Abstract
Objective
Anatomic fundoplication failure occurs after antireflux surgery and may be more common in the learning curve of laparoscopic antireflux surgery (LARS). The authors’ aims were to assess the incidence, presentation, precipitating factors, and management of anatomic fundoplication failures after LARS.
Summary Background Data
The advent of LARS has increased the frequency with which antireflux surgery is performed for the treatment of gastroesophageal reflux disease. Postoperative symptoms frequently occur and may result from physiologic abnormalities or anatomic failure of the fundoplication (e.g., displacement or disruption). Few data exist on the potential causes or best treatment of anatomic fundoplication failures.
Method
LARS was performed in 290 patients by one of the authors over a 6-year period. In the first 53 patients (group 1), the short gastric vessels were divided on a selective basis and the diaphragmatic crura were closed only when large hiatal hernias were present. In the subsequent 237 patients (group 2), the crura were always approximated posterior to the short gastric vessels and full fundic mobilization was performed. Clinical postoperative evaluation was performed on a regular basis, with detailed tests of anatomy and physiology when untoward symptoms developed. Postoperative foregut symptoms were reported by 26% of the patients, of whom 73% were found to have an intact fundoplication. In 7% of the entire group, anatomic failure of the fundoplication was demonstrated, with the majority exhibiting intrathoracic migration of the wrap with or without disruption of the fundoplication. New-onset postoperative epigastric or substernal chest pain frequently heralded fundoplication failure. Factors correlated with the development of anatomic fundoplication failure included presence in group 1, early postoperative vomiting, other diaphragm “stressors,” and large hiatal hernias. Repeat operation has been performed in 8 of the 20 patients (40%), with 5 patients successfully treated using laparoscopic techniques.
Conclusions
Anatomic fundoplication failure occurred in 7% of patients undergoing LARS, with the majority occurring in patients who underwent surgery during the learning curve. Anatomic failure is associated with technical shortcomings, large hiatal hernias, and early postoperative vomiting. Full esophageal mobilization and meticulous closure of the diaphragmatic crura posterior to the esophagus should minimize anatomic functional failure after LARS.
Since its introduction in 1991, 1 laparoscopic antireflux surgery (LARS) has rapidly been incorporated into the management algorithm of gastroesophageal reflux disease (GERD) by both physicians and surgeons. Given the apparently lower morbidity rate of the laparoscopic approach versus conventional open procedures for antireflux surgery, 2,3 patients and physicians have become more willing to consider surgery as an alternative to lifelong medical therapy of GERD. Correspondingly, numerous reports of the early outcomes of LARS have been published, with >3000 such procedures reported in the literature. 2 The results of most of these clinical series have been excellent, with good symptomatic outcomes and lower rates of incidental splenectomy and mortality than with open surgery. 2,4,5 However, in all published series, there is a small cohort of failed procedures or disrupted fundoplications.
LARS is technically challenging, and surgeons may occasionally “cut corners” from the fastidious technical details suggested for the appropriate performance of open antireflux operations. 6,7 Given that the surgical procedures are functional and reconstructive in nature, patients must be followed after surgery for long periods to assess outcome. Few series have reported physiologic measurements of foregut function (pH studies or esophageal manometry) after surgery, 4,5,8,9 because it is challenging to convince a satisfied and asymptomatic patient to undergo uncomfortable and time-consuming tests. Postoperative symptoms may be an effective way of screening patients to focus more detailed diagnostic evaluation. 10 When untoward symptoms occur after surgery, it is appropriate to assess the cause of the symptoms, which may result from physiologic problems (e.g., foregut motility disturbances, non-GERD disease processes, recurrent GERD with an intact fundoplication) or secondary to anatomic failure of the fundoplication (e.g., intrathoracic migration, slippage down onto the stomach, disruption of the fundoplication). 10–13
The aim of the current study was to assess the incidence, presentation, precipitating factors, and management of anatomic fundoplication failure after LARS.
PATIENTS AND METHODS
Patient Population
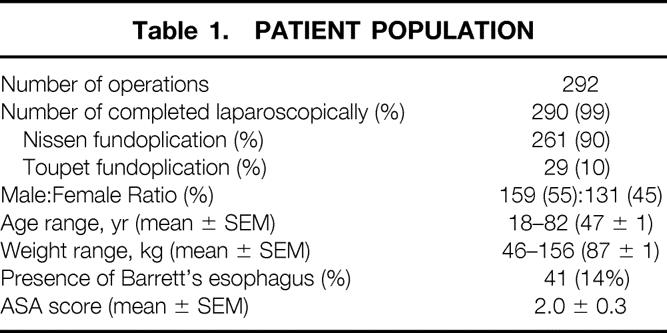
The patient population consisted of 292 consecutive patients undergoing LARS by the primary author between May 1992 and August 1998 (Table 1). Patient data were entered prospectively into a computerized database. Preoperative evaluation of all patients included a detailed history assessing global health status and symptoms of GERD. All patients underwent upper gastrointestinal endoscopy and esophageal manometry. Most patients underwent 24-hour pH tests to establish the diagnosis of GERD. 14 Conversion to open antireflux surgery occurred in two patients (<1%), leaving a total of 290 procedures completed laparoscopically. Of these, 90% were total, or 360°, fundoplications (Nissen fundoplication); the remaining 10% were partial posterior, or 270°, fundoplications (Toupet fundoplication). Barrett’s esophagus was diagnosed in 14% of patients.
Table 1. Patient Population
Operative Technique
Patients were divided into two groups based on the date and technique of LARS. In all patients, the fundoplication was constructed around a 50 to 60 F Maloney dilator. For Nissen fundoplications, the wrap was created using three sutures of heavy-gauge nonabsorbable suture and was sutured to the esophageal wall; the wrap was ≤2.5 cm long. In the first 53 patients (group 1), all procedures were total fundoplications, and mean (± SEM) operative time was 177 ± 9 minutes (Table 2). In group 1, details of the operative technique were variable. The crura were reapproximated only if a large defect existed, and the short gastric vessels were divided selectively.
Table 2. Comparison of Group 1 and Group 2
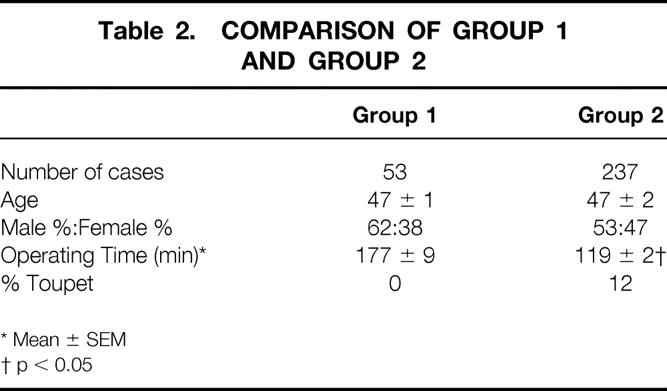
In the subsequent 237 patients (group 2), the crura were always approximated posterior to the esophagus and the short gastric vessels were divided routinely, allowing full fundic mobilization. 15 An extra suture was placed between the posterior aspect of the fundoplication and the right crus of the diaphragm. Mean operating time in group 2 dropped to 119 ± 2 minutes (p < 0.05 vs. group 1) as a result of the learning curve.
