Abstract
Objective
To determine whether alteration in wound exudate cell immune function occurs after trauma-hemorrhage.
Background
Although clinical and experimental studies indicate that the rate of wound infection is increased after trauma and hemorrhagic shock, the underlying mechanism for this increased susceptibility remains unknown.
Methods
Male C3H/HeN mice were subjected to a midline laparotomy and polyvinyl alcohol sponges were implanted subcutaneously in the abdominal wound before hemorrhage (35 ± 5 mm Hg for 90 minutes and resuscitation) or sham operation. The wound exudate cells from the sponges were harvested on the first, third, and fifth postoperative day and cultured for 24 hours in the presence of lipopolysaccharide (10 μg/ml) or heat-killed Staphylococcus aureus. Interleukin (IL)-1β, IL-6, monocyte chemotactic protein 1, macrophage inflammatory protein 2, and nitrite levels were determined in the supernatants. The distribution of macrophages and polymorphonuclear leukocytes was assessed in the sponge with and without in vivo injection of S. aureus. The phagocytic activity of isolated wound exudate cells was determined using fluorescent S. aureus.
Results
The composition of exudate cells was unaltered by hemorrhagic shock; however, in vivo injection of S. aureus significantly decreased the percentage of macrophages under such conditions. Wound exudate cell phagocytic activity and the release of IL-1β, IL-6, monocyte chemotactic protein 1, and macrophage inflammatory protein 2 was decreased on the first postoperative day. The release of IL-1β and IL-6 was also decreased on the third postoperative day in hemorrhaged mice. On the fifth postoperative day, wound exudate cell cytokine production was comparable to that in shams.
Conclusions
Because most wound infections occur early after severe trauma, these results suggest that the dysfunction of wound exudate cells after hemorrhage might contribute to the increased incidence of wound infections. Therefore, attempts to enhance or restore wound cell immune function might be helpful for decreasing the incidence of wound infections in trauma victims.
Several studies have indicated that cell-mediated immune responses are markedly depressed in male patients after trauma and hemorrhagic shock, and that these changes persist for ≥5 days after resuscitation. 1,2 In this respect, a decreased release of proinflammatory cytokines by splenic and peritoneal macrophages (Mφ) in response to lipopolysaccharide (LPS) has been demonstrated. Further, this hyporesponsiveness of immune cells has been shown to be associated with an increased morbidity and mortality rate from subsequent sepsis. 3,4
In addition to an increased susceptibility to polymicrobial sepsis, an increased rate of wound infection has also been reported in clinical and experimental studies after severe trauma and blood loss. 5–8 In trauma patients, blood loss, shock, and the duration of hypotension have been identified as significant risk factors for the development of wound infection. 9,10 Similarly, a significantly higher number of wound complications have been documented after surgery for bleeding peptic ulceration versus nonbleeding ulceration. 11 Livingston and Malangoni 5 noted an increased susceptibility to wound infection after hemorrhagic shock in rats, as evidenced by an increased presence of gross purulence at the wound site after Staphylococcus aureus injection. Moreover, antibiotic prophylactics failed to reduce the incidence of wound infection. 5 Further, hypovolemic shock and the local administration of epinephrine have been shown to increase the virulence of a dermal staphylococcal infection. 6
The above studies suggest that defense mechanisms at the wound site may be compromised after hemorrhagic shock. We hypothesize that hemorrhagic shock might depress the function of wound immune cells, thereby increasing the rate of wound infections. The aim of this study, therefore, was to determine whether hemorrhagic shock produces any alterations in wound exudate cell function.
MATERIALS AND METHODS
Animals
Inbred male C3H/HeN mice (Charles River Laboratories, Wilmington, MA), 7 weeks old and weighing 24 to 27 g, were used in this study. All procedures were carried out in accordance with the guidelines set forth in the Animal Welfare Act and The Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. This project was approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital and Brown University.
Experimental Groups
Male mice were randomized into two groups (six to eight per group). The mice in group 1 underwent trauma alone (sham animals). The mice in group 2 were subjected to the trauma-hemorrhage procedure (hemorrhage animals).
Trauma Procedure and Sponge Implantation
Mice were lightly anesthetized with methoxyflurane (Metofane, Pitman-Moore, Mundelein, IL) and restrained in a supine position, and the skin was disinfected using 75% ethanol. A 2.5-cm midline laparotomy (i.e., trauma induced) was performed and the muscular layer was then closed aseptically using 6-0 Ethilon sutures (Ethicon, Inc., Somerville, NJ). Polyvinyl alcohol sponges (0.8 × 0.5 × 0.5 cm) were then aseptically implanted subcutaneously (four per animal) next to the incision site, avoiding contamination or infection of the wound site. The skin incision was then closed using 9-mm surgical clips (MikRon Autoclip, Clay Adams, Parsippany, NJ).
Hemorrhage Procedure
After the midline laparotomy and sponge implantation, both femoral arteries were aseptically cannulated with polyethylene 10 tubing (Clay Adams) using a minimal dissection technique. Blood pressure was constantly monitored by attaching one of the catheters to a blood-pressure analyzer (Digi-Med, Louisville, KY). On awakening, the animals were bled rapidly through the other catheter to a mean arterial blood pressure of 35 ± 5 mm Hg (prehemorrhage pressure was 95 ± 5 mm Hg), which was maintained for 90 minutes. At the end of that period, the animals were resuscitated with lactated Ringer’s solution (four times the shed blood volume over 30 minutes) to provide adequate fluid resuscitation. Lidocaine was applied to the groin incision sites, the catheters were removed, the vessels were ligated, and the groin incisions were closed.
Sham-operated animals underwent the same groin dissection, which included ligation of both femoral arteries; however, neither hemorrhage nor fluid resuscitation was carried out.
No deaths were observed in this trauma-hemorrhage model.
Preparation of Wound Exudate Cells From the Sponges
The animals were killed by methoxyflurane overdose on the first, third, or fifth postoperative day after hemorrhage and resuscitation or sham operation. The sponges were dissected free from the surrounding connective tissue using sterile surgical methods. Wound exudate from the sponges was harvested as previously described. 12,13 In brief, the sponges from each animal were combined in ice-cold phosphate-buffered saline solution in a plastic bag and then placed in a Stomacher (Tekmar Company, Cincinnati, OH) for 30 seconds; this provides repeated compression of the sponges. The resultant cell suspension was centrifuged at 300g for 15 minutes at 4°C. The cell pellet of wound exudate cells was diluted to 1.5 × 106 cells/ml in RPMI media containing 10% fetal calf serum. One milliliter of this cell suspension was cultured on a 24-well plate for 24 hours at 37°C, 5% CO2 and 90% humidity, in the presence of 10 μg/ml of LPS W. In addition to LPS stimulation, separate wells containing 1.5 × 106 wound exudate cells per milliliter in RPMI media harvested on the first postoperative day were cultured in the presence of heat-killed S. aureus (0.001% w/v or 0.01% w/v; Calbiochem, San Diego, CA). After incubation, the cell suspension was centrifuged at 300g for 15 minutes and the supernatants were harvested and stored at −80°C until assayed for interleukin (IL)-1β, IL-6, monocyte chemotactic protein 1 (MCP-1), and macrophage inflammatory protein 2 (MIP-2) levels.
