Abstract
Objective
To study the efficacy of gastric surgery-induced weight loss for the treatment of pseudotumor cerebri (PTC).
Summary Background Data
Pseudotumor cerebri (also called idiopathic intracranial hypertension), a known complication of severe obesity, is associated with severe headaches, pulsatile tinnitus, elevated cerebrospinal fluid (CSF) pressures, and normal brain imaging. The authors have found in previous clinical and animal studies that PTC in obese persons is probably secondary to a chronic increase in intraabdominal pressure leading to increased intrathoracic pressure. CSF–peritoneal shunts have a high failure rate, probably because they involve shunting from a high-pressure system to another high-pressure zone. In an earlier study of gastric bypass surgery in eight patients, CSF pressure decreased from 353 ± 35 to 168 ± 12 mm H2O at 34 ± 8 months after surgery, with resolution of headaches in all.
Methods
Twenty-four severely obese women underwent bariatric surgery—23 gastric bypasses and one laparoscopic adjustable gastric banding—62 ± 52 months ago for the control of severe obesity associated with PTC. CSF pressures were 324 ± 83 mm H2O. Additional PTC central nervous system and cranial nerve problems included peripheral visual field loss, trigeminal neuralgia, recurrent Bell’s palsy, and pulsatile tinnitus. Spontaneous CSF rhinorrhea occurred in one patient, and hemiplegia with homonymous hemianopsia developed as a complication of ventriculoperitoneal shunt placement in another. There were two occluded lumboperitoneal shunts and another functional but ineffective lumboperitoneal shunt. Additional obesity comorbidity in these patients included degenerative joint disease, gastroesophageal reflux disease, hypertension, urinary stress incontinence, sleep apnea, obesity hypoventilation, and type II diabetes mellitus.
Results
At 1 year after bariatric surgery, 19 patients lost an average of 45 ± 12 kg, which was 71 ± 18% of their excess weight. Their body mass index and percentage of ideal body weight had fallen to 30 ± 5 kg/m2 and 133 ± 22%, respectively. In four patients, less than 1 year had elapsed since surgery. Five patients were lost to follow-up. Surgically induced weight loss was associated with resolution of headache and pulsatile tinnitus in all but one patient within 4 months of the procedure. The cranial nerve dysfunctions resolved in all patients. The patient with CSF rhinorrhea had resolution within 4 weeks of gastric bypass. Of the 19 patients not lost to follow-up, 2 regained weight, with recurrence of headache and pulsatile tinnitus. Additional resolved associated comorbidities were 6/14 degenerative joint disease, 9/10 gastroesophageal reflux disorder, 2/6 hypertension, and all with sleep apnea, hypoventilation, type II diabetes mellitus, and urinary incontinence.
Conclusions
Bariatric surgery is the long-term procedure of choice for severely obese patients with PTC and is shown to have a much higher rate of success than CSF–peritoneal shunting reported in the literature, as well as providing resolution of additional obesity comorbidity. Increased intraabdominal pressure associated with central obesity is the probable etiology of PTC, a condition that should no longer be considered idiopathic.
Pseudotumor cerebri (PTC), also known as idiopathic intracranial hypertension, is a condition associated with headache, blurred vision, and often pulsatile tinnitus (an annoying auditory swishing sound synchronous with the heartbeat). Cranial imaging studies are normal, with the exception of small ventricles and/or an empty sella. Cerebrospinal fluid (CSF) pressures are always elevated. These patients may also have other central nervous system and cranial nerve dysfunction, including visual disturbances and rarely Bell’s palsy and trigeminal neuralgia. Several case reports have documented that major weight loss, by either bariatric surgery or an aggressive dietary program, was associated with resolution of headache and reduction in CSF pressure. 1–3 In a previous study, 4 we found that gastric bypass surgery in eight patients decreased CSF pressure from 353 ± 35 to 168 ± 12 mm H2O (p < 0.001) at 34 ± 8 months after surgery, with resolution of headache in all. This report extends our clinical observations of bariatric surgery for the treatment of severe obesity associated with PTC.
METHODS
Patients entered the study if they had symptoms of PTC that included persistent, severe headache, a negative brain imaging study, and elevated CSF pressures (>200 mm H2O). Patients were referred by their primary care physician, a neurologist, or a neuroophthalmologist, or the diagnosis was considered by otolaryngologists for symptoms of pulsatile tinnitus associated with headache or at the time of initial evaluation for bariatric surgery. Persistent headache associated with annoying pulsatile tinnitus, probably secondary to increased sagittal sinus venous pressure, is suggestive of PTC. 5 CSF pressures were obtained by the referring neurologist or by a neuroophthalmologist coinvestigator (WLF). All patients underwent ophthalmologic examination for evidence of papilledema and visual field defects. 4 Additional symptoms of cranial nerve dysfunction elicited were trigeminal neuralgia (tic douloureux), fifth cranial nerve; diplopia, sixth cranial nerve; and facial nerve paralysis (Bell’s palsy), seventh cranial nerve.