Postoperative Care and Evaluation
In the early part of our experience, postoperative antiemetics were ordered on an as-needed basis. In the last 150 cases, intravenous ondansetron was routinely administered during surgery and every 6 hours for three doses after surgery. Nasogastric tubes were not used and oral clear liquids were offered on the first postoperative morning. Patients were discharged when tolerating a soft diet, which occurred at an average of 1.6 ± 0.1 days after surgery. The patients were allowed to assume full, unrestricted activity as tolerated.
Patients were evaluated as outpatients at 2 to 4 weeks and 6 and 12 months after surgery; thereafter, they were evaluated annually. They completed detailed questionnaires regarding satisfaction, overall quality of life, GERD-related symptomatology, and occurrence of “diaphragmatic stressors” (e.g., repeated coughing or sneezing, vomiting, motor vehicle accidents, weight-lifting). Patients unable to return to the medical center were contacted by a nurse who administered the same questionnaire by telephone or mail.
Radiologic and physiologic tests of upper gut function were not performed routinely. However, patients who reported unusual abdominal or chest discomfort, GERD-related symptoms, or dysphagia underwent diagnostic testing. Generally, the first diagnostic test performed was a barium swallow to assess the overall morphologic appearance of the fundoplication and to obtain qualitative information regarding esophageal function. Additional testing (endoscopy, pH monitoring, and/or manometry) was performed as necessary to provide a diagnosis and guide therapy.
Anatomic fundoplication failure was defined as displacement (intrathoracic migration or slippage aborally onto the proximal stomach) or disruption of the fundoplication.
Data were analyzed using chi square analysis, Student’s t test, and logistic regression multivariate analysis as appropriate, using CLINFO and SAS (Carey, NC) software programs. Summary data were expressed as median and mean ± SEM. Significant differences were assumed to exist at p < 0.05.
RESULTS
Perioperative and Early Postoperative Outcomes
There were no deaths. Grade II or III complications 16 occurred in 14 patients (5%), with 3 patients (1%) requiring a second surgical procedure within 2 weeks. One patient required a second surgical procedure within the first week for a delayed gastric perforation, probably caused by a cautery serosal burn. Early postoperative (≤4 weeks) esophageal dilatation was required in four patients as a result of severe dysphagia, which resolved in all cases. Postoperative vomiting occurred within a week of surgery in 18 patients. In the first such patient, substernal chest pain developed 10 days after surgery; this patient was discovered to have intrathoracic migration of the fundoplication on a barium swallow. The patient was asymptomatic at the time and initially deferred therapy, but 3 years later was discovered to have a marked increase in the amount of fundus above the diaphragm associated with recurring symptoms. A second laparoscopic procedure was attempted, but conversion to a thoracotomy was needed for satisfactory repair. Subsequently, all patients with early postoperative emesis underwent immediate barium swallow to assess the status of the wrap. Two patients who were discovered to have intrathoracic migration of the fundoplication after a bout of postoperative vomiting underwent a second laparoscopic procedure within the first 10 days, with satisfactory repair of the defect (Fig. 1).
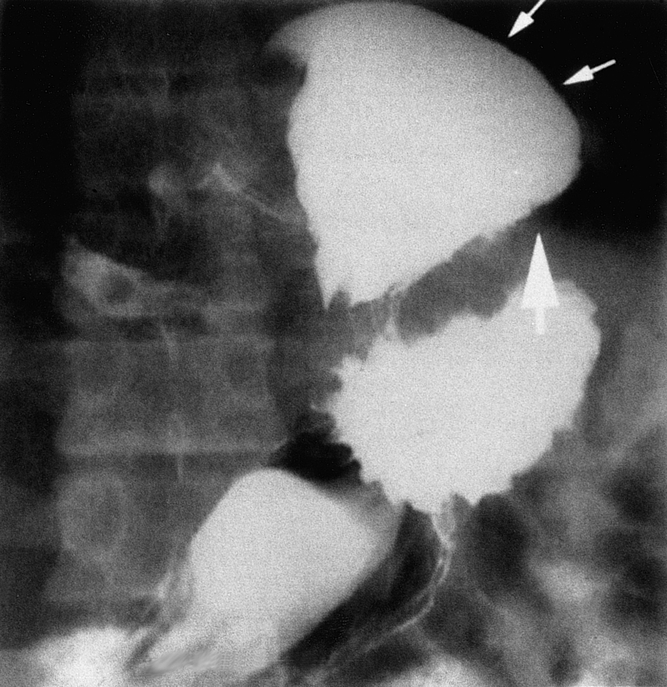
Figure 1. Barium swallow obtained 24 hours after a laparoscopic Nissen fundoplication in a patient who vomited after surgery. The fundoplication has herniated through the diaphragmatic hiatus into the mediastinum (small arrows), and the impression of the diaphragm on the herniated fundus can be clearly seen (heavy arrow).
Subsequent Follow-Up
Of the 290 patients undergoing successful LARS, 2 have died from unrelated causes; 2 are profoundly mentally retarded, precluding clinical evaluation; and 4 have been lost to follow-up. Thus, clinical follow-up is 98.6% complete and ranges from 1 to 76 months (Table 3). Of these patients, 74% are asymptomatic and have not undergone further diagnostic evaluation. In 74 patients (26%), diagnostic testing was performed because of postoperative symptoms. The most common symptom was epigastric or substernal chest pain, which occurred in 66% of the symptomatic group. In descending order of frequency, dysphagia (7%), heartburn (5%), and regurgitation (3%) were also reported. The ultimate diagnosis in the 74 symptomatic patients is shown in Table 4. In 54 of them (73%, or 19% of the entire group), the fundoplication was intact. In 26 of these patients, the workup was negative and the symptoms subsequently resolved. Recurrent GERD despite an intact fundoplication was diagnosed in 10 patients (3.4%). A stricture or narrowing of the distal esophagus was found in a total of five patients (including the four patients who underwent dilatation within the first month); it responded to intraluminal dilatation in all cases. In the other 13 symptomatic patients with intact fundoplications, symptoms were ultimately ascribed to functional disorders of the foregut and were managed medically.
