Abstract
Objective
To examine the levels of a Th1 IgA-inhibiting cytokine (interferon γ) and the Th2 IgA-stimulating cytokines (interleukin [IL]-4, IL-5, IL-6, and IL-10) within the intestine of animals manipulated with enteral or parenteral nutrition, and to correlate these cytokine alterations with intestinal IgA levels.
Summary Background Data
Enteral feeding significantly reduces the incidence of pneumonia in critically injured patients compared with intravenous total parenteral nutrition (IV TPN) or no nutritional support. Experimentally, complex diets prevent impairments in mucosal immunity induced by IV TPN. These impairments include decreases in intestinal and respiratory tract IgA levels, impaired IgA-mediated antiviral defenses, and increases in the mortality rate against established immunity to Pseudomonas pneumonia. Intragastric (IG) TPN maintains antiviral defenses but only partially preserves protection against Pseudomonas pneumonia. Because IgA levels depend on interactions between Th1 IgA-inhibiting and Th2 IgA-stimulating cytokines, the authors postulated differences in gut cytokine balance in enterally and parenterally fed mice.
Methods
Sixty-one mice were randomized to receive chow, IV TPN, IG TPN, or an isocaloric, complex enteral diet. After 5 days of feeding, animals were killed and supernatants from samples of intestine were harvested, homogenized, and assayed for Th1 and Th2 cytokines by enzyme-linked immunosorbent assay.
Results
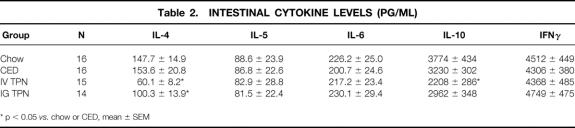
The Th2 cytokines, IL-5 and IL-6, and the Th1 cytokine, interferon γ, remained unchanged by diet. IL-4 levels decreased significantly in both IV and IG TPN groups versus the chow or complex enteral diet groups, whereas IL-10 decreased only in IV TPN mice. Decreases in Th2 cytokines correlated with intestinal IgA levels.
Conclusion
Chow and complex enteral diets maintain a normal balance between IgA-stimulating and IgA-inhibiting cytokines while preserving normal antibacterial and antiviral immunity. The IgA-stimulating cytokine IL-4 drops significantly in mice receiving IG and IV TPN in association with reduced IgA levels, whereas IL-10 decreases significantly only in mice receiving IV TPN. These data are consistent with severely impaired mucosal immunity with IV TPN and partial impairment with IG TPN and provide a cytokine-mediated explanation for reduction in diet-induced mucosal immunity.
Intravenous total parenteral nutrition (IV TPN) is associated with an increased incidence of infectious complications, especially pneumonia and intraabdominal abscess, in critically ill and critically injured patients compared with enteral feeding. 1–4 Previous investigators hypothesized a breakdown in gut barrier function resulting from increased mucosal permeability, 5–7 overgrowth of bacteria, and/or increased bacterial translocation, 8,9 but none of these reasons have provided satisfactory explanations for the increased susceptibility to pneumonia. The gut-associated lymphoid tissue (GALT) is the primary immunologic defense of mucosal surfaces. Our prior work demonstrated that IV TPN significantly reduces GALT mass by depleting Peyer’s patches, lamina propria, and the intraepithelial space of T and/or B cells and decreasing the CD4+/CD8+ ratio within the lamina propria. 10 Reduction in GALT cell populations occurred simultaneously with drops in both intestinal and respiratory IgA levels. 11 Although chow and a complex enteral diet (CED) preserve normal GALT cell populations, IgA levels, and antiviral 12 and antibacterial immunity, 13 the administration of intragastric (IG) TPN (as a model of an elemental formula) produces GALT atrophy and reductions in CD4+/CD8+ ratio similar to IV TPN animals. IG TPN feeding, however, maintains effective antiviral defenses and partially, but not completely, preserves established respiratory defenses against intratracheal Pseudomonas.13
The mucosal-associated lymphoid tissue is one of the largest immune organs in the body and contains 70% to 80% of all immunoglobulin-secreting cells. 14 IgA, the primary immunologic product of this system, is a critical component in mucosal immunity and barrier integrity and accounts for 50% of the body’s total immunoglobulin production. 15 IgA is secreted from IgA+ plasma cells after sensitization in the Peyer’s patches and homing to the lamina propria. The IgA produced in the lamina propria is immediately transported by means of mucosal epithelial cells by secretory component onto the mucosal surface. 16 Secretory IgA (sIgA) prevents adherence of bacteria, viruses, and other toxic molecules to the mucosal surfaces and may also play a role in eliminating infectious agents that have penetrated epithelial cell layers. 17 Intestinal IgA levels correlate inversely with bacterial overgrowth, bacterial translocation, and changes in intestinal permeability in animal models. 18,19
Production of IgA is controlled by cytokine-producing T cells within the GALT and possibly by cytokine released from the mucosa. T cells, a major producer of cytokines, can be separated into two distinct subsets, Th1 and Th2 cells, on the basis of the pattern of cytokines secreted. 20 Within the GALT, the Th1 cytokines, interferon γ (IFNγ) and tumor necrosis factor β (TNFβ), downregulate IgA production, whereas the Th2 cytokines, interleukin (IL)-4, IL-5, IL-6, and IL-10, upregulate IgA production. 21–23 A balance between Th1 and Th2 cytokines is perceived to be necessary for maintaining normal IgA immune responses.