Patients were considered eligible for bariatric surgery based on the criteria in the 1992 National Institutes of Health Consensus Conference. These included a body mass index of >35 kg/m2 with severe obesity comorbidity (sleep apnea, obesity hypoventilation, type II diabetes, venous stasis ulcers, or severe degenerative joint disease pain), or >40 kg/m2 without comorbidity. 6 Although PTC was not mentioned in the Consensus Conference statement, we considered it to be a severe comorbidity factor. Additional comorbidity was determined by clinical evaluation. A diagnosis of sleep apnea required >10 respiratory disturbance events (apneas and hypopneas) per hour of sleep, documented with sleep polysomnography. Indications for obtaining a sleep polysomnogram included symptoms of daytime somnolence (falling asleep while driving or at work), loud snoring, and frequent nocturnal awakenings. Gastroesophageal reflux disease (GERD) was based on the patient’s symptoms, although an occasional patient underwent measurement of 24-hour esophageal pH. Degenerative joint disease was presumed if the patient complained of pain in the hips, knees, ankles, or back. The diagnosis of hypertension required a systolic blood pressure of >140 mm Hg and/or a diastolic blood pressure of >90 mm Hg, or use of antihypertensive medications prescribed by the primary care physician. Urinary stress incontinence was considered to be present if the patient complained of wetting herself with straining; it was considered to be severe if a perineal pad was necessary. The diagnosis of type II diabetes required the use of insulin or oral hypoglycemic agents to maintain normal fasting blood sugar levels. Routine preoperative glucose tolerance tests were not obtained.
The bariatric procedures used included the standard Roux-en-Y gastric bypass, as previously described, 7 or the “long-limb” gastric bypass for superobese patients with a body mass index ≥50 kg/m2, 8 and a laparoscopic adjustable Silastic gastric band. 9 Patients were asked to return for follow-up evaluation 1 week, 3 months, 6 months, 12 months, and 18 months after surgery and at yearly intervals thereafter. Except for the eight patients in the previously mentioned study, 4 repeat CSF measurements were not obtained unless the patient complained of recurrent headache 1 year or more after surgery. Statistical analysis included the paired Student’s t test.
RESULTS
Twenty-four severely obese women (127 ± 17 kg, body mass index 47 ± 6 kg/m2, 208 ± 27% ideal body weight) underwent bariatric surgery—23 gastric bypasses (a long-limb bypass in 6) and one laparoscopic adjustable gastric banding—62 ± 52 months (range 8 months to 14 years) ago for the control of severe obesity associated with PTC (Table 1). Two patients underwent conversion from a failed horizontal gastroplasty to gastric bypass. All patients had persistent, severe headaches and had a negative preoperative brain imaging study (either magnetic resonance imaging or computed tomography). Preoperative CSF pressures averaged 324 ± 83 mm H2O (Table 1). Twelve patients had papilledema. Additional PTC manifestations (Table 2) included pulsatile tinnitus (VIII) in 22, peripheral visual field loss (II) in 13, trigeminal neuralgia (V) in 2, and recurrent Bell’s palsy (VII) in 2. Spontaneous CSF rhinorrhea occurred in one patient, and hemiplegia with homonymous hemianopsia, associated with refractory axillary and groin ulcerations on the hemiparetic side, developed as a complication after placement of a ventriculoperitoneal shunt in one. Two patients had an occluded lumboperitoneal shunt, and another had persistent headache, pulsatile tinnitus, and CSF opening pressure of 235 mm H2O despite a lumboperitoneal shunt shown by scintigraphy to be functional. Additional obesity comorbidity in these patients included hypertension (n = 6), GERD (n = 10), sleep apnea (n = 3), obesity hypoventilation (n = 1), urinary stress incontinence (n = 3), and degenerative joint disease (n = 14). One patient had type II diabetes mellitus (Table 3).
Table 1. Demographics of Patients with Pseudotumor Cerebri
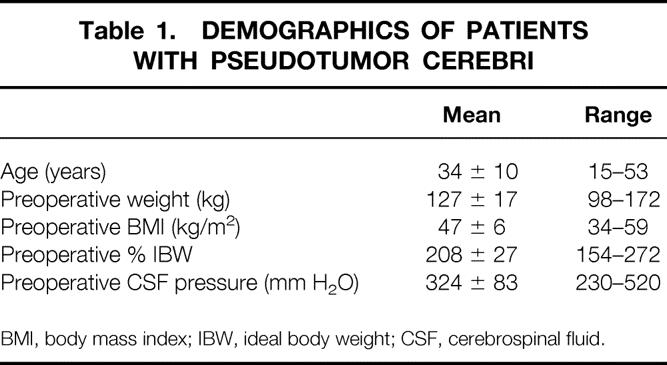
Table 2. Preoperative Central Nervous System Symptoms in 24 Patients with Pseudotumor Cerebri
Table 3. Additional Preoperative Obesity Comorbidity in 24 Patients with Pseudotumor Cerebri

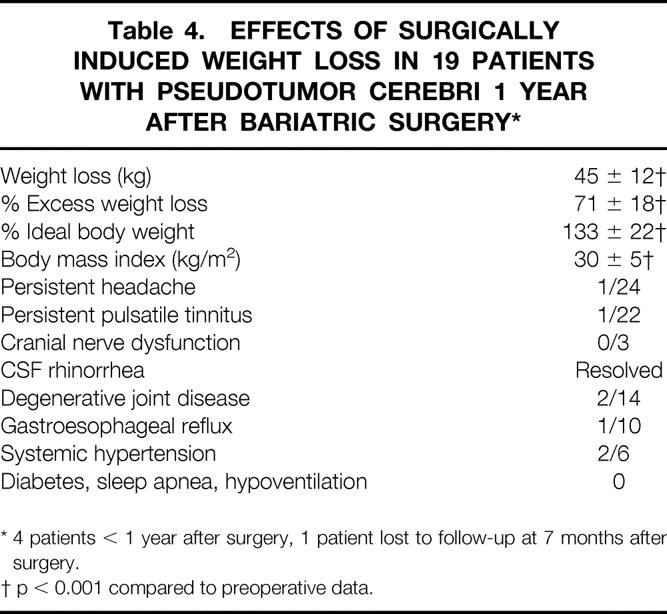
At 1 year after bariatric surgery, 19 patients had lost an average of 45 ± 12 kg (p < 0.01), which was 71 ± 18% of their excess weight. Their body mass index and percentage of ideal body weight had fallen to 30 ± 5 kg/m2 and 133 ± 22, respectively. In four patients, <1 year had elapsed since surgery, and one patient was lost to follow-up at 7 months after surgery. Five patients were lost to follow-up at 7 months to 7 years after gastric bypass. Surgically induced weight loss was associated with resolution (p < 0.01) of headache and pulsatile tinnitus in all but one patient within 4 months of the procedure, including the patient with the functioning but ineffective lumboperitoneal shunt (Table 4). Papilledema and cranial nerve dysfunctions resolved in all patients. The patient with CSF rhinorrhea had resolution within 4 weeks of gastric bypass, and the axillary and groin ulcerations healed at 6 months after weight loss in the patient with hemiplegia. Weight regain occurred in 2/19 patients not lost to follow-up, with recurrence of headache and pulsatile tinnitus. Additional comorbidities that resolved were degenerative joint disease (5/14), GERD (9/10), and hypertension (2/6); diabetes, sleep apnea, hypoventilation, and urinary incontinence resolved in all patients who had these conditions before surgery.