Table 3. Clinical Follow-up
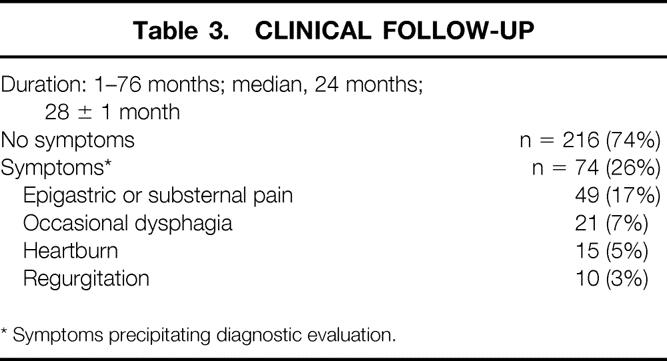
Table 4. Diagnosis in Symptomatic Patients
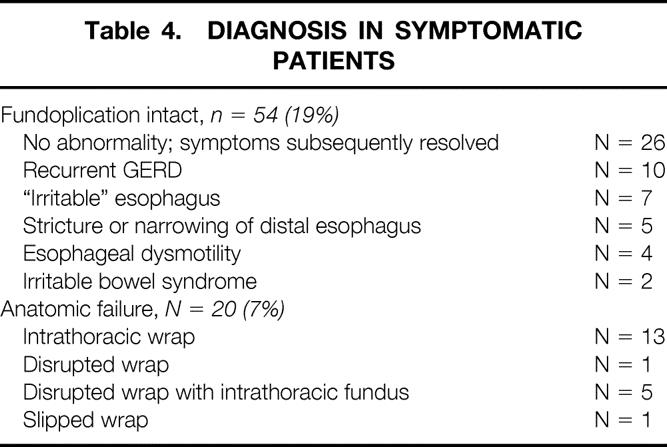
In the remaining 20 symptomatic patients (7%), anatomic failure of the fundoplication was demonstrated (Table 4). In 13 of these patients, the fundoplication was intact but was located superior to (above) the diaphragmatic hiatus (Figs. 1 and 2). In one patient the wrap was disrupted, and in five patients the fundoplication was partially or completely disrupted, along with a portion of the fundus being located above the diaphragm (Figs. 3 and 4). In a single patient, the fundoplication had slipped down onto the proximal stomach in association with recurrent hiatal hernia (Fig. 5). The interval between surgery and the diagnosis of fundoplication failure ranged from 1 day to 60 months, with a median of 24 months (20 ± 4 months).
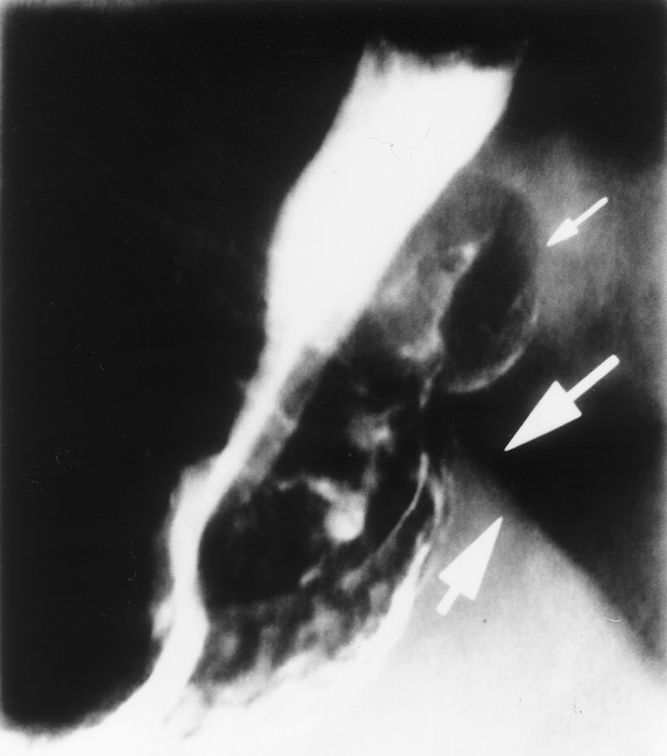
Figure 2. Barium swallow obtained 6 months after laparoscopic Nissen fundoplication in a patient with mild substernal discomfort. The fundoplication with air and barium within it (small arrow) can be seen protruding alongside the esophagus above the diaphragm (heavy arrows).
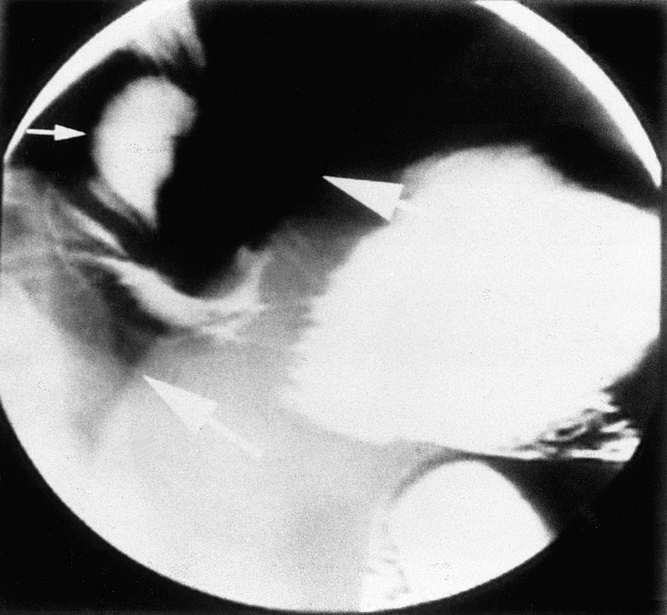
Figure 3. This patient vomited within the first 24 hours after a laparoscopic Nissen fundoplication. The wrap can be seen to be partially disrupted and located in the mediastinum (small arrow) above the level of the left hemidiaphragm (heavy arrows).
