Abstract
Objective
Distal pancreatectomy is performed for a variety of benign and malignant conditions. In recent years, significant improvements in perioperative results have been observed at high-volume centers after pancreaticoduodenectomy. Little data, however, are available concerning the current indications and outcomes after distal pancreatectomy. This single-institution experience reviews the recent indications, complications, and outcomes after distal pancreatectomy.
Methods
A retrospective analysis was performed of the hospital records of all patients undergoing distal pancreatectomy between January 1994 and December 1997, inclusive.
Results
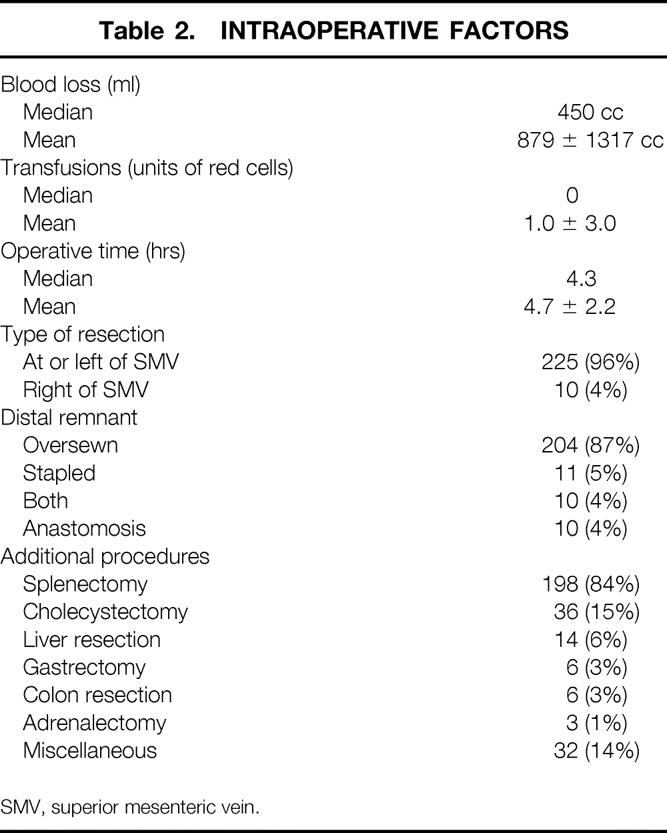
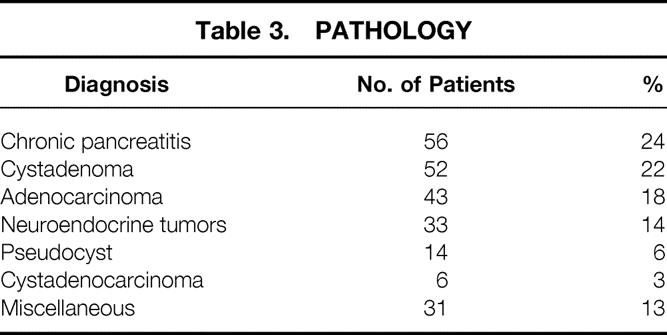
The patient population (n = 235) had a mean age of 51 years, (range 1 month to 82 years); 43% were male and 84% white. The final diagnoses included chronic pancreatitis (24%), benign pancreatic cystadenoma (22%), pancreatic adenocarcinoma (18%), neuroendocrine tumor (14%), pancreatic pseudocyst (6%), cystadenocarcinoma (3%), and miscellaneous (13%). The level of resection was at or to the left of the superior mesenteric vein in 96% of patients. A splenectomy was performed in 84% and a cholecystectomy in 15% of patients. The median intraoperative blood loss was 450 ml, the median number of red blood cell units transfused was zero, and the median operative time was 4.3 hours. Two deaths occurred in the hospital or within 30 days of surgery for a perioperative mortality rate of 0.9%. The overall postoperative complication rate was 31%; the most common complications were new-onset insulin-dependent diabetes (8%), pancreatic fistula (5%), intraabdominal abscess (4%), small bowel obstruction (4%), and postoperative hemorrhage (4%). Fourteen patients (6%) required a second surgical procedure; the most common indication was postoperative bleeding. The median length of postoperative hospital stay was 10 days. Patients who underwent a distal pancreatectomy with splenectomy (n = 198) had a similar complication rate (30% vs. 29%), operative time (4.6 vs. 5.1 hours), and intraoperative blood loss (500 vs. 350 ml) and a shorter postoperative length of stay (13 vs. 21 days) than the patients who had splenic preservation (n = 37).
Conclusions
This series represents the largest single-institution experience with distal pancreatectomy. These data demonstrate that elective distal pancreatectomy is associated with a mortality rate of <1%. These results demonstrate that, as with pancreaticoduodenectomy, distal pancreatectomy can be performed with minimal perioperative mortality and acceptable morbidity.