The reduction in the CD4+/CD8+ ratio within the lamina propria of mice receiving IG and IV TPN occurs largely through reductions in CD4+ cells, and we speculated a reduction in the Th1/Th2 ratios with IV and IG TPN feeding, recognizing that although both antiviral and antibacterial immunity is lost with IV TPN, this loss is only partial with IG TPN. We studied this hypothesis in small-intestine samples.
MATERIALS AND METHODS
Studies conformed to the guidelines for the care and use of laboratory animals established by the Animal Care and Use Committee of the University of Tennessee. Protocols were approved by that committee. Healthy male ICR (Institute of Cancer Research) mice were purchased from Harlan (Indianapolis, IN) and were housed in an conventional facility accredited by the American Association for the Accreditation of Laboratory Animal Care under controlled conditions of temperature and humidity with a 12/12 hour light/dark cycle. Animals were fed ad libitum water and chow except when receiving experimental diets. During the experiments, the mice were housed in metal metabolic cages with wire-grid bottoms to eliminate coprophagia and the ingestion of bedding.
Experimental Protocol
Male ICR mice, age 6 to 8 weeks, were randomized to receive chow with an IV catheter (n = 16), IV TPN (n = 15), IG TPN (n = 14) via gastrostomy, or Nutren (CED; Clintec, Chicago, IL) via gastrostomy (n = 16).
In animals randomized to gastrostomy, a sham neck incision was performed; animals with IV lines underwent sham laparotomy. Under general anesthesia (ketamine 100 mg/kg and acepromazine maleate 10 mg/kg mixture), a silicone rubber catheter (0.012″ ID/0.25″ OD; Baxter, Chicago, IL) was inserted into the vena cava through the right jugular vein or directly into the stomach. Lines were tunneled subcutaneously from either the right jugular vein or the gastrostomy site and exited the tail at its midpoint. Animals were partially immobilized by tail restraint during the infusion, a model that does not produce physical or chemical evidence of stress.
Catheterized animals were infused with saline at the rate of 4 ml/day, with an increase in goal rate to 10 ml/day in chow, IV TPN, and IG TPN animals and 15 ml/day in the CED group. For the first 2 days after surgery, animals were allowed ad libitum access to chow, and on the third day after surgery they resumed their assigned diets. The TPN solution contained 4.1% amino acids, 34.3% glucose (4859 kJ/L), electrolytes, and multivitamins with a nonprotein calorie/nitrogen ratio of 740 kJ/g nitrogen. The CED contained 12.7% carbohydrate and 3.8% fat (3250 kJ/L), and 4.0% protein (nonprotein calorie/nitrogen ratio of 508 kJ/g nitrogen), along with electrolytes and vitamins. Because of the more dilute solution, animals initially received 4 ml/day and were advanced to a goal rate of 15 ml/day by the third day of feeding. These feedings met the calculated nutritional requirements of mice weighing 25 to 30 g. TPN mice received 1619 kJ/kg/day of nonprotein calories and 14 g protein/kg/day. CED mice received 1625 kJ/kg/day of nonprotein calories and 20 g protein/kg/day.
Mice given intranasal liposomes without the antigen served as unmolested controls and did not undergo surgery. They received ad libitum chow and water throughout the study.
After their respective diets, animals were killed, the intestine was removed and homogenized, and the supernatant was stored for enzyme-linked immonosorbent assay.