Table 4. Effects of Surgically Induced Weight Loss in 19 Patients with Pseudotumor Cerebri 1 Year After Bariatric Surgery*
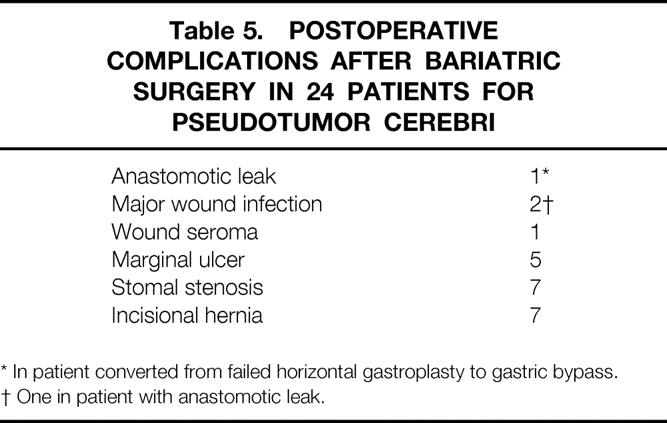
Complications seen after surgery in these patients (Table 5) included two major wound infections (one in the patient with the leak); one minor wound seroma; five patients with a marginal ulcer that responded to treatment with a hydrogen-pump inhibitor; seven patients with stenosis at the gastrojejunal anastomosis, all of whom responded to endoscopic dilatation; seven patients with incisional hernia necessitating herniorrhaphy with polypropylene mesh; and two patients with anastomotic leaks who required emergent surgery, one after conversion from a failed horizontal gastroplasty to gastric bypass. There were no clinical episodes of thrombophlebitis or pulmonary embolism, major wound infections (defined as requiring readmission to the hospital or a delay in discharge), or small bowel obstructions necessitating adhesiolysis. There were no known deaths in this group of patients followed for up to 14 years.
Table 5. Postoperative Complications After Bariatric Surgery in 24 Patients for Pseudotumor Cerebri
DISCUSSION
In a previous study of eight patients with PTC, 4 we noted a significant decrease in CSF pressure from 353 ± 35 to 168 ± 12 mm H2O (p < 0.001) at 34 ± 8 months after gastric bypass surgery, associated with resolution of headaches and pulsatile tinnitus in all patients (Fig. 1). The current study supports the efficacy of surgically induced weight loss for the treatment of PTC in morbidly obese patients. Ventriculo- or lumboperitoneal shunts are widely used for this condition but have a high frequency of failure. 10 In a study of shunts placed in 21 patients, 8 patients required >3 revisions: 1 had 6, 2 required 10, 1 required 29, and 1 patient had 38 shunt revisions! 11 It is probable that the high rate of shunt failure in these patients is secondary to shunting from one high-pressure system (the CSF) to another (the abdomen). Further, as occurred in one patient in the current study, there is a risk of bleeding and hemiplegia associated with the intracranial placement of a ventriculoperitoneal shunt.
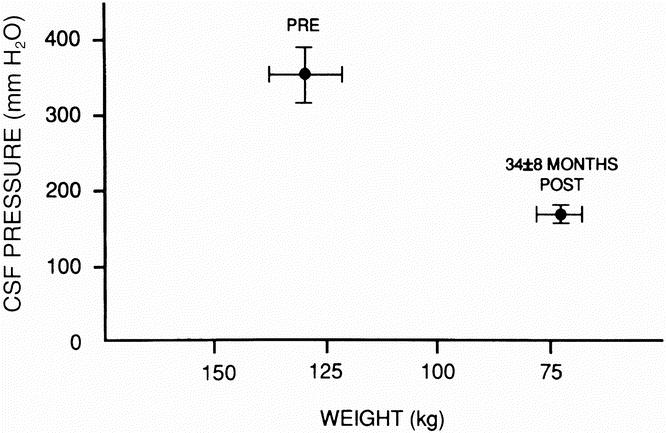
Figure 1. Mean cerebrospinal fluid pressure and weight before and 34 ± 8 months after surgically induced weight loss (p < 0.001, postoperative weight and cerebrospinal fluid pressure vs. preoperative values. (Reprinted with permission from Sugerman HJ, Felton WL III, Salvant JB Jr, Sismanis MD, Kellum JM. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology 1995; 45:1655–1659.)