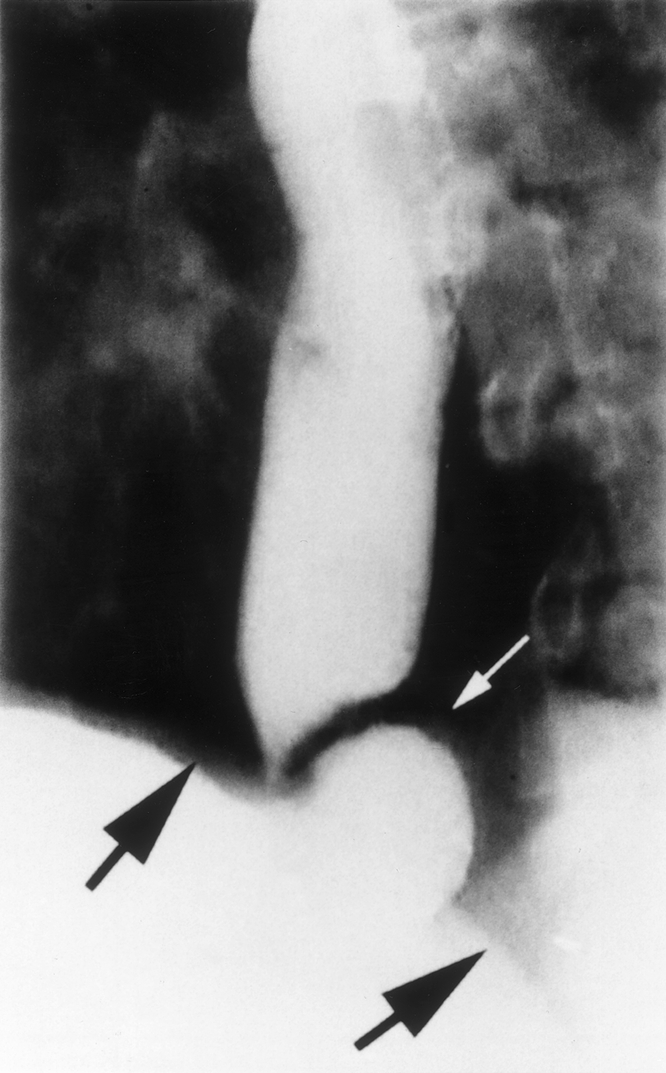
Figure 4. In this patient, recurrent heartburn and regurgitation developed 1 year after a laparoscopic Toupet fundoplication. A barium swallow revealed partial unwrapping of the fundoplication, with the fundus (small arrow) protruding above the level of the diaphragm (heavy arrows).
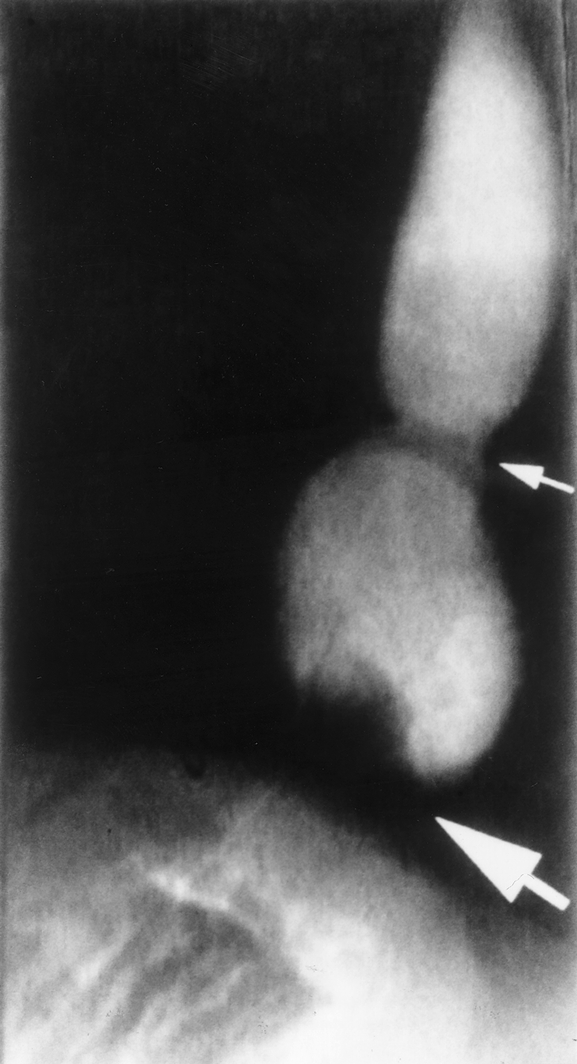
Figure 5. This patient had a large (5-cm) hiatal hernia with extensive periesophageal scarring at the time of laparoscopic Nissen fundoplication. After a bout of sneezing 6 months after surgery, dysphagia developed. A barium swallow revealed a recurrent hiatal hernia, with the gastroesophageal junction (small arrow) located well above the diaphragm, and the fundoplication defect can be seen around the proximal stomach (heavy arrow) at the level of the diaphragm.
In the entire group of 74 patients with untoward symptoms after LARS, there was an equal distribution of typical GERD-type symptoms (heartburn or regurgitation) between patients with and without anatomic failure of the fundoplication (Table 5). However, the development of new epigastric or substernal chest pain after LARS was reported by 85% of the patients with anatomic fundoplication failure versus 60% of those with an intact fundoplication (p < 0.05).
Table 5. Symptoms Associated With Anatomic Fundoplication Failure
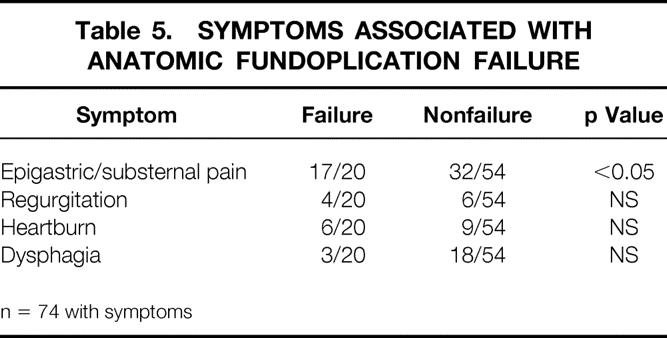
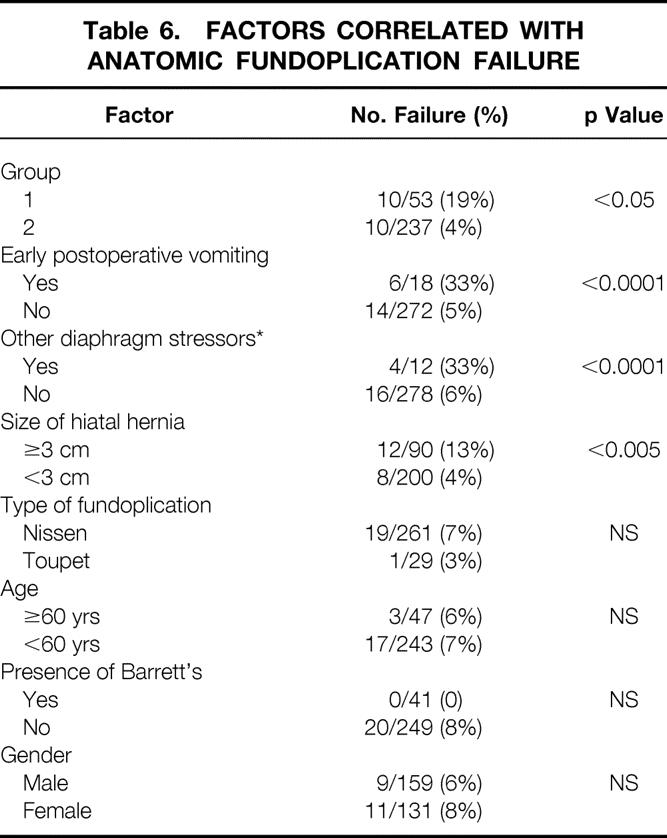
Table 6 details various factors that may be involved in anatomic functional failure. Patient age and gender, the presence of Barrett’s esophagus, and the type of fundoplication were not correlated with anatomic failure. However, patients in group 1 were much more likely to have failures (19%) than those in group 2 (4%; p < 0.05). Early postoperative vomiting and other subsequent diaphragm stressors were both associated with fundoplication failure (p < 0.0001 for each). Finally, the size of the hiatal hernia at the time of the original surgical procedure was significantly correlated with fundoplication failure: 13% of hiatal hernias >3 cm ultimately failed, compared with 4% of those with small or no hiatal hernias (p < 0.005). Multivariate analysis revealed that all four of these factors (operative group, postoperative vomiting, diaphragmatic stressors, hiatal hernia size) were significantly related (p < 0.05) to anatomic fundoplication failure.
