Abstract
Objective
This prospective, randomized, single-institution trial was designed to evaluate the end points of mortality, morbidity, and survival in patients undergoing standard versus radical (extended) pancreaticoduodenectomy (including distal gastrectomy and retroperitoneal lymphadenectomy).
Summary Background Data
Numerous retrospective reports and one prospective randomized trial have suggested that the performance of an extended lymphadenectomy in association with a pancreaticoduodenal resection may improve long-term survival for some patients with pancreatic and other periampullary adenocarcinomas. Many of these previously published studies can be criticized for their retrospective and nonrandomized designs, for the inclusion of nonconcurrent control groups, and for their small numbers.
Methods
Between April 1996 and December 1997, 114 patients with periampullary adenocarcinoma were enrolled in an ongoing, prospective, randomized trial at The Johns Hopkins Hospital. After intraoperative verification of completely resected periampullary adenocarcinoma, the patients were randomized to receive either a standard pancreaticoduodenectomy (removing only the peripancreatic lymph nodes en bloc with the specimen) or a radical pancreaticoduodenectomy (standard resection plus distal gastrectomy and retroperitoneal lymphadenectomy). All pathology specimens were reviewed and categorized. The postoperative morbidity, mortality, and short-term outcomes were examined.
Results
Of the 114 patients randomized, 56 underwent a standard pancreaticoduodenectomy and 58 a radical pancreaticoduodenectomy. The two groups were statistically similar with regard to age and gender, but there was a higher percentage of white patients in the radical group. All the patients in the radical group underwent distal gastric resection, whereas 86% of the patients in the standard group underwent pylorus preservation. The mean operative time in the radical group was 6.8 hours, compared with 6.2 hours in the standard group. There were no significant differences between the two groups with respect to the intraoperative blood loss, transfusion requirements, location of primary tumor, mean tumor size, positive lymph node status, or positive margin status. There were three deaths in the standard group and two in the radical group. The complication rates were 34% for the standard group and 40% for the radical group. Patients undergoing radical resection had a higher incidence of early delayed gastric emptying but had similar rates of other complications, such as pancreatic fistula, wound infection, intraabdominal abscess, and need for reoperation. The mean total number of lymph nodes resected was higher in the radical group. Of the 58 patients in the radical group, only 10% had metastatic carcinoma in the resected retroperitoneal lymph nodes, and none of those patients had the retroperitoneal nodes as the only site of lymph node involvement. The 1-year actuarial survival rate for patients surviving the immediate postoperative periods was 77% for the standard resection group and 83% for the radical resection group.
Conclusions
These data demonstrate that radical pancreaticoduodenectomy (with the addition of a distal gastrectomy and extended retroperitoneal lymphadenectomy to a standard pancreaticoduodenectomy) can be performed with similar morbidity and mortality to standard pancreaticoduodenectomy. However, the survival data are not sufficiently mature and the numbers of patients enrolled are not adequate to allow firm conclusions to be drawn regarding survival benefit.
Periampullary adenocarcinoma (carcinoma of the head of the pancreas, distal common bile duct, ampulla of Vater, and peri-Vaterian duodenum) is a common cause of cancer death in the United States, with >30,000 new cases identified annually. Surgical resection by means of pancreaticoduodenectomy provides the only chance for cure for patients with periampullary adenocarcinoma. Several recent reports have documented that various factors serve as important predictors of prognosis for patients with resected periampullary adenocarcinoma. 1–12 In most analyses, the prognostic factors include size of the primary tumor, degree of tumor differentiation, status of resected lymph nodes, and status of resection margins. Other prognostic factors include tumor DNA content, 13 use of adjuvant chemoradiation therapy, 14 and postresection CA19-9 levels. 15–17 The outcomes reported in most series of patients treated for periampullary adenocarcinoma have been obtained using standard pancreaticoduodenal resection without radical (extended) retroperitoneal lymph node resection.
Radical pancreatic resection was proposed by Fortner in 1973 as a means of improving the resectability rates and cure rates for patients with cancer of the pancreas. 18 Initially described as “regional resection of the pancreas,” this procedure was intended to remove the entire pancreas, the adjacent tissues, and the primary lymphatic drainage of the pancreas. It was to be performed as an en bloc total pancreaticoduodenectomy with subtotal distal gastrectomy and was to include resection of the transpancreatic portion of the portal vein (and occasional resection of the celiac axis, superior mesenteric artery, and middle colic artery with vascular reconstruction), with biliary-enteric drainage and restoration of gastrointestinal tract continuity. 19–21 Radical pancreaticoduodenectomy has evolved in the last two decades, and it is now most commonly defined as a wide en blocpancreaticoduodenal resection, gaining a wide soft-tissue resection margin, combined with a retroperitoneal lymphadenectomy.
Radical pancreaticoduodenectomy has been used most extensively in Japan 22–25 and has gained some advocates in Europe 26 and the United States. 27 Most data in support of radical pancreaticoduodenal resection are from retrospective nonrandomized studies, which often compare patients treated with radical resection to historical controls who underwent standard resection. The conclusions that can be drawn from these studies are clearly limited. Recently, a multicenter, prospective, randomized study was reported by Pedrazzoli et al from several institutions in Italy. 28 This study accrued 81 patients over a 3-year period, stratified patients based on tumor size, and allocated patients to standard versus radical lymphadenectomy. The two groups were similar with respect to demographic and histopathologic characteristics and had similar morbidity, mortality, and overall survival. A subgroup analysis using small patient numbers indicated that node-positive patients had a significantly (p < 0.05) better survival rate after radical rather than standard lymphadenectomy.
The current prospective, randomized, single-institution study has been designed to evaluate the impact of standard versus radical pancreaticoduodenal resection on short-term outcomes (mortality and postoperative complications) and long-term outcomes (survival and quality-of-life issues). In this article we present an interim analysis of this ongoing trial.
PATIENTS AND METHODS
This study was approved by the Joint Committee on Clinical Investigation of The Johns Hopkins University School of Medicine. Patients were recruited into the study before surgery on the basis of the anticipation of pancreaticoduodenal resection for adenocarcinoma of the periampullary region, and appropriate informed consent was obtained. Between April 1996 and December 1997, 276 patients underwent pancreaticoduodenectomy at The Johns Hopkins Hospital, with 190 of these patients undergoing resection for periampullary adenocarcinoma. Of these, 114 patients were enrolled into this study. Specific exclusion criteria included preoperative chemoradiation therapy, pathology revealing tumor other than adenocarcinoma primary to the periampullary region, the presence of gross tumor left behind at the conclusion of the standard pancreaticoduodenal resection, and absence of informed consent.