Many other severely obese patients in our center who underwent gastric bypass and also complained of persistent headache and mild pulsatile tinnitus may have had PTC but did not undergo a complete evaluation for this problem. Other possible causes for headache in this population include systemic hypertension and obstructive sleep apnea. The true incidence of PTC would be difficult to ascertain because of the unpleasant and invasive nature of measuring CSF or intracranial pressure. Almost half of our patients were considered to have PTC in the absence of papilledema, including the patient with the functioning lumboperitoneal shunt. There have been several reports of PTC in the absence of papilledema. 12–14 Headache was present in all and pulsatile tinnitus in all but two of our patients with PTC without papilledema; both symptoms resolved in all but one of these patients after surgically induced weight loss.
One of the potential complications of PTC is the risk of blindness. Although most of the patients had visual field defects, no patient was considered to have an imminent risk of total loss of vision. Treatment for this condition would be emergent optic nerve fenestration 15 rather than gastric surgery for obesity. One patient in this study had both intermittent Bell’s palsy and trigeminal neuralgia. There were two other patients who had each of these problems. The Bell’s palsy responded to periodic spinal taps and CSF decompression. Trigeminal neuralgia was treated in both instances with neurovascular decompression. Bell’s palsy and trigeminal neuralgia are reported to be rare complications of PTC. 16,17
In addition to the benefit of weight loss on PTC, this study, as have others, 18–20 also found marked improvement in other obesity comorbidities shared by these patients, including hypertension, degenerative joint disease symptoms, GERD, obesity hypoventilation, sleep apnea, and type II diabetes mellitus. The long-term follow-up in this study was poor. However, other studies with 80% to 95% follow-up have documented that the percentage of excess weight loss averages 60% at 5 years and 50% at 10 years after surgery, far better than any dietary weight-reduction program. 6,21 Staple-line disruption, a previously reported frequent cause of surgical failure, is now approximately 1% in our experience using three or four superimposed firings of a two-row horizontal stapler. 6,19 The primary cause for failure, defined as the loss of <40% of excess weight and seen in approximately 15% of patients, is the frequent ingestion of high-fat junk foods such as french-fried potatoes or potato chips, which crumble quickly and are not restricted by the small gastrojejunal anastomosis.
The hypothesis that increased intraabdominal pressure is responsible for increased CSF pressures in obese patients was derived from several lines of evidence. In a previous study, we noted extremely high central venous, pulmonary artery, and pulmonary artery occlusion pressures in severely obese patients with obesity hypoventilation syndrome. 22 These pressures remained elevated after gastric bypass surgery and mechanical ventilation. Further, the pulmonary artery occlusion pressure was almost always within 10 mm Hg of pulmonary artery diastolic pressure; therefore, it did not seem reasonable that these pressures were secondary to hypoxemic pulmonary artery vasoconstriction. The first clue to the probable pathophysiology of these phenomena came from a study of urinary bladder pressures in obese women with stress overflow urinary incontinence, measured before and 1 year after gastric bypass. 23 These pressures were noted to be extremely high before weight-reduction surgery, as high as or higher than urinary bladder pressures seen in severely ill nonobese patients with an acute abdominal compartment syndrome. Emergent abdominal decompression is thought to be warranted in these patients with an acute abdominal compartment syndrome when the urinary bladder pressure exceeds 25 cm H2O to treat acute pulmonary and renal failure. In another study of 84 morbidly obese patients and 5 patients with ulcerative colitis, urinary bladder pressure was noted to be 19 ± 1.9 cm H2O before gastric bypass surgery for obesity, significantly higher than the nonobese patients with colitis, in whom it averaged 7 ± 1.6 cm H2O before a colectomy, ileoanal pouch procedure. 24 Additionally, the urinary bladder pressure correlated (r = 0.68, p < 0.001) with the sagittal abdominal diameter in these obese patients (Fig. 2). One year after gastric bypass surgery, weight loss was associated with a significant decrease in both abdominal diameter and urinary bladder pressure. 25 We hypothesized that centrally obese patients have a chronic abdominal compartment syndrome. Presumed comorbidity related to chronically increased intraabdominal pressure included obesity hypoventilation syndrome, GERD, venous stasis disease, systemic hypertension, and an increased risk of incisional hernia (as seen in the patients in this study).
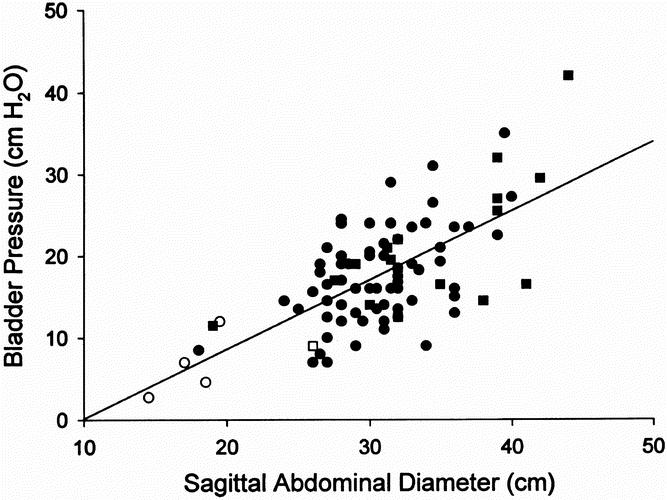
Figure 2. Correlation between urinary bladder pressure and sagittal abdominal diameter in 84 morbidly obese patients (filled square, men; filled circle, women) and 5 control nonobese patients (open square, men; open circle, women) with ulcerative colitis (r = 0.67, p < 0.0001). (Reprinted with permission from Sugerman HJ, Windsor ACJ, Bessos MK, Wolfe L. Abdominal pressure, sagittal abdominal diameter and obesity co-morbidity. J Int Med 1997; 241:71–79.)