Distal pancreatectomy is a term applied to resection of that portion of the pancreas extending to the left of the midline and not including the duodenum and distal bile duct. Distal pancreatectomy is performed for various indications, including benign inflammatory conditions and benign and malignant neoplasms. In the past decade, pancreaticoduodenectomy, or resection of the head of the pancreas, has been performed with increased frequency because of a marked reduction in procedure-related morbidity and mortality rates. 1–4 The frequency of resection of the distal pancreas has not risen as dramatically during this period, primarily because of the relative infrequency of resectable malignant pancreatic neoplasms involving the body and tail of the gland 5–7 and a general dissatisfaction with distal resection for the management of chronic pancreatitis. 8,9 The purpose of this report is to review a single high-volume institution’s experience with distal pancreatectomy and to define the current indications and short-term outcome associated with the procedure.
PATIENTS AND METHODS
From January 1984 to December 1997, 265 patients underwent distal pancreatectomy at The Johns Hopkins Hospital. A retrospective review of hospital records was completed. Thirty patients were excluded from the reported patient population because distal pancreatectomy was performed emergently for trauma or as part of a radical resection for gastric cancer. Statistical analysis was performed using a commercially available statistical program using the chi square test and Student’s t test as appropriate. Results are reported as mean ± standard deviation and/or median. Statistical significance was accepted at p < 0.05.
RESULTS
Patient Demographics and Intraoperative Factors
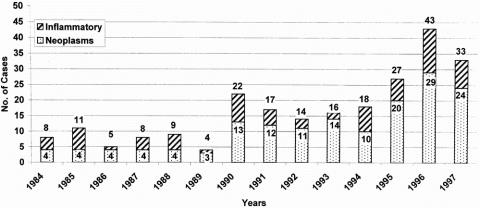
In the 14 years of this study, 235 patients underwent elective distal pancreatectomy. The annual distribution of these resections is depicted in Figure 1. A steady increase has been observed during the past decade. Resection of pancreatic neoplasms, both benign and malignant, has accounted for most of the increase seen over this period. The patient demographics and intraoperative factors are presented in Tables 1 and 2. The mean age of the patients was 51 ± 18 years (median age 50 years; range 1 month to 82 years). Nine patients (4%) were younger than 18 years; 54 patients (23%) were older than 65 years. There were 134 women (57%) and 101 men (43%). The racial distribution in the series was 196 whites (84%), 24 blacks (10%), and 13 other (6%). The mean operative time was 4.7 ± 2.2 hours, the mean intraoperative blood loss was 879 ± 1317 ml (median 450 ml, range 20 to 12,000 ml), and the median number of units of red blood cells transfused was zero.
Figure 1. Number of distal pancreatectomies per year from 1984 to 1997. For each vertical bar, the total number of resections is shown, as is the number of resections performed for pancreatic neoplasms, both benign and malignant.
Table 1. Demographics
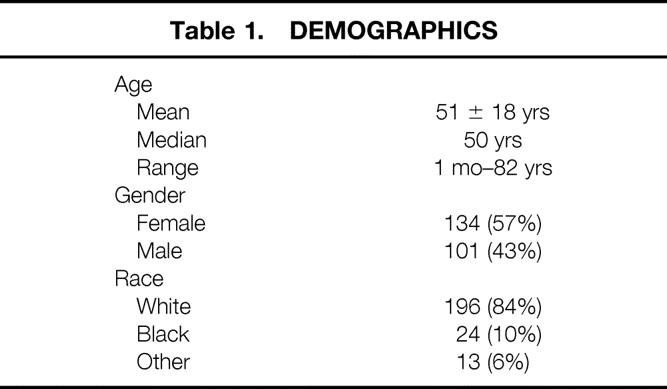
Table 2. Intraoperative Factors
Distal pancreatectomy was performed with a concomitant splenectomy in 198 patients (84%). A cholecystectomy was performed in 36 patients (15%), liver resection in 14 patients (6%), gastric resection in 6 patients (3%), and colon resection in 6 patients (3%). Miscellaneous additional procedures were performed in 32 patients (14%): nephrectomies (4), Nissen fundoplication (1), appendectomy (1), salpingo-oophorectomy (1), and transduodenal accessory pancreatic duct sphincteroplasty (1).
Distal pancreatic resection was performed at or to the left of the superior mesenteric vein in 225 patients (96%). In the remaining 10 patients, the resection approached the duodenum, encompassing up to 85% of the gland. The distal end of the retained pancreas was oversewn in 204 patients (87%), stapled in 11 patients (5%), and both stapled and oversewn in 10 patients (4%). In 10 patients (4%), the distal gland was anastomosed to a Roux-en-Y jejunal limb, creating a pancreaticojejunostomy.