Surgical Technique
Patients were randomized (using a computer-generated random number pattern) during surgery after standard pancreaticoduodenal resection to one of two surgical procedures: standard pancreaticoduodenal resection (i.e., resection complete: nodal groups resected include the anterior pancreaticoduodenal lymph nodes, posterior pancreaticoduodenal lymph nodes, nodes in the lower hepatoduodenal ligament, and nodes along the right lateral aspect of the superior mesenteric vessels) or radical pancreaticoduodenal resection (Figs. 1 and 2). For the purposes of this study, radical pancreaticoduodenal resection included a 30% to 40% distal gastrectomy, including lymphatic tissue within the lesser omentum and greater omentum along the course of the right gastric artery and right gastroepiploic artery, respectively, and a retroperitoneal lymph node dissection extending from the hilum of the right kidney to the left lateral border of the aorta in the horizontal axis, and from the portal vein to below the third portion of the duodenum in the vertical axis (the origin of the inferior mesenteric artery is a near-constant anatomic landmark for the most inferior aspect of the dissection). The procedure includes removal of regional lymph nodes and connective tissue by extensive dissection of the portal vein, superior mesenteric vein, superior mesenteric artery, and retroperitoneum anterior to the hilum of the right kidney, the vena cava, and the aorta. Resection of the portal, superior mesenteric, aortic, and vena caval lymph nodes is performed, and celiac nodes are sampled.
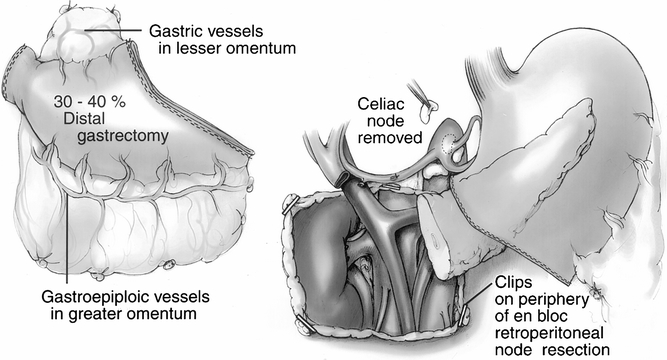
Figure 1. Components of the radical procedure. (Left) The 30% to 40% distal gastrectomy specimen, which includes the pylorus and a 1- to 2-cm cuff of the duodenum. (Right) The retained stomach, the pancreatic body and tail, and an overview of the retroperitoneal dissection. Titanium clips have been placed to mark the extent of the retroperitoneal dissection. A celiac node is removed for histologic analysis.
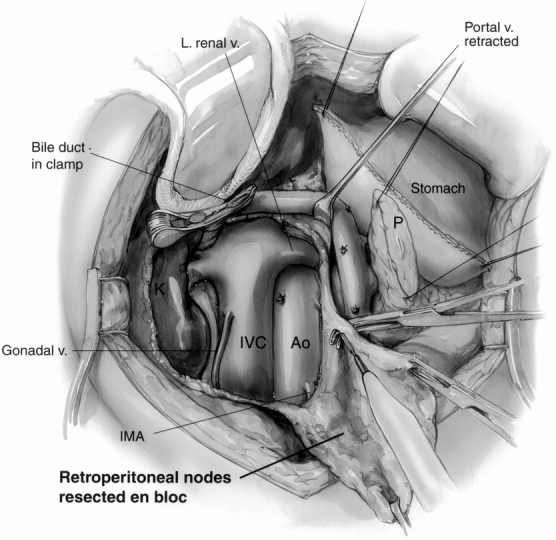
Figure 2. The retroperitoneal dissection component of the radical procedure. The retroperitoneum is dissected from the hilum of the right kidney (K) to the left lateral border of the aorta (Ao) in the horizontal axis, exposing the left renal vein. In the vertical axis, the dissection extends from the level of the portal vein to below the level of the third portion of the duodenum (level of the inferior mesenteric artery [IMA] origin). Here, the gastric staple line and pancreatic remnant (P) are being retracted toward the upper right. The inferior vena cava (IVC) and aorta are fully exposed, and the right gonadal vein has been preserved. A curved vascular clamp gently occludes the inferior aspect of the bile duct. The retroperitoneal fat and lymph nodes are being resected en bloc (bottom right).
All pancreaticoduodenal resections were performed favoring partial pancreatectomy and pancreaticojejunostomy. 29 For the patients entered into the standard group, we favored a pylorus-preserving resection. Vagotomy, tube gastrostomy, and feeding jejunostomy were not used.
Postoperative Management
All patients received histamine H2-receptor antagonists during their postoperative hospitalization as prophylaxis for stress and marginal ulceration. As part of an ongoing clinical trial evaluating pancreatic fistula and other complications, approximately 25% of the patients in this series received postoperative octreotide (250 μg subcutaneously every 8 hours) for 7 days. Further, most patients received erythromycin lactobionate (200 mg intravenously every 6 hours from postoperative day 2 to 10) as prophylaxis against delayed gastric emptying. 30 Operatively placed drains left in the vicinity of the pancreatic and bile duct anastomoses were removed at the discretion of the attending surgeon, usually between postoperative days 5 and 8, depending on drain output. All patients were evaluated after surgery by medical oncology and radiation oncology personnel and given recommendations regarding treatment with adjuvant chemoradiation therapy 14,31 and immunotherapy. 32,33 Approximately 75% of the patients received postoperative chemoradiation therapy.
Data Collection
Data were collected prospectively on all patients and included historical information, details of the operative procedure, a surgeon questionnaire (detailing such factors as type of resection performed, presence or absence of gross tumor after resection, and pancreatic texture), and clinical information regarding postoperative course (both in-hospital and after discharge). Follow-up was complete through July 1998 and was obtained from office records, letter or telephone contact with patients, or the U.S. Social Security Administration.
Pathologic Review
All pathology specimens were reviewed to determine the primary pathologic diagnosis and the extent of disease, as previously described. 1 Two criteria were used to define the origin of the carcinoma. First, the location of the bulk of the tumor was determined grossly and microscopically. Second, all four periampullary components were carefully evaluated for an in situ component. The retroperitoneal lymph node dissection specimens were submitted in their entirety for histologic examination. The perigastric and peripancreatic lymph nodes were examined as previously described. 1
Study End Points
Multiple end points are being evaluated in this ongoing study. For the purposes of this report, the primary end points include assessment of intraoperative complications, postoperative complications, and length of postoperative hospital stay. The secondary end point of survival can be reported only on a preliminary basis because of the number of patients and short follow-up.