Assessment of IL-1β Release
IL-1β levels in the wound exudate cell supernatants were determined using the sandwich enzyme-linked immunoassay technique (ELISA) described by us previously. 14 In brief, 96-well plates (Inter-Med/Nunc VWR Scientific, Batavia, IL) were coated overnight (4°C) with 2.0 μg/ml monoclonal hamster antimouse IL-1β (Genzyme Diagnostics, Cambridge, MA). After repeated washings, the samples and the standard (1000 pg/ml recombinant mouse IL-1β, Genzyme Diagnostics) were added and then incubated overnight at 4°C. After repeated washings, the plates were incubated at 37°C for 1 hour with a biotinylated polyclonal rabbit antimouse IL-1β (Genzyme Diagnostics) at a concentration of 0.8 μg/ml. The plates were washed and then incubated with horseradish peroxidase for 15 minutes at 37°C. After washing, 100 μl of tetramethylbenzidine (Sigma Chemical, St. Louis, MO) was added to initiate color development. After 15 minutes, 100 μl of stop solution (2N H2SO4) was added, and the optical density at 450 nm for each well was then measured on a microplate reader (EL-311, Bio-Tek Instruments Inc., Winooski, VT). The concentration of IL-1β present in the samples was determined by interpolation against the standard curve.
Assessment of IL-6 Release
IL-6 activity in culture supernatant was determined by the degree of proliferation of the murine B-cell hybridoma cell line 7TD1, which grows only in the presence of IL-6. 15 The IL-6–sensitive murine B-cell hybridoma (7TD1) (a gift from Dr. Jacques Van Snick) was maintained as previously described. 16 Serial dilutions of Mφ supernatants were added to 4 × 104 7TD1 cells/ml, and the cells were incubated for 72 hours at 37°C in 5% CO2. For the last 4 hours of incubation, 20 μl of a 3-(4,5-dimethythiazol-2-L)-2,5-diphenyltetrazolium-bromide solution (MTT; 5 mg/ml in RPMI-1640, Sigma) was added to each well (only viable cells incorporate MTT). The assay was stopped by aspiration of 150 μl supernatant from each well, with subsequent replacement by 100 μl of 10% sodium dodecyl sulfate solution in phosphate-buffered saline (lauryl sulfate, Sigma) to dissolve the dark-blue formazan crystals. Using an automated microplate reader (EL-311, Bio-Tek Instruments), the light absorbance was measured at 570 nm.
Assessment of MIP-2 Release
MIP-2 levels in the Mφ supernatants were determined using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN) as described by the manufacturer. In brief, 50 μl of assay diluent and 50 μl of standard and sample were added to 96-well plates precoated with a polyclonal antibody specific for mouse MIP-2 and incubated for 2 hours at room temperature. After washing, 100 μl of a enzyme-linked polyclonal antibody specific for mouse MIP-2 was added to all wells and incubated for 2 hours at room temperature. After washing, 100 μl of substrate solution was added, and the color development was stopped after 30 minutes by the addition of 100 μl of stop solution. The optical density at 450 nm was then determined on a microplate reader, and the concentration of MIP-2 present in the samples was determined by interpolation against the standard curve.
Assessment of MCP-1 Release
MCP-1 levels in the wound exudate cell supernatants were determined using a mouse MCP-1 ELISA kit (Pharmingen, San Diego, CA) as described by the manufacturer. In brief, 96-well plates (Inter-Med/Nunc VWR Scientific) were coated overnight (4°C) with antimouse MCP-1 monoclonal antibody. After washing, 100 μl of samples or standard was added and incubated for 2 hours at room temperature. After washing, antimouse MCP-1 monoclonal antibody conjugated to horseradish peroxidase was added and incubated for 1 hour at room temperature. After 15 minutes, 100 μl stop solution (2N H2SO4) was added, and the optical density at 450 nm for each well was then determined on a microplate reader. The concentration of MCP-1 present in the samples was determined by interpolation against the standard curve.
Assessment of Nitrite Release
The concentration of nitrite (NO2−), a stable degradation product of nitric oxide, was measured in the supernatants of wound exudate cells by a microplate method based on the formation of chromophore after reaction with Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2.5% phosphoric acid), as previously described. 17 Fifty microliters of Griess reagent and 50 μl of supernatants were added to a 96-well plate and incubated at room temperature for 10 minutes. Absorbance was measured at 550 nm, and the concentration of NO2− in the samples was determined by interpolation against the standard curve.
Determination of the Phagocytic Activity of Wound Exudate Cells
Before the experiment, S. aureus BioParticles (Molecular Probes, Eugene, OR) were suspended in mouse plasma and incubated for 1 hour at 37°C for opsonization. To determine the phagocytic activity of wound exudate cells on the first postoperative day, 0.5 ml of 1 × 106/ml wound cells were cultured for 1 hour at 37°C, 5% CO2 and 90% humidity, in the absence or presence of 10 μg/ml of LPS. After this, 500 μl of a suspension containing 1 × 10 7 /ml opsonized fluorescent S. aureus BioParticles were added to the cell suspension and incubated in a shaking water bath for 30 minutes. The cells were washed twice with ice-cold phosphate-buffered saline to remove nonphagocytosed bacteria. Analysis of fluorescein isothiocyanate (FITC)-positive cells was performed using a FACS Vantage flow cytometer (Becton Dickinson Inc., San Jose, CA). DAPI was excited with a Coherent ANOVA-70S Spectrum laser (Spectrum Product Division, Palo Alto, CA) set to 360 nm, and fluorescent emission was detected with a 424 ± 22 nm blue reflecting dichroic filter. Cells were excited with an argon laser set to 488 nm, using a 530 ± 15-nm-wide band pass filter. No fewer than 10,000 gated granular cells were assessed per sample. Using PC-Lysis 1.0 software (Becton-Dickinson, Inc., San Jose, CA), the percentage of FITC-positive staining cells and the mean channel fluorescence of the positive staining cells were determined for each sample.
Injection of Heat-Killed S. Aureus in the Wound Site
To assess the in vivo effect of a microbial challenge at the wound site, 100 μl of a 0.01% (w/v) solution containing 1 × 108 heat-killed S. aureus per milliliter (Calbiochem) was injected in the sponges on the first postoperative day. On the second postoperative day, the animals were killed and wound exudate cells harvested. Absolute and relative numbers of wound exudate cells isolated from each animal were calculated from hemocytometer counts (total wound cells per animal) and from differential counts on LeukoStat-stained (Fisher Scientific, Pittsburgh, PA) cytospins (\f105 cells per slide) (Shandon, Pittsburgh, PA).
Statistical Analysis
The results are presented as mean ± SEM. One-way analysis of variance followed by the Student-Newman-Keuls test as a post hoc test for multiple comparisons was used to determine the significance of the differences between experimental means. Significance between the colony-forming units was determined using the Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Distribution of Mφ and Polymorphonuclear Leukocytes at the Wound Site
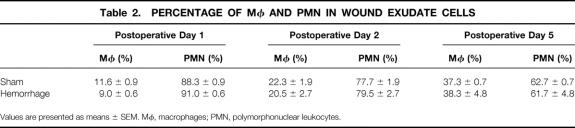
The number of wound exudate cells (Table 1) and the percentage of Mφ and polymorphonuclear leukocytes (PMNs) was similar in hemorrhaged and sham-operated animals (Table 2). The percentage of Mφ continuously increased from the first to the fifth postoperative day in both groups.