Cytokine Immunoassays
From the proximal, middle, and distal small intestine, 0.5 g of intestines were removed and placed in 10 ml of lysis buffer containing protease inhibitors (2 mM phenylmethysulfonyl fluoride and 2 μg/ml of leupeptin, pepstatin A, and aprotinin; Sigma, St. Louis, MO) at 4°C. Samples were homogenized for 30 seconds and homogenates were ultracentrifuged at 15,000 RPM for 45 minutes at 4°C. The amount of cytokines (IL-4, IL-5, IL-6, IL-10, and IFNγ) in the supernatants was measured using enzyme-linked immunosorbent assay. Briefly, 96-well plates were coated with coating antibodies (Endogen, Woburn, MA). After inhibition of nonspecific binding, prediluted cytokine standard (mIL-10, BioSource, Camarillo, CA; mIL-5, R&D Systems, Minneapolis, MN; IL-4, IL-6, and IFNγ, Endogen) and experiment samples were added for 1 to 18 hours at room temperature, depending on the cytokine, and then biotin-labeled antibodies (Endogen) were added for 1 hour at room temperature. Color was developed by TMB substrate (3,3′, 5,5′-tetramethylbenzidine) for 30 minutes and stopped by 2N H2SO4. Absorbance was read at 450 and 550 nm. The amount of cytokines was determined from a standard curve and expressed as pg/ml.
Antibody Quantitative Analysis
IgA was measured in intestinal washings in the sandwich enzyme-linked immunosorbent assay using a polyclonal goat antimouse IgA (Sigma) to coat the plate, a purified mouse IgA (Sigma) as standard, and horseradish peroxidase conjugated goat antimouse IgA. IgA values are reported in μg.
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analysis of differences among groups was carried out by analysis of variance with Scheffe’s method of adjusting for multiple comparisons, using StatView software (Brain Power, Calabasas, CA). After transforming values with natural logarithms, Pearson correlation coefficients were estimated to quantify relations among individual cytokines and IgA levels.
RESULTS
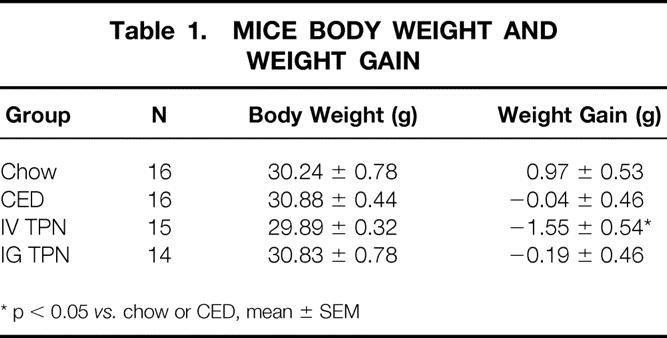
The weights of all groups were similar before the experiment. The weight gains of the chow and CED groups were significantly greater than the IV TPN group, but no differences between the IG-TPN and any other group (Table 1).
Table 1. Mice Body Weight and Weight Gain
There were significant decreases in IL-4 levels from the IV TPN and IG TPN groups compared with the chow or CED animals. IL-10 decreased in IV TPN mice only compared with the chow or CED mice. There were no significant changes in the Th2 cytokines, IL-5 and IL-6, in any group. The Th1 cytokine, IFNγ, also remained stable in all groups (Table 2).
Table 2. Intestinal Cytokine Levels (pg/ml)
Intestinal IgA levels decreased significantly in the IV TPN (35.2 ± 7.9 μg) and IG TPN (27.8 ± 7.4 μg) groups compared with the chow group (87.1 ± 26.0 μg; Fig. 1). Although the IgA level in the CED group (73.3 ± 13.9 μg) was greater than in the IV TPN or IG TPN mice, this value failed to reach statistical significance. The log of the IgA levels correlated with both the log IL-4 (Fig. 2; r = 0.65, p < 0.0001) values and the log IL-10 values (Fig. 3; r = 0.54, p < 0.0002). An IgA value from a single chow-fed animal appeared to be an outlier, residing five standard deviations from the mean. If this single data point were eliminated, the correlations changed slightly for IL-4 (r = 0.73, p < 0.0001) and IL-10 (r = 0.5, p < 0.0005).
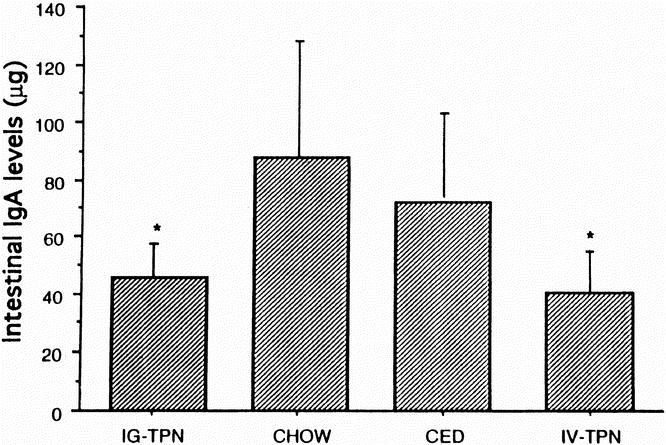
Figure 1. Intestinal IgA levels in different diet groups. IgA levels were significantly lower in intravenous TPN and intragastric TPN animals than in animals fed chow. Data are expressed as mean±SEM. * p < 0.05 vs. chow.