Statistical Analyses
The number of patients predicted to be necessary for statistical validity (one-sided) was based on the premise of improving the overall 5-year survival rate from 20% to 35%, with set at 0.05 and set at 0.2, yielding a power of 80%. We calculated that 121 patients would be required in each arm of the study, for a total projected study population of 242 patients.
Comparability of the standard and radical groups was verified with Student’s t test and chi square statistics. Differences in survival between subsets were compared using the log-rank test. Results are reported as mean ± SEM. Significance was accepted at the 5% level.
RESULTS
Patient Population
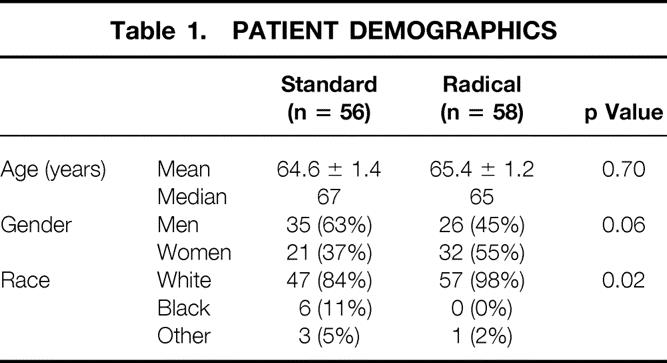
The study population consisted of 114 patients, with 56 patients in the standard group and 58 in the radical group (Table 1). The mean patient age was 65 years and was similar between the two groups. In the standard group, 63% of the patients were men, compared with 45% in the radical group (p = 0.06). The racial distribution was dissimilar despite the randomization: 98% of the patients in the radical group were white, 84% in the standard group (p = 0.02).
Table 1. Patient Demographics
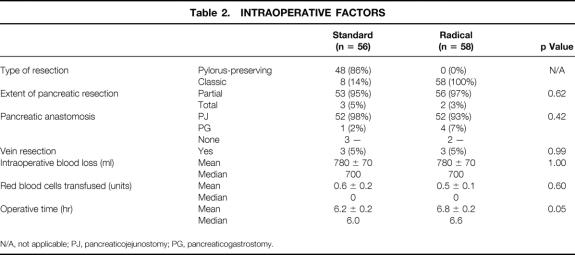
Intraoperative data are shown in Table 2. In the standard group, 86% of patients underwent pylorus preservation, whereas all patients in the radical group underwent distal gastrectomy. The two groups were comparable with respect to the extent of pancreatic resection (96% underwent partial pancreatectomy), type of pancreatic anastomosis (95% received a pancreaticojejunostomy), estimated intraoperative blood loss (median = 700 ml), and units of red blood cells transfused (median = 0 units). The mean operative time was 6.2 hours for the standard group, and significantly longer at 6.8 hours for the radical group (p = 0.05), reflecting the additional time for the distal gastrectomy and retroperitoneal lymphadenectomy.
Table 2. Intraoperative Factors
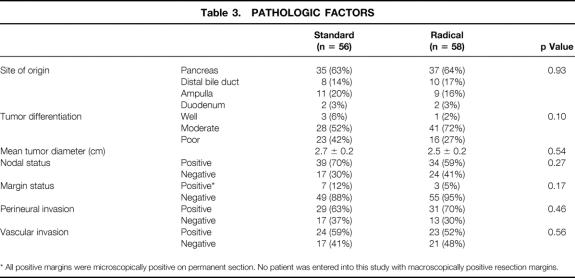
Table 3 depicts the final pathologic analyses of the resected specimens. The specimens were comparable between the two groups with respect to site of tumor origin (63% pancreatic, 16% distal common bile duct, 18% ampulla of Vater, and 3% duodenum), tumor differentiation, and mean tumor diameter (2.6 cm). Lymph node status and resection margin status were also similar between the two groups. The standard group had lymph node metastases in 70% of patients and microscopic carcinoma at a margin in 12% of patients; the radical group had lymph node metastases in 59% of patients and microscopic carcinoma at a margin in 5% of patients. No patient was entered into this study with macroscopically positive resection margins. Of the 10 patients with microscopically positive resection margins (9%), 7 had tumor involvement at the level of the uncinate process, 2 in the standard group had microscopic involvement close to the duodenal margin, and 1 had a positive microscopic margin at the pancreatic neck transection site (frozen section at the margin had been misinterpreted as negative). Perineural or vascular invasion was identified in the majority of patients, with no differences between the standard and radical groups.
Table 3. Pathologic Factors
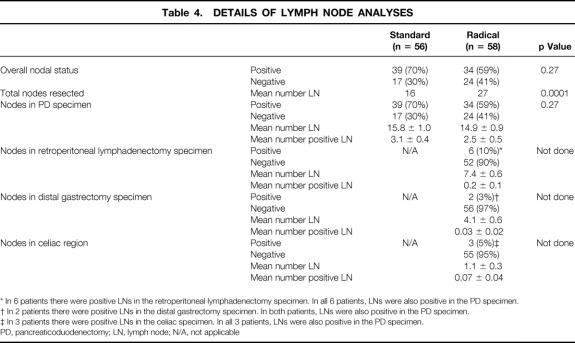
A detailed analysis of the resected lymph node status is depicted in Table 4. For the standard group, 70% of patients had histologically positive lymph node metastases in the resection specimen, and the mean total number of nodes resected was 16. The mean total number of lymph nodes identified in the standard group was 15.8 nodes; the mean number of positive lymph node metastases was 3.1 nodes. For the radical group, 59% of patients had histologically positive lymph node metastases in the resection specimen. The mean total number of lymph nodes resected was 27 in the radical group, significantly greater than in the standard group (p < 0.0001). The mean number of lymph nodes identified in the pancreaticoduodenectomy specimen was 14.9 nodes; the mean number of positive nodes was 2.5. The retroperitoneal lymphadenectomy specimen yielded a mean of 7.4 nodes, but only six patients (10%) had histologic evidence of lymph node metastases in these retroperitoneal nodes. In all six of these patients, nodes within the pancreaticoduodenectomy specimen also had microscopic evidence of metastatic tumor. The distal gastrectomy specimen yielded a mean of 4.1 nodes, but only two patients (3%) had histologic evidence of tumor in these perigastric nodes. In both of these patients, nodes within the pancreaticoduodenectomy specimen also revealed microscopic evidence of tumor. The celiac node sampling yielded a mean of 1.1 nodes, with three patients (5%) having histologic evidence of a metastasis in these celiac nodes. In all three of these patients, nodes within the pancreaticoduodenectomy specimen also contained tumor. Thus, in no patient was there histologic evidence of metastatic adenocarcinoma in lymph nodes in the retroperitoneal, perigastric, or celiac region without there also being nodal disease in the pancreaticoduodenectomy specimen. As a result, the radical procedure did not alter the UICC stage of any patient.