Table 1. Cells Harvested from Sponges After Hemorrhage or Sham Operation
Table 2. Percentage of Mφ and PMN in Wound Exudate Cells
After the injection of S. aureus in vivo, the number of wound exudate cells was found to be lower in hemorrhaged animals (−30% vs. sham animals, p > 0.05; Table 1). Moreover, the percentage of Mφ and the total number of Mφ in the wound exudate cells were significantly decreased (p < 0.05) after hemorrhage (Fig. 1). In contrast, the percentage of PMNs in the wound exudate cells was not significantly altered after hemorrhage versusshams(Table 2).
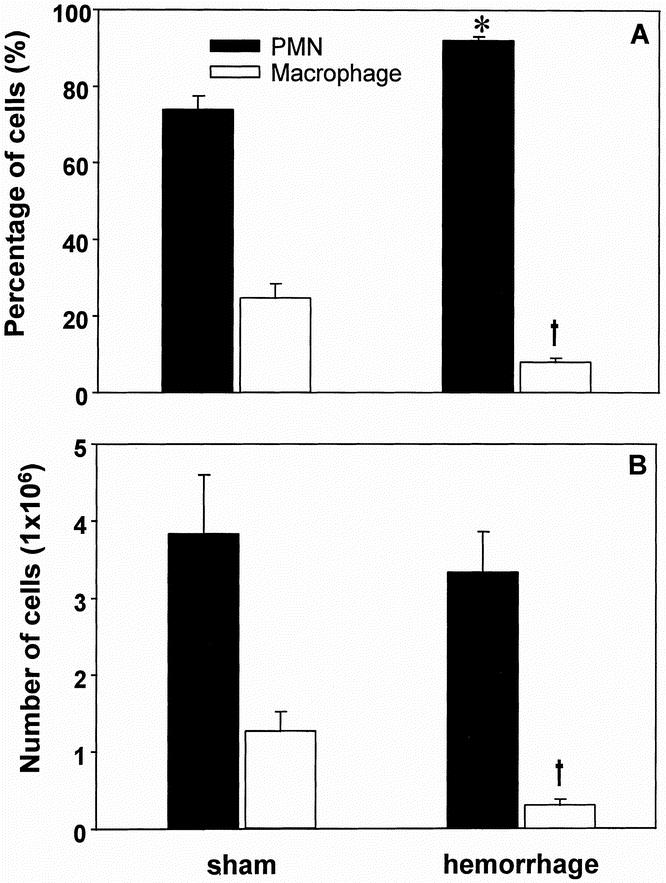
Figure 1. Differential count of wound exudate cells (percentage [A] and absolute number [B]) harvested on the second postoperative day from animals that were subjected to a subcutaneous injection with heat-killed S. aureus on the first postoperative day. Cytospins of the wound exudate cells were obtained and 300 cells were counted for differential counts. Analysis of variance, *p < 0.05 vs. PMNs from sham mice, †p < 0.05 vs. Mφ from sham mice.
Proinflammatory Cytokine Release by Wound Exudate Cells
There was a significant decrease in the LPS-stimulated release of IL-1β from wound exudate cells on the first and third postoperative days (−29.6% and −52.8% vs. sham animals, respectively, p < 0.05) after hemorrhage (Fig. 2A). On the fifth postoperative day, the capacity of wound exudate cells to release IL-1β was not different between sham and hemorrhage animals.
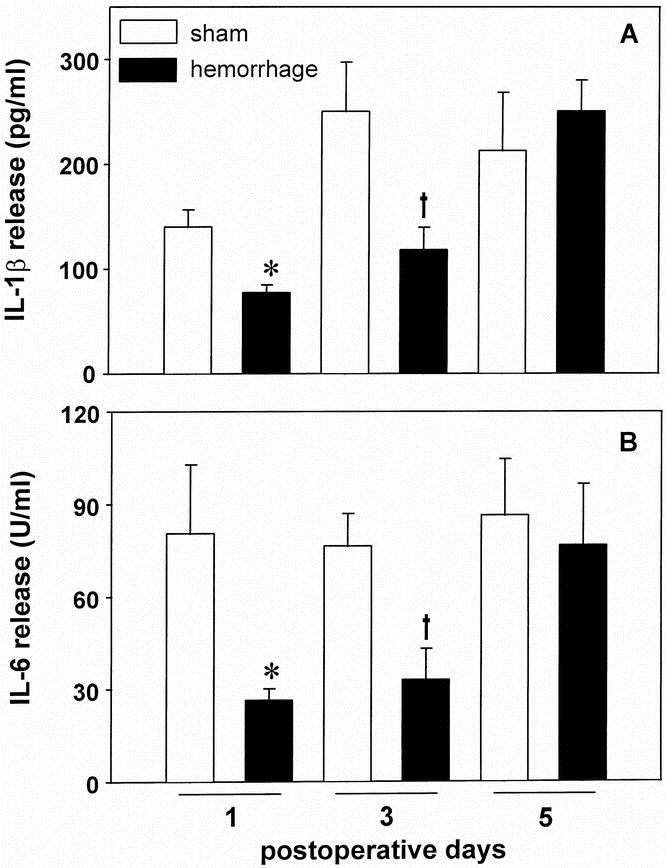
Figure 2. Release of IL-1β (A) and IL-6 (B) by wound exudate cells harvested from subcutaneous sponges on the first, third, and fifth postoperative days after hemorrhage or sham operation. The cells were cultured for 24 hours in the presence of 10 μg/ml lipopolysaccharide A. The release of IL-1β was determined by ELISA, the release of IL-6 by a specific bioassay (7TD1). Analysis of variance, *p < 0.05 vs. sham mice on the first postoperative day, †p < 0.05 vs. sham mice on the third postoperative day.
The LPS-stimulated release of IL-6 was markedly depressed on the first and third postoperative days after hemorrhagic shock (−67.2% and −57.0% vs. sham animals, respectively, p < 0.05; Fig. 2B). On the fifth postoperative day, similar to IL-1β, the release of IL-6 was not different between sham and hemorrhage animals.
Release of Chemokines by Wound Exudate Cells
The release of the chemokines MIP-2 and MCP-1 (Fig. 3) was significantly depressed on the first postoperative day after trauma-hemorrhage (−88.8% for MIP-2 and −71.6% for MCP-1 vs. sham animals, p < 0.05). On days 3 and 5 after hemorrhage, the release of MIP-2 and MCP-1 was restored to sham levels.