Table 6. Factors Correlated with Anatomic Fundoplication Failure
Treatment of Anatomic Fundoplication Failure
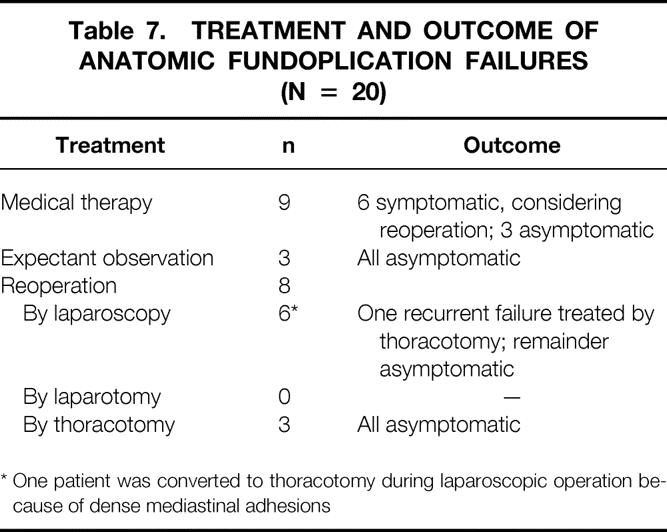
The treatment and outcome of anatomic fundoplication failures are shown in Table 7. Medical therapy or expectant observation has been employed in 12 patients; half of these patients have intermittent symptoms and are considering a second surgical procedure, and the other half are asymptomatic and declining surgical reintervention at this time. Surgical reintervention has been performed in eight patients at 2 days to 3 years after LARS. Laparoscopic treatment was attempted in six patients and completed successfully with excellent outcome in four of the six. One patient had dense mediastinal adhesions, requiring conversion of laparoscopy to thoracotomy for definitive repair. This patient vomited early after surgery and had had a longstanding intrathoracic migration of the Nissen fundoplication, with a large portion of the gastric fundus above the hiatus. The other patient in whom laparoscopic re-repair ultimately failed was a young male weight-lifter in whom acute intrathoracic migration of the wrap developed while he was bench-pressing >300 lb. The defect was re-repaired laparoscopically without complication. He subsequently did well for 6 months until he returned to weight-lifting, at which point the fundoplication again migrated into the chest. Surgical reintervention was performed by thoracotomy. The other three patients underwent thoracotomy as the initial reoperation because of a short esophagus, with the need for an esophageal lengthening procedure. All of these patients have done well in the postoperative interval.
Table 7. Treatment and Outcome of Anatomic Fundoplication Failures (N = 20)
DISCUSSION
Many publications have assessed the outcomes of open (conventional) procedures for GERD over the last several decades. Anatomic fundoplication failures have been reported in <5% to 50% of patients. 6,10,17–19 There are fewer reports objectively documenting failed procedures after LARS, but the rates range from 4% to 8% of cases. 4,5,20,21 In our experience, symptomatic anatomic fundoplication failure developed in 7% of patients. Most of these involved migration of the wrap into the chest, with subsequent pain or GERD symptoms.
In many patients, foregut symptoms develop or are retained after antireflux surgery. 22 We maintained a low threshold for investigating any untoward postoperative foregut symptoms. Of the symptomatic patients studied, 35% had no demonstrable abnormalities, and the symptoms resolved without therapy. It could be argued that other patients also have anatomic functional failure, because asymptomatic patients did not undergo routine postoperative diagnostic tests in this series. However, symptomatic outcome is generally a good indicator of fundoplication anatomy and function, as has been reported by others. 10 The primary reason for reviewing our experience with LARS was to identify possible causes of anatomic fundoplication failure to minimize such complications in the future.
Several potential elements associated with fundoplication failure became readily apparent from this series. These included technical factors, events causing marked increases in intraabdominal pressure and diaphragmatic tension, and the size of the initial hiatal hernia. We have previously reported the influence of surgical technique on early clinical outcome of laparoscopic Nissen fundoplication. 15 The current study confirms our previous observations: patients in group 1, in whom routine mobilization of the fundus and closure of the hiatus were not performed, had a nearly fivefold increased incidence of fundoplication failure compared with patients who routinely underwent these technical steps. Because these two groups were sequential, it is also likely that surgical inexperience played a role in the markedly increased number of failures in the earlier group. 11,23 Regardless of the specific cause, the incidence of anatomic functional failure decreased from 19% in group 1 to only 4% in group 2.
One of the major anatomic abnormalities seen in patients with anatomic fundoplication failure was migration of the wrap into the chest, with or without disruption of the repair. Contributing factors may include inadequate closure of the diaphragmatic crura, a short esophagus, and/or inadequate mobilization of the esophagus and physiologic factors that would tend to increase the pressure or tension at the esophageal hiatus. Our data support these possibilities. The occurrence of early postoperative vomiting should be taken seriously and investigated promptly. In 18 patients who retched or vomited within the first week after surgery, anatomic fundoplication failure developed in one third; many of these patients were not diagnosed until much later. When prompt barium x-rays were obtained after a bout of postoperative vomiting, we were easily able to re-repair the herniated fundoplication laparoscopically in the early postoperative period in two patients. The occurrence of early postoperative nausea and vomiting seems to have been lessened by the routine use of intravenous ondansetron in patients undergoing LARS.