Table 4. Details of Lymph Node Analyses
Table 5 depicts the postoperative course and complications, comparing the standard and radical groups. The overall postoperative in-hospital and 30-day mortality rate was 4.4%, with three deaths in the standard group (5.4%) and two in the radical group (3.4%). Sepsis and multisystem organ failure contributed to all five deaths. Eight patients underwent reoperation during their index admission: three for wound problems, one for postoperative bleeding, two for anastomotic disruptions and sepsis, one for repair of hepatic artery thrombosis, and one for late gastrointestinal bleeding.
Table 5. Postoperative Course
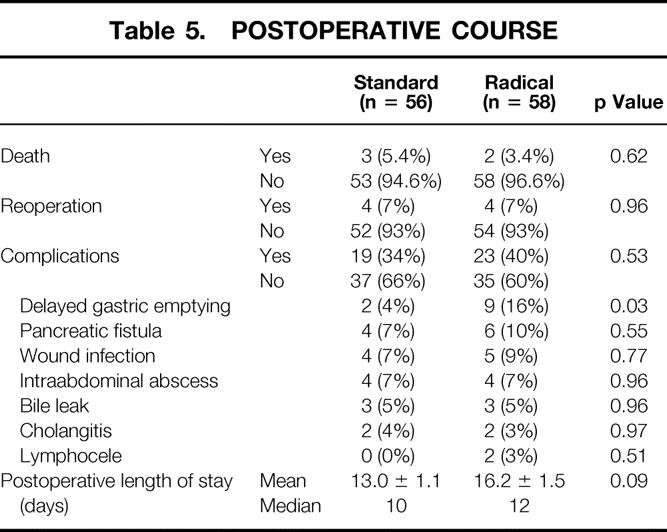
Sixty-three percent of patients had an uncomplicated postoperative hospitalization. The overall complication rate was 37% (34% for the standard group and 40% for the radical group). The most common postoperative complication was early delayed gastric emptying, which was observed in 11 patients (10%). This was the only complication with a statistically significant difference in occurrence between the two groups (4% in the standard group and 16% in the radical group, p = 0.03). Other complications, such as pancreatic fistula (9%), wound infection (8%), intraabdominal abscess (7%), bile leak (5%), and cholangitis (3.5%), occurred with similar frequency between the two groups. Two patients (3%) in the radical group developed postoperative lymph collections. Both patients presented several weeks after discharge with symptoms of right upper quadrant pressure and pain. In both patients, these sterile collections were ablated with the use of percutaneous catheter drainage.
The mean postoperative hospital length of stay was 13.0 days in the standard group (median = 10 days) and 16.2 days in the radical group (median = 12 days), a difference that approached significance (p = 0.09).
Actuarial survival curves for the patients surviving the immediate postoperative period (n = 109) are depicted in Figures 3 through 6. Figure 3 shows the survival curves for all patients, comparing the standard group (n = 53) with the radical group (n = 56). The 1- and 2-year survival rates were 77% and 47% for the standard group and 83% and 56% for the radical group (p = 0.6; NS). Patients with a diagnosis of pancreatic adenocarcinoma represented 63% of all patients in the study. Figure 4 depicts the survival curve for patients with pancreatic cancer, comparing the standard group (n = 34) with the radical group (n = 36). The 1- and 2-year survival rates were 71% and 39% for the standard group and 80% and 48% for the radical group (p = 0.5; NS). Figures 5 and 6 provide subgroup analyses of patients with node-positive and node-negative pancreatic adenocarcinoma, respectively. For the node-positive patients, the 1-year survival rate was 72% for the standard group (n = 25) and 72% for the radical group (n = 24; p = 0.9; NS). For the node-negative patients, the 1- and 2-year survival rates were 71% and 71% in the standard group (n = 9) and 92% and 71% in the radical group (n = 12; p = 0.9; NS).
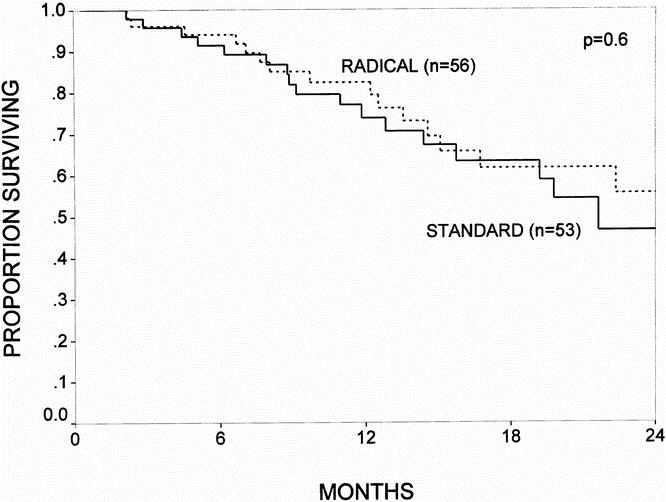
Figure 3. The actuarial survival curves for all patients who survived the immediate postoperative period, comparing the standard resection (n = 53) and the radical resection (n = 56) groups. The 1- and 2-year survival rates were 77% and 47% for the standard group and 83% and 56% for the radical group (p = 0.6; NS).
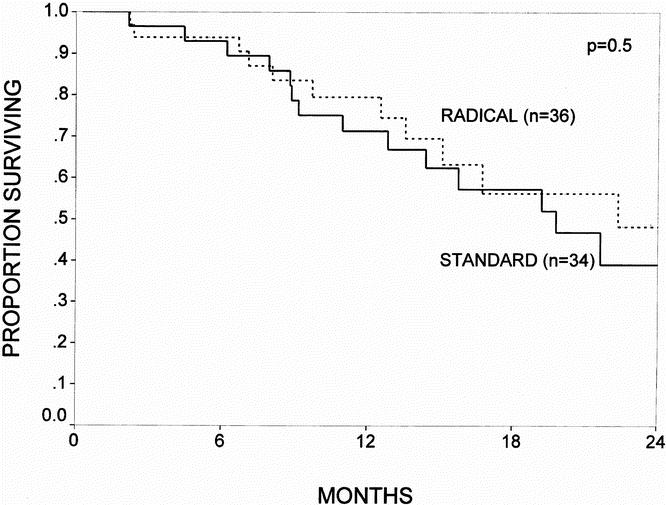
Figure 4. The actuarial survival curves for all patients with pancreatic adenocarcinoma who survived the immediate postoperative period, comparing the standard resection (n = 34) and the radical resection (n = 36) groups. The 1- and 2-year survival rates were 71% and 39% for the standard group and 80% and 48% for the radical group (p = 0.5; NS).