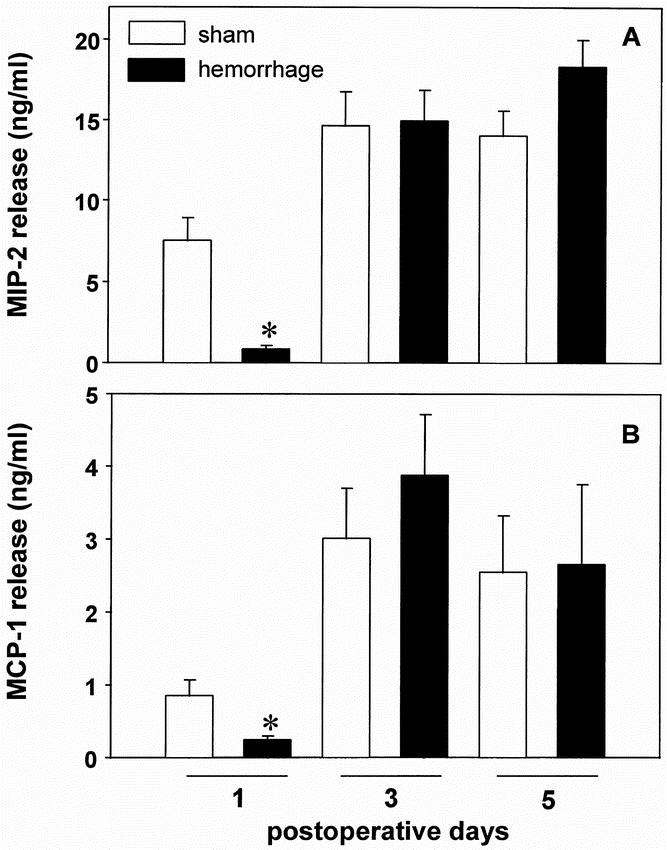
Figure 3. Release of MIP-2 (A) and MCP-1 (B) by wound exudate cells harvested from subcutaneous sponges on the first, third, and fifth postoperative days after hemorrhage or sham operation. The cells were cultured for 24 hours in the presence of 10 μg/ml lipopolysaccharide A. The release of MIP-2 and MCP-1 was determined by ELISA. Analysis of variance, *p < 0.05 vs. sham mice on the first postoperative day.
Nitrite Production by Wound Exudate Cells
The concentration of NO2−, a stable degradation product of nitric oxide, in the supernatants of wound exudate cells was significantly decreased on the first postoperative day after hemorrhage (−88.9% vs. sham animals, p < 0.05; Fig. 4). On the third and fifth postoperative days, no difference was evident in the concentration of NO2− between sham and hemorrhaged animals.
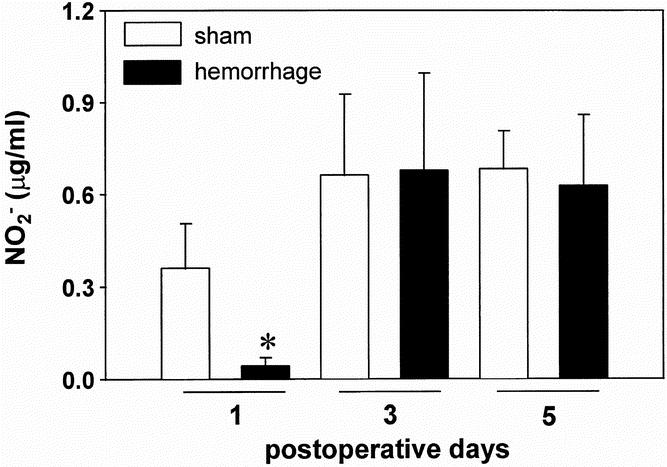
Figure 4. Concentration of nitrite in supernatants of wound exudate cells harvested from subcutaneous sponges on the first, third, and fifth postoperative days after hemorrhage or sham operation and cultured for 24 hours in the presence of 10 μg/ml lipopolysaccharide A. The concentration was determined by Griess reagent. Analysis of variance, *p < 0.05 vs. sham mice on the first postoperative day.
Cytokine Production in Response to S. aureus
In addition to culturing wound exudate cells in the presence of LPS, cells harvested on the first postoperative day were stimulated with either 0.001% (w/v) or 0.01% (w/v) heat-killed S. aureus. Wound cells from shams and hemorrhaged mice did not release different amounts of IL-6 when stimulated with 0.001% S. aureus. However, stimulation with 0.01% S. aureuscaused an approximately fourfold increase in IL-6 release by cells from shams but not from hemorrhaged animals (Fig. 5A).
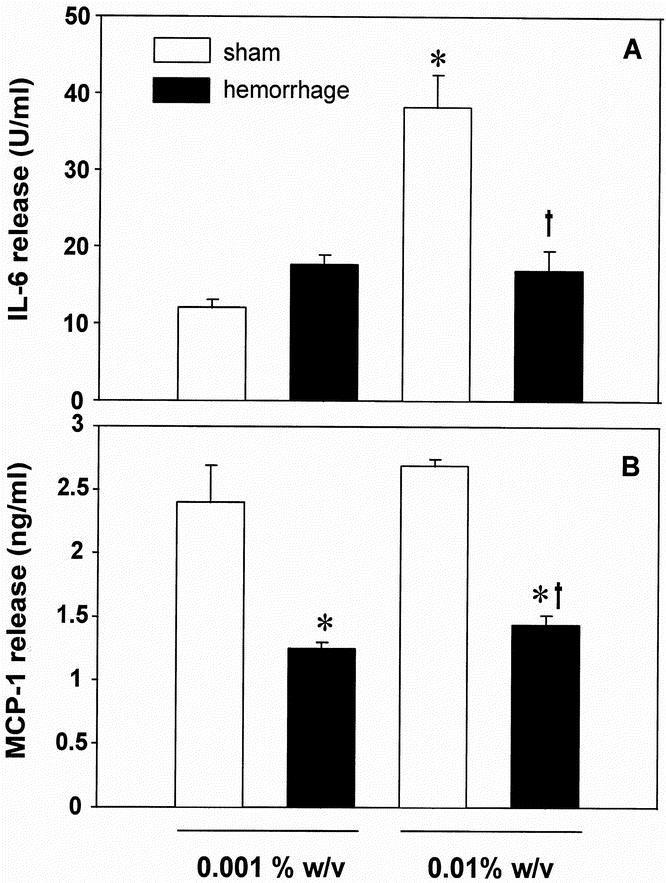
Figure 5. Release of IL-6 (A) and MCP-1 (B) by wound exudate cells harvested from subcutaneous sponges on the first postoperative day after hemorrhage or sham operation. The cells were cultured for 24 hours in the presence of 0.001% or 0.01% heat-killed S. aureus. The release of IL-6 was determined by a specific bioassay (7TD1), the release of MCP-1 by ELISA. Analysis of variance, *p < 0.05 vs. sham mice stimulated with 0.001% S. aureus, †p < 0.05 vs. sham mice stimulated with 0.01% S. aureus.
The release of the chemokine MCP-1 by wound cells from hemorrhaged animals in response to S. aureus stimulation was significantly depressed compared with sham levels (−48.0% when stimulated with 0.001% S. aureus and −46.6% when stimulated with 0.01% S. aureus vs. shams, p < 0.05; Fig. 5B).
Phagocytic Activity of Wound Exudate Cells
There was no significant difference in the percentage of phagocytic wound exudate cells (i.e., FITC-positive) without stimulation on the first postoperative day (Fig. 6A). After LPS stimulation, the percentage of wound exudate cells staining positive for FITC significantly increased in sham animals (+57% vs. nonstimulated cells, p < 0.05) but not in hemorrhaged mice (Fig. 6A).
