Abstract
Objective
To determine which mammographically guided breast biopsy technique is the most efficient in making a diagnosis in women with suspicious mammograms.
Summary Background Data
Mammographically guided biopsy techniques include stereotactic 14-gauge core-needle biopsy (SC bx), stereotactic 11-gauge suction-assisted core biopsy (Mammotome [Mbx]), stereotactic coring excisional biopsy (Advanced Breast Biopsy Instrument [ABBI]), and wire-localized biopsy (WL bx). Controversy exists over which technique is best.
Methods
All patients undergoing any one of these biopsy methods over a 15-month period were reviewed, totaling 245 SC bx, 107 Mbx, 104 ABBI, and 520 WL bx. Information obtained included technical success, pathology, discordant pathology, and need for open biopsy.
Results
Technical success was achieved in 94.3% of SC bx, 96.4% of Mbx, 92.5% of ABBI, and 98.7% of WL bx. The sensitivity and specificity were 87.5% and 98.6% for SC bx, 87.5% and 100% for Mbx, and 100% and 100% for ABBI. Discordant results or need for a repeat biopsy occurred in 25.7% of SC bx, 23.2% of Mbx, and 7.5% of ABBI biopsies. In 63.6% of ABBI and 50.9% of WL bx, positive margins required reexcision; of the cases with positive margins, 71.4% of ABBI and 70.4% of WL bx had residual tumor in the definitive treatment specimen.
Conclusion
Although sensitivities and specificities of SC bx and Mbx are good, 20% to 25% of patients will require an open biopsy because a definitive diagnosis could not be reached. This does not occur with the ABBI excisional biopsy specimen. The positive margin rates and residual tumor rates are comparable between the ABBI and WL bx. The ABBI avoids operating room and reexcision costs; therefore, in appropriately selected patients, this appears to be the most efficient method of biopsy.
Screening mammography has led not only to early detection of breast cancers, but also to an increase in the number of abnormal mammograms requiring evaluation. With present technology, abnormal mammograms can be dealt with in one of two basic ways: observation or biopsy. An evaluation of management options using a decision analysis model demonstrated that for a typical 50-year-old woman, quality adjusted life expectancy is maximized by early wire-localized biopsy (WL bx) when the risk of malignancy exceeds 30%. 1 However, this threshold takes into account the clinical outcomes and costs of WL bx versus observation. With newer technologies, which purportedly decrease the untoward effects and costs of breast biopsy, this 30% threshold may be lowered, implying that more breast cancers would be diagnosed earlier.
At present, there are several options available to obtain tissue for pathologic evaluation of nonpalpable mammographic lesions. These include ultrasound-guided fine-needle aspiration (FNA) or core-needle biopsy, 2 stereotactic FNA 3 or stereotactic core-needle biopsy (SC bx), 4–6 stereotactic suction-assisted large core-needle biopsy (Mammotome [Mbx]), 7 stereotactic coring excisional biopsy (Advanced Breast Biopsy Instrument [ABBI]), 8,9 and open WL bx. 10 Ultrasound-guided biopsy is useful only if the lesion can be seen sonographically; therefore, microcalcifications cannot be sampled using this technique. Ultrasound or stereotactic FNA is operator- and cytopathologist-dependent, with insufficient aspirates reported in up to 50% of cases. 11 Therefore, FNA has largely been abandoned. Nevertheless, much debate exists over the most appropriate method of biopsy.
The purpose of this study was to clarify which breast biopsy technique most efficiently evaluates patients with suspicious mammographic lesions requiring biopsy.
METHODS AND MATERIALS
Patients
All patients within the Henry Ford Health System requiring mammographically guided breast biopsy from January 1, 1997, to March 31, 1998, were included in this study. This time period was chosen because it marked the introduction of both the Mammotome (Ethicon, Cincinnati, OH) and ABBI (United States Surgical Corp., Norwalk, CT) into our practice. Patients who underwent ultrasound-guided biopsies were excluded from analysis.
Stereotactic Core-Needle Biopsy
Selection criteria for SC bx are as follows:
• Mass, asymmetry, or clustered microcalcifications that can be targeted using digital imaging equipment
• Patients must be able to lie prone and still for approximately 30 to 60 minutes
• The breast must be ≥20 mm thick.
The patient lies prone on the Lorad mammography table with the breast suspended through an aperture. The breast is then compressed with a paddle with the targeted lesion within the working window. Two digital images are obtained at 30° angles and processed by the computer. The lesion is targeted by the physician, and the computer calculates the position of the lesion in three-dimensional space. After appropriate antisepsis and anesthesia are achieved, a puncture is made in the skin and the 14-gauge core needle is advanced to the lesion. The automated core needle is fired, and confirmatory mammographic images are obtained. Three to nine core biopsies are obtained. If the biopsy is performed for microcalcifications, a mammogram is obtained to confirm microcalcifications within the cores sampled.
Stereotactic Suction-Assisted Large Core Biopsy
Positioning and stereotactic guidance are done exactly as described above. The instrument that obtains the biopsy, however, is the 11-gauge Mammotome instrument (Ethicon). The tip of the instrument is advanced through a skin puncture to the mammographic lesion under stereotactic guidance. A core biopsy is obtained. The instrument is then rotated to a different region of the lesion and a repeat biopsy is obtained. This process is repeated until five to nine core biopsies are obtained. Once again, a mammogram is obtained to determine if microcalcifications are found within the core samples.
