Abstract
Objective
To determine the effects of recombinant human insulin-like growth factor-1 (IGF-1) complexed with its principal binding protein, IGFBP-3, on skeletal muscle metabolism in severely burned children.
Summary Background Data
Severe burns are associated with a persistent hypermetabolic response characterized by hyperdynamic circulation and severe muscle catabolism and wasting. Previous studies showed that nutritional support and pharmacologic intervention with anabolic agents such as growth hormone and insulin abrogated muscle wasting and improved net protein synthesis in the severely burned. The use of these agents, however, has several adverse side effects. A new combination of IGF-1 and IGFBP-3 is now available for clinical study.
Methods
Twenty-nine severely burned children were prospectively studied before and after treatment with 0.5, 1, 2, or 4 mg/kg/day IGF-1/IGFBP-3 to determine net balance of protein across the leg, muscle protein fractional synthetic rates, and glucose metabolism. Another group was studied in a similar fashion without IGF-1/IGFBP-3 treatment as time controls.
Results
Seventeen of 29 children were catabolic before starting treatment. The infusion of 1.0 mg/kg/day IGF-1/IGFBP-3 increased serum IGF-1, which did not further increase with 2.0 and 4.0 mg/kg/day. IGF-1/IGFBP-3 treatment at 1 to 4 mg/kg/day improved net protein balance and increased muscle protein fractional synthetic rates. This effect was more pronounced in catabolic children. IGF-1/IGFBP-3 did not affect glucose uptake across the leg or change substrate utilization.
Conclusions
IGF-1/IGFBP-3 at doses of 1 to 4 mg/kg/day attenuates catabolism in catabolic burned children with negligible clinical side effects.
Severe burns are associated with a persistent hypermetabolic response characterized by hyperdynamic circulation and increased circulating levels of catabolic hormones such as catecholamines, glucagon, and cortisol. 1 High energy expenditures are met by heightened energy substrate release from available protein and fat stores. Protein breakdown is primarily from active muscle tissue, which leads to a loss of lean body mass and severe muscle wasting. This muscle wasting leaves severely burned patients with insufficient strength to recover from their injuries in a timely fashion.
Previous studies in severely burned patients showed that nutritional support 2 and pharmacologic intervention with anabolic agents such as growth hormone and insulin abrogated muscle wasting. Growth hormone was shown to improve whole-body and isolated limb net protein synthesis in severely burned children. 3 Chronic insulin infusion produced similar improvements in net protein synthesis and fractional synthetic rate (FSR) in isolated limbs of severely burned adults. 4,5 The use of anabolic agents, however, has several adverse side effects, notably hyperglycemia associated with growth hormone 6,7 and hypoglycemia associated with insulin, in which exogenous glucose well above calculated caloric needs was sometimes required. 4 Growth hormone was further shown to increase the mortality rate in critically ill nonburned adults, 8 an effect not seen in severely burned children. 6
Many of the effects of growth hormone are mediated through insulin-like growth factor-1 (IGF-1). IGF-1 alone has been infused in burned adults with improvements in protein kinetics identified, but at the effective dose, hypoglycemia occurred. 9 The purpose of this study was to determine the effects of a new recombinant compound of IGF-1 complexed with its principal binding protein, IGF binding protein-3, on skeletal muscle metabolism in children during the hypermetabolic phase of a severe burn. We hypothesized that IGF-1/IGFBP-3 administration would improve net protein balance in the muscle of fed children who were catabolic after severe burn without the side effects often noted with other anabolic agents. We further postulated that the improvement in net protein balance is mediated through an increase in muscle protein synthesis.
MATERIALS AND METHODS
Twenty-nine severely burned children were prospectively studied to determine the effects of IGF-1/IGFBP-3 on skeletal muscle during recovery from the burn injury. Patients younger than 15 years of age with >40% total body surface area (TBSA) burn without evidence of organ failure admitted to our hospital within 20 days of injury were enrolled. Informed consent in accordance with the Institutional Review Board of the University of Texas Medical Branch at Galveston was obtained from all patients and/or parents.
Each patient was studied during the acute hospital stay after injury in the Shriners Burns Hospital-Galveston. Resuscitation given immediately after burn was guided by the Galveston formula of 5000 cc/m2 TBSA burned + 2000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and the wounds were closed with available autograft skin and allograft for the remaining open areas. Patients returned to the operating room when autograft donor sites healed and became available for reharvest. Sequential staged surgical procedures for repeat excision and grafting were undertaken until the wounds were 95% healed.