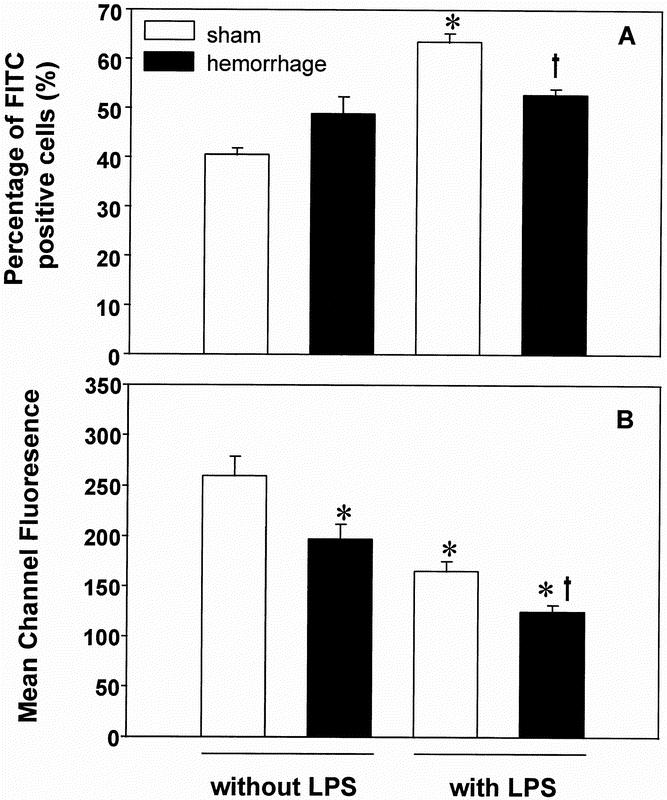
Figure 6. Percentage of FITC-positive staining wound exudate cells (A) and the mean channel fluorescence of positive-staining cells (B) harvested on the first postoperative day and stimulated with or without 10 μg/ml lipopolysaccharide A for 1 hour. FITC-positive cells represent cells that have incorporated fluorescent S. aureus BioParticles that had been incubated together with the wound exudate cells for 30 minutes. Analysis of variance, *p < 0.05 vs. sham mice without stimulation, †p < 0.05 vs. sham mice with stimulation.
Bacterial phagocytosis, as determined by mean channel fluorescence (a measure of total phagocytic activity), however, was decreased in nonstimulated wound exudate cells harvested from hemorrhaged animals compared with shams (−24.8% vs. shams, p < 0.05; Fig. 6B). Although LPS stimulation increased the percentage of FITC-positive cells harvested from sham animals, the total phagocytic activity of the cell population decreased under those conditions (−36.4 vs. nonstimulated cells harvested from shams, p < 0.05; Fig. 6B). Stimulation of wound exudate cells harvested from hemorrhaged animals also further reduced the phagocytic activity (−24.5% vs. shams stimulated with LPS, p < 0.05; Fig. 6B).
DISCUSSION
Wound infection remains one of the most frequent complications after severe trauma, 7 accounting for approximately 25% of the total number of nosocomial infections. 8 Further, surgical wound infections continue to consume a considerable portion of health care finances. 18 In this respect, Olson and Lee reported that the reduction of the overall wound infection rate from 4.2% to 2.5% at one medical center saved approximately $3 million in hospital room costs during a 10-year period. 19 Moreover, the wound can become a potential cause of considerable morbidity and mortality as a result of subsequent tissue inflammation and infection; the latter is especially common given the compromised immune function seen in the critically ill patient. 20–22 Studies by Livingston and Malangoni suggested an increased susceptibility of hemorrhaged animals to wound infection, as defined by the presence of gross purulence and bacterial growth in culture, 7 days after hemorrhage. 5 Moreover, antibiotics failed to decrease the rate of wound infection in hemorrhaged animals, suggesting alterations in the host defense mechanisms after hemorrhagic shock. 6 The aim of the present study, therefore, was to determine whether a dysfunction of immune cells at the wound site occurs after severe blood loss.
Polyvinyl alcohol sponges were implanted subcutaneously adjacent to the laparotomy wound incision site, and wound exudate cells were harvested on the first, third, or fifth postoperative day to determine whether immune cell dysfunction occurs at the wound site. Sponges were used because wound exudate cells cannot be readily harvested directly from the wound site. Further, implantation of sponges is a commonly used and well-described experimental technique for obtaining wound exudate cells. 12,13 The wound exudate cells assessed in this study represent an unpurified (mixed) cellular sample composed of primarily PMNs and Mφ. This was done in an attempt to simulate the in vivo setting of wound exudate cells and their immune response to a second stimulus in vitro. In future studies, however, targeted cell population depletion should be considered to elucidate which cell population specifically contributes to the observed alterations in the immune response at the wound site after hemorrhagic shock.
The results indicate that the release of the proinflammatory cytokines IL-1β and IL-6 in response to LPS was significantly decreased on the first and third postoperative days but returned to normal on the fifth postoperative day. Because the percentage of wound exudate cells that were macrophages or PMNs was not significantly affected by hemorrhage, the observed differences in the cytokine pattern do not appear to be attributable to variations in the distribution of wound exudate cells. IL-1β and IL-6 have been shown to be involved in the wound-healing process in both experimental models using sponges and in surgical patients. 13,23 IL-1 and IL-6 stimulate leukocyte chemotaxis, T- and B-cell activity, and PMN bactericidal activity. 24–26 A decreased release of proinflammatory cytokines in glucocorticoid-treated as well as diabetic mice has been reported to be associated with alterations in wound healing. 27,28 In addition, the capacity of wound exudate cells to release proinflammatory cytokines in response to LPS was used as a marker to determine the responsiveness of wound exudate cells to a second stimulus after hemorrhagic shock or sham operation. In this regard, a decreased release of proinflammatory cytokines by splenic and peritoneal Mφ in response to subsequent stimulation has been demonstrated after hemorrhagic shock. 2 This hyporesponsiveness of splenic and peritoneal Mφ after hemorrhage has been shown to be associated with dysfunction of these cells (i.e., impaired antigen presentation and cytotoxicity). 29,30 The present study indicates that the phagocytic capacity of wound exudate cells subjected to LPS stimulation was also diminished in hemorrhaged animals versus shams. Whether other functional capacities of wound exudate cells (i.e., bactericidal activity) are compromised after severe blood loss remains to be determined. Comparable to LPS stimulation, the capacity of wound exudate cells to release IL-6 was also diminished in response to stimulation with heat-killed S. aureus. Thus, the dysfunction of wound exudate cells does not appear to be restricted to stimulation through the CD14 receptor by LPS. Because wound exudate cells were not separated in the present study, it can only be speculated which cell type is responsible for the release of proinflammatory cytokines by wound exudate cells in response to LPS. In this regard, a number of investigators have shown that proinflammatory cytokine production can be induced in both PMNs and Mφ by LPS stimulation. 12,2,31 Thus, it is plausible that PMNs might be a major contributor to the release of proinflammatory cytokine seen on the first postoperative day. Alternatively, on the third and fifth postoperative days, Mφ are likely to be the predominant contributor to the released proinflammatory cytokines, because the percentage of Mφ increased at these times after surgery. This hypothesis, however, remains to be verified in additional studies using purified PMN and Mφ populations from wound exudate cells.