Advanced Breast Biopsy Instrument
Criteria for patient selection are similar to those described above, with the additional criteria that the breast must be ≥30 mm thick when compressed, the lesion must be ≤1 cm in diameter, and the lesion must be ≥1 cm from the chest wall and skin. Stereotactic localization is the same as described above. After appropriate antisepsis and anesthesia are achieved, a skin incision 1 to 2.5 cm long is made to accommodate the chosen cannula size (10, 15, or 20 mm in diameter). With the circular knife oscillating, the cannula is advanced through the breast tissue until the appropriate depth is achieved. A cautery snare is activated to complete the excisional biopsy. After the cylinder of breast tissue is removed, a mammogram is obtained to confirm the presence of the lesion in the specimen. Hemostasis is achieved as necessary. The skin is closed with absorbable, intradermal suture.
Open Wire-Localized Biopsy
Unlike the first three techniques described, this is a two-stage procedure. In the first stage, the patient is sent to the mammography suite, where a standard hooked wire is placed to localize the lesion of interest by the mammographer. The patient is then sent to the operating room, where the surgeon performs an excisional biopsy as guided by the localizing wire. The specimen is then sent back to mammography, where a specimen mammogram is obtained to determine if the lesion is within the specimen. The skin is then closed per the surgeon’s preference.
Mammographic/Pathologic Evaluation
For all lesions obtained by stereotactic-guided techniques, the pathologic results are correlated with the mammographic image in a formal conference of mammographers and breast pathologists. The purpose of this conference is to determine whether the pathologic diagnosis is in concordance with the mammographic image. If the results do not appear to explain each other, then the biopsy is considered discordant. With discordant biopsy results, open surgical biopsy is usually recommended.
Occasionally, the treating surgeon may not be satisfied with the biopsy results and may recommend open WL bx for confirmation. In addition, repeat biopsies are routinely performed in lesions that contain atypical ductal hyperplasia (ADH) and radial scar because of the high rate of associated breast malignancies. These outcomes are tabulated under the rubric of discordant/rebiopsy result.
Treatment and Follow-Up
Patients found to have malignancies were subsequently presented at the Henry Ford Health System Breast Cancer Tumor Board. Appropriate treatment options were formulated in this forum, including observation alone, adjuvant radiotherapy, reexcision and radiotherapy, lumpectomy with axillary lymph node dissection, simple mastectomy, or modified radical mastectomy, depending on tumor and patient characteristics.
For patients with benign biopsy results who did not undergo confirmatory open biopsy, follow-up was per the surgeon’s discretion. Most patients had 6-month follow-up mammograms, some had a clinical examination only, and some were simply referred back to their primary care physician.
Data Analysis
For SC bx and Mbx, results were considered to be true positive if any malignancy was present at subsequent lumpectomy or mastectomy. No distinction for the purposes of this study was made between ductal carcinoma in situ and invasive ductal or lobular carcinoma. Lobular carcinoma in situ, for the purposes of this study, was categorized as a benign finding. Results were considered false positive if any malignancy was present in the core specimen, without malignancy being found in the lumpectomy or mastectomy specimen. Results were considered false negative if a core biopsy showed no malignancy, but any subsequent biopsy of the same lesion demonstrated malignancy. Results were considered true negative if there was benign pathology in the biopsy specimen with subsequent confirmation by excisional biopsy, the 6-month mammogram demonstrated stability of the lesion, or a follow-up clinical examination demonstrated no clinical evidence of malignancy. This definition of true negatives probably overestimates the number of true negatives because some breast malignancies are very indolent, and 6-month mammographic or clinical examination may not detect these.
For the ABBI and WL bx techniques, the definition of true positive, false negative, and true negative results are the same as described above; however, because these are excisional biopsies, there were no false positive results. Sensitivity is defined as true positive/(true positive + false negative), specificity as true negative/(true negative + false positive).
Nominal data were analyzed for statistical significance using the chi square test with Yates’s continuity correction. A p value of 0.05 was considered statistically significant.
RESULTS
Over the 15-month study period, 245 SC bx, 107 Mbx, 104 ABBI, and 520 WL bx were performed.
Technical Success, Cancer Yield, and Atypical Ductal Hyperplasia
Technical success was achieved if the radiologist or surgeon performing the procedure was satisfied that enough tissue had been removed to produce a pathologic diagnosis. Using this definition, 94.3% of SC bx, 96.4% of Mbx, 92.5% of ABBI, and 98.7% of WL bx were considered technically successful (p = NS). Procedures that were not technically successful were repeated and are included in the discordant/rebiopsy category.
Cancer yield (Fig. 1) was similar among SC bx, Mbx, and ABBI techniques. However, there was a substantially higher cancer yield in the WL bx group (p < 0.01). This implies that patients with very suspicious lesions may have been preferentially offered open WL bx.
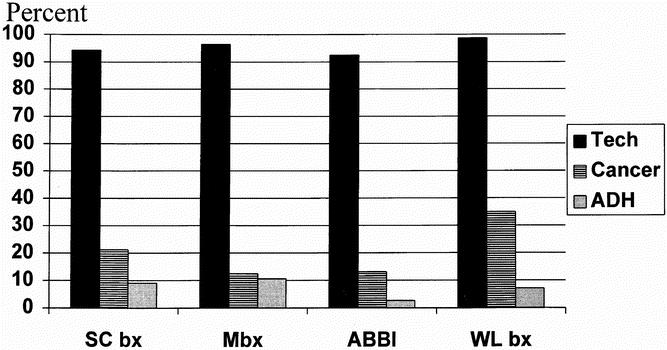
Figure 1. Comparison of technical success, cancer yield, and yield of atypical ductal hyperplasia among the four biopsy techniques. Cancer yield was statistically significantly higher for wire-localized biopsy vs. the other three techniques. Otherwise, no statistically significant differences are found. Tech, technique; Cancer, cancer yield; ADH, atypical ductal hyperplasia yield.