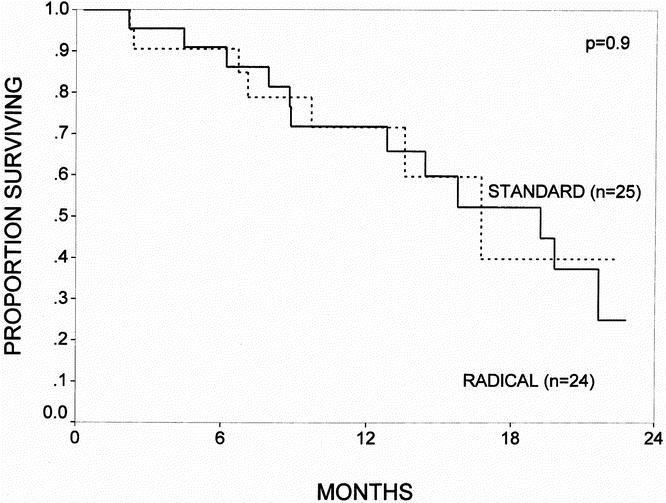
Figure 5. The actuarial survival curves for all patients with node-positive pancreatic adenocarcinoma (UICC stage 3) who survived the immediate postoperative period, comparing the standard resection (n = 25) and the radical resection (n = 24) groups. The 1-year survival rate was 72% for the standard group and 72% for the radical group (p = 0.9; NS).
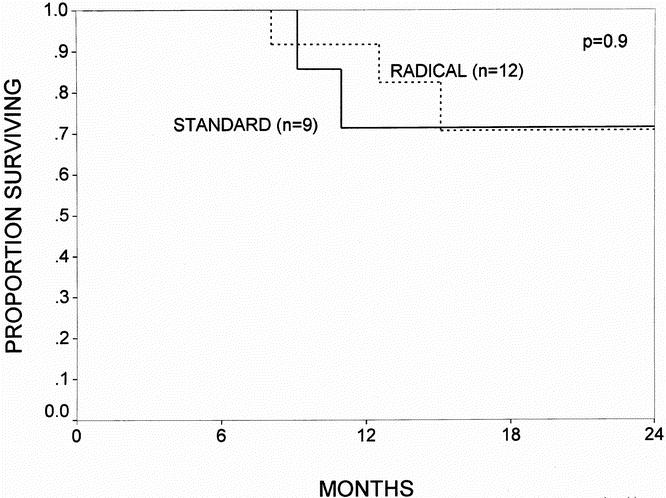
Figure 6. The actuarial survival curves for all patients with node-negative pancreatic adenocarcinoma (UICC stage 1 and 2) who survived the immediate postoperative period, comparing the standard resection (n = 9) and the radical resection (n = 12) groups. The 1- and 2-year survival rates were 71% and 71% for the standard group and 92% and 71% for the radical group (p = 0.9; NS).
For the nonpancreatic tumors, the numbers of patients in each subgroup were small, and comparisons of survival data should be cautiously interpreted. For the patients with distal common bile duct cancer, the 1-year survival rate was 67% in the standard group (n = 7) and 71% in the radical group (n = 8); for ampullary cancer, the 1-year survival rate was 78% in the standard group (n = 10) and 83% in the radical group (n = 9). For duodenal cancer, the 1-year survival rate was 100% in both the standard and radical groups (n = 2 for each group).
DISCUSSION
This is the largest series reported to date involving a prospective randomized comparison between standard and radical pancreaticoduodenectomy. We acknowledge that our enrollment is not yet complete and that the randomization has not yet made the two groups statistically similar. For example, there is an excess of men in the standard group, an excess of white patients in the radical group, and an excess of poorly differentiated tumors in the standard group. It can be anticipated that the accrual of additional patients to the trial will cause most of these current discrepancies to be less impressive. Nonetheless, with a few exceptions, the current data support the premise that the addition of a distal gastrectomy and retroperitoneal lymph node dissection to a standard pancreaticoduodenal resection is relatively safe and does not significantly affect overall morbidity and mortality. Of note, patients treated with radical resection had significantly longer operative times and an increased incidence of early delayed gastric emptying, with a commensurate trend toward an increased median postoperative length of stay (10 days standard vs. 12 days radical).
It is important to elaborate on the actual procedure that we have chosen to perform and that we term a radical pancreaticoduodenectomy. This is not the same procedure described by Fortner in 1973 18 that was termed a “regional resection” of the pancreas. 18–21 The radical procedure described in this study differs in several points: a total pancreatectomy is not performed, requisite portal vein resection is not performed, a subtotal gastrectomy is not performed, splenectomy is not performed, the transverse mesocolon and middle colic vessels are not resected, and the branches of the celiac axis are not skeletonized. We believed that such an extensive “regional resection” was neither logical from an oncologic standpoint nor justifiable for patients undergoing margin-negative standard pancreaticoduodenal resection. We therefore reduced the scale of the additional resection (i.e., reduced the radicality), bringing our radical resection more in line with current cancer-directed pancreatic resection techniques.
Many of the past reports extolling the virtues of radical pancreaticoduodenectomy have shortcomings, with the main criticisms being that the reports present nonconcurrent retrospective data, without comparable numbers of patients at identical pathologic stages being treated with each of the two operations. For example, Ishikawa et al retrospectively reviewed 59 pancreatic resections for cancer of the head of the pancreas, comparing 37 consecutive patients from 1971 to 1981 treated using the standard technique with 22 subsequent patients from 1981 to 1983 treated using the radical approach. 23 The two groups, although nonrandomized and nonconcurrent, did not differ with regard to tumor staging. The operative mortality rate was 14% in the standard group and 5% in the radical group (p = NS). The 3-year survival rate was 13% in the standard group and 38% in the radical group, a difference that achieved statistical significance (p < 0.05).
Manabe et al retrospectively studied patients treated either with standard or radical pancreaticoduodenectomy for cancer of the head of the pancreas. 22 Their data showed a significant improvement in survival for 18 stage 3 or 4 patients treated with radical resection (5-year survival = 21%), compared with 35 similar-stage patients treated with standard resection (5-year survival = 0%; p < 0.05). However, the two groups were not comparable: 50% of the patients in the radical group were node-negative, and none had metastases to N2 or N3 nodes, whereas only 35% of the patients in the standard group were node-negative, and 37% had metastases to N2 or N3 nodes.