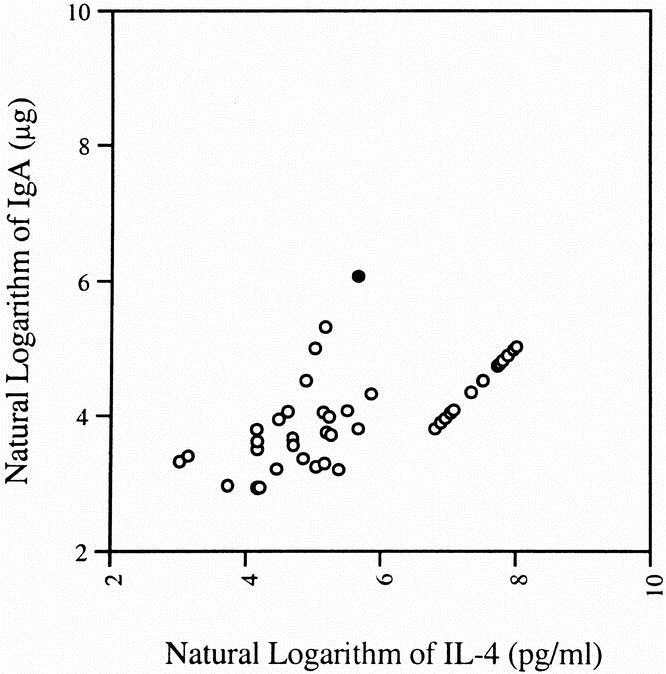
Figure 2. There was a significant correlation between IL-4 concentrations, and intestinal IgA levels correlated well. These correlations improved when the outlier (filled circle) was removed from the analysis.
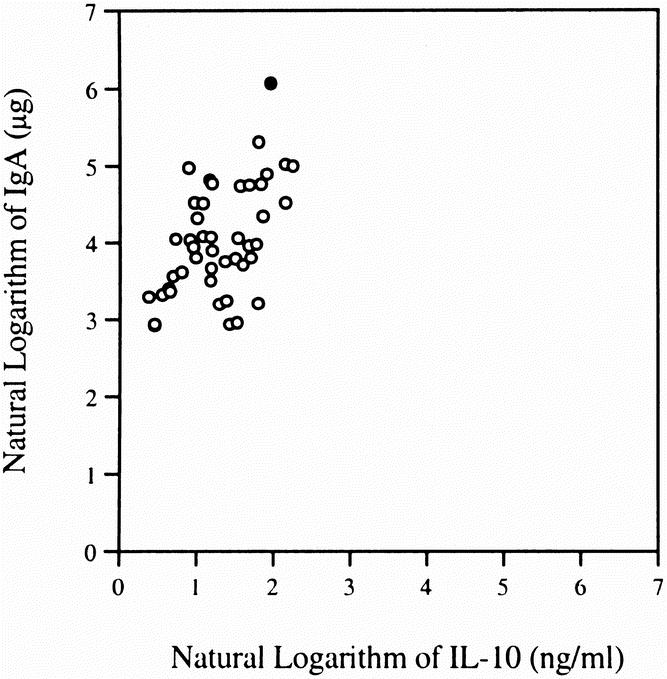
Figure 3. There was a significant correlation between IL-10 concentrations in intestinal samples and luminal IgA levels. The correlations remained relatively stable when the data from the single outlier (filled circle) was removed.