Pathologic Analysis of Resected Specimens
The final pathologic diagnoses of the resected specimens are shown in Table 3. Chronic pancreatitis was the final diagnosis in 56 patients (24%). Pancreatic neoplasms included benign cystadenoma in 52 patients (22%), adenocarcinoma in 43 patients (18%), neuroendocrine tumors in 33 patients (14%), and cystadenocarcinoma in 6 patients (3%). Pancreatectomy was performed for a pancreatic pseudocyst in 14 patients (6%). Thirty-one patients (13%) were classified as having a miscellaneous pathologic diagnosis, including four patients with papillary cystic and solid neoplasms (Hamoudi tumors), three patients with nesidioblastosis, and two patients with metastatic renal cell carcinomas. The pathologic diagnoses for the nine children included nesidioblastosis (2), chronic pancreatitis (2), pancreaticoblastoma (1), pancreatic pseudocyst (1), neuroendocrine tumor (1), ganglioneuroma (1), and Gaucher’s disease (1).
Table 3. Pathology
Postoperative Results
Two deaths occurred in the hospital or within 30 days of the pancreatic resection, resulting in a 0.9% mortality rate (Table 4). One death followed a thrombotic stroke; intraabdominal hemorrhage contributed to the death of the second patient. There were no deaths in the pediatric age group. One death occurred in a patient older than 65 years of age, for a perioperative mortality rate in the older age group of 2%.
Table 4. Postoperative Results
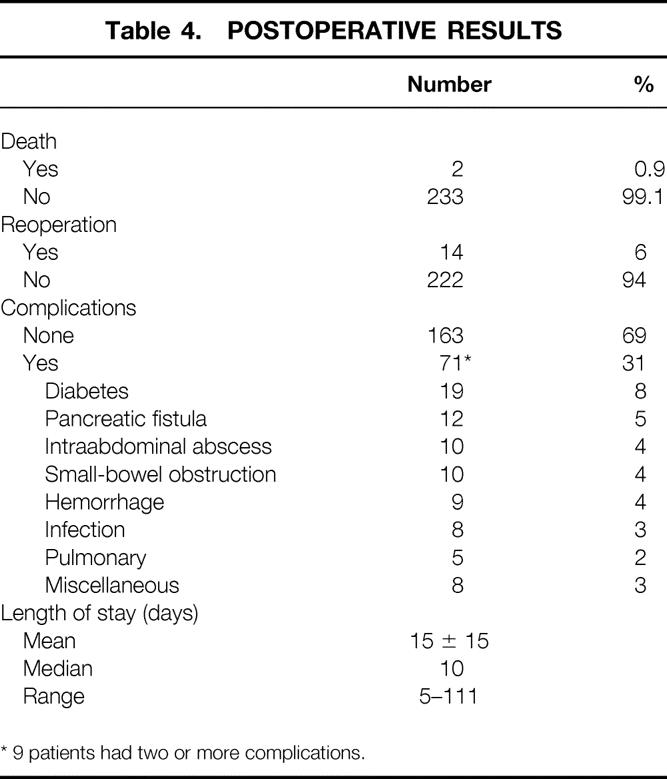
One hundred sixty-three patients (69%) had no postoperative complications. Seventy-one patients (31%) had at least one postoperative complication. Nine patients had two or more complications. The most common complications were new-onset insulin-dependent diabetes in 13 patients (8%), pancreatic fistula in 12 patients (5%), intraabdominal abscess in 10 patients (4%), small-bowel obstruction in 10 patients (4%), and postoperative hemorrhage in 9 patients (4%). All pancreatic fistulas closed spontaneously, and all intraabdominal abscesses were managed with percutaneous drainage.
Fourteen patients (6%) required a second surgical procedure within 30 days of the initial one. The indications for reoperation included hemorrhage (5), small-bowel obstruction (5), pancreatic débridement (2), drainage of peripancreatic hematoma (1), and tracheostomy (1). The mean postoperative length of stay was 15 ± 15 days (median postoperative stay 10 days).