These findings led to the hypothesis that increased intraabdominal pressure pushes the diaphragm superiorly and increases intrathoracic pressure. This increased intrathoracic pressure raises cardiac filling pressures (central venous, pulmonary artery, pulmonary artery occlusion pressures) and decreases venous drainage from the brain. 26 We postulated that this is the cause for elevated intracranial pressure, not only in PTC but also in patients with the acute abdominal compartment syndrome. 27 In an acute porcine study, 28 when abdominal pressure was increased, intrathoracic pressure, cardiac filling pressures, and intracranial pressure also increased, but they were prevented from increasing in another group of animals with a median sternotomy and pleuropericardiotomy (Fig. 3). In another study, we found that five patients with PTC had increased urinary bladder pressures (22 ± 3 cm H2O), transesophageal pleural pressures (15 ± 10 mm Hg), and increased cardiac filling pressures (central venous pressure 20 ± 6 mm Hg, pulmonary artery pressure 31 ± 6 mm Hg, pulmonary artery occlusion pressure 21 ± 7 mm Hg) at the time of gastric bypass surgery. 29
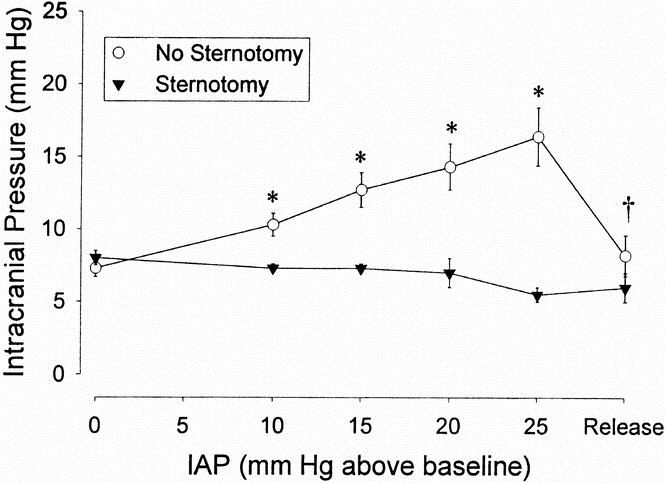
Figure 3. Increased intracranial pressure secondary to increased intraabdominal pressure in an acute porcine model with the intraabdominal instillation of isosmotic polyethylene glycol and prevention of the increased intracranial pressure with median sternotomy and pleuropericardiotomy. (Reprinted with permission from Bloomfield GL, Ridings PC, Blocher CR, Sugerman HJ. Increased pleural pressure mediates the effects of elevated intra-abdominal pressure upon the central nervous and cardiovascular systems. Crit Care Med 1997; 25:496–503.)
Finally, we published a case report of a patient with combined abdominal and head trauma in whom an acute abdominal compartment syndrome developed associated with markedly elevated cardiac filling pressures and low cardiac and urine outputs; this patient required drainage of fluid from a ventriculostomy catheter to lower a markedly increased intracranial pressure. 30 All pressures returned to normal after abdominal decompression, including intracranial pressure, and CSF drainage by ventriculostomy was no longer required.
The data from these studies strongly support the hypothesis that increased intraabdominal pressure is the cause of the increased intracranial pressure in PTC associated with obesity.
Many of the patients in this study had a rapid relief of their headache, often within 3 months of their surgery, when they had lost only approximately 20% of their excess weight. The patient with CSF rhinorrhea had resolution of this problem within 4 weeks of surgery. Similar rapid improvements in lower extremity venous stasis, obesity hypoventilation, and systemic hypertension may be explained by the hypothesis that the abdomen in these patients is similar to a tense balloon in which, according to LaPlace’s law, only a modest deflation is required to produce a significant decrease in pressure. We have developed an externally applied continuous negative abdominal pressure device and plan to measure its effects in centrally obese patients with PTC on headache and pulsatile tinnitus symptoms, papilledema, and urinary bladder pressures before, during, and after its application.
There are some concerns with the presented hypothesis. Why is PTC rarely seen in severely obese men, who usually have central obesity and an increased intraabdominal pressure, as reflected in an elevated urinary bladder pressure? 23 Why do some severely obese women have PTC and some do not? The latter may be partly explained by the more prevalent female gynoid distribution of fat in the buttocks and hips, which is not likely to raise intraabdominal pressure. Many of our morbidly obese patients had headaches before surgery, which resolved after surgically induced weight loss. Some of these headaches may be vascular or tension in origin, some related to obstructive sleep apnea syndrome, but some may be associated with an increased intracranial pressure without papilledema, or with a combination of these factors.
We have hypothesized that systemic hypertension associated with obesity is secondary to increased intraabdominal pressure. There are three potential mechanisms for this hypothesis, each of which could lead to activation of the renin-angiotensin-aldosterone system, resulting in salt and water retention as well as vasoconstriction:
• Increased renal venous pressure, causing back-pressure on the glomerulus with glomerular capillary damage associated with the proteinuria often seen in severe obesity
• Direct pressure on the kidneys
• Increased intrathoracic pressure, decreasing venous return to the heart and, therefore, cardiac output.
Animal studies are in progress to evaluate these potential mechanisms. If these hypotheses are correct, then it is also possible that increased intraabdominal pressure is responsible for the hypertension, proteinuria, headaches, seizures, and potential hepatic rupture seen in toxemia of pregnancy.
In conclusion, gastric bariatric surgery was associated with resolution of PTC in morbidly obese women in the current study. Further, additional obesity comorbidity, such as hypertension, degenerative joint disease, GERD, obesity hypoventilation, sleep apnea, venous stasis disease, and diabetes, also resolved with surgically induced weight loss. Previous studies support the hypothesis that PTC associated with obesity is secondary to increased intraabdominal pressure. This is probably the explanation for the high failure rate of ventriculo- or lumboperitoneal shunts for the treatment of this condition. Further studies are needed to evaluate the hypothesis that increased intraabdominal pressure is the cause of systemic hypertension in obesity and toxemia of pregnancy.