DISCUSSION
There is substantial clinical evidence that enteral feeding reduces the incidence of pneumonia and intraabdominal abscess in critically ill patients. Our laboratory has focused on the respiratory mechanisms of defense because of the much higher incidence of pneumonia in this patient population. Although increased gut permeability, bacterial overgrowth, and increased bacterial translocation have been proposed as causes of such infections, these concepts have not provided a cohesive explanation for respiratory infectious complications. Our work supports GALT maintenance as an important factor in the protection of both the gastrointestinal and respiratory tract. 11–13
In health, mucosal immunity provides a strategic barrier against invading pathogens and toxic substances for all moist mucosal surfaces. The source of this immunity, the GALT, is one of the largest immune organs within the body, producing 70% to 80% of all immunoglobulin-secreting cells and 50% of all immunoglobulin produced by the body. As luminal antigens are taken up by M cells overlying Peyer’s patches, naïve B cells and T cells are sensitized to antigens processed within the Peyer’s patches and mature within the mesenteric lymph nodes. Daughter cells are released into the thoracic duct to distribute themselves beneath mucosa sites, where these sensitized B and T cells, in coordination, produce antigen-specific IgA. The IgA is immediately transported by overlying mucosal epithelial cells onto the epithelial surface. Although defensins, lactoferrin, mucin, and other substances play an important role, IgA appears to play a strategic role against bacterial and viral colonization and invasion. 14,16,24
Our work has shown that this defensive system is affected by the route and type of nutrition. Significant reductions in GALT cell populations and IgA levels are induced by IG TPN and IV TPN and correlate with functional changes. Antibacterial defenses are also affected. For example, immunization with Pseudomonas aeruginosa antigens and liposomes stimulates production of Pseudomonas-specific IgA within the respiratory secretions of animals. Immunization also provides an effective defense to a subsequent lethal tracheal dose of Pseudomonas, reducing the mortality rate from approximately 90% in nonimmunized animals to approximately 10% to 20% in animals fed chow or CED. This protection is completely lost in animals receiving IV TPN for 5 days, resulting in a 90% mortality rate, but it is partially preserved in animals receiving IG TPN, with a mortality rate of approximately 50%. Although this antibacterial defense may involve other mechanisms besides IgA, we believe that mucosal immunity is an important cofactor in effective respiratory defense. After immunization and establishment of IgA-mediated antiviral defenses against the H1N1 virus, immunity is lost in approximately 50% of the animals fed intravenously but is maintained in the animals receiving chow, CED, or IG TPN. 12 Because IgA production by plasma cells is influenced by cytokine production from the associated T cells within the GALT, we characterized the cytokine profile in animals fed CED or chow and examined changes in the cytokine profile induced by IV TPN and IG TPN. We speculated a predictable interplay of Th cells and cytokines because of the reduction in CD4+/CD8+ ratios in animals receiving IG TPN and IV TPN compared with animals fed chow or CED.
For the first time, this work demonstrates that diet-induced changes in intestinal IgA levels are associated with a change in Th1/Th2 cytokines within the intestine. Compared with “normal” levels of Th1 and Th2 cytokines in intestinal samples of chow- and CED-fed mice (diets that maintain antibacterial and antiviral defenses), both IL-4 and IL-10 levels drop significantly with IV TPN, a diet that both impairs IgA-mediated antiviral defenses and destroys established antibacterial defenses against Pseudomonas pneumonia. Only IL-4 drops significantly in IG TPN mice; IL-10 levels are preserved. This is consistent with our observations that IG TPN preserves established IgA-mediated antiviral defenses and partially preserves antibacterial defenses—in other words, mucosal immunity is partially but not completely impaired.
These changes in IV TPN and IG TPN cytokine levels confirm an imbalance in favor of the IgA-inhibiting cytokine, IFNγ, by Th1 cells relative to the IgA-stimulating cytokines, IL-4 and/or IL-10, by the Th2 cells in total gut samples. The correlation between IL-4 and IL-10 and IgA levels, although significant, is in the 0.5 to 0.6 range, suggesting other important factors. This is expected, because IgA levels are almost certainly influenced by the number of B cells able to produce IgA within the GALT, which we have shown to decrease by 40% to 50% with IG TPN and IV TPN. 10 Because one would expect that IgA production would be influenced by both the cytokine milieu and by the absolute number of cells available for IgA production, this limited correlation between cytokines alone is not surprising.
The cytokines produced by CD4+ helper T cells (Th cells) play a key role in regulating mucosal and systemic immunity. Th cells can be divided into two general subsets, Th1 and Th2 cells, according to the different cytokine profiles they express. Th1 cells produce IL-2, IFNγ, and TNFβ, which mainly promote cell-mediated immunity. Within the GALT, both IFNγ and TNFβ are IgA-inhibiting cytokines, but unfortunately no antibodies for TNFβ currently exist to test tissue levels. Th2 cells produce IL-4, IL-5, IL-6, and IL-10, which promote antibody response and are IgA-stimulating within the GALT. There is cross-reactivity between the two types of cytokines. The Th1 cytokine, IFNγ, inhibits the growth of Th2 cells; the Th2 cytokine, IL-10, inhibits cytokine production by Th1 cells. 25 In mucosal immunity, these cytokines act on different stages of B-cell development. IL-4 stimulates sIgA− B cells to switch to sIgA+ B cells in Peyer’s patches, whereas IL-5, IL-6, and IL-10 promote sIgA+ B cells to mature to sIgA-secreting plasma cells in the lamina propria. 21,22,26–29 There appear to be synergistic effects between cytokines as well, because IL-10 and IL-4 cooperate to stimulate IgA secretion in vitro from CD40-activated peripheral blood mononuclear cells drawn from IgA-deficient individuals. 30 Interestingly, IL-10 alone produces maximal stimulation of IgA in normal individuals.