Study Design
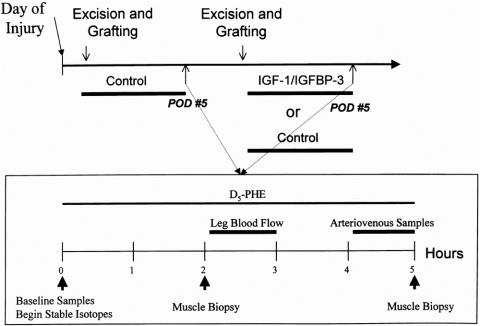
After one of the sequenced surgical procedures, patients were studied without drug to determine baseline protein metabolism. On the fifth postoperative day, net phenylalanine balance across the leg and FSR of skeletal muscle protein were measured. Arteriovenous glucose differences were used to determine glucose uptake, and indirect calorimetry was performed to determine any changes in substrate utilization. When the donor sites healed at 5 to 10 days, patients returned to the operating room for another excision and grafting procedure. After this next procedure, patients were randomized to treatment with continuous intravenous IGF-1/IGFBP-3 at 0.5, 1.0, 2.0, or 4.0 mg/kg/day for 5 days. Another group served as time controls and received a second period of 0.9% NaCl. A second series of studies were repeated on postoperative day 5 of the later surgical procedure to determine any differences with IGF-1/IGFBP-3 treatment or time (Fig. 1).
Figure 1. Experimental design of stable isotype design infusion protocol. The upper line depicts the study time periods. The lower line shows the stable isotopic infusion study design.
All patients received nasoduodenal feedings with Vivonex TEN (Sandoz Nutrition, Minneapolis, MN), an elemental formula containing 82.3% carbohydrate, 3% fat (linoleic acid), and 14.7% protein. Caloric intake was given at a rate calculated to deliver 1500 kcal/m2 TBSA burned + 1500 kcal/m2 TBSA. This feeding regimen was started at admission and continued at a constant rate until the wounds were 95% healed. Caloric intake was kept constant throughout the study periods.
Patients were at bed rest after excision and grafting procedures for 5 days. After this, patients ambulated daily until the next excision and grafting procedure. Patients were treated in an identical fashion in terms of mobilization and rehabilitation in both study periods.
IGF-1/IGFBP-3
The rhIGF-1/IGFBP-3 complex was provided by Celtrix Pharmaceuticals, Inc. (Santa Clara, CA) in a 1:1 molar ratio of rhIGF-1 to rhIGFBP-3 corresponding to the naturally occurring protein complex purified by cation exchange column chromatography. Infusions were prepared from frozen vials containing 10.8 ml rhIGF-I/IGFBP-3 (10 mg/ml) in sterile buffered solutions with 50 mM sodium acetate and 105 mM sodium chloride at pH 5.5. Infusions were started at 8 AM the day after staged excision and grafting of the burn wound and continued until the donor sites had healed.
Stable Isotope Infusion Protocol
On postoperative day 5, 3 F 8-cm single-lumen catheters were inserted into the femoral artery and vein in the study leg and into the subclavian vein using intravenous sedation and local anesthesia. Catheters were used for blood sampling to determine the arteriovenous balance of phenylalanine and glucose across the leg and leg blood flow. The subclavian central venous catheter was used for systemic blood sampling and infusion of stable isotopes.
Baseline blood samples were obtained for background amino acid enrichment and systemic indocyanine green concentrations. A primed constant infusion of L-[ring-2H5]-phenylalanine was given through the subclavian central venous catheter for 5 hours, using a priming dose of 2 μmol/kg followed by 0.08 μmol/kg/min. Vastus lateralis muscle biopsies were taken from the study leg at 2 and 5 hours into the study. These biopsies were performed using a Bergstrom needle (Depuy, Chicago, IL) attached to a suction device. Samples were immediately blotted dry and snap-frozen in liquid nitrogen for storage at −70°C. Between hours 3 and 4, leg blood flow was determined by indocyanine green infusion into the femoral artery. Blood samples from the femoral and subclavian veins were taken for this determination. Between hours 4 and 5, blood samples were obtained from the femoral artery and vein to determine arteriovenous phenylalanine concentration differences across the leg. After the last muscle biopsy, the stable isotope infusion was stopped. Catheters were left in place for use at the next excision and grafting operation (Fig. 1).