Several reports document relatively high operative mortality and morbidity rates after radical resection for pancreatic cancer. For example, Sindelar reported on his experience with 20 patients, noting a 20% treatment-related mortality rate, a 55% incidence of complications, and only a 10% 3-year survival rate. 34 Nagakawa et al reported on 53 patients treated with radical resection in Japan, noting a 15% postoperative mortality rate, a 33% complication rate, and a 30% 3-year survival rate. 35
There have been previous retrospective analyses that have shown similar outcomes when comparing standard and radical resection. For example, Satake et al reported on 185 patients undergoing resection for tumors <2 cm at 59 institutions in Japan. 24 No differences in survival were noted between the two types of pancreaticoduodenal resection, with a 5-year survival rate of 27% for each. Similarly, a retrospective comparison between standard and radical pancreaticoduodenectomy was reported from Memorial Sloan-Kettering Cancer Center comparing 79 standard resections with 35 radical resections; there was no difference in median survival between the two groups. 2
There are two prospective studies that seem to support the premise that radical pancreaticoduodenectomy may have a benefit in terms of overall outcome and survival. A small study by Henne-Bruns et al from Hamburg reported a nonrandomized comparison between 17 patients undergoing radical resection and 17 patients undergoing standard resection. 26 In this study, the perioperative mortality rates were unacceptably high by today’s standards (18% radical and 12% standard), but the cumulative 3-year survival rates for patients with a diagnosis of pancreatic cancer who survived the operative procedure were 50% for the radical resection group and 0% for the standard resection group. Although this study is clearly underpowered, it can be interpreted as suggesting that extended lymph node clearance may benefit patients with early-stage pancreatic adenocarcinoma.
An Italian multicenter, prospective, randomized study reported by Pedrazzoli et al in 1998 accrued 81 patients with adenocarcinoma of the head of the pancreas over a 3-year period. 28 Patients were stratified based on tumor size and allocated to standard or radical lymphadenectomy. Standard lymphadenectomy included removal of the anterior and posterior pancreaticoduodenal, pyloric, bile duct, and superior and inferior pancreatic head and body lymph node stations. The extended lymphadenectomy added removal of the lymph nodes from the hepatic hilum, along the aorta from the diaphragmatic hiatus to the inferior mesenteric artery, laterally to both renal hila, with circumferential clearance of the origin of the celiac trunk and superior mesenteric artery. Postoperative adjuvant therapy was not administered to any patient. The two groups were similar with respect to demographic and histopathologic characteristics, with 60% of patients having node-positive resections. The postoperative length of stay was similar for both groups (approximately 20 to 21 days), and there were no significant differences in the postoperative mortality (5%) and morbidity (approximately 40% to 50%) rates in each group. The overall 1-, 2-, 3-, and 4-year survival rates were 51%, 22%, 9%, and 7%, respectively, with no significant difference between the standard and the radical groups. When survival was analyzed based on the presence or absence of metastatic lymph nodes using an a posteriori analysis not planned at study design, it was found that node-positive patients had a significantly better survival rate after undergoing an extended lymphadenectomy (p < 0.05). This important study has indicated that radical resection does not affect morbidity or mortality rates and does not influence overall survival, but may improve outcome in node-positive patients (who make up the majority of patients). However, several criticisms can be raised about this study, including the relatively small number of patients enrolled (the study is closed and no longer accruing patients), the concern that approximately 25% of patients underwent resection with positive resection margins, the low 2-year survival rate of only 22%, and the fact that the number of lymph nodes harvested was only minimally different between the two groups (total nodes retrieved was 13.3 in the standard group and 19.8 in the radical group). Further, recent molecular analyses of resected lymph nodes for K-ras oncogene mutations using polymerase chain reaction and analysis for restriction fragment length polymorphisms have indicated that approximately two thirds of patients with histologically negative lymph nodes harbor molecular micrometastases in at least one regional lymph node. 36 Thus, standard histologic staging understates the percentage of node-positive patients.
This interim analysis of our ongoing trial has failed to confirm an apparent survival benefit for patients with node-positive pancreatic cancer treated with the radical procedure, as reported in the Italian multicenter trial. Although we acknowledge that we may observe changes in survival with further patient accrual and maturation of the survival curves, our current analyses reveal nearly identical short-term outcomes for all subgroups of patients with periampullary adenocarcinoma. It appears clear that radical pancreaticoduodenectomy, as defined in this study, can be performed without increased mortality or serious increased morbidity. We anticipate that the completion of this trial will allow further careful analysis of postoperative complications and long-term survival. It is likely that improvements in outcome for patients with periampullary cancer also will come from improvements in early detection, 37 safer surgery, 38 and advances in various adjuvant therapy regimens, such as chemotherapy, radiation therapy, and immunotherapy. 14,31–33,39
Acknowledgments
The authors thank Corinne Sandone for the superb illustrations and the surgical house staff and nurses at the Johns Hopkins Hospital, without whose dedication and expertise this study would not be possible.
Discussion
Dr. Murray F. Brennan (New York, New York): The authors are the most experienced group and institution in the surgical management of pancreatic malignancy in this country, probably in the world. For me it’s enormously gratifying to see them ask a surgically demanding question in a prospective randomized trial. Surgeons have long been criticized for their reluctance to critically evaluate surgical technique.
The concept of an extended node dissection or extended radical pancreaticoduodenectomy was espoused most strongly by Joseph Fortner of our institution in the late seventies and early eighties. In the 1990s in a retrospective view of a prospective database, we’re able to show in a nonrandom fashion that such extended operations did not improve long-term survival.
Other authors, as Dr. Yeo pointed out, have suggested there is a benefit to more extended operations. But it’s important to emphasize that the present radical pancreatectomy is clearly much less than that proposed initially by Fortner and subsequently by the Japanese.
I would certainly agree with the present authors that extended resections involving major vascular resection, skeletonization of vessels, is unlikely, as they are not involved in nodal drainage to justify. In most situations, however, more extended dissections have not changed survival, contributing only in the main to better staging.
Conversely, such trials can only be done in experienced centers. As in other situations, such as gastric cancer, more extended lymph node dissection done in multicenter fashion has been accompanied by markedly increased mortality and morbidity.
The authors show very clearly that such extended resections can be done in experienced hands with no greater morbidity and in fact even less, although not significant, mortality. They ought to be congratulated. Lightheartedly, however, there is another benefit from this prospective randomized trial: the authors now have similar complications and similar mortality to us other mortals.
I have only two questions. Can the authors reinforce just how different were the two procedures? The total nodes resected were increased almost 100% compared to the standard resection. Were the pathologists who examined the nodes aware of which group was which? Was there a standard approach to the evaluation of nodes? Was there a size limit to the nodes looked at?