Because total gut cytokine levels were measured, the site of the individual cytokine production remains unclear: the mucosa, GALT T cells, and other cells may produce cytokines and induce local effects. IL-4 is a pleiotropic cytokine with a variety of effects on the growth and regulation of both B and T cells. 31 IL-4 influences the balance of Th1 and Th2 cells 32 and has potent antiinflammatory activity. 33,34 It downregulates IL-1, IL-6, IL-8, and TNF as well as superoxide anion secretion. In addition to T cells, IL-4 is also produced by the basophils and mast cells. IL-10 is produced by T cells, B lymphocytes, macrophages, and epithelial cells. It inhibits the secretion of IL-1, IL-2, IL-6, IL-8, IFNγ, and TNF and inhibits the production of tissue-destructive free radical superoxides produced by polymorphonuclear cells. 35,36 Because fatal enterocolitis of the intestine and colon develops in IL-10-deficient mice under the stimulation of luminal antigens, 37 it appears to play a crucial role in maintaining the balance of antiinflammatory and proinflammatory cytokines and the integrity of the intestinal barrier. It is interesting that unstimulated lamina propria T cells are not a major source of IL-10, whereas activated lamina propria T cells produce a large amount. 38 Whether IV TPN and the associated increases in permeability and mucosal atrophy lead to inactivation of lamina propria T cells with impaired production of IL-10, or whether epithelial cells are responsible for this IL-10 secretion with IV feeding is unknown, but close interaction and regulation of the GALT by the overlying mucosa has been speculated. Epithelial cells may be responsible for the basic IL-10 secretion involved in mediated permanent “immuno-unresponsiveness” in the normal mucosa. With atrophy, this relation may change.
In summary, cytokine changes within the GALT and/or mucosa influence intestinal and respiratory immunity. With IV TPN and, to a lesser extent, IG TPN, there are significant changes in cytokines within the small intestine, associated with intraluminal intestinal IgA levels. Although IFNγ, a potent IgA-inhibitory cytokine, remains unchanged with the route and type of nutrition, a significant reduction in two important IgA-stimulating cytokines, IL-4 and IL-10, occurs in association with IV TPN. These findings are consistent with a loss of immunity in the respiratory tract to viruses and a lethal Pseudomonas challenge. IG TPN completely preserves effective immunity against the IgA-mediated antiviral challenge, partially preserves defenses against Pseudomonas bacteria, and is associated with significant decreases in IL-4 but not IL-10. IV TPN induces significant decreases in both IL-4 and IL-10 and is reflected in greater losses in antiviral and antibacterial defenses.
Discussion
Dr. John A. Mannick (Boston, Massachusetts): This latest piece of work shows us, in answer to questions we have asked a couple of years ago at this meeting, what are the cells that are left in the gut making in the way of cytokines, because this might throw some light on what has been going on to the detriment of the host, particularly those hosts receiving TPN. And (Dr. Kudsk) has shown us some interesting but, I think, confusing results.
He has shown us that one of the factors that tends to induce IgA synthesis is not being normal amounts in the gut after TPN. But strangely enough, in spite of the fact that he has proven previously that there is a great diminution in several of the cell types that one would expect to make, the other cytokines that he is measuring, namely, T lymphocytes, and that he has got normal amounts of IL-5 produced, we think, by the same subset of lymphocytes that ordinarily make IL-4. And he has also shown us he has perfectly normal levels of interferon gamma being made in the gut.
All of this is a little hard to square anatomically with what cell types are left. So I’d like to ask him if he has any data at all about the cytokines that are made by specific cell types.
His lab is one of the few that can harvest enough cells out of the gut to show us what the specific cell types are making, and I’d like to ask him if he has any data on this subject.
I think that what he has shown us may have a role to play, as he has said, in the diminished IgA production after parenteral feeding. On the other hand, I suspect that he is right in his final comments in his presentation that there is more than that going on. Certainly, the fact that we have a great diminution in the cells available to make IgA antibody must have something to do with the problem, not just the cytokines they are exposed to.