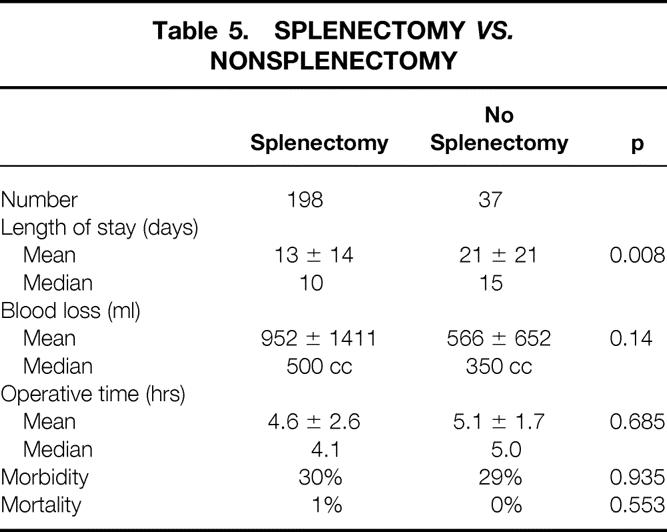
Patients who underwent distal pancreatectomy with splenectomy (n = 198) had a postoperative complication rate similar to that of patients who had a distal pancreatectomy with splenic preservation (n = 37) (30% vs. 30%) (Table 5). In addition, there were no significant differences between the two groups in terms of operative time (4.6 ± 2.6 hours vs. 5.1 ± 1.7 hours) or intraoperative blood loss (952 ± 1411 ml vs. 566 ± 652 ml). Patients undergoing splenectomy had a shorter length of postoperative stay (13 ± 14 days vs. 21 ± 21 days, p = 0.008). Both deaths occurred in patients who underwent a splenectomy.
Table 5. Splenectomy vs. Nonsplenectomy
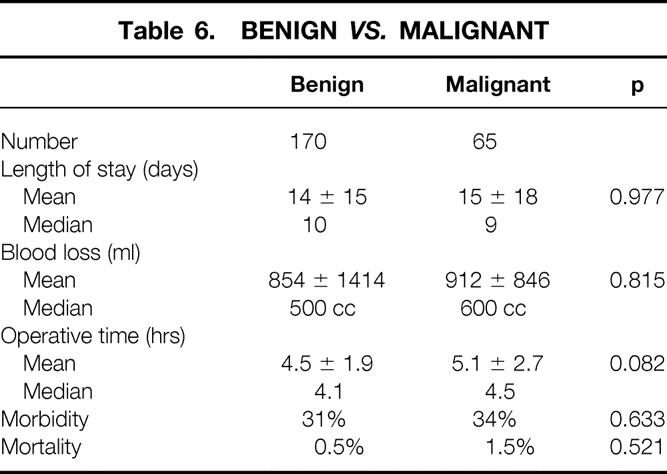
The cohort of patients who underwent a distal pancreatectomy for benign disease (n = 170) was compared with those with malignant conditions (n = 65) (Table 6). There was no significant difference in terms of complication rate (31% vs. 34%), operative time (4.5 ± 1.9 hours vs. 5.1 ± 2.7 hours), intraoperative blood loss (854 ± 1414 ml vs. 912 ± 846 ml), or length of postoperative stay (14 ± 14 days vs. 15 ± 18 days). One death occurred in each group.
Table 6. Benign vs. Malignant
DISCUSSION
Resection of the distal pancreas dates back more than a century to the first reported resection of a malignant pancreatic tumor by Billroth in 1884. 10 By early in the 20th century, case reports by Finney at Hopkins 10 and Mayo in Rochester 11 had described the surgical technique and short-term outcome for distal pancreatic resection. In a review of the published literature by Finney, 10 only 9 of 17 patients survived the procedure. All deaths occurred in patients with pancreatic cancer. This trend continued through most of this century: pancreatic resection was associated with significant perioperative morbidity and mortality rates, 12 leading many groups to suggest that pancreatic resection for pancreatic cancer be abandoned.
During the past decade, however, significant improvement in the short-term outcome after pancreaticoduodenectomy has been reported at a number of high-volume centers. 1–4,13,14 These results have also been extended to distal pancreatic resection, as evidenced by a report by Fernandez-del Castillo et al. 3 They reported a 1.4% perioperative mortality rate and a 20% incidence of complications in a series of 71 patients.
In the current series, a 0.9% perioperative mortality rate was observed, with a complication rate of 31%. The most prevalent complication was new-onset insulin-dependent diabetes mellitus, which was observed in 19 patients (8%). This sequelae reflects the loss of pancreatic islet cell mass associated with surgical resection, as well as the underlying disease process. Two of the other common postoperative complications, pancreatic fistula and intraabdominal abscess, were managed without surgery, although they extended the length of postoperative stay. Although significant intraoperative blood loss occurred in selected patients (blood loss ranged from 20 to 12,000 ml), the median number of transfusions in this series was zero. Postoperative hemorrhage occurred in nine patients (4%), with five patients requiring urgent reexploration for bleeding and a sixth patient requiring a delayed second surgical procedure to evacuate a peripancreatic hematoma.