Because ADH produces a unique pathologic dilemma on core-needle biopsy, 12 these results were reviewed separately (Fig. 1). Although there were slightly higher rates of ADH in SC bx and Mbx, these were not statistically different than those in ABBI and WL bx. This implies that the incisional core biopsy does not lead to an inordinate difficulty in the pathologic diagnosis of ADH.
Sensitivity and Specificity
The sensitivities and specificities of each biopsy technique are shown in Figure 2. The sensitivities for SC bx and Mbx are relatively lower than for the ABBI method. However, specificity is nearly perfect for each technique, implying a very low false-positive rate.
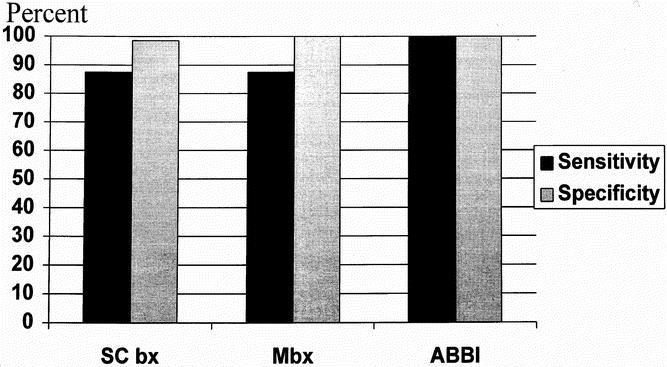
Figure 2. Comparison of the sensitivities and specificities of the stereotactic breast biopsy techniques.
Discordant/Rebiopsy Rate
There was a substantial difference in the discordant/rebiopsy rate among the techniques evaluated (p = 0.009) (Fig. 3). These results include rebiopsy because of technical failure, the presence of ADH and radial scar on core specimens, and discordant mammographic/pathologic interpretations. In fact, all the ABBI rebiopsies resulted from technical failure in the first 30 cases. As our experience has grown, we have not had a technical failure in our last 75 cases. There were no discordant cases in the ABBI group when the lesion of interest was within the specimen radiograph. However, technical failure accounted for a minority of the rebiopsies in the SC bx and Mbx groups. The majority were discordant, as determined by mammographic/pathologic interpretation.
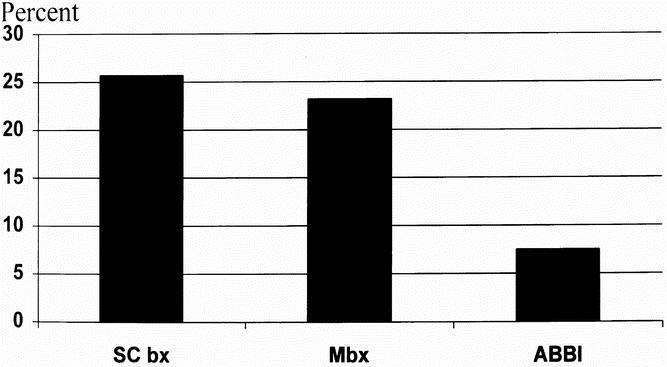
Figure 3. Comparison of the mammographic/pathologic discordant and/or rebiopsy rate of the three stereotactic breast biopsy techniques. The rate for the Advanced Breast Biopsy Instrument (ABBI) technique was statistically significantly lower than those for the incisional biopsy techniques.
Positive Margins and Residual Carcinoma
Because SC bx and Mbx are incisional biopsy techniques, by definition all cancers diagnosed using these methods have positive margins and require additional lumpectomy or mastectomy for definitive treatment.
ABBI and WL bx are excisional biopsies and potentially could produce clear margins, not requiring lumpectomy. Therefore, the frequency of positive margins becomes important. In patients with malignant lesions, 63.6% of ABBI biopsies had positive margins. Of those with positive margins, 71.4% had residual carcinoma on reexcision or mastectomy. By comparison, of the malignant lesions diagnosed with WL bx, 50.9% had positive margins (p = NS vs. ABBI), and of these 70.4% had residual carcinoma in the definitive lumpectomy or mastectomy specimen (Fig. 4).
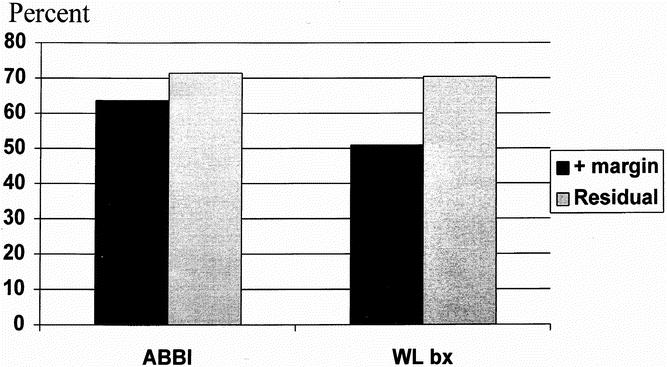
Figure 4. Comparison of positive-margin rate and residual cancer rate of specimens with breast malignancy of the excisional breast biopsy techniques. There were no statistically significant differences.
DISCUSSION
Screening mammography has led to an explosion in the number of patients with abnormal findings requiring evaluation. In general, the approach to these patients has been observation with a 6-month follow-up mammogram or surgical biopsy. Many studies have addressed the issue of which lesions can be safely watched. These studies have focused on mammographic criteria for malignancy 13,14 and a second opinion from an expert mammographer, 15 among others. Recently, computer-generated decisions 16,17 and fractal analysis 18 have been studied. Although promising, these are still considered research areas and are not meant for routine clinical decisions. Therefore, in most cases the choice of lesions selected for biopsy is based on the mammographer’s interpretation.