An increased rate of wound infection has been previously demonstrated on the first and third postoperative days after hemorrhagic shock; however, by the fifth postoperative day, the susceptibility to this infection was comparable to that in sham animals. 5 Moreover, Miles 6 demonstrated increased virulence of a dermal S. aureus injection after hypovolemic shock. Although the susceptibility of hemorrhaged animals to subsequent wound infection was not determined in the present study, these earlier reports suggest that the hyporesponsiveness of wound exudate cells after hemorrhage might contribute to an increased susceptibility to wound infection. This hypothesis is further supported by the finding in the present study that the restored cytokine release on the fifth postoperative day after hemorrhage is associated with an unaltered rate of wound infection in the studies of Livingston and Malangoni. 5 In addition to alterations in the cytokine production, decreased phagocytic activity of wound exudate cells after trauma-hemorrhage was evident on the first postoperative day. Recent studies by Simms and D’Amico 32 indicate that a decline in the phagocytic activity of PMNs in response to hypoxia was associated with a decreased bactericidal activity. Although the bactericidal activity of wound exudate cells was not determined in the present study, the above findings would suggest that the decreased phagocytic activity of wound exudate cells after hemorrhage might be associated with a decreased bactericidal activity.
On the first postoperative day, the release of the chemokines MIP-2 and MCP-1 by wound exudate cells harvested from hemorrhaged animals was decreased in response to LPS. MIP-2, which is similar to human IL-8, exhibits potent PMN and T-lymphocyte chemotactic activity. 33–35 Although the release of MIP-2 in vitro was decreased in hemorrhaged animals in response to stimulation, the number of PMNs in vivo was unchanged after S. aureus injection. Recent studies suggest that PMN apoptosis is delayed in septic patients versus healthy volunteers, 36 indicating that the unchanged number of PMNs in the wound after hemorrhagic shock might result from decreased apoptosis. IL-8 has been shown to increase the bactericidal activity of activated PMNs against S. aureus and Escherichia coli. 26 Thus, it is possible that the decreased release of MIP-2 by stimulated wound exudate cells might lead to altered PMN function in vivo, thereby increasing the susceptibility of the wound to infection. However, further studies are required to verify this hypothesis. MCP-1 belongs to the CC family of chemotactic cytokines, which stimulate the migration of monocytic cells. 37 We found a decreased number of Mφ 24 hours after S. aureus injection in the wound site of hemorrhaged animals, suggesting that a decreased release of MCP-1 after stimulation results in an impaired Mφ influx. Whether decreased release of other chemokines or mediators also contributes to the lower Mφ number at the wound site after severe blood loss is unknown.
The results of the present study also indicate that the release of nitric oxide is decreased on the first postoperative day after trauma-hemorrhage. Although the precise role of nitric oxide in the wound is unclear, studies indicate that depletion of nitric oxide by the inhibition of inducible nitric oxide synthase activity or genetically in inducible nitric oxide synthase–deficient mice impairs wound repair. 38,39
The precise mechanisms responsible for inducing the hyporesponsiveness of wound cells after hemorrhagic shock seen in the present study remains unknown. Hemorrhage has been shown to cause a decrease in blood flow, which results in regional hypoxia. 40 Decreased subcutaneous wound oxygen tension is associated with an increased rate of wound infection 41 and impaired wound healing in surgical patients. 42 Similarly, the resistance to infection of musculocutaneous flaps in dogs was decreased in animals housed in 12% oxygen versus normoxic conditions. 43 Thus, attempts to increase tissue perfusion after trauma and hemorrhagic shock with agents such as L-arginine 44 might improve or restore the depressed immune response of cells at the wound site. Studies have also shown that plasma glucocorticoid levels increase after hemorrhagic shock. 2 Further, a decreased release of proinflammatory cytokines in glucocorticoid-treated mice has been reported. 27 It is thus possible that the increased plasma glucocorticoid levels in hemorrhaged animals contribute to the altered cytokine release in those mice.
Only male animals were used in the present study. In this respect, several studies from our laboratory indicate that females in the proestrus state of the estrus cycle showed enhanced cell-mediated immune responses as opposed to depressed immune functions in males after hemorrhagic shock. 45 It is therefore tempting to speculate that exudate cells present at the wound site might also exhibit gender-specific immune responses. This, however, needs to be determined.
In summary, the present study demonstrates that wound exudate cells harvested on the first and third postoperative days from hemorrhaged animals exhibit an impaired release of proinflammatory cytokines and chemokines in response to a second stimulus in vitro. This dysfunction of wound exudate cells could provide a possible mechanism by which it contributes to the imbalance of host resistance to pathogens in the wounds of trauma patients, leading to an increased rate of wound infection. Therefore, improvements in or restoration of immune cell function at the wound site might be helpful in decreasing the incidence of wound infection in trauma victims.
Acknowledgments
The authors thank Adriana J. Meszaros for her valuable assistance in the determination of wound cellular infiltrate composition. They also acknowledge the valuable advice of Dr. Jorge E. Albina in these studies.
Discussion
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): I rise to compliment Dr. Cioffi and Dr. Bland and their colleagues on another in their series of studies identifying factors that increase the risk of infection and sepsis following injury. To help us evaluate the importance of their finding of decreased production of inflammatory cytokines and chemokines by the cells that enter a foreign body and implanted near a surgical wound in animals with superimposed hemorrhage, we need additional information. To that end, I ask the following questions:
Since the implanted sponges represent foreign bodies which may alter cell function per se, have you assayed cytokine and chemokine production from cells harvested from the incisions of the wound per se? And do they show the same dysfunction?
Were any of the sponges infected or show bacterial growth?
Could the differential response to heat-killed staphylococci be due to exotoxin content?
The constant suppression of MCP-1 response, regardless of the number of organisms, is consistent with a toxic effect. Are the temporal changes in inflammatory cytokine levels merely a reflection of the decrease in the number of macrophages seen with hemorrhage when challenged by heat-killed staphylococci, since macrophage number was diminished on postinjury day 1 and rose progressively thereafter?
You carefully specified that all study animals were male mice. In the past, you have found that female gender is a protective factor. Have you repeated these studies using female mice? And, if so, did they manifest the same findings?
Have you explored the mechanism causing these changes? It is known that glucocorticoids mediate gene suppression of other cytokine-induced chemokines such as CINC-gro through impairment of nuclear factor kappa-B activation? Have you assayed cytokine-induced formation of NF kappa-B complexes?
Have you used this model to test susceptibility to infection? Your thesis was that these changes altered infection.
Since this is proposed as a trauma hemorrhage model, it seems to me that a contemporaneous control group of just hemorrhage is missing.
Lastly, does this represent a beneficial mechanism to reduce local inflammation and promote unhindered healing of the wound?
And, finally, does this speak for reinstitution of 3-day postoperative antibiotic therapy? Since all the changes were improved at 3 and 5 days, do we need additional coverage during the first 3 days, rather than just the perioperative period?
Dr. R. Neal Garrison (Louisville, Kentucky): Drs. Cioffi, Chaudry, and their research colleagues have presented further detailed evidence that following trauma and hemorrhagic shock there exists depressed immunologic capacity to resist a subsequent infectious challenge.
In their current study, they have extended their findings of systemic malfunction of the macrophage and leukocyte to the local battlefield of the surgical wound.
The overall theme of this extensive series of experiments dating back to the 1980s is that the white cell is injured during injury and, therefore, is less efficient in combating an infectious challenge following shock and severe hemorrhage.
I have several questions or comments for the authors:
What, in your opinion, is the stimulus for this depression? You mention tissue hypoxia and wound perfusion as a potential etiologic factor in your manuscript, but is that the overriding stimulus for the systemic depression?