Other diaphragmatic stressors occurring later after surgery are more difficult to predict and prevent, and are subject to recall bias. In one patient, the sport of weight-lifting was temporally related to the occurrence of two episodes of intrathoracic migration of the fundoplication. At the time of the first reoperation, one of the 0-gauge braided polyester sutures had broken, allowing herniation of the wrap through the hiatus. It is difficult to predict the type of stress placed on the diaphragm with heavy weight-lifting, and it may be wise to advise patients who have undergone LARS to avoid these types of activities. In a second patient, wrap herniation and disruption developed after a high-speed motor vehicle accident that also caused a pulmonary contusion and shoulder separation.
The size of the hiatal hernia at the time of the initial surgical procedure was also associated with anatomic fundoplication failure: fundoplications performed in the presence of larger hiatal hernias were three times more likely to fail than those with negligible or no hiatal hernias. This difference may relate to anatomic foreshortening of the esophagus, inadequate mobilization of the esophagus, or a combination of these two factors leading to axial tension on the gastroesophageal junction. Although a markedly foreshortened esophagus can sometimes be detected on preoperative studies (and these patients can undergo elective Collis–Nissen fundoplication), the surgeon must be prepared to perform a lengthening procedure at the time of LARS if the esophagus cannot be adequately mobilized to allow 3 or 4 cm to remain intraabdominally without tension before the repair. 24,25 It is hoped that preoperative means of measuring the effective length of the esophagus will be developed in the near future.
Management of anatomic fundoplication failures is challenging. Several small reviews of laparoscopic reoperations have been published. 20,26 Certainly, an intrathoracic fundoplication effectively represents a paraesophageal hiatal hernia and may predispose to strangulation and intrathoracic perforation. 27 Therefore, it is prudent to advise operative reintervention. Before a second surgical procedure, barium swallow and endoscopy should be performed to rule out significant mucosal abnormalities and to assess for esophageal foreshortening. Esophageal manometry should also be performed to measure the amplitude of proximal esophageal peristalsis. If the gastroesophageal junction appears to be intraabdominal, a laparoscopic procedure may be attempted, with the knowledge that a significant percentage of cases will need to be converted to open conventional surgery.
The risk of complications with these reoperations is probably greater than for primary antireflux surgery. If a patient is found to have a short esophagus in association with anatomic fundoplication failure, we advocate performance of a thoracotomy with a Collis lengthening procedure as the preferred treatment.
CONCLUSION AND RECOMMENDATIONS
Anatomic fundoplication failure occurred in 7% of patients undergoing LARS in this series, with the majority occurring in patients who underwent surgery during the learning curve. Anatomic failure is associated with technical shortcomings, large hiatal hernias, and early postoperative vomiting, which should be prevented as much as possible. Full esophageal mobilization, a loose wrap, and meticulous closure of the diaphragmatic crura posterior to the esophagus should minimize anatomic functional failure after LARS.
Acknowledgment
The authors thank Mrs. Judith Stroer for secretarial assistance.
Discussion
Dr. Bruce D. Schirmer (Charlottesville, Virginia): This paper documents what has arisen and is true about laparoscopy in terms of antireflux surgery, and that is this procedure has now become commonly done by surgeons who have expertise in laparoscopic surgery.
While a series such as Dr. Soper’s may not be common for the average gastrointestinal surgeon, I think there are reasons why there is an increasing incidence of surgical treatment of this disease in the last few years. And these are two. The first is our referring physicians perceive laparoscopic surgery as less traumatic and less morbid for their patients. And the second is that the results of these operations done laparoscopically, to this point, have been excellent. Dr. Soper’s paper is an illustration of that second point. However, I would quickly add that it is only through our continued adherence to the track record that he has established that we will continue to see this pattern of referrals continue.
Dr. Soper’s paper points out several important lessons for those of us who would perform these operations, and I think they deserve special emphasis.
The first is, it is important to take down the short gastric vessels. Arguments aside as to whether this is necessary for complete fundic mobilization, I routinely teach my residents to do the operation by starting with this part of the procedure. By marching up along the fundus of the stomach, you run right into the left cruris of the diaphragm. You can’t get lost. It is much less likely that you are going to inadvertently dissect into the side of the esophagus. And I think this is a good way to start the operation, particularly for the novice surgeon who is first undertaking an experience with this procedure laparoscopically.
The second, as the data have well illustrated, is to adequately close the crura.
Third, and very importantly, is the fundoplication must be sutured to the diaphragm.
Fourth is that large hiatal hernias are technically difficult challenges and should be undertaken after a significant laparoscopic experience has been already experienced. And the surgeon needs to follow the tenets of reducing the sac, adequately mobilizing the esophagus, and completely closing the crura.
And, finally, Dr. Soper has shown us the fifth point that has not previously been well described in the literature. And that is patients who develop early postoperative emesis are candidates for slippage of their fundus up into the chest and should be studied early and aggressively to determine whether or not this has occurred.
I commend Dr. Soper particularly, also, on his high percentage of follow-up in his patients, and I have two questions for him.
The first is I’d like you to define what you mean by early postoperative vomiting. In other words, how many days postoperative is this? To what extent? And how many times must a patient vomit before you will study a patient?
And the second is, there were a large number of patients that were studied for symptoms postoperatively. About two thirds of those did not have any disruption of the wrap. Would you please comment on the cost effectiveness of aggressively studying all of these patients versus simply following the natural history of what would happen to those patients if they were unstudied and suddenly their symptoms progressed, and only those who really had wrap slippage then were studied later?
Dr. William O. Richards (Nashville, Tennessee): Laparoscopic antireflux surgery is a technically demanding procedure. Not only does experience reduce the time to perform the procedure, but it also reduces the anatomic failure rate. The number of procedures that Dr. Soper has identified to produce these results is 53 cases. This is four times the number of laparoscopic cholecystectomies that the Southern Surgeons club identified as a critical number of procedures in the learning curve to reduce common bile duct injuries. I doubt that few or any chief residents complete 53 laparoscopic fundoplications during their chief residency. You have identified that as the break point in reducing postoperative failure.
My first question is two-fold: is it the number of cases in the surgeon’s experience, or is it application of the technical modifications that you have elaborated—i.e., takedown of the short gastric, closure of the hiatus in all cases, and suture of the wrap to the crura—that makes the difference in postoperative failure? The second part of the question is whether or not you can teach technical skills outside of the operating room that will effectively reduce the postoperative failure rate.
My third question concerns hernia size and recurrence. We have learned that hernia repair, inguinal hernia repair, with prosthetic materials can reduce recurrence rate for inguinal hernias. Should we not adopt the same philosophy with large hiatal hernias that have attenuated tissues, and use prosthetic materials to reinforce or assist in the repair?