Dr. Kenneth R. Sirinek (San Antonio, Texas): This work by Dr. Kudsk and his colleagues represents another piece of the puzzle toward our understanding of the homeostatic mechanisms of the gut immune system and enteral versus parenteral nutrition. I would like to ask the following technical and theoretical questions:
Could the results be secondary to differences in the fuel substrates in the various diets? Compared to the TPN solutions, the complex enteral diet had a lower nonprotein calorie-to-nitrogen ratio, 508 versus 740, and also contained fat. And I was wondering if you could address that.
Similar reductions in IgA occurred in mice fed TPN solutions by either the intravenous or intragastric routes compared to animals fed the chow or complex enteral diets. IL-4 was significantly reduced in both TPN groups, but IL-10 was only decreased in the intravenous TPN group, compared to the other fed controls. Therefore, is IL-4 a more potent upregulator of IgA than IL-10?
It would appear that IL-4 is a more potent upregulator of IL-5 and 6, because their levels remained constant while IgA levels decreased. Therefore, is there a hierarchy among these upregulators for IgA production?
Your prior studies demonstrated a 40% to 50% decrease in the number of B cells of the gut associated with lymphoid tissue with parenteral nutrition. This study demonstrates a change in the cytokine milieu.
Could you speculate as to which adverse effect on IgA production is more detrimental, or do they appear equal? Are they both contributing to this problem that we see with parenteral nutrition?
Dr. Kenneth A. Kudsk (Memphis, Tennessee): What we are measuring here in this assay is total gut cytokine level. We are looking at both the mucosa, which Wes Alexander and his group have shown is a contributor. And there is a known or at least a suspected interaction between what is happening in the mucosa and the underlying GALT system. And so right now we are in the process of harvesting the cells from the lamina propria. Dr. Wu has perfected the PCR analysis, and we are starting to look at within the GALT itself at what is happening with these cytokine levels. But that does not mean that the mucosal cytokine levels are not playing a role.
Eventually, we are working toward cell separation techniques and using LE spot to add cytokines in for the ability of the cells to produce this IgA, to see if it is a cell defect that occurs, a cytokine defect, or exactly what the problem is. And we will be embarking on those experiments probably within a year.
Dr. Sirinek, you asked a question about the difference in fuel substrates. There is a difference. We have added fat to the TPN solution when the animals are fed intragastrically, and there does seem to be a little bit of a change in the cell profile, but that certainly does not answer, it doesn’t bring it back to normal.
We have deliberately not put fat into the intravenous TPN because of the controversy of whether fat is, of itself, an immunosuppressant. And, unfortunately, there aren’t any enteral products that are available other than using IV TPN which does not have any fat, so we are sort of caught between the devil and the deep blue sea.
Is IL-4 a more potent stimulant than the others? I am not sure. Perhaps we will get some insight into that when we do the LE spots. One thing I do know, and it is the result of some work that we have been doing with the neuropeptides and with glutamine. You can give glutamine, and you can bring most of these effects about two thirds of the way back to normal, specific gut enterocyte and lamina propria fuel. You can bring it 100% back to normal by administering gastrin-releasing peptide or the model that we have used, bombesin, which is essentially the same thing.
Which leads me to wonder whether it is really the nutrition itself which is having an effect upon the GALT and the mucosa, et cetera, or whether it is the ability of the nutrients to stimulate the release of hormones by the gut, which is then maintaining our immunity. Totally different mechanisms, but we are starting to work our way to that point.
Footnotes
Correspondence: Kenneth A. Kudsk, MD, 956 Court Avenue, Suite E228, Memphis, TN 38163.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Supported by NIH grant 1 R01 GM53439.
Accepted for publication December 1998.
References
- 1.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 1992; 215: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg 1992; 216: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma—reduced septic morbidity. J Trauma 1989; 29: 916–922. [DOI] [PubMed] [Google Scholar]
- 4.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma—a prospective, randomized study. J Trauma 1986; 26: 874–880. [DOI] [PubMed] [Google Scholar]
- 5.Buckman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J Parenter Enteral Nutr 1995; 19: 453–460. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. J Parenter Enteral Nutr 1994; 18: 303–307. [DOI] [PubMed] [Google Scholar]
- 7.Purandare S, Offenbartl K, Westrom B, Bengmark S. Increased gut permeability to fluorescein isothiocyanate-dextran after total parenteral nutrition in the rat. Scand J Gastroenterol 1989; 24: 678–682. [DOI] [PubMed] [Google Scholar]
- 8.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988; 104: 185–190. [PubMed] [Google Scholar]
- 9.Deitch EA. Bacterial translocation of the gut flora. J Trauma 1990; S184–S189. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 1995; 39: 44–52. [DOI] [PubMed] [Google Scholar]
- 11.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg 1997; 132: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 12.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg 1996; 223: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg 1999; 229: 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langkamp-Henken B, Glezer JA, Kudsk KA. Immunologic structure and function of the gastrointestinal tract. Nutr Clin Pract 1992; 7: 100–108. [DOI] [PubMed] [Google Scholar]
- 15.Brandtzaeg P. distribution and characteristics of mucosal immunoglobulin-producing cells. In Ogra PL, Lamm ME, McGhee JR, et al, eds. Handbook of mucosal immunology. San Diego: Academic Press; 1994: 251–262.