Discussion
Dr. J. Patrick O’Leary (New Orleans, Louisiana): The presentation today deals with a rare phenomenon which may not be so rare after all. My own personal experience with this as a primary diagnosis from which bariatric surgery was contemplated and performed goes back to 1975 with a JI bypass, and there are 7 patients in this group.
I called the neurologist who sent me that patient in 1975 and talked with him recently after I received the manuscript, and 22 years later that patient is alive and well with weight controlled and does not have symptoms of pseudotumor.
The authors propose that this is a compartment syndrome related to increased intraabdominal pressure, which produces increased intrathoracic pressure, thereby impeding flow in the venous system from the brain. There are several questions that I would like to ask.
Firstly, there are other models now of increased impedance to venous from the brain such as tricuspid insufficiency, congestive heart failure, superior vena cava syndrome, and primary pulmonary hypertension in humans. Do they have an increased incidence of pseudotumor cerebri?
Secondly, women are affected much more commonly than are males, and yet males more commonly have an android type of distribution of fat. And if you saw Dr. Sugerman’s slide, they actually have increased intraabdominal pressures, more so than women. So why, if that’s the case, Harvey, do men not get pseudotumor cerebri?
Thirdly, when did the symptoms disappear? Is it a body mass index thing, or is it simply a matter of pounds lost?
And, finally, there is another compartment syndrome that women are exposed to, and that is pregnancy. Is there increased incidence of pseudotumor cerebri in the pregnant woman, or are they protected by some other mechanism?
Dr. Walter J. Pories (Greenville, North Carolina): At lunch the other day, a medical student asked several of us to define the terms “surgery” and “surgeon.” We had a lively discussion that even included a call to the American College, but we were stumped. The best we could do was to agree that a “surgeon cares for surgical diseases.”
It wasn’t a very good answer. What is a surgical disease? In our lives, the definition of surgery has changed to a remarkable degree. Many of you here will remember when Hodgkin’s disease of the neck and tuberculosis of the lung were surgical diseases, and when heart attacks and renal failure were the province of the nonsurgeons.
Dr. Sugerman has expanded our horizon even more today. Who would have thought that pseudotumor cerebri with its severe headaches, cranial nerve disorders, and even blindness would end up as a general surgical disease? And his concepts are correct: this is not an uncommon disorder.
Even a brief review of our series at East Carolina revealed seven cases who were mirror images to his cohort: similar in age, all were women ranging from 29 to 48, and differing in only one respect—six of our seven patients, or 86%, had abnormal glucose tolerance curves compared to the sole diabetic in his group, or 4%. I believe the difference relates to our different interests: ours in diabetes and his in neurologic disease.
So I rise to congratulate Dr. Sugerman for emphasizing still another aspect of morbid obesity, that increasingly common, serious, and still unexplained disease. Yes, who would have thought it? General surgeons successfully treating diabetes, infertility, Pickwickian syndrome, arthritis, and now even diseases of the brain just with an operation on the gut!
And, oh yes, we finally did come up with a definition of a surgeon: A real surgeon is a physician who also operates.
Dr. Ward O. Griffen, Jr. (Frankfort, Michigan): I won’t ask any questions that have been asked previously, but I do have some difficulty with the hypothesis that has been proposed. As I understood the manuscript, none of these patients had their intraabdominal pressure measured either before or after the procedure. I think you need to do that if you’re going to conclude that the etiology has some relationship to the intraabdominal pressure.
Secondly, it is interesting that these patients’ symptoms resolved after 3 or 4 months, but clearly they have not had their permanent weight reduction. Although it is true that they maintain their amelioration of symptoms, if they continue to have satisfactory weight reduction, but if they regain their weight, their symptoms return.
Thirdly, I would like to ask you the questions about the five patients with marginal ulcer. I notice in the manuscript that, I believe, six of your patients had the super-obese operation, which is a longer Roux-en-Y limb. And I wondered if the margin ulcerations occurred in those patients with the longer limb or if it was spread over the entire group.
There was one patient also on whom you did a gastric banding, which I think is sort of a Band-Aid kind of approach to this disease. And I wondered that patient had any kind of satisfactory weight reduction.
And I would echo what Dr. Pories has said: Who would have “thunk” that surgeons would be treating a whole host of what were formerly regarded as internal medicine diseases?
Dr. Sugerman also provided me with a comment from a neurosurgeon in Miami by the name of Philip Villaneuva, who is an associate professor in the department of neurosurgery. And I think he asked some interesting questions which I would like to include as a part of the discussion under his name.
Dr. Philip A. Villanueva (Miami, Florida): This is a very interesting paper by Dr. Sugerman and his team from the Medical College of Virginia. The problem of pseudotumor cerebri has remained a significant one in the neurosurgical field. The currently utilized procedures, i.e., lumboperitoneal shunt or ventriculoperitoneal or ventriculoatrial shunt, certainly have been characterized by a significant degree of failure and a need for multiple revisions. The concept advanced by Dr. Sugerman and his group, that of shunting one high-pressure system into another, would seem to explain this.
The high pressure of the intraabdominal compartment clearly can reduce the venous flow from the intracranial space, leading to progressively elevated intracranial pressures. This has been seen not only in chronic conditions but also in the acute setting. The other interesting thing in this group of patients is the relatively rapid reduction of intracranial pressure symptoms and findings following bypass surgery.