The results from this series, which is believed to be the largest series of distal pancreatectomy reported, nicely complement the prior report by Fernandez-del Castillo et al. 3 Other institutions have reported small series with distal pancreatectomy for either pancreatic cancer 6,7 or chronic pancreatitis 8,9,15 without a perioperative death. These results probably represent the experience of high-volume centers, which has recently been recognized in the management of complex surgical procedures. 16–18 Published data from two large statewide registries have shown that perioperative morbidity and mortality rates and hospital costs were significantly reduced in high-volume centers when compared with centers performing a low volume of pancreatic resection. These data have suggested that the experience of a high-volume institution lowers perioperative mortality and morbidity rates and duration of hospital stay, even when controlling for patient characteristics and comorbidities. The results of this current study and other reports of distal pancreatectomy would suggest that the regionalization of care for this complex surgical resection might also have a substantial impact on the outcome for patients undergoing this procedure.
In recent years, a trend has been observed at our institution toward an increased frequency in the performance of distal pancreatectomy (Fig. 1). This increase, however, is not as dramatic as that observed for the performance of pancreaticoduodenectomy. 4 This is in part the result of the limited indications for distal pancreatic resection. Although resection of pancreatic neoplasms (both benign and malignant) has accounted for most of the increase in distal pancreatectomy at our institution, the most common pancreatic tumor, ductal adenocarcinoma, when arising in the body or tail of the gland usually presents at a late stage, often with evidence of either metastatic or locally unresectable disease. In the current series, only 49 patients (21%) underwent resection for adenocarcinoma of the pancreas, including both ductal carcinoma and cystadenocarcinoma. Although this series would seem small, it surpasses the number of resections performed for pancreatic cancer of the body and tail in two recent series from the Mayo Clinic and Memorial Sloan-Kettering Cancer Center. 6,7 Long-term survival rates are not available for patients who underwent resection in our series; therefore, the potential curative benefit of this procedure in our hands has yet to be defined. Analysis of short-term outcome would suggest, however, that resection for a malignant indication is not associated with a significant increase in perioperative morbidity or mortality rates, operative time, or postoperative length of stay.
Similarly, the role of pancreatic resection for chronic pancreatitis has been questioned. 8,9 Rattner et al 8 have concluded that even when chronic pancreatitis appears to involve primarily the body and tail of the pancreas, distal pancreatectomy seldom provides sustained pain relief. Similarly, Sawyer and Frey 9 have concluded that distal pancreatectomy is indicated in only 5% to 15% of patients with chronic pancreatitis. Although in their experience 90% of patients with disease limited to the body and tail of the gland had good results in terms of pain relief, nutrition, and preservation of endocrine function, follow-up was limited and long-term outcome remains in question. The current series also does not provide extended follow-up of the symptomatic relief of chronic pancreatitis and therefore also leaves in question the long-term benefits of this procedure.
Recently, various modifications of pancreatic resection techniques have been offered in hopes of preserving both pancreatic parenchyma and the need for adjacent organ resection (duodenum, bile duct, spleen). 19–22 These procedures include both pancreatic head resections that spare both the duodenum and biliary tree,performed for chronic pancreatitis, 17,18 and lesser resections of the midsegment of the gland 21 or simple enucleation of benign cystic or islet cell tumors. 22 Although excellent short-term results have been provided with these procedures, in almost all cases they require significant surgical experience and are likely best confined to high-volume centers because of their relatively limited indications.
Finally, this series has addressed the role of splenic preservation during distal pancreatectomy. The traditional distal pancreatectomy performed with en bloc resection of the spleen was thought to be necessary because of the close relation of the splenic artery and vein to the body of the pancreas. In 1943, Mallett-Guy and Vachon described the technique for splenic preservation during distal pancreatectomy. 23 Recently, three series have reported a considerable experience with splenic preservation. 15,24,25 The surgical procedure can be performed with either splenic artery and vein division distal to the tail of the gland at the splenic hilum 24 or with preservation of the entire length of these structures, which is a more time-consuming procedure.
In two series, comparisons were performed between patients undergoing distal pancreatectomy with and without splenectomy. In the first series of 21 patients reported by Richardson and Scott-Conner, 25 there were no deaths in either the splenectomy or splenic-preservation groups. Splenic preservation was accomplished with no increase in complication rate, operative time, or length of postoperative stay. In a similar series reported by Aldridge and Williamson, 15 conventional pancreatectomy with splenectomy was performed in 42 patients, resection with splenic preservation in 35 patients. There were no postoperative deaths in either group and similar complication rates. The authors of both series concluded that the spleen can be safely preserved in many patients undergoing distal resection, and this technique should be more liberally applied.
In the current series, only 16% of patients had splenic preservation. As with other series, retrospective comparisons suggest no difference in operative time, blood loss, or postoperative morbidity and mortality rates. Patients undergoing distal pancreatectomy with splenectomy did, however, have a significantly shorter postoperative stay. In general, it is the policy at our institution to apply splenic preservation selectively based on the anatomic relation of the structures, the presence or absence of splenic vein involvement with the disease process, and the extent of resection necessary based on the underlying pathology. An en bloc splenectomy is always performed when malignancy is suspected.