Once a decision is made for biopsy, there are several techniques available. The gold standard is WL bx. This technique has a very low failure rate, generally <2%. 10 In addition, in small cancers, it can serve as the definitive lumpectomy for patients wishing breast conservation, if the oncologic criteria of negative margins is met. Nevertheless, it is a surgical procedure and leaves a scar that is not cosmetically appealing, it can cause psychological and emotional stress, and it definitely entails substantial financial costs. 1 Therefore, “minimally invasive” alternatives to open WL bx have been explored.
Several issues arise with these newer image-guided techniques. The first and most important issue is the accuracy of diagnosis—not missing a cancer. Several studies have been published by both radiologic and surgical groups demonstrating that stereotactic core-needle biopsy has both a high sensitivity and specificity with breast cancer diagnosis, 4–6 which is in agreement with our data. In large series, cancer miss rates have been reported from 1.5% to 7.6%. 4–6,19 Nevertheless, concern over “nondiagnostic” core biopsies has led to the development of instruments to improve accuracy. One of these is the suction-assisted large core-needle instrument (Mammotome). A recent study reported a technical success rate for this procedure of 95%, with only one cancer missed in 112 biopsies. 7 Unfortunately, this article made no comment on discordant/rebiopsy rates.
With these techniques, cancers may be missed because of the anatomic relation of the malignant cells to the microcalcifications. Selim and Tahan 20 studied 32 nonpalpable breast cancers associated with microcalcifications diagnosed by open biopsy. They found that in 34% of the cases, microcalcifications were found only within the benign tissue surrounding the malignant lesion. Therefore, they expressed concern that mammographically guided core biopsies of microcalcifications may not adequately sample the breast tissue that contains the malignancy.
Another concern with SC bx is follow-up. In the largest series reported, only approximately 60% of patients returned for follow-up; therefore, in 40% it is impossible to know if these benign biopsy results are true negatives or not. 4 Therefore, we do not know whether the cancer miss rate is as low as these studies report. In addition, in those who returned for follow-up, slow-growing tumors may stay mammographically stable for some time, which is why it has been our practice to obtain 6-month mammograms over a 2-year period to ensure stability.
Another issue is that of discordant results or the need for a repeat biopsy. This may result from an inadequate sample, mammographic/pathologic discrepancy, lack of confidence in the biopsy results on the part of the surgeon, radiologist, or pathologist, or patient preference. 21,22 In addition, specific pathologic findings on the core-needle biopsy are highly associated with malignancy. Bonzanini et al 23 found that 14% of patients with radial scars had carcinoma. Moore et al 12 documented that 7 of 21 patients (33%) had carcinoma with a finding on SC bx of ADH. In our study, the pathologic finding of ADH was not inordinately high compared to excisional biopsy techniques, implying that the pathologic diagnosis is not more difficult. Discordant results and a need for rebiopsy, however, have been reported in 18% to 22% of cases by other authors. 21,22,24 A discordant/rebiopsy rate of 15% to 25% is confirmed by our study as well. More troubling, as Dershaw et al 21 showed, is the fact that up to 50% of patients with discordant results may be harboring a malignancy. Therefore, programs that primarily rely on SC bx or Mbx for mammographically guided biopsy must formally review each biopsy in a mammographic/pathologic forum to confirm concordance so as not to miss cancers. 25
The advantage of the ABBI technique is that it is an excisional biopsy, just like WL bx. Therefore, as long as the procedure is technically successful, the pathologist will have the entire lesion to examine. This eliminates the difficulties associated with discordance, ADH, and radial scars. The advantage of the ABBI (as performed by surgeons) over WL bx is that it avoids operating room, anesthesia, radiology, and hospital room costs, which can account for up to 50% of the costs of WL bx. 5,22,26 In addition, it takes only approximately 2 hours from the time the patient arrives for the procedure to when she leaves, clearly a time advantage when compared to a procedure requiring operating room, anesthesia, and recovery room services.
Nevertheless, we have been troubled by what we believed was an unacceptably high positive-margin rate in patients with malignancies (Fig. 4). If most patients with cancers require reexcision after the ABBI, then perhaps the advantages compared to SC bx and Mbx would be negligible. However, the positive-margin rate for ABBI is only slightly higher than WL bx (p = NS). Although the criticism can be levied that our positive-margin rate for WL bx is high, it is in keeping with previously published reports of 49% to 55%. 5,26,27 In our study, we also demonstrated that the incidence of residual cancer in these patients with positive margins exceeds 70% in both the ABBI and WL bx groups. Therefore, the ABBI achieves the same positive-margin and residual rates as open WL bx, without the associated operating room, nursing, and anesthesia costs.
Given these data, the core issue is which procedure would entail the fewest episodes requiring reexcision or rebiopsy. If we assume a cancer yield of 20%, an ABBI reexcision rate of 60%, and an SC bx rebiopsy rate of 20%, then 12% of ABBI patients will require reexcisionversus 36% of SC bx patients. This clearly favors the ABBI.
In conclusion, for patients who meet the criteria, an ABBI mammographically guided biopsy appears to be the most efficient means for definitive diagnosis of nonpalpable breast lesions. Some women, however, do not fit the inclusion criteria, and one of the other techniques may be appropriate. For programs that use SC bx or Mbx, a formal mammographic/pathologic assessment is required to ensure that discordant lesions undergo surgical biopsy to avoid cancer misses. Lastly, the ABBI should be used as the lumpectomy for malignancies only after a formal tumor board review of the pathologic specimen to ensure that the ABBI excision met appropriate oncologic criteria.
Discussion
Dr. Edward G. Copeland III (Gainesville, Florida): The discordant results for stereotactic and Mammotome biopsies are disconcerting since these biopsy techniques are now so commonly used nationwide. A technique with a 25% error rate should be questioned, as the authors have done both in their institution and in their manuscript.
