, and commented on the analysis and interpretation. GDS helped design the review, provided a methodological perspective and general advice, and commented on the analysis and interpretation. PND provided a methodological perspective and expertise on omega 3 biochemistry, and commented on the analysis and interpretation. SBJE helped design the review, provided a methodological perspective and general advice, and commented on the analysis and interpretation. RAH screened retrieved papers against inclusion criteria, appraised quality of papers, abstracted data from papers, provided general advice, and commented on the analysis and interpretation. JPTH helped design the review, provided a statistical perspective and general advice, and commented on the analysis and interpretation. LH conceived the review, designed and coordinated the review, developed the search strategy and undertook searches, screened the search results, organised retrieval of papers, screened retrieved papers against inclusion criteria, appraised study quality, abstracted data from included papers, wrote to authors and experts for additional information, managed the review data, entered data into RevMan, analysed and interpreted the data, and was the primary author. HJM screened retrieved papers against inclusion criteria, appraised quality of papers, abstracted data from papers and commented on the analysis and interpretation, ARN commented on the protocol, provided additional relevant articles, screened retrieved papers against inclusion criteria, appraised quality of papers, abstracted data from papers, and was involved in discussing the findings, interpreting the data, and writing up. RAR helped design the review, screen retrieved papers against inclusion criteria, appraise quality of papers, and interpret the data, provided general advice, and commented on the analysis and interpretation. CDS helped design the review, screen retrieved papers against inclusion criteria, appraise quality of papers, and abstract data from papers, and commented on the analysis and interpretation. RLT helped design the review, screen retrieved papers against inclusion criteria, appraise quality of papers, abstract data frompapers, provide general advice, and comment on the analysis and interpretation. HVW helped in the analysis and interpretation of the data and provided a methodological and statistical perspective and general advice. LH is guarantor.
Funding: A northwest research and development research fellowship (UK Department of Health) and the British Dietetic Association.
Competing interests: NC has received fees for speaking by Solvay Healthcare, who market Omacor.
Ethical approval: Not required.
References
- 1.Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med Scand 1972;192: 85-94. [DOI] [PubMed] [Google Scholar]
- 2.Nettleton JA. Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. J Am Diet Assoc 1991;91: 331-7. [PubMed] [Google Scholar]
- 3.Bhatnagar D, Durrington PN. Omega-3 fatty acids: their role in the prevention and treatment of atherosclerosis related risk factors and complications. Int J Clin Pract 2003;57: 305-14. [PubMed] [Google Scholar]
- 4.Thies F, Garry JM, Yaqoob P, Rerkasm K, Williams J, Shearman CP, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 2003;361: 477-85. [DOI] [PubMed] [Google Scholar]
- 5.Geelen A, Brouwer IA, Zock PL, Katan MB. Antiarrhythmic effects of n-3 fatty acids: evidence from human studies. Curr Opin Lipidol 2004;15: 25-30. [DOI] [PubMed] [Google Scholar]
- 6.Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease—fishing for a natural treatment. BMJ 2004;328: 30-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food Standards Agency. Dioxins and PCBs in the UK diet: 1997. Total diet study samples. London: Food Standards Agency, 2000. www.foodrisk.org/dioxin_pcb_exposure.cfm (accessed 13 Mar 2006).
- 8.Ministry of Agriculture, Fisheries and Food. Concentrations of metals and other elements in marine fish and shellfish. London: MAFF, 1998. archive.food.gov.uk/maff/archive/food/infsheet/1998/no166/166vege.htm (accessed 13 Mar 2006).
- 9.Liem AKD, Theelen RMC. Dioxins: chemical analysis, exposure and risk assessment. Utrecht: Universiteit Utrecht, 1997.
- 10.Steenland K, Bertazzi P, Baccarelli A, Kogevinas M. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ Health Perspect 2004;112: 1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guallar E, Sanz-Gallardo I, van't Veer P, Bode P, Aro A, Gomez-Aracena J, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 2002;347: 1746-53. [DOI] [PubMed] [Google Scholar]
- 12.Risher JF, Murray HE, Prince GR. Organic mercury compounds: human exposure and its relevance to public health. Toxicol Ind Health 2002;18: 109-60. [DOI] [PubMed] [Google Scholar]
- 13.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 2002;112: 298-304. [DOI] [PubMed] [Google Scholar]
- 14.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr 2003;57: 193-200. [DOI] [PubMed] [Google Scholar]
- 15.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 2004;4: CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7: 177-88. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327: 557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Expression of concern. BMJ 2005;331: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Chung M, Balk E, Kupelnick B, DeVine D, Lawrence A, et al. Effects of omega-3 fatty acids on cardiovascular disease. Rockville, MD, USA: Agency for Healthcare Research and Quality, 2004. Evidence Report/Technology Assessment 94. www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1a.chapter.38290 (accessed 13 Mar 2006).
- 20.Scientific Advisory Committee on Nutrition. Advice on fish consumption: benefits and risks. London: Stationery Office, 2004. www.food.gov.uk/multimedia/pdfs/fishreport200401.pdf (accessed 13 Mar 2006).
- 21.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354: 447-55. [PubMed] [Google Scholar]
- 22.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio DDMR, Franzosi MG, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105: 1897-903. [DOI] [PubMed] [Google Scholar]
- 23.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2: 757-61. [DOI] [PubMed] [Google Scholar]
- 24.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke 2004;35: 1538-42. [DOI] [PubMed] [Google Scholar]
- 25.Wood D, Durrington P, Poulter N, McInnes G, Rees A, Wray R, et al. Joint British recommendations on prevention of coronary heart disease in clinical practice. Heart 1998;80: S1-29. [PMC free article] [PubMed] [Google Scholar]
- 26.Scottish Intercollegiate Guidelines Network. Cardiac rehabilitation: a national clinical guideline. Edinburgh: SIGN, 2002. www.sign.ac.uk/guidelines/fulltext/57/index.html (accessed 24 Feb 2006).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.