Rather surprisingly in the radical group, only 10% had metastatic carcinoma in previously unresected nodes. If that’s true, then we can only predict it would be very unlikely to ever show a benefit. My second question then: if the potential benefit can be to only 10% of the patients, what numbers would they require in a trial to make a significant difference?
And, finally, of particular interest is that the extended node dissection accompanied by distal gastrectomy resulted in greater, rather than lesser, delayed gastric emptying when compared to a pylorus-preserving procedure. Would you comment?
The management of pancreatic cancer continues to be a surgical disease, but the results in all hands have shown very little improvement in 20 years beyond the improvements in technical expertise and decreased perioperative mortality. The authors are to be congratulated for asking a surgical question within the confines of extended resection.
I would argue, given the long-term mortality from this disease, that any patient undergoing a resection for parapancreatic malignancy should be entered into a trial that addresses either a technical question as in here or an adjuvant trial designed to evaluate potential improvements in survival.
Dr. Russell G. Postier (Oklahoma City, Oklahoma): This is a prospective randomized trial comparing standard pancreaticoduodenectomy, usually pyloric preserving, with a more extensive procedure which includes a distal one third gastrectomy and an extended lymphadenectomy. The more extensive procedure was outlined by Dr. Yeo, and I won’t detail that.
This more extensive procedure does vary considerably from that reported by Fortner and is also somewhat less extensive than that reported by groups in Japan and Europe. However, it is a well-designed and reasonable extension of the standard resection. This is obviously a well-designed and well-performed study by, as Dr. Brennan says, the preeminent pancreatic surgical group in probably the world.
The distribution of patients in both groups was comparable except for a preponderance of white patients in the more extensive resection group, which is detailed in the manuscript. As expected, more nodes were removed in the more radical procedure, 27 versus 16.
The operating time was significantly longer—but not excessively so—in the more extensive operation. The length of stay was somewhat longer, but again not significantly so. There were no differences, as he pointed out, in mortality and in most of the major morbidities except for what was referred to as early delayed gastric emptying.
Again they show that this extensive procedure can be performed with no increase in mortality and with little difference in early morbidity, which is similar to that of a standard resection. The mean follow-up, I think, is too short for us to make significant comments about long-term survival. I have four questions.
This study as outlined in the manuscript appears to overlap with the study of the use of octreotide to prevent leakage at a pancreatic-enteric anastomosis. If this is true, were those patients randomized to ensure equal numbers of patients in the placebo or treatment group so that any differences that octreotide may make would be shown in your morbidity statistics?
Similarly, some of the patients, but not all, received erythromycin as a motility agonist. Were those patients similarly distributed between the two groups?
Thirdly, at least some of the patients received adjuvant chemoradiation. And were those patients equally distributed?
Finally, a number of studies have corroborated my anecdotal experience that patients who undergo a pyloric-preserving pancreaticoduodenectomy do well long term and have very little operative-related morbidity. Have you done or do you plan to do quality-of-life studies comparing those who undergo pyloric-preserving pancreaticoduodenectomy with your more extended procedures? Quality-of-life issues may become the tie-breaker unless your overall survival data on longer-term analysis becomes more statistically significant.
Dr. Charles R. Sachatello (Lexington, Kentucky): I have just several questions.
What role does carbohydrate antigen 19-9 play in your assessment of these patients?
Number two: Have you observed a small subgroup of patients who have unusually high levels who seem to do much better than you might think otherwise?
And number three: Have you abandoned the role of pancreaticogastroscopy for reanastomosis and, if so, why?
Dr. Ward O. Griffen, Jr. (Frankfort, Michigan): Dr. Yeo, I have one question. I noticed in one of your first slides you said you had 190 patients who had periampullary carcinoma, but you only analyzed 114. Is that because the others hadn’t been entered yet, or is there some other magic reason?
I hope you will answer all of the questions that were posed to you accurately and briefly.
Dr. Charles J. Yeo (Closing Discussion): Yes, sir. I thank all the discussants for their comments, and I really do appreciate to opportunity to present these data. I remind you it was an interim report; it’s an ongoing study. We’ve now accrued 173 patients in the trial, and our goal is to accrue at least 300.
The first question dealt with the analysis of the pathology specimen. Obviously, the pathologists do know when patients just have the standard resection versus the radical because they get additional material.
The retroperitoneal specimen is completely analyzed, sectioned entirely and analyzed, and the nodes are counted very carefully. Our pathologists—and it’s referenced in the manuscript—have reported exactly how they do this in a little manuscript that’s been published.
It is important to note that only 10% of patients in the radical group had nodes in the retroperitoneal or distal gastrectomy specimen, so they do have nodes outside of the N1 zone, if you will.
Therefore, you would suspect that perhaps those would be the only patients to have the benefit. We would caution you, though, that this is a histopathologic node analysis, and in reality a large proportion of patients who have node-negative pancreatic cancer by histopathologic analysis, in fact have K-RAS mutations or malignant DNA in nodes that appear histologically negative. So, in fact, other than just 65% of patients being node-positive, it’s probably more like 85% are node-positive and, therefore, that skews the data even further.
The delayed gastric emptying issue that both Dr. Brennan and Dr. Postier asked about, that issue is that clearly the patients in the radical group had a four-fold higher incidence of delayed gastric emptying. That may be related to the neural dissection in the retroperitoneum. It may, I think, more likely be related to the retention of the pyloric sphincter. The fact that some of what we call delayed gastric emptying is really bile reflux gastritis.
Dr. Postier aptly pointed out that in the manuscript about a quarter of these patients participated in a prospective ongoing randomized trial of octreotide to decrease the rate of pancreatic fistula. They are similarly distributed between both groups. Similarly, 100% of our patients receive erythromycin postoperatively in an effort to decrease the gastric stasis and decrease the incidence of delayed gastric emptying, and those are, of course, equally distributed.
About 75% of patients do receive chemoradiation therapy, and about 15% receive immunotherapy as well. Our therapies are typically 5-FU–based with radiation therapy, and we have some arms that receive mitomycin C leucovorin dipyramidal. Our immunotherapy is a killed allogeneic tumor vaccine that’s been transfected with the plasma that carries the GM-CSF gene in an effort to stimulate, if you will, improved autoimmunity.
The quality-of-life issues are very important. We will definitely present those in our final manuscript regarding this. We have submitted a manuscript to the American Surgical dealing with that. And I think from the preliminary analysis there does not seem to be any long-term differences in quality of life.
And for those of you that are asked the question, which is a common question, “What is my life going to be like after a Whipple operation?” if you take normal healthy people in the audience and rate them on a zero-to-100 scale, the quality of life comes out to be about 88 percentile. People after a Whipple are at the 83rd percentile. So I think 83rd percentile is not quite as good as 88 percentile, but it’s much better than zero percentile, which is what you are when you are dead.