- 16.Hanson LA, Ahlstedt S, Anderson B, et al. The biologic properties of secretory IgA. J Reticuloendothelial Soc 1980; 28: 1S–9S. [PubMed] [Google Scholar]
- 17.Lamm ME, Mazaneca MB, Nedrud JG, Kaetzel CS. New functions for mucosal IgA. In Mestecky J, Russell MW, Jackson S, et al, eds. Advances in mucosal immunology. New York: Plenum Press; 1995: 647–650. [DOI] [PubMed]
- 18.Deitch EA, Xu D, Qi L, Berg R. Elemental diet-induced immune suppression is caused by both bacterial and dietary factors. J Parenter Enteral Nutr 1993; 17: 332–336. [DOI] [PubMed] [Google Scholar]
- 19.Haskel Y, Xu D, Xu D, Lu Q, Deitch E. Elemental diet-induced bacterial translocation can be hormonally modulated. Ann Surg 1993; 217: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol 1989; 7: 145–173. [DOI] [PubMed] [Google Scholar]
- 21.Kramer DR, Sutherland RM, Bao S, Husband AJ. Cytokine-mediated effects in mucosal immunity. Immunol Cell Biol 1995; 73: 389–396. [DOI] [PubMed] [Google Scholar]
- 22.Ramsay AJ. Genetic approaches to the study of cytokine regulation of mucosal immunity. Immunol Cell Biol 1995; 73: 484–488. [DOI] [PubMed] [Google Scholar]
- 23.Lebman DA, Coffman RL. Cytokines in the mucosal immune system. In Ogra PL, Lamm ME, McGhee Jr, Mestecky J, Strober W, Bienenstock J, eds. Handbook of mucosal immunology. San Diego: Academic Press; 1994: 243–249.
- 24.Brandtzaeg P. Molecular and cellular aspects of the secretory immunoglobulin system. APMIS 1995; 103: 1–19. [DOI] [PubMed] [Google Scholar]
- 25.Fishman MA, Perelson AS. Th1/Th2 cross regulation. J Theor Bio 1994; 170: 25–56. [DOI] [PubMed] [Google Scholar]
- 26.Lebman DA, Coffman RL. The effects of IL-4 and IL-5 on the IgA response by murine Peyer’s patch B cell subpopulations. J Immunol 1988; 141: 2050–2056. [PubMed] [Google Scholar]
- 27.Murray PD, McKenzie DT, Swain SL, Kagnoff MF. Interleukin 5 and interleukin 4 produced by Peyer’s patch T cells selectively enhance immunoglobulin A expression. J Immunol 1987; 139: 2669–2674. [PubMed] [Google Scholar]
- 28.Harriman GR, Kunimoto DY, Elliott JF, et al. The role of IL-5 in IgA B cell differentiation. J Immunol 1988; 140: 3033–3039. [PubMed] [Google Scholar]
- 29.Beagley KW, Eldridge JH, Lee F, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high-rate IgA secretion in IgA-committed B cells. J Exp Med 1989; 169: 2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marconi M, Plebani A, Avanzini MA, et al. IL-10 and IL-4 co-operate to normalize in vitro IgA production in IgA-deficient (IgAD) patients. Clin Exp Immunol 1998; 112: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood 1991; 9: 1859–1870. [PubMed] [Google Scholar]
- 32.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell 1994; 76: 241–251. [DOI] [PubMed] [Google Scholar]
- 33.Hart PH, Vitti GF, Burgess DR, et al. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor α, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci USA 1989; 86: 3803–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci USA 1992; 89: 4076–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard M, O’Garra A. Biological properties of interleukin 10. Immunol Today 1992; 13: 198–201. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann TR. Properties and functions of interleukin-10. In Dixon FJ, ed. Advances in immunology. San Diego: Academic Press; 1994: 1–23. [PubMed]
- 37.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993; 75: 263–274. [DOI] [PubMed] [Google Scholar]
- 38.Braunstein J, Qiao L, Autschbach F, et al. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut 1997; 41: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]