In my experience at the University of Florida, this error rate seems high. The ABBI technique would be expected to be more accurate when compared to stereotactic biopsy since the criteria for biopsy are stricter and a much larger piece of tissue is available for pathologic review. However, a 2.5-cm incision might be needed and hemorrhagic complications might be increased.
We have not utilized the ABBI in our institution because patients with extensive ductal carcinoma in situ or T1 cancers are candidates for sentinel lymph node biopsy protocols. We have hypothesized that lymphatic flow is disrupted least by stereotactic biopsy. This hypothesis has not been proven, however.
In my practice, patients who are candidates for needle-wire localization have frozen section intraoperatively. If a malignant diagnosis is confirmed, additional margins are taken and pathologically confirmed to be negative. This technique eliminates the need for future re-excision to obtain negative margins. Almost all patients biopsied by ABBI with a malignant diagnosis, in my opinion, should have the biopsy site re-excised to insure negativity.
My questions for Drs. Ferrara and Velanovich are as follows:
Who does the stereotactic biopsies at your institution?
Do you use sentinel lymph node biopsy and, if so, have you noted any one biopsy technique to negatively impact the ability to find the sentinel node, either by the blue dye technique or radioscintigraphy?
And, last, for those 30% of patients biopsied by ABBI and having negative margins, do you recommend re-excision of the biopsy site?
Dr. Kirby I. Bland (Providence, Rhode Island): The authors have acknowledged that the diagnostic sensitivities and specificities that they report with stereotactic core biopsies are good to excellent. Like their series, in our own we find that about one of every four or five patients who actually have stereotactic biopsy will still require an open biopsy. And we also typically would use a needle-localized technique. However, the authors have noted that the additional need does not occur with the ABBI excisional biopsy system and have concluded that it is the most efficient system, as it avoids operating room re-excision costs.
I first query the authors on their criteria for biopsy selection. How is each method selected? What are the lesion sizes, types, and lesion numbers for which a physician would choose the ABBI approach? What are the criteria for the number of cores as well as the needle size, based on this prospective nonrandomized analysis?
Now as the ABBI system typically requires a biopsy for a lesion that is less than 1 cm, in other words, a T1B or smaller in size, a greater frequency of sampling error with larger lesions would be expected. As many of you realize that do this technique, the comparisons between these two techniques are somewhat different if you compare it with a Mammotome instrument.
For instance, in the Mammotome instruments, you can compress 20 mm of breast tissue and can biopsy with a 14-gauge needle, whereas in the ABBI system, it requires 30 mm more volume of tissue to compress such that you can obtain this biopsy. Therefore, an essential question, do the results of non-ABBI core biopsy system, which is done on greater than 30-mm tissue samples, compare favorably with the ABBI system? Did you select patients with smaller lesions required to receive the ABBI because, first, you were more concerned about the outcomes of the sampling, you wanted to avoid “seeding” the biopsy track, or you were trying to remove the entire lesion this approach?
The learning curve for performing imaging-guided biopsy techniques is steep. It has been advocated that outcomes should not be tabulated until the surgeon or radiologist has performed at least 50 procedures. In reviewing the manuscript, I could not determine the experience of the surgeons and/or radiologists who performed the biopsies. Did all surgeons or radiologists have sufficient experience to assure accurate data? How many different physicians performed biopsies with the ABBI and non-ABBI techniques, and how many of each did each surgeon perform? In your view, does the experience with the procedure have a bearing on the outcomes, and could uneven experience of the practitioners have biased your results?
The sensitivity of the stereotactic core biopsy has been confirmed in the literature to be directly related to the number of cores that have been obtained. Sensitivities of the non-ABBI methods ranged in the high 90s with at least five cores. Obviously, taking too few cores could bias the data. In your study, three to six cores were actually obtained, if I reviewed your manuscript correctly. So were at least five cores obtained with a non-ABBI method before these comparisons were completed?
As the selection of patients was not randomized, this could be said to be a flaw in your protocol design and could bias your data in favor of the ABBI. But, clearly, the ABBI requires a small lesion, it has to be deep in the skin and 1 cm off the chest wall, and you have used those other approaches to use it, so there does seem to be some bias in this type of selection, John.
And as we all know, today the cost effectiveness represents the “sound bite” of our managed-care environment. In the analyses that I am aware of, ABBI disposable are considerably more costly than those used in other core or Mammotome biopsy methods. When diagnoses can be made with less-expensive and/or less-invasive methods, how do you justify the use of the ABBI? Further, since a significant number of patients who have been either diagnosed with cancer or who have discordant or indeterminate biopsy results will require re-excision, have you done a cost analysis of the various modalities? In Providence, Rhode Island, believe me, cost is a big issue.
How do the cosmetic results compare in patients who have had diagnostic core biopsies and/or Mammotome biopsies, especially in those who do not require re-excision, to those who have had ABBI? How do the actual biopsy times compare for each technique?
Follow-up outcomes are reported and true negatives are reported in this study; however, data from follow-ups of less than or equal to 6 months’ duration were included in these outcomes. How do you determine true negatives, that is, specificity, as follow-up at 6-month intervals for at least 2 years is recommended to substantiate any conclusions? Do you have more follow-up data?
Dr. John S. Bolton (New Orleans, Louisiana): President Griffen, Secretary Copeland, Members, and Guests. I do not have direct experience with the ABBI system or the Mammotome, but I have quite a bit of experience with 14-gauge stereotactic core biopsies.
And I want to try to understand a little better the reasons for the difference between your results and ours, where in over 1400 consecutive core biopsies with an 85% mammogram follow-up rate out to 2 years, we reported a 0.1% false-negative rate, had a discordant rate requiring repeat core for wire-localized biopsy in only 2% of patients, and were then able to complete definitive one-stage surgical therapy with negative margins in 90% of patients based on a core biopsy diagnosis of invasive cancer.