In conclusion, this series has demonstrated that distal pancreatectomy can be safely performed for a variety of pancreatic diseases, including chronic pancreatitis and benign and malignant pancreatic neoplasms. The procedure can be completed with a minimal mortality rate and an acceptable perioperative morbidity rate. The majority of postoperative complications do not require invasive treatment, although the hospital stay is generally prolonged in association with complications. There appears to be minimal difference in short-term outcome when distal pancreatectomy is performed either with or without splenectomy or for benign or malignant disease.
Discussion
Dr. Andrew L. Warshaw (Boston, Massachusetts): This is, as Dr. Lillemoe said, an experience of 235 patients over 14 years—roughly 17 per year on average but most recently, 30 to 40 per year, reflecting the increasing volume of pancreatic surgery which is attracted to this premier institution. It clearly demonstrates that distal pancreatectomy can be done with very low mortality, the lowest reported as far as I know, and acceptable morbidity. As any good study should, it is a door-opener by stimulating many more questions, which I would like to ask the authors.
The 14-year time span will allow longitudinal observations, not only of the case members but evolution of case finding and case selection. How much has the common use of imaging tests increased the discovery of asymptomatic lesions such as cyst adenomas? Or decreased the fruitless attempted resection of carcinomas which are local, extensive, or metastatic?
Your length of stay averaged 15 days with a median of 10, almost equal to the length of stay which you have reported for Whipple operations. But what is it down to now? I suspect it is considerably lower in recent years.
There were 9 significant intraabdominal hemorrhages and 14 patients required early reexploration. Are these complications on the decline with modern technique and can they be prevented?
One fourth of your patients were resected for chronic pancreatitis—a small absolute number, all things considered. Does this reflect your disappointment with the success rate in treating pain in this disease with distal pancreatectomy, as we found in the absence of localized pathology such as a pseudocyst in the tail?
Twenty-five percent had a cystic neoplasm, but only 3% were cystadenocarcinomas. Since mucinous cystadenocarcinoma is more prevalent than the benign or borderline mucinous cystic neoplasms and 70% of mucinous cystadenocarcinomas are in fact resectable in most series, including ours, does this indicate that most of your cystic neoplasms were serous cystadenomas which did not become malignant? Or might your pathologists be less likely than ours to call a borderline mucinous cystic neoplasm cancer?
Eighteen percent of your patients had a ductal adenocarcinoma. This is in fact higher than I would have expected, since resectability of these lesions in general is so low. What is the denominator for these patients who are somewhere about 48 patients? What’s the denominator for that 48? Is your resection rate higher than the 5% to 15% reported by other institutions? Or do you do an extended dissection such as you are advocating for the Whipple operation? Or are you willing to leave gross tumor behind for a palliative excision?
You show that splenic preservation does not add risk, but does it provide substantial benefit in the adult? How do you balance such benefit against potential compromise of a cancer operation?
Finally, what are your indications for lesser procedures, either enucleation of smaller tumors or middle segment resection with Roux-en-Y reconstruction for greater preservation of pancreatic tissue?
While only 8% of your patients developed new insulin-dependent diabetes, surely more of them became diet-controlled diabetics or required oral agents, and, certainly, more of them will become insulin-dependent over time.
We accept enucleation of small islet cell tumors but worry about enucleation of the mucinous cystic neoplasms, especially since their malignancy may be and remain uncertain at the operating table.
We have now done 20 middle segment reductions and found them safe and suitable for selective lesions and for the purpose of preserving pancreatic tissue.
The size and quality of the Hopkins experience qualifies this as a benchmark report for perioperative outcomes.
Dr. William H. Nealon (Galveston, Texas): In the current report, the outcomes of 235 patients who have undergone distal pancreatectomy from 1984 through 1997, only 45 of which were performed prior to 1990 and thus, 190 of which have been performed since that date, are considered.
The authors describe the same sort of superb operative outcomes that we have all come to expect from this center with a mortality below 1%.
The fascinating mix of diagnoses managed by the procedure is consistent with the experiences of other major centers and is dominated by chronic pancreatitis and benign and malignant neoplasms, primarily cystic.
I have four questions:
First, in view of the fact that the largest percentage of patients underwent operation for chronic pancreatitis, what was the indication for this operation in that subset?
Since pseudocysts were listed as a separate entity for indication for operation, I presume these represent patients who were thought to have primarily disease in the body and tail of the pancreas. I wonder if you have any information—Dr. Warshaw already asked about the success rate for pain relief–but if you have any information on the degree of disease in the head of the gland in this subset of patients.
Second, you list diabetes as the most common complication with this operation. I wonder if there is any correlation between that outcome after operation and the diagnosis of chronic pancreatitis?