On a long-term basis, one can easily understand the reduction in intracranial pressure. However, at the early stages, is it possible to postulate a direct mechanical effect of surgery, i.e., fenestration of the abdominal cavity and hence intracranial pressure reduction on an immediate basis, to be followed by progressive and permanent reduction?
The long-term success rate cited by Dr. Sugerman and his team is very impressive. He compares extremely favorably with the neurosurgical experience in terms of shunting, and indeed the results certainly seem better. The direct risk factors to the central nervous system are obviously greatly reduced in this procedure. Also, the concept of being able to reduce intracranial pressure without placing a prosthetic device (shunt system) is quite attractive.
I note that CSF dynamic studies were not carried out on all patients. Once relieved of their symptoms, one would not normally expect a need to repeat dynamic studies. However, Galbraith and Cardoso have demonstrated three peaks of the intracranial pressures wave form. The first was referable to the arterial pulse, the second to intracranial compliance, and the third to extracranial factors. It would be extremely interesting to record the wave forms of these patients before and after their abdominal procedures. I would imagine that the third component, i.e., the extracranial component of the intracranial pressure wave form, would be initially elevated and decreased postoperatively. I would also be very interested in seeing if the second peak, that of the intracranial compliance, eventually declined after the procedure. This information would likely put to rest any questions regarding the efficacy of this procedure.
In conclusion, Dr. Sugerman and his team have very elegantly demonstrated the efficacy of an extracranial procedure in treating a neurological complication of morbid obesity. The data presented are clear and cogent and certainly oblige the neurosurgical community to recognize this as a definitive if not the definitive treatment for pseudotumor cerebri.
Dr. Harvey J. Sugerman (Closing Discussion): To answer the questions, starting with Pat: superior vena cava thrombosis and the superior vena cava syndrome are associated with headaches and pulsatile tinnitus and symptoms of pseudotumor. In addition, sagittal sinus thrombosis or compression associated with mastoiditis and acoustic neuromas are also causes of pseudotumor. Thus, any cause of decreased venous drainage from the brain can lead to pseudotumor cerebri.
Why don’t men, who have a higher prevalence of central obesity, have a greater frequency of pseudotumor, rather than the other way around? And this is to partially respond to Ward Griffen’s question about these patients. Clearly this is an important question to which I don’t know the answer. It is hypothesized by our neurosurgical colleagues that there may be a valve in the internal jugular vein in men that prevents transmission of the intrathoracic pressure to the venous system of the brain, but further studies are clearly needed in that regard.
Pat, I’m glad you asked about pregnancy. Pseudotumor cerebri is definitely increased in pregnancy, and furthermore it is my hypothesis that increased intraabdominal pressure in these women produces a subacute abdominal compartment syndrome, in contrast to a chronic abdominal compartment syndrome in obesity, and I think that this is the cause of preeclampsia and eclamptic seizures.
We have developed a device which we call the Ab Shell that fits over the abdomen and uses vacuum to lower intraabdominal pressure. We have had approval from the Food and Drug Administration to study this device in morbidly obese patients. In five of these women with pseudotumor, the device led to rapid relief of their headache, which remained absent through most of the following day after sleeping in the device at night. We are currently trying to get FDA approval to study the device in preeclampsia.
One of our patients with pseudotumor is undergoing gastric bypass by Dr. John Kellum, a member of this organization, today. She had a lumboperitoneal shunt placed that threatened blindness; however, it didn’t relieve her headaches or tinnitus. In studies with the device and in her this past week, we found her urinary bladder pressures to be 220 mm of water and her shunt pressure to be 210 mm, further supporting the hypothesis of the problems with lumboperitoneal or ventriculoperitoneal shunts.
With regards to Dr. Griffen’s question about the marginal ulcer incidence, it didn’t seem to be restricted to the long limb bypass; it was distributed throughout the population. And we don’t know why the incidence was higher in this group than in our average incidence, which runs about 12%. We think maybe it could be related to tension at the anastomosis, but we’re not sure.
This patient who had the gastric banding did have an excellent weight loss. In fact, she had the best weight loss of all the gastric band patients we’ve studied to date. But many of the gastric band patients that we’ve studied have not lost any weight, and I think that this is a procedure that needs to be critically and thoroughly evaluated by the Food and Drug Administration before it is proposed for everybody to have it.
A subset of these patients were studied and the data published in Neurology. They were found to have increased urinary bladder pressure, sagittal abdominal diameter, central venous pulmonary artery and pulmonary artery occlusion pressures.
In another study published in the International Journal of Obesity, we found a significant decrease in sagittal abdominal diameter, urinary bladder pressure, and presumed intraabdominal pressure-related morbidity 1 year following gastric-bypass–induced weight loss.
Dr. Villanueva is probably correct regarding the dynamic CSF-pressure response to decreasing intraabdominal pressure. These wave forms can be deduced from ventriculostomy catheters placed in head-injury patients, but would be markedly dampened when measuring these pressures through a lumbar CSF catheter. The ventricles in pseudotumor patients are very narrow, and it would be technically very difficult as well as unethical to place a ventriculostomy catheter in these patients.
With regards to Dr. Villanueva’s comments, although the pressures may go down during the operation with the abdomen open, it would return to baseline or even higher with bowel edema after closing the abdomen.
And again, to answer your question, Ward, I believe the rapid improvements in symptoms is probably due to the rapid decrease in abdominal and therefore intrathoracic and intracranial pressures with weight losses that we often see within a month of surgery of 25 to 50 pounds. That may be analogous to the rapid decrease in pressure and wall tension after letting a little air out of a very tense balloon, as shown in LaPlace’s Law.
Footnotes
Correspondence: Harvey J. Sugerman, MD, P.O. Box 980519, MCV/VCU, Richmond, VA 23298-0519.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Accepted for publication December 1998.