My questions are, why did you require as few as three cores, especially when you set a minimum of five for the larger biopsy systems? That just seems inconsistent to me.
Second, why would you ever attempt a stereotactic core needle biopsy for a radial scar? My understanding and our practice is that it’s contraindicated in that situation.
Third, why do you characterize a core biopsy diagnosis of atypia with the discordant/rebiopsy group? There isn’t any discordance here. The core biopsy has identified significant epithelial pathology that requires further evaluation and has provided clinically useful relevant information. The same can be said for lobular carcinoma in situ, and this is why your “false-negative” rate is so high for stereotactic core biopsy and why your discordant rate is also high.
And, fourth, I was surprised by the high positive margin rate for the wire-localized biopsy group. I know you can find similar reports in the literature, but it is possible to do better, Particularly if you’re guided by core biopsy results indicating invasive cancer, you should be able to obtain negative margins at the first excision 90% of the time.
As a final comment, your numbers of cases are small—245 stereotactic core biopsies, 107 Mammotome biopsies, 104 ABBIs—and this works out to be only about 15 cancers diagnosed by ABBI and by Mammotome. And it may be that the message here is to pick one technique, and probably any one of the techniques, stick with it, work out the kinks in your institution, and you’ll get good results. But I think it’s premature on this analysis to decide that one technique is better than the other.
Dr. David S. Robinson (Kansas City, Missouri): The authors are to be congratulated for having taken a topic in the comparison of techniques in what is considered to be a very rapidly moving and expanding area, breast cancer stereotactic biopsy, and of having presented probably the first comparison study.
To date in this country, much of the technology has been industry-driven rather than user-driven. This has been by two major manufacturers and now two major biopsy approaches. And to further complicate the issue, surgeons and radiologists have gone into battle with one another over the ownership of the image breast biopsy territory. This is important enough that the American College of Surgeons has been proactive both in the teaching of the technique to surgeons and in the determination of credentialing with the American College of Radiologists for surgeons and radiologists.
The issue of technical preference is complicated further by several factors, including patient selection, biopsy instrument selection, and the technical requirements of the instruments themselves, that is, how many sample should be taken, as we’ve heard from the other questioners. The authors have concluded that the ABBI is a preferable instrument because of a lower rebiopsy rate and because of the greater number of biopsies that have free margins.
In regard to the first conclusion, did radiologists and surgeons both use the instruments? Were both sets of instruments available to both groups of practitioners? And were there preferences by the two groups, and did this make a difference?
The selection criteria of patients for the ABBI were given as a lesion of 10 mm or less. Were the same criteria used for other instrumentation? Clearly, there are retrospective data with a great many variables here. Was consideration given in the future to a randomized prospective study?
Regarding the biopsy instruments themselves and their use, very few practitioners are actually now using the 14-gauge Tru-Cut. Many people have moved on to either the 11-gauge needle-suction–assisted device or the single-core ABBI device. These have replaced many of the smaller sampling devices just as the fine-needle aspiration was replaced by the 14-gauge Tru-Cut about 5 years ago.
This paper states that about 5- to 9-suction–assisted 11-gauge cores were taken, yet the standard for many people has now become 12 to even 24 cores. And the cavity may be up to about 1.5 cm in diameter, close to the 20-mm ABBI-created cavity, with an accuracy equal to that of needle-localization open biopsy in a larger series by Dr. Burbank, and confirmed by another by Dr. Jackman, both radiologists. The results have not been compared, however, to ABBI.
With consideration of the margins, this has really not been an issue so far in the United States, because at the FDA hearings in November of 1996, it was made very clear that all of these diagnostic instruments are strictly to be used for diagnosis only and not for therapy. Yet with needle localization and open biopsy in this country, there’s a growing trend toward treating the very small lesion that we have not yet diagnosed as a potential cancer, placing the wire and performing an excisional biopsy as a lumpectomy.
In that regard, there are two series that have looked at lesions that are somewhat larger than these, one from Fox Chase and one from the John Wayne Cancer Center. And in those two series, each of several hundred patients, one found 49% needed rebiopsy after the excisional biopsy with needle localization, and in the other series, about 75%. So it looks in many of these series that if it’s important, to go back.
Certainly, in this particular series, the lesions were smaller. They were 10 mm or less and were using a 20-mm core device. That would mean that if you were dead-on perfectly accurate, you’d have 5 mm to either side. And some studies have now suggested that that may not be quite enough, the NSABP studies notwithstanding.
With regard to the issue of positive margins, 63.6% were found to be positive on the ABBI, leaving a remainder of 26.4%. Were these re-excised with lumpectomy or mastectomy, or were they simply left alone? If they were re-excised, what was the percentage of involvement of those negative margins? What kind of confirmation was performed?
And, finally, with regard to margins and the issue of using the ABBI as a therapeutic device, many of us share your hope for a minimally invasive interstitial approach to stereotactically imaged breast cancers. There’s ongoing work in several institutions, including my own, using stereotactic interstitial laser treatment. Other people are using radio frequency, and still others are using cryotherapy in an effort to produce a minimally approach to small breast cancers.
We share your enthusiasm for refinement of the stereotactic biopsy technique, but wonder if the diagnostic efficacy of these several different approaches in taking large samples might be similar if put to a randomized prospective study.
Dr. Charles E. Cox (Tampa, Florida): Though not the focus of this presentation, I was discouraged by the dismissal of the FNA as a biopsy method, and I wish to go on record that it is a very cost-effective and sensitive method of determination of pathology. In our own series, the use of FNA results comprised approximately 37.8% of the positive diagnoses obtained, for which we perform definitive surgical care. The qualifying statement made regarding the need for excellent cytopathologists is not to be taken lightly. My only concern is that we as surgeons may have to drag some of the cytopathologists kicking and screaming into the enlightened era that their well-trained and aggressive colleagues have pioneered.