The issues about CA 19-9, we don’t rely on CA 19-9 for anything critical. We, of course, rely on the history and physical taking, the jaundice that most of these patients present with. The better marker is probably TIMP-1, the tissue inhibitor metalloproteinase 1, which has been recently reported out of Burt Vogelstein’s and Ken Kensler’s group.
And one other question comes back to pancreaticogastrostomy. We use pancreaticogastrostomy occasionally for large bulky pancreatic remnants that do not invaginate well into the jejunum. But, remember, when you do a pancreaticogastrostomy, you open another suture line, that is, the posterior gastric suture line, so it actually is a little bit more work to do that. So we’ve basically stuck to the pancreaticojejunostomy.
Footnotes
Correspondence: Charles J. Yeo, MD, Department of Surgery, The Johns Hopkins Hospital, Blalock 606, 600 N. Wolfe St., Baltimore, MD 21287-4606.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Supported in part by grants from the National Institutes of Health (R01-CA56130 and P50-CA62924).
Accepted for publication December 1998.
References
- 1.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg 1998; 227: 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–73. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg 1995; 221: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delcore R, Rodriquez FJ, Forster J, et al. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. Am J Surg 1996; 172: 463–469. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Lillemoe KD, Cameron JL, et al. Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg 1998; 2: 79–87. [DOI] [PubMed] [Google Scholar]
- 6.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection of pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar and distal tumors. Ann Surg 1996; 224: 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washburn WK, Lewis WO, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg 1995; 130: 270–276. [DOI] [PubMed] [Google Scholar]
- 9.Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater: a 28-year experience. Ann Surg 1997; 225: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett CG, Warshaw AL, Convery K, Compton CC. Patterns of failure after pancreaticoduodenectomy for ampullary adenocarcinoma. Surg Gynecol Obstet 1993; 176: 33–38. [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL. Review topic: prognostic factors in ductal pancreatic cancer. Langenbeck’s Arch Surg 1998; 383: 129–133. [DOI] [PubMed] [Google Scholar]
- 12.Pickleman J, Koelsch M, Chejfec G. Node-positive duodenal carcinoma is curable. Arch Surg 1997; 132: 241–244. [DOI] [PubMed] [Google Scholar]
- 13.Allison DC, Piantadosi S, Hruban RH, et al. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol 1998; 67: 151–159. [DOI] [PubMed] [Google Scholar]
- 14.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997; 225: 621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluemke DA, Abrams RA, Yeo CJ, et al. Recurrent pancreatic adenocarcinoma: spiral CT evaluation following the Whipple procedure. RadioGraphics 1997; 17: 303–313. [DOI] [PubMed] [Google Scholar]
- 16.Tian F, Appert HE, Myles J, Howard JM. Prognostic value of serum CA19–9 levels in pancreatic adenocarcinoma. Ann Surg 1992; 215: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of recurrence and survival by postresection CA19–9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol 1997; 4: 551–556. [DOI] [PubMed] [Google Scholar]
- 18.Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery 1973; 73: 307–320. [PubMed] [Google Scholar]
- 19.Fortner JG. Recent advances in pancreatic cancer. Surg Clin North Am 1974; 54: 859–863. [DOI] [PubMed] [Google Scholar]
- 20.Fortner JG, Kim DK, Cubilla A, et al. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg 1977; 186: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortner JG, Klimstra DS, Senie RT, Maclean BJ. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg 1996; 223: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manabe T, Ohshio G, Baba N, et al. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer 1989; 64: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa O, Ohhigashi H, Sasaki Y, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg 1988; 208: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satake K, Niskiwaki H, Yokomatsu H, et al. Surgical curability and prognosis for standard versus extended resections for T1 carcinoma of the pancreas. Surg Gynecol Obstet 1992; 175: 259–265. [PubMed] [Google Scholar]
- 25.Kayahara M, Nagakawa T, Ueno K, et al. Surgical strategy for carcinoma of the pancreas head area based on clinicopathologic analysis of nodal involvement and plexus invasion. Surgery 1995; 117: 616–623. [DOI] [PubMed] [Google Scholar]
- 26.Henne-Bruns, Kremer B, Meyer-Pannwitt U, et al. Partial duodenopancreatectomy with radical lymphadenectomy in patients with pancreatic and periampullary carcinomas: initial results. Hepato-Gastroenterol 1993; 40: 145–149. [PubMed] [Google Scholar]
- 27.McFadden DW, Reber HA. Cancer of the pancreas: radical resection—supporting view. Adv Surg 1994; 27: 257–272. [PubMed] [Google Scholar]
- 28.Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreaticoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas. A multicenter, prospective, randomized study. Ann Surg 1998; 228: 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, outcomes. Ann Surg 1997; 226: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying following pancreaticoduodenectomy: a prospective, randomized placebo-controlled trial. Ann Surg 1993; 218: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carducci MA, Abrams RA, Yeo CJ, et al. Early evaluation of abdominal/hepatic irradiation and 5-FU/leucovorin infusion after pancreaticoduodenectomy. Int J Rad Onc Biol Phys 1996; 35: 143–150. [DOI] [PubMed] [Google Scholar]
- 32.Jaffee EM, Schutte M, Gossett J, et al. Development and characterization of cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am 1998; 4: 194–203. [PubMed] [Google Scholar]
- 33.Jaffee EM, Abrams R, Cameron J, et al. A phase I trial of lethally irradiated allogeneic pancreatic tumor cells transfected with the GM-CSF gene for the treatment of pancreatic adenocarcinoma. Human Gene Therapy 1998; 9: 1951–1971. [DOI] [PubMed] [Google Scholar]
- 34.Sindelar WF. Clinical experience with regional pancreatectomy for adenocarcinoma of the pancreas. Arch Surg 1989; 124: 127–132. [DOI] [PubMed] [Google Scholar]
- 35.Nagakawa T, Nagamori M, Futakami F, et al. Results of extensive surgery for pancreatic carcinoma. Cancer 1996; 77: 640–645. [PubMed] [Google Scholar]
- 36.Demeure MJ, Doffek KM, Komorowski RA, et al. Molecular metastases in stage I pancreatic cancer: improved survival with adjuvant chemoradiation. Surgery 1998; 124: 663–669. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Sokoll LJ, Bruzek DJ, et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev 1998; 7: 109–112. [PubMed] [Google Scholar]
- 38.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AR, Robinson EK, Lee JE, et al. Neoadjuvant chemoradiation for adenocarcinoma of the pancreas. Surg Oncol Clin North Am 1998; 7: 183–197. [PubMed] [Google Scholar]