Secondly, it seems that the surgical wound simply reflects the systemic depression. Are there differences in depression seen between organ systems such as the lung, intestine, or wounded soft tissue?
Thirdly, in the majority of your experiments, dating for over 10 years now, you resuscitate with four volumes of crystalloid solution and no blood or colloid, clearly, an artificial resuscitating regimen from what we do clinically. Does this severe acute hemodilution play a factor in your findings? I suspect that such an acute hemodilution leads to a significant degree of cellular and tissue edema. Can you account for this as a variable in your findings?
Finally, do any of the previously reported therapeutic agents that you have studied, such as chloroquine or pentoxifylline, when added to the resuscitation regimen, have an effect on the inflammatory wound infiltrate in this model?
Dr. John A. Mannick (Boston, Massachusetts): I do think that the authors have very clearly shown that after hemorrhage and wounding, there is certainly something going wrong with cytokine production in the local environment of the wound, as well as systemically.
One of the problems with the data, it seems to me, is that while the production of both IL-6 and IL-1β was clearly down for several days after hemorrhage and wounding, and the chemokine production was definitely decreased in the first postwounding or posthemorrhage day, when you look at the environment of the wound as reflected in the cells coming out of the polyvinyl sponges, not a whole lot was going on. The same cell numbers were there. Pretty much the same cell types were there. There was, in fact, some diminution in the ability to phagocytize bacteria, but it wasn’t great. And I guess I am wondering whether or not the authors are convinced that they have found the significant factor in the wound that might allow the wound to be more susceptible to infection.
It seems to me that what they really need to do now is to find out whether, in their hands, the prior experiments of Livingstone and Malangoni, which they referred to, actually hold true. In other words, in this model, are these wounds in fact more susceptible to infection with Staphylococcus aureus, for example, in the first posthemorrhage day or are they not?
So far, the data is very convincing as far as the diminished production of cytokines and chemokines, but I am wondering whether it all adds up to something that is biologically significant. And I wonder if Dr. Cioffi has further data about the effect of all of this on true resistance to infection or whether he plans to do such experiments.
Dr. Gregory B. Bulkley (Baltimore, Maryland): There were two points that I thought were worth perhaps elaborating on a bit.
The first was related to the fact that we know, back from even the studies of Jack Burke quite a few years ago, that patients in shock don’t heal wounds as well. And Jack’s interpretation of that 25 years ago when he did those studies was that it was related to decreased perfusion in the wound at the time because of the sympathetic discharge and the vasoconstriction that takes place in the wound during that time. And I am just wondering. The authors were looking at the cells that actually collected within this little sponge. Were the cells in the animals different or were they just different cells getting collected in the sponge? In other words, was there a difference in the fundamental immune system or a difference in delivery?
My second point is just a little bit of an elaboration on what Dr. Mannick said. The most common logical fallacy in the medical literature is what Aristotle called the post hoc igitur propter hocfallacy—the jumping from association to cause and effect. Dr. Cioffi is very careful, I think, in stating his conclusions, but I just wonder if he would elaborate. Does he believe that these specific cytokine defects in these cells are actually the cause of the problem? And, if so, is he going to do experiments where he perturbs the system by adding them back or stimulating them? Or is he suggesting that this is merely one of the many manifestations of this?
Dr. William G. Cioffi (Closing Discussion): One of the pivotal questions by three of the discussants dealt with what is the stimulus for observed findings. Dr. Pruitt mentioned the possibility of glucocorticoids being the driving force and did mention that glucocorticoids and their effect on both CINC and kappa B. In our opinion, it is probably not glucocorticoids. A more likely stimulus for this might well be the presence of IL-10 in wounds. Hauser reported over the past 5 to 6 years a marked increase in IL-10 and fracture hematomas from patients, and they went on to describe the effect of the presence of IL-10 in these fracture hematomas on cell function and found some things that were quite similar to what we found. The stimulus for the IL-10 release in these fracture hematomas was thought by some authors to be related to the stress response in epinephrine. And, indeed, in the Surgical Forum in 1995, one author reported that epinephrine is capable of increasing IL-10 release by monocytes.
Dr. Pruitt asked several other questions about foreign body and was there infection present. We did culture the sponges; there was no infection. We need to use sponges or we can’t get enough cells, but other authors like Hauser have used fracture hematomas, and other people have tried to use blister fluid and have found similar results.
Is it an exotoxin result? I don’t think so, but we did use LPS and staph and we could, of course, use other bacteria to prove that.
The question of males versus females: we have not looked at that in this study population, but we have in other kinds of infectious challenges, and we have noted that females are better able to handle infection posttrauma hemorrhage, and we need to do the same in this set of experiments.
Dr. Garrison asked is this a systemic effect or a local effect. We are most interested in the wound, although we have found similar findings in other compartmental analyses of splenocyte function, as well as, I have heard, neomacrophages. And so I think some of the changes are similar, depending on the compartment we are looking at. We were most interested in the wound and what would affect the ability of the cells to migrate into the wound and fight infection.
He did ask about crystalloid only, and that has been a long argument with this model. We have resuscitated animals in the past with blood that did not contain heparin, and we don’t see any significant physiologic differences between those resuscitated with blood versus those with crystalloid only, so we choose crystalloid for a cleaner experimental model.
We have not performed any experiments with chloroquine or pentoxifylline, but other investigators in the lab have looked at the effect of betaglucon given to the wound, and that might be a better way to go as a topical rather than a systemic therapy.
In closing, I’d just like to respond to Dr. Bulkley’s comments, are these cause or effect? Is this just something that we happen to see in the wounds?
Livingstone’s group did use a model very similar to ours. It was a murine hemorrhage model, and they noticed an increased susceptibility to infection. We need to do the same.
Will we add or subtract cytokines? I don’t think we would add or subtract the cytokines or chemokines that we measured; however, I think it would be wise to start at possible etiologic things like glucocorticoids, IL-10, and that, to see if we can abrogate the changes that we found in our model.
Footnotes
Correspondence: Irshad H. Chaudry, PhD, Center for Surgical Research, Brown University School of Medicine and Rhode Island Hospital, Middle House II, 593 Eddy Street, Providence, RI 02903.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Supported by NIH grant R01 GM 37127.
Dr. Angele is currently with the Department of Surgery, Klinikum Grosshadern, Munich, Germany.
Accepted for publication December 1998.
References
- 1.Zellweger R, Ayala A, DeMaso CM, Chaudry IH. Trauma-hemorrhage causes prolonged depression in cellular immunity. Shock 1995; 4: 149–153. [DOI] [PubMed] [Google Scholar]
- 2.Chaudry IH, Ayala A. Immunological aspects of hemorrhage. Austin, TX: Medical Intelligence Unit, R.G. Landes Co.; 1992.