Dr. Velanovich has clearly pointed out that any studies which involve breast biopsy require careful follow-up of the patients who had a negative biopsy to insure accurate assessment of false-negative results. Their data demonstrates some clear advantages of the ABBI biopsies done by surgeons. It provides the pathologist with the entire specimen, eliminates discordance of ADH and radial scar, avoids operating room, anesthesia, and radiology costs, reduces overall time for the patient, and—not mentioned in the study, but yet another advantage of the ABBI technique—is the ability to verify adequacy of removal of the lesion both in the specimen and in the breast prior to termination of the procedure.
Another problem of a stereotactic core and Mammotome biopsy is the high discordance rate. This is not inconsequential in that re-excision was required in 20% to 25% of patients. These cases then incurred the expense of the original and the secondary biopsy. I would recommend that the authors comment on this. Additionally, have they done a cost analysis of the various biopsy methods, including the secondary cost of rebiopsy?
The residual tumor at the margins following biopsy is neither dramatic nor unusual and validates our own previously published experience. However, if rapid, reliable topographic analysis of suspicious ABBI or wire-localization biopsy specimens were available, could effective lumpectomy and possible sentinel node biopsies be simultaneously performed?
I was extremely interested in the comment about rebiopsy rate for the ABBI procedure. In the first 30 cases, all documented technical failures occurred. The fact that no technical failures were recorded in the last 75 cases implies a specific learning curve for the development of this new technology in the hands of trained surgeons. Have the authors calculated individual or institutional learning curves for this new technology?
Also, one omission in the methods section I would suggest the authors comment on would be the use of postbiopsy clips. Do you routinely place clips following core needle and Mammotome breast biopsies, and if not, why not?
Dr. Vic Velanovich (Closing Discussion): I will try to be as brief as possible.
Dr. Copeland—who does the stereo core biopsies? The radiologists do the stereo core biopsies and the Mammotomes, and we do the ABBI biopsies as well as the wire-localization biopsies.
Do we re-excise ABBIs with negative margins? We mostly do. We have watched elderly patients with low-grade small DCIS lesions as per Breast Tumor Board protocol.
Does the type of biopsy change your need for sentinel node? I don’t have that data so I can’t answer that question.
Moving on the Dr. Bland’s comments: criteria selection, basically, as was mentioned before, the ABBI requires a patient to be able to lie prone for anywhere from 30 minutes to an hour and a breast thickness of 30 mm and, most importantly, to be able to see the lesion on the digital mammographic equipment. That’s where we found most of our technical failures was just not being able to see the lesion on the digital equipment.
With regard to the core biopsies for those patients who had breasts with greater than 30 mm of thickness, I do not have the answer to that question. Selection criteria with regard to lesions less than 1 cm, we did this in order to be able to completely excise the lesion.
The number of core biopsies in the manuscript and in the presentation was listed as anywhere from three to nine. Mostly, it is now greater than five and closer to 10 core samples that are being obtained. Early on, the radiologists did obtain only three or so samples, but that has changed.
With regard to the cost analysis, this also goes with Dr. Cox’s question. We find that, when you look at the number that requires rebiopsy, the difference actually favors ABBI by about $500 per patient, and there is about an $1800 difference between ABBI and wire-localized biopsies.
Determination of the false negatives: I think this is going to be an interesting thing in the long run because I don’t believe that 6-month follow-up is adequate to determine the true false-negative rate. Many cancers are indolent and may take a while, up to 2 years or so, before we would be able to know for sure whether they are in fact true false-negatives or not.
With regard the Dr. Bolton’s comments, I congratulate him on his excellent results, and clearly we are not able to obtain a discordant rate of less than 2%. I suspect that this is related to a good working relationship that they have with their radiology colleagues and possibly a control over how it’s done. Now why were there only three samples? Again, that was early on in the experience, we don’t do that anymore, and it’s mostly anywhere between five to 10.
Radial scar: again, that was done early on in the experience, and we no longer recommend it. With regard to the issue of an atypical ductal hyperplasia, this did require rebiopsy, so that’s why we put it into the rebiopsy group, in order to make it uniform throughout all four techniques.
To answer Dr. Robinson’s questions, radiologists did the stereo core biopsies and the Mammotome biopsies; we did the ABBI biopsies. There was no cross-fertilization.
The difference in criteria with regard to the size. Basically, all ABBI biopsies needed to be done with lesions less than 1 cm in diameter, whereas there were no such size criteria for either the stereo core or the Mammotome.
When we re-excised the cancers after the ABBI with the negative margins, we actually found no residual cancers in the lumpectomy specimen; however, we only would opt to watch patients who are elderly and who have low-grade DCIS lesions in that regard.
Finally, for Dr. Cox, I think I previously mentioned the cost data analysis. With regard to the topographical analysis with the frozen sections, since the ABBI is not done in the operating room, it’s not done with anesthesia. We do not have that ability to be able to quickly check the margins and then go back and redo the biopsy.
The learning curve, although it was listed as 30 for the entire institution, when you break it down by surgeon, it occurred within the first 10 for each surgeon. And this is again primarily selection criteria with regard to picking patients that you can easily see the lesion on the digital mammographic equipment.
Our radiologists do in fact leave a clip with both the Mammotome and the stereo core biopsy, and that is done as a routine.
Footnotes
Correspondence: Vic Velanovich, MD, Division of General Surgery, K-8, Henry Ford Hospital, 2799 West Grand Blvd., Detroit, MI 48202-2689.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Accepted for publication December 1998.