I also will mention that I have some concern when a report like this includes a morbidity rate of 30%, when this specific complication of glucose intolerance is included as a primary source of complication. Isn’t glucose intolerance quite predictable when the body and the tail of the pancreas are known to be anatomically where the majority of beta cells reside? And isn’t some element of glucose intolerance, therefore, predictable and possibly represent something you might term as a predictable outcome as opposed to an actual complication?
Third, I wonder if you have looked closely at your patients who have had massive hemorrhage. Although it’s a small number, was the management of the splenic artery or vein different in any way in that subset? Were any of those patients the ones that were managed by a stapling device across the body of the pancreas?
And my fourth question similarly relates to the complication of fistula formation: was any of the smaller subset of patients managed in a different fashion for the divided end of the pancreas, those that were managed by some of the alternative methods, particularly the placement of the stapling device?
Dr. James A. O’Neill, Jr. (Nashville, Tennessee): Since you included children in your report, it raises a question as to whether you and your colleagues make any effort toward splenic preservation in that regard.
I might share this experience with you. We recently reported 51 patients with various endocrine disorders that had varying degrees of what one might call distal or extended distal pancreatectomy. Now this is from a group of 84 patients that had various forms of distal pancreatectomy. And in the childhood age group, the entities are ordinarily various neuroendocrine disorders, including adenomas and diffuse islet cell dysplasia, trauma, usually with pancreatic pseudocysts associated, and chronic familial forms of pancreatitis. And in this group of 84 patients, there were 6 patients who had splenectomy performed. All of these patients had, at some time during their course, prophylactic therapy. It is of interest that in the follow-up of those patients—and these span about 25 years—there were two deaths due to postsplenectomy sepsis at 2 and 5 years following operation.
It certainly is the case that our results were similar to yours in terms of a very low fistula rate. There was no mortality, operative mortality, in this group of patients. So distal splenectomy, at least for benign disorders, is a relatively safe operation. But the long-term outlook relative to splenectomy and postsplenectomy sepsis, certainly in the childhood age group and in infancy, there is no argument about that. The risk in adults is less, but it isn’t zero. And, therefore, the question is whether with long-term follow-up you might see more problems with postsplenectomy sepsis.
And the question: Other than for malignant disease, should efforts be made to preserve the spleen? We will continue to do so in the age group that we deal with. I would be interested in your views of the young adult.
Dr. Keith D. Lillemoe (Closing Discussion): I’d like to thank the discussants for their comments. Dr. Warshaw, I really do agree with you that the new technology has brought forth a lot of previously asymptomatic benign cystadenomas of the pancreas that we have been referred probably because of our increasing experience with pancreatic surgery. Other new technology which has been helpful—we generally perform diagnostic laparoscopy in people with suspected adenocarcinoma of the body and tail of the pancreas in order to stage those patients to rule out peritoneal or liver metastasis, feeling that most of these patients have nothing to gain from a laparotomy in terms of palliation.
Both Dr. Warshaw and Dr. Nealon asked about the intraoperative hemorrhage. We really can’t quantitate the operative techniques very well. You can’t read between the lines of most operative notes. Certainly portal hypertension, occlusion of the splenic vein by cancer is a factor that may very well have contributed, or a benign inflammatory condition could very well have contributed, to the portal hypertension in these cases.
We, too, do not consider distal pancreatectomy a primary operation for most patients with chronic pancreatitis. We feel, as do most, that the heart of chronic pancreatitis begins in the head of the pancreas and, particularly in later years, where our increasing experience with pancreaticoduodenectomy has gone after this as being the sole problem with chronic pancreatitis. Most of our indications for distal pancreatectomy have involved an isolated distal stricture in the body or tail of the gland that has been seen on ERCP, which also raises the potential for a malignancy at that point.
Dr. Nealon asked about our outcome. We are concurrently evaluating a number of surgical options for chronic pancreatitis as part of a separate investigation and, therefore, we do not have those results to report today.
Andy, we do not know our absolute denominator for pancreatic cancer of the body and tail. Clearly, this number is small compared to that. Both your group, the group at Mayo, the group at Memorial Sloan-Kettering, and we previously have reported that these cases are frequently unresectable, both at the time that they present with simply CT scans as well as with our staging. I don’t have that number, nor do I have the follow-up in terms of the survival of those patients.
We certainly do not attempt a palliative resection for pancreatic cancer of the body and tail, although I would have to acknowledge that in some cases there was a positive margin, usually at the celiac axis.
The splenectomy is always performed when cancer is suspected, so we do not compromise our cancer operation with splenic preservation.
We have an interest in the lesser operations. Last year at this meeting, Dr. Pitt reported our limited experience with approximately 14 patients with enucleation of serous lesions. Many of these were located in the head of the pancreas—trying to avoid a Whipple in these otherwise normal glands. Whereas, if we are in the body and tail, we will more likely than not go with a distal pancreatectomy.