Dr. David B. Adams (Charleston, South Carolina): This is a topic, I think, that is especially critical to the general surgeon because of the increasing numbers of these procedures that are being done. And it is my view that the patient morbidity and mortality that you can incur from complications of this operation is as big as those you get from lap-chole common bile duct injuries. So my questions relate chiefly to the technique that you espouse, focusing on the problem of the intrathoracic migration fundoplication.
Dr. Soper, why is it that when we were doing fundoplications with an open technique, selective division of the short gastrics achieved excellent results, and now the conventional wisdom with the lap/Nissen is that the short gastrics must be divided? Could you elaborate on the technique of short gastric division? Where do you start? Where do you end? Is it really necessary to mobilize the fundus completely posteriorly? Is it possible to mobilize too much fundus and create a “too loose” fundoplication, which is prone to herniation? Do you close the crura over your esophageal dilator? Do you use pledgets in your crural closure, and can the crural closure be too tight?
And another issue that came up in the manuscript is the issue of the short esophagus and its role in intrathoracic migration. Can this diagnosis be made successfully preoperatively? And, if so, how do you manage it?
Dr. C. Randle Voyles (Jackson, Mississippi): I stand to make some comment about the financial morbidity, about the decisions that we as surgeons make when we elect to do our operations, and specifically I want to address instrumentation.
You know, it was only 8 years ago that 90% of us were doing our laparoscopic cholecystectomies with the lasers. Only 7 years ago, we realized that the laser was completely unnecessary. I am concerned that we have a similar scenario now with the ultrasonic dissector or so-called harmonic scalpel for taking down the short gastric vessels. Monopolar electrode surgery doesn’t work well, clips don’t work well, and the medical industry has provided us with the so-called harmonic scalpel.
I think there is a better solution, and this is a direct parallel with a laparoscopic cholecystectomy. Bipolar electrosurgery works very, very well. It is quicker. There is good laboratory evidence that shows that you get a stronger seal. And even if you don’t accept all of that, it has to be compounded by the fact that it is at least $300 less expensive compared to the ultrasonic dissector.
So at this point in time, at least in my practice, I feel very comfortable in saying that the bipolar devices are superior to the ultrasonic devices for taking down the short gastric vessels, but I couch that in the overall picture, that reducing your patient morbidity is, obviously, the most important thing.
So in closing, I’d like to ask the authors if—they have given us excellent direction in dealing with some of the morbid problems that can occur with complications—I would like them to shortly address some of the financial morbidity with instrument selection.
Dr. Nathaniel J. Soper (Closing Discussion): Airing dirty laundry in front of people, I thought that in fact I would get some different comments. But I think there are two things to do, either talk about your good results—which is commendable—but I think if you talk about your bad results, I think it is ultimately more educational. Given the number of surgeons on the learning curve, I do think there is value in a report such as this.
Dr. Schirmer, you asked about the definition of early postoperative vomiting. In general, they were all vomits within the hospital, except for two out of the 18 that occurred on their first or second day at home, so we defined it as within the first week postoperatively. How long that is a problem in terms of really making the wrap go up, I don’t think anybody really knows, but I think certainly early in the postoperative period—the first few days—I think the wrap is at risk.
In terms of the cost effectiveness of work-up, again, we have no good data on that. Certainly, early on, we were very nervous about the outcomes of this operation, and we worked up people at the drop of a hat. Now we did not bring people back for routine physiologic follow-up, as has been attempted in a number of studies. The problem is it is very difficult to get a happy, satisfied customer back for uncomfortable and very time-consuming tests.
So in my mind, barium swallow is cheap, it is easily tolerated. And in a patient who has untoward symptoms, it is a good way to diagnose anatomic failure. It may not give you all the information, but it is a good starting point.
Dr. Richards asked about the learning curve. Well, certainly, the learning curve doesn’t go to 53 and then change. That was the point at which I realized we were doing something wrong and changed to doing it the same way every time, as I tell the people in the operating room.
There are a number of others, such as Jamison in Australia, who have looked at their results. And they looked at their first 50, in fact, in the institution and saw a higher complication rate, reoperation, et cetera. So that was 50, but it was within the institution, and that is similar to what our results show. After the institution had gotten expertise and experience, then with each individual surgeon learning after that, the learning curve was somewhere between 10 and 20, and I think that comes with getting the appropriate techniques and then mandating that things be done that way.
Can skills be taught outside of the operating room? Sure, it can—sure they can, I mean, at least in terms of two-handed eye-hand coordination, some of the video skills, and that sort of thing. But in terms of really being able to put sutures in and feel the tension of the wrap, et cetera, I think there is nothing like the operating room itself. Animal models are okay, and it is a good place to start, but I think the human is really where it counts, obviously.
In terms of the hernia size, large hiatal hernias are very difficult, particularly periesophageal hiatal hernias, which are separate from this. But large ones can be a real problem, in that I believe there can be tension, there may be a short esophagus. You can have a big hole.
And I agree, with inguinal hernias, most of us now have cottoned to the fact that you need to put some material in to make it without tension. We are very nervous putting prostheses around the esophagus. There are a number of unreported accidents now out there in practice, where people have put in polypropylene mesh at the hiatus, people have had to do esophagectomies, gastrectomies to remove these things that have grown into the stomach and esophagus. So I would very much warn against using polypropylene mesh. Perhaps Gor-Tex material or polytetrafluoroethylene would be a reasonable alternative. And there are several prospective randomized trials where periesophageal hiatal hernias right now are going on, looking at using PTFE or not. And I think we need more data from that.
In terms of the technique, Dr. Adams asked about how to divide the short gastric vessels and why. Why? I think it is more because you get a good fundic mobilization than dividing the short gastric vessels themselves. And, yes, we fully mobilize the fundus from its posterior and lateral attachments so that we can literally wrap it around two or three times if we wanted. Can you make it too loose? I suppose you probably can, although I am not 100% sure of that. I don’t think anybody has ever shown what too loose of a wrap really is.
We close the hiatus without pledgets, unless it is a large hiatal hernia, and then we use Teflon pledgets. How large is large? I don’t know. When I eyeball and it looks big, we put in pledgets, thinking that may help reduce the tearing-through of the tissue.
We do not do it over a dilator. What we try to do is close the hiatus so that without a dilator in place, it is just right up to the esophageal wall. We put the dilator in then subsequently, while we do the wrap itself. If you have a dilator in at the time of trying to close the crura posteriorly, it is very difficult to actually access that area.
In terms of short esophagus, the diagnosis of that can be difficult. Most hiatal hernias, no matter how big they look on preoperative evaluations, can have full esophageal mobilization and bring the GE junction into the abdomen, but some you just can’t.