References
- 1.Newborg B. Pseudotumor cerebri treated by rice-reduction diet. Arch Intern Med 1974; 133: 802–807. [PubMed] [Google Scholar]
- 2.Amaral JF, Tsiaris W, Morgan T, Thompson WR. Reversal of benign intracranial hypertension by surgically induced weight loss. Arch Surg 1987; 122: 946–949. [DOI] [PubMed] [Google Scholar]
- 3.Noggle JD, Rodning CB. Rapidly advancing pseudotumor cerebri associated with morbid obesity: an indication for gastric exclusion. South Med J 1986; 79: 761–763. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman HJ, Felton WL III, Salvant JB Jr, et al. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology 1995; 45: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 5.Sismanis A, Butts FM, Hughes GB. Objective tinnitus in benign intracranial hypertension: an update. Laryngoscope 1990; 100: 331–336. [DOI] [PubMed] [Google Scholar]
- 6.NIH Conference: Gastrointestinal surgery for severe obesity: Consensus Development Conference Panel. Ann Intern Med 1991; 115:956–961. [PubMed]
- 7.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg 1987; 205: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brolin RE, Kenler HA, Gorman JH, Cody RP. Long-limb gastric bypass in the superobese: a prospective randomized study. Ann Surg 1992; 215: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belachew M, Legrand M, Vincent V, et al. Laparoscopic adjustable gastric banding. World J Surg 1998; 22: 955–963. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg ML, Corbett JJ, Smith C, et al. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology 1993; 43: 1071–1072. [DOI] [PubMed] [Google Scholar]
- 11.Burgett RA, Purvin VA, Kawasaki A. Lumboperitoneal shunting for pseudotumor cerebri. Neurology 1997; 49: 734–739. [DOI] [PubMed] [Google Scholar]
- 12.Scanarini M, Mingrino S, d’Avella D, DellaCorte V. Benign intracranial hypertension without papilledema: case report. Neurosurgery 1979; 5: 376–377. [PubMed] [Google Scholar]
- 13.Spence JD, Amacher AL, Willis NR. Benign intracranial hypertension without papilledema: role of 24-hour cerebrospinal fluid pressure monitoring in diagnosis and management. Neurosurgery 1980; 7: 326–336. [DOI] [PubMed] [Google Scholar]
- 14.Marcellis J, Silberstein SD. Idiopathic intracranial hypertension without papilledema. Arch Neurol 1991; 48: 392–399. [DOI] [PubMed] [Google Scholar]
- 15.Corbett JJ, Nerad JA, Tse DT, Anderson RL. Results of optic nerve fenestration for pseudotumor cerebri. Arch Ophthalmol 1988; 106: 1391–1397. [DOI] [PubMed] [Google Scholar]
- 16.Capobianco DJ, Brazis PW, Cheshire WP. Idiopathic intracranial hypertension and seventh nerve palsy. Headache 1997; 37: 286–288. [DOI] [PubMed] [Google Scholar]
- 17.Davenport RJ, Will RG, Galloway PJ. Isolated intracranial hypertension presenting with trigeminal neuropathy. J Neurol Neurosurg Psychiatry 1994; 57: 381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugerman HJ, Fairman RP, Sood RK, et al. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr 1992; 167: 392S–399S. [DOI] [PubMed] [Google Scholar]
- 19.Foley EF, Benotti PN, Borlase BC, et al. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg 1992; 163: 294–297. [DOI] [PubMed] [Google Scholar]
- 20.Carson JL, Ruddy ME, Duff AE, et al. The effect of gastric bypass surgery on hypertension in morbidly obese patients. Arch Intern Med 1994; 154: 193–200. [PubMed] [Google Scholar]
- 21.Pories WJ, MacDonald KG, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-year follow-up. Am J Clin Nutr 1992; 55: 582S–585S. [DOI] [PubMed] [Google Scholar]
- 22.Sugerman HJ, Baron PL, Fairman RP, et al. Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss. Ann Surg 1988; 207: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bump RC, Sugerman HJ, Fantl JA, McClish DK. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol 1992; 167: 392–399. [DOI] [PubMed] [Google Scholar]
- 24.Sugerman HJ, Windsor ACJ, Bessos MK, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity co-morbidity. J Int Med 1997; 241: 71–79. [DOI] [PubMed] [Google Scholar]
- 25.Sugerman H, Windsor A, DeMaria E, Kellum J. Effects of surgically induced weight loss on urinary bladder pressure, sagittal abdominal diameter and obesity co-morbidity. Int J Obesity Metabol Disord 1998; 22: 230–235. [DOI] [PubMed] [Google Scholar]
- 26.Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after volume expansion. J Trauma 1995; 39: 1168–1172. [DOI] [PubMed] [Google Scholar]
- 27.Bloomfield GL, Ridings PC, Blocher CR, et al. Effects of increased intra-abdominal pressure upon intracranial and cerebral perfusion pressure before and after volume expansion. J Trauma 1996; 40: 936–943. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfield GL, Ridings PC, Blocher CR, Sugerman HJ. Increased pleural pressure mediates the effects of elevated intra-abdominal pressure upon the central nervous and cardiovascular systems. Crit Care Med 1997; 25: 496–503. [DOI] [PubMed] [Google Scholar]
- 29.Sugerman HJ, DeMaria EJ, Felton WL III, et al. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology 1997; 49: 507–511. [DOI] [PubMed] [Google Scholar]
- 30.Bloomfield GL, Dalton JM, Sugerman HJ, et al. Treatment of increasing intracranial pressure secondary to the acute abdominal compartment syndrome in a patient with combined abdominal and head trauma. J Trauma 1995; 30: 1101–1103. [DOI] [PubMed] [Google Scholar]