We have no experience with your midsegment pancreatectomy. I certainly have seen your work and think it is an excellent option in those selected patients with tumors that fall into that area.
We acknowledge, for both Bill and Dr. Warshaw, that a longer follow-up is necessary to determine the absolute incidence of diabetes mellitus. And, Bill, we struggled whether to consider this a complication or just the extent of the disease process. Certainly, the patients with chronic pancreatitis had a greater incidence of postoperative diabetes.
Dr. Nealon, in addition to the questions I have tried to answer in conjunction with Dr. Warshaw’s, asked if there was any correlation with fistulas and the use of the stapling device, and we do not have that analysis.
Dr. O’Neill, we are certainly interested in splenic preservation in all our young children. We are aware that postsplenectomy sepsis is not isolated only to children. As I said, certainly if there is the specter of malignant disease, either for a big cystic neoplasm or what is suspected to be an adenocarcinoma, we do not consider splenic preservation. But in many of the other cases, we will do that. Of course, a lot of times for malignant and sometimes benign disease with chronic pancreatitis, we will run into cases of splenic vein thrombosis, and we will plan to take the vein routinely in that situation.
Footnotes
Correspondence: Keith D. Lillemoe, MD, Professor of Surgery, The Johns Hopkins Medical Institutions, 600 N. Wolfe Street, Baltimore, MD 21287.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Dr. Pitt is currently at the Department of Surgery, Medical College of Wisconsin, Madison, WI.
Accepted for publication December 1998.
References
- 1.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg 1987; 206: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trede M, Schwall G, Saeger H. Survival after pancreaticoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 195; 130: 295–300. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Ann Surg 1997; 246: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norback IH, Hruban RH, Boitnott JK, et al. Carcinoma of the body and tail of the pancreas. Am J Surg 1992; 164: 26–31. [DOI] [PubMed] [Google Scholar]
- 6.Dalton RR, Sarr MG, van Heerden JA, Colby TV. Carcinoma of the body and tail of the pancreas: is curative resection justified? Surgery 1992; 111: 489–494. [PubMed] [Google Scholar]
- 7.Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg 1996; 223: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rattner DW, Fernandez-del Castillo C, Warshaw AL. Pitfall of distal pancreatectomy for relief of pain in chronic pancreatitis. Am J Surg 1996; 171: 142–146. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer, R, Frey CF. Is there still a role for distal pancreatectomy in surgery for chronic pancreatitis? Am J Surg 1994; 168: 6–9. [DOI] [PubMed] [Google Scholar]
- 10.Finney JMT. Resections of the pancreas. Trans Am Surg Assoc 1910; 315–330. [Google Scholar]
- 11.Mayo WJ. The surgery of the pancreas. Ann Surg 1913; 58: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudjousson B. Cancer of the pancreas: 50 years of surgery. Cancer 1987; 60: 2284–2303. [DOI] [PubMed] [Google Scholar]
- 13.Braasch JW, Rossi RL, Watkins E Jr, et al. Pyloric and gastric preserving pancreatic resection. Experience with 87 patients. Ann Surg 1986; 204: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grace PA, Pitt HA, Tompkins RK, et al. Decreased morbidity and mortality after pancreaticoduodenectomy. Am J Surg 1986; 151: 141–149. [DOI] [PubMed] [Google Scholar]
- 15.Aldridge MC, Williamson RCN. Distal pancreatectomy with and without splenectomy. Br J Surg 1991; 78: 976–979. [DOI] [PubMed] [Google Scholar]
- 16.Gordon TA, Burleyson GP, Tielsch, JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg 1995; 221: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patient undergoing pancreatic resection for malignancy. Ann Surg 1995; 222: 685–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosa, JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beger HG, Krautzberger W, Bittner R, Buchler M, Limmer J. Duodenum-preserving resection of the head of the pancreas in patients with severe chronic pancreatitis. Surgery 1985; 98: 467–472. [PubMed] [Google Scholar]
- 20.Frey CF, Schmith GJ. Description and rationale of a new operation for chronic pancreatitis. Pancreas 1987; 2: 701–707. [DOI] [PubMed] [Google Scholar]
- 21.Warshaw AL, Rattner DW, Fernandez-del Castillo C, Zgraggen K. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg 1998; 133: 327–331. [DOI] [PubMed] [Google Scholar]
- 22.Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg 1998; 227: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallet-Guy P, Vachon A. Pancreatites chroniques gauches. Paris: Masson; 1943.
- 24.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988; 123: 550–553. [DOI] [PubMed] [Google Scholar]
- 25.Richardson DQ, Scott-Conner CEH. Distal pancreatectomy with and without splenectomy. Am Surg 1989; 55: 21–25. [PubMed] [Google Scholar]