We look at a barium swallow, we measure the distance, we see if there is any laxity of the esophagus upstream. We also do esophageal manometry and localize the LES in relation to the esophageal hiatus. And if there is a clear separation, I am worried about a short esophagus, I would tend to treat those patients with Collis procedures. If you can do them laparoscopically, great. If not, then I think the patient needs a thoracotomy for a good outcome.
In terms of the technology, Dr. Voyles asked, we actually did a small prospective randomized trial comparing the harmonic scissors to the bipolar technology. We could show no difference in any measured outcomes, but we had one cautery burn of the stomach with the bipolar. There is some lateral heat transmitted, but not much. And we also found it slightly more awkward to use in terms of the bipolar and cutting between it, compared to the harmonic scalpel. We tend to use the harmonic scalpel. I realize that that does accrue an expense that goes along with it. I think in each individual’s hand, you must find what you feel comfortable with and use that and use it well. I think any energy source we have is potentially dangerous, and I think we must just use them in a responsible fashion.
Footnotes
Correspondence: Nathaniel J. Soper, MD, Department of Surgery, Washington University School of Medicine, One Barnes Hospital Plaza, Box 8109, St. Louis, MO 63110.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Supported by Washington University Institute for Minimally Invasive Surgery as funded by Ethicon Endosurgery, Inc.
Accepted for publication December 1998.
References
- 1.Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc 1991; 1: 138–143. [PubMed] [Google Scholar]
- 2.Perdikis G, Hinder RA, Lund RJ, et al. Laparoscopic Nissen fundoplication: where do we stand? Surg Laparosc Endosc 1997; 7: 17–21. [PubMed] [Google Scholar]
- 3.Laine S, Rantala A, Gullichsen R, Ovaska J. Laparoscopic vs. conventional Nissen fundoplication. Surg Endosc 1977; 11: 441–444. [DOI] [PubMed] [Google Scholar]
- 4.Hunter JG, Truss TL, Branum GD. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg 1996; 223: 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinder RA, Filipi CJ, Wetscher G. Laparoscopic Nissen fundoplication is an effective treatment for gastroesophageal reflux disease. Ann Surg 1994; 220: 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesophageal reflux disease. Ann Surg 1986; 204: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunnington GL, DeMeester TR, and the Dept. Of V.A. GERD Study Group. Outcome effect of adherence to operative principles of Nissen fundoplication by multiple surgeons. Am J Surg 1993; 166: 654–659. [DOI] [PubMed] [Google Scholar]
- 8.Patti MG, De Pinto M, de Bellis M, et al. Comparison of laparoscopic total and partial fundoplication for gastroesophageal reflux. J Gastro Surg 1997; 1: 309–315. [DOI] [PubMed] [Google Scholar]
- 9.Anvari M, Allen C. Esophageal and lower esophageal sphincter pressure profiles 6 and 24 months after laparoscopic fundoplication and their association with postoperative dysphagia. Surg Endosc 1998; 12: 421–426. [DOI] [PubMed] [Google Scholar]
- 10.O’Hanrahan T, Marples M, Bancewicz J. Recurrent reflux and wrap disruption after Nissen fundoplication: detection, incidence and timing. Br J Surg 1990; 77: 545–547. [DOI] [PubMed] [Google Scholar]
- 11.Watson DI, Jamieson GG, Devitt PG, et al. Changing strategies in the performance of laparoscopic Nissen fundoplication as a result of experience with 230 operations. Surg Endosc 1995; 9: 961–966. [DOI] [PubMed] [Google Scholar]
- 12.Stein HJ, Feussner H, Siewert JR. Failure of antireflux surgery: causes and management strategies. Am J Surg 1996; 171: 36–40. [DOI] [PubMed] [Google Scholar]
- 13.Dallemagne B, Weerts JM, Jehaes C, Markiewicz S. Causes of failures of laparoscopic antireflux operations. Surg Endosc 1996; 10: 305–310. [DOI] [PubMed] [Google Scholar]
- 14.Waring JP, Hunter JG, Oddsdottir M, Wo J, Katz E. The preoperative evaluation of patients considered for laparoscopic antireflux surgery. Am J Gastroenterol 1995; 90: 35–38. [PubMed] [Google Scholar]
- 15.Wu JS, Dunnegan DL, Luttmann DR, Soper NJ. The influence of surgical technique on clinical outcome of laparoscopic Nissen fundoplication. Surg Endosc 1996; 10: 1164–1170. [DOI] [PubMed] [Google Scholar]
- 16.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992; 111: 518–526. [PubMed] [Google Scholar]
- 17.Low DE, Mercer CD, James EC, Hill LD. Post-Nissen syndrome. Surg Gynecol Obstet 1988; 167: 1–5. [PubMed] [Google Scholar]
- 18.Thor KBA, Silander T. A long-term randomized prospective trial of the Nissen procedure versus a modified Toupet technique. Ann Surg 1989; 210: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand DL, Eastwood IR, Martin D, Carter WB, Pope CE. Esophageal symptoms, manometry, and histology before and after antireflux surgery. Gastroenterology 1979; 76: 1393–1401. [PubMed] [Google Scholar]
- 20.DePaula AL, Hushiba K, Bafutto M, Machado CA. Laparoscopic re-operations after failed and complicated antireflux operations. Surg Endosc 1995; 9: 681–686. [DOI] [PubMed] [Google Scholar]
- 21.Lundel L, Abrahamsson H, Rydberg L, et al. Total (Nissen-Rosetti) or semi-fundoplication (Toupet)? Long-term results from a prospective randomized study. Swedish Surg Soc 1994; 103: 217. [Google Scholar]
- 22.Swanstrom L, Wayne R. Spectrum of gastrointestinal symptoms after laparoscopic fundoplication. Am J Surg 1994; 167: 538–541. [DOI] [PubMed] [Google Scholar]
- 23.Watson DI, Baigrie RJ, Jamieson GG. A learning curve for laparoscopic fundoplication: definable, avoidable, or a waste of time? Ann Surg 1996; 224: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication. Surg Endosc 1998; 12: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 25.Jobe BA, Horvath KD, Swanstrom LL. Postoperative function following laparoscopic Collis gastroplasty for shortened esophagus. Arch Surg 1998; 133: 867–874. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly MJ, Mullins S, Reddick EJ. Laparoscopic management of failed antireflux surgery. Surg Laparosc Endosc 1997; 7: 90–93. [PubMed] [Google Scholar]
- 27.Johansson B, Glise H, Hallerback B. Thoracic herniation and intrathoracic gastric perforation after laparoscopic fundoplication. Surg Endosc 1995; 9: 917–918. [DOI] [PubMed] [Google Scholar]