- 3.Stephan RN, Kupper TS, Geha AS, Baue AS, Chaudry IH. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg 1987; 122: 62–68. [DOI] [PubMed] [Google Scholar]
- 4.Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males: restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg 1997; 132: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 5.Livingston DH, Malangoni MA. An experimental study of susceptibility to infection after hemorrhagic shock. Surg Gynecol Obstet 1989; 168: 138–142. [PubMed] [Google Scholar]
- 6.Miles AA. Nonspecific defense reactions in bacterial infections. Ann NY Acad Sci 1956; 66: 356–369. [Google Scholar]
- 7.O’Keefe GE, Maier RV, Diehr P, Grossman D, Jurkovich GJ, Conrad D. The complications of trauma and their associated costs in a level I trauma center. Arch Surg 1997; 132: 920–924. [DOI] [PubMed] [Google Scholar]
- 8.Nichols RL. Surgical wound infection. Am J Med 1991; 91: 54S–64S. [DOI] [PubMed] [Google Scholar]
- 9.Weigelt JA, Haley RW, Seibert B. Factors which influence the risk of wound infection in trauma patients. J Trauma 1987; 27: 774–781. [DOI] [PubMed] [Google Scholar]
- 10.Polk HCJ. Factors influencing the risk of infection after trauma. Am J Surg 1993; 165: 2S–7S. [DOI] [PubMed] [Google Scholar]
- 11.McGinn FP. Effects of hemorrhage upon surgical operations. Br J Surg 1976; 63: 742–746. [DOI] [PubMed] [Google Scholar]
- 12.Mateo RB, Reichner JS, Albina JE. Interleukin-6 activity in wounds. Am J Physiol 1994; 266: R1840–R1844. [DOI] [PubMed] [Google Scholar]
- 13.Ford HR, Hoffman RA, Wing EJ, Magee DM, McIntyre L, Simmons RL. Characterization of wound cytokines in the sponge matrix model. Arch Surg 1989; 124: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 14.Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. J Surg Res 1994; 56: 579–585. [DOI] [PubMed] [Google Scholar]
- 15.Angele MK, Ayala A, Monfils BA, Cioffi WG, Bland KI, Chaudry IH. Testosterone and/or low estradiol: normally required but harmful immunologically for males after trauma-hemorrhage. J Trauma 1998; 44: 78–85. [DOI] [PubMed] [Google Scholar]
- 16.Ihle JN, Keller J, Greenberger JS, Henderson L, Yetter RA, Morse HC. Phenotypic characteristics of cell lines requiring IL-3 for growth. J Immunol 1982; 129: 1377–1383. [PubMed] [Google Scholar]
- 17.Schwacha MG, Somers SD. Thermal injury-injury immunosuppression in mice: the role of macrophage-derived reactive nitrogen intermediates. J Leuko Biol 1998; 63: 51–58. [DOI] [PubMed] [Google Scholar]
- 18.Sawyer RG, Pruett TL. Wound infections. Surg Clin North Am 1994; 74: 519–536. [PubMed] [Google Scholar]
- 19.Olson MM, Lee JTJ. Continuous, 10-year wound infection surveillance. Results, advantages, and unanswered questions. Arch Surg 1990; 125: 794–803. [DOI] [PubMed] [Google Scholar]
- 20.Goris R, Te Boekhorst T, Nuytinck J. Multiple-organ failure: generalized autodestructive inflammation. Arch Surg 1985; 120: 1109. [DOI] [PubMed] [Google Scholar]
- 21.Hunt TK, Knighton D, Goodson W. Wound healing in current surgical diagnosis and treatment. Crit Care Med 1988; 16: 86–98. [Google Scholar]
- 22.Baue AE. Multiple organ failure. In Baue AE, ed. Multiple organ failure: patient care and prevention. St. Louis, MO: Mosby Year Book; 1990: 421–470.
- 23.Ueo H, Inoue H, Honda M, et al. Production of interleukin-6 at operative wound sites in surgical patients. J Am Coll Surg 1994; 179: 326–332. [PubMed] [Google Scholar]
- 24.Schaffer MR, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg 1998; 85: 444–460. [DOI] [PubMed] [Google Scholar]
- 25.Thornton FJ, Schaffer MR, Barbul A. Wound healing in sepsis and trauma. Shock 1997; 8: 391–401. [PubMed] [Google Scholar]
- 26.Simms HH, D’Amico R. Studies on polymorphonuclear leukocytes bactericidal function. III: The role of extracellular matrix proteins. J Surg Res 1997; 72: 123–128. [DOI] [PubMed] [Google Scholar]
- 27.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996; 8: 548–556. [DOI] [PubMed] [Google Scholar]
- 28.Fahey TJ, Sadaty A, Jones WG, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res 1991; 50: 308–313. [DOI] [PubMed] [Google Scholar]
- 29.Ayala A, Ertel W, Chaudry IH. Trauma-induced suppression of antigen presentation and expression of major histocompatibility class II antigen complex in leukocytes. Shock 1996; 5: 79–90. [DOI] [PubMed] [Google Scholar]
- 30.Ayala A, Perrin MM, Wang P, Ertel W, Chaudry IH. Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity: involvement of cell-associated TNF and reactive nitrogen. J Immunol 1991; 147: 4147–4154. [PubMed] [Google Scholar]
- 31.Palma C, Cassone A, Serbousek D, Pearson CA, Djeu JY. Lactoferrin release and interleukin-1, interleukin-6 and its receptor are expressed by human polymorphonuclear cells stimulated by various lipopolysaccharides: relationship to growth inhibition of Candida albicans. Infect Immun 1992; 60: 4604–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simms HH, D’Amico R. Studies on polymorphonuclear leukocyte bactericidal function. II: The role of oxidative stress. Shock 1997; 7: 339–344. [PubMed] [Google Scholar]
- 33.Yoshimura T, Matsushima K, Tanaka S. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA 1987; 84: 9233–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen CG, Anderson AO, Appella E. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science 1989; 243: 1464–1466. [DOI] [PubMed] [Google Scholar]
- 35.Wolpe SD, Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J 1989; 3: 2565–2573. [DOI] [PubMed] [Google Scholar]
- 36.Ertel W, Keel M, Infanger M, Ungethüm U, Steckholzer U, Trentz O. Circulating mediators in serum of injured patients with septic complications inhibit neutrophil apoptosis through up-regulation of protein-tyrosine phosphorylation. J Trauma 1998; 44: 767–776. [DOI] [PubMed] [Google Scholar]
- 37.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol 1994; 6: 865–873. [DOI] [PubMed] [Google Scholar]
- 38.Schaffer MR, Tantry U, Gross SS. Nitric oxide regulates wound healing. J Surg Res 1996; 63: 237–240. [DOI] [PubMed] [Google Scholar]
- 39.Yamasaki K, Edington HD, McClosky C, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest 1998; 101: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudry IH, Ayala A. Immune consequences of hypovolemic shock and resuscitation. Curr Opin Anaesthesiol 1993; 6: 385–392. [Google Scholar]
- 41.Williams-Hopf H, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg 1997; 132: 997–1004. [DOI] [PubMed] [Google Scholar]
- 42.Jonsson K, Jensen JA, Goodson WH III, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg 1991; 214: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonsson K, Hunt TK, Mathes SJ. Oxygen as an isolated variable influences resistance to infection. Ann Surg 1988; 208: 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angele MK, Smail N, Wang P, Cioffi WG, Bland KI, Chaudry IH. L-arginine restores the depressed cardiac output and regional perfusion following trauma-hemorrhage. Surgery 1998; 124: 394–401. [PubMed] [Google Scholar]
- 45.Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Enhanced immune responses in females as opposed to decreased responses in males following hemorrhagic shock. Cytokine 1996; 8: 853–863. [DOI] [PubMed] [Google Scholar]