References
- 1.Velanovich V. Immediate biopsy versus observation for abnormal findings on mammograms: an analysis of potential outcomes and costs. Am J Surg 1995; 170: 327–332. [DOI] [PubMed] [Google Scholar]
- 2.Staren ED. Ultrasound guided biopsy of nonpalpable breast masses by surgeons. Ann Surg Oncol 1996; 3: 476–482. [DOI] [PubMed] [Google Scholar]
- 3.Sarfati MR, Fox KA, Warneke JA, et al. Stereotactic fine-needle aspiration cytology of nonpalpable breast lesions: an analysis of 258 consecutive aspirates. Am J Surg 1994; 168: 529–532. [DOI] [PubMed] [Google Scholar]
- 4.Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 1994; 193: 359–364. [DOI] [PubMed] [Google Scholar]
- 5.Yim JH, Premsti W, Weber B, et al. Mammographically detected breast cancer: benefits of stereotactic versus wire localization biopsy. Ann Surg 1996; 223: 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuhrman GM, Cedarbom GJ, Bolton JS, et al. Image-guided core-needle breast biopsy is an accurate technique to evaluate patients with nonpalpable imaging abnormalities. Ann Surg 1998; 227: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberman L, Smolkin JH, Dershaw DD, et al. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology 1998; 208: 251–260. [DOI] [PubMed] [Google Scholar]
- 8.Kelley WE, Bailey R, Bertelson C, et al. Stereotactic automated surgical biopsy using the ABBI biopsy device: a multicenter study. Breast J 1998; 4: 302–306. [Google Scholar]
- 9.Shuler FW, White JG, Wilson RA, et al. Advanced breast biopsy instrumentation: an early prospective series. Proceedings of the 6th World Congress of Endoscopic Surgery. Bologna, Italy: Monduzzi Editore; 1998: 751–755.
- 10.Jackman RJ, Marzoni FA. Needle-localized breast biopsy: why do we fail? Radiology 1997; 204: 677–684. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt RA. Stereotactic breast biopsy. CA Cancer J Clin 1994; 44: 172–191. [DOI] [PubMed] [Google Scholar]
- 12.Moore MM, Hargett CW III, Hanks JB, et al. Association of breast cancer with finding of atypical ductal hyperplasia at core needle biopsy. Ann Surg 1997; 225: 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson EJ, McGreevy JM, Muskett A. Selective nonoperative management of patients referred with abnormal mammograms. Am J Surg 1990; 160: 659–663. [DOI] [PubMed] [Google Scholar]
- 14.Hasselgren P-O, Hummel RP, Fieler MA. Breast biopsy with needle localization: influence of age and mammographic features on the rate of malignancy in 350 nonpalpable breast lesions. Surgery 1991; 110: 623–628. [PubMed] [Google Scholar]
- 15.McManus V, DeSautels JEL, Beneditsson H, et al. Enhancement of true-positive rates for nonpalpable carcinoma of the breast through mammographic selection. Surg Gynecol Obstet 1992; 175: 212–218. [PubMed] [Google Scholar]
- 16.Wu YC, Freedman MT, Hasegawa A, et al. Classification of microcalcifications in radiographs of pathologic specimens for the diagnosis of breast cancer. Acad Radiol 1995; 2: 199–204. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim N, Fujita H, Hara T, Endo T. Automated detection of clustered microcalcifications on mammograms: CAD system application of MIAS database. Phys Biol Med 1997; 42: 2577–2589. [DOI] [PubMed] [Google Scholar]
- 18.Velanovich V. Fractal analysis of mammographic lesions: a prospective, blinded trial. Breast Cancer Res Treat 1998; 49: 245–249. [DOI] [PubMed] [Google Scholar]
- 19.Dowlatshahi K, Yaremko ML, Kluskans LF, Jokich PM. Nonpalpable breast lesions: findings of stereotaxic needle-core biopsy and fine-needle aspiration cytology. Radiology 1991; 181: 745–750. [DOI] [PubMed] [Google Scholar]
- 20.Selim A, Tahan SR. Microscopic localization of calcifications in and around breast carcinoma: a cautionary note for needle core biopsies. Ann Surg 1998; 228: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dershaw DD, Morris EA, Liberman L, Abramson AF. Nondiagnostic stereotaxic core breast biopsy: results of rebiopsy. Radiology 1996; 198: 323–325. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Egglin TK, Phipotts L, et al. Cost-effectiveness of stereotactic core needle biopsy: analysis by means of mammographic findings. Radiology 1997; 202: 849–854. [DOI] [PubMed] [Google Scholar]
- 23.Bonzanini M, Gilioli E, Brancato B, et al. Cytologic features of 22 radial scar/complex sclerosing lesions of the breast, three of which associated with carcinoma: clinical, mammographic, and histologic correlation. Diag Cytopathol 1997; 17: 353–362. [DOI] [PubMed] [Google Scholar]
- 24.Meyer JE, Smith DN, Lester SC, et al. Large-needle core biopsy: nonmalignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology 1998; 206: 717–720. [DOI] [PubMed] [Google Scholar]
- 25.Berg WA, Ruban RH, Kumar D, et al. Lessons from mammographic–histopathologic correlation of large-core needle breast biopsy. Radiographics 1996; 16: 1111–1130. [DOI] [PubMed] [Google Scholar]
- 26.Lind DS, Minter R, Steinbach B, et al. Stereotactic core biopsy reduces the re-excision rate and the costs of mammographically detected cancer. J Surg Res 1998; 78: 23–26. [DOI] [PubMed] [Google Scholar]
- 27.Acosta JA, Greenlee JA, Gubler D, et al. Surgical margins after needle-localization breast biopsy. Am J Surg 1995; 170: 643–646. [DOI] [PubMed] [Google Scholar]