Net Phenylalanine Balance
Sample concentrations of whole-blood total phenylalanine were determined by the internal standard approach. 10 Briefly, 1-ml whole-blood samples were collected in ice-cold tubes containing sulfosalicylic acid and a known amount of internal standard solution (L-[ring-13C6]-phenylalanine at 30 μmol/L). Amino acids in the supernatant were isolated in cation exchange column and processed for the n-acetyl, n-propyl ester derivatives of amino acids. Isotope enrichment was measured on a Hewlett-Packard 5989B gas chromatograph/mass spectrometer (Hewlett-Packard, Palo Alto, CA) with chemical ionization. Ions were selectively monitored at mass-to-charge ratios 250 to 255 and 256 for phenylalanine. The values of phenylalanine enrichment were used to calculate the phenylalanine concentration in the blood.
Net balance of blood free phenylalanine across the leg was determined using the Fick principle:
where Ca is the concentration of phenylalanine in the arterial blood, Cv is the concentration of phenylalanine in the venous blood from the same leg, and BF is leg blood flow. Leg volume was determined independently by a nomogram related to circumference and length measures at defined anatomic sites on the leg. This allowed us to index our results to body volume.
Fractional Synthetic Rate
FSR in the vastus lateralis muscle was determined by measuring incorporation of labeled phenylalanine into the protein-bound portion of the muscle. 11 Muscle samples were weighed and precipitated with perchloric acid. The tissue was homogenized with separation of the supernatant and bound protein by centrifugation and ethanol washes. Enrichment of intracellular phenylalanine was measured using the gas chromatograph/mass spectrometer with the same derivatives and ionization techniques described above. The muscle pellet was washed, dried, and then hydrolyzed in 6N HCl. Amino acids were collected in cation exchange columns. Phenylalanine enrichment was determined using hydrogen bromide derivatives and chemical ionization in an MD-800 gas chromatograph/mass spectrometer (Finnigan, San Jose, CA). Ions were selectively monitored at mass-to-charge ratios 407 and 409.
FSRs were calculated using the following:
where Ep1 and Ep2 are the enrichments of the bound protein amino acids at 2 and 5 hours respectively, Em represents the average intracellular enrichment of the 2- and 5-hour samples, and t is the time in minutes between samples.
Leg Blood Flow
During hour 3 of the stable isotope infusion, indocyanine green was infused through the femoral artery catheter at 0.5 mg/min. Blood samples were simultaneously obtained from the femoral vein and subclavian vein for determination of leg blood flow. Serum was analyzed by spectrophotometry (Beckman, Palo Alto, CA) at 805 nm to determine the indocyanine green concentrations. Blood flow was determined using the Fick principle. Four blood flow determinations 5 minutes apart were averaged.
Serum Hormone Measurements
Serum was collected on postoperative day 5 for measurement of IGF-1, IGFBP-3, growth hormone, and insulin just before beginning the stable isotope studies. Levels were measured by radioimmunoassay (Endocrine Sciences, Calabasas Hills, CA).
Net Glucose Balance
Arteriovenous glucose concentration differences were measured and used to calculate glucose uptake across the leg. Serum glucose concentrations were measured from the femoral artery and vein with each sampling from 4 to 5 hours. Glucose levels were determined on a Stat 5 Analyzer (Nova Biomedical, Waltham, MA) with the average of four values reported as the concentration over the sampling hour.
Indirect Calorimetry
Resting energy expenditure and respiratory quotient were calculated from O2 and CO2 concentrations in expired gases. A metabolic cart calorimeter (Sensormedics, Yorba Linda, CA) and standard equations were used.
Statistical Analysis
Demographic comparisons between groups were made using one-way analysis of variance. Ordinal data between groups were compared using chi square or Fisher’s exact test. Serum levels were tested for significant differences with Kruskal-Wallis one-way analysis of variance on ranks, with Dunn’s method of multiple comparisonsversus the control group. The comparison of phenylalanine balance and FSR for the effects of IGF-1/IGFBP-3 in the treatment groups, and in the control-control group, were made a priori by the paired one-tailed t test because previous studies in burned patients with stimulated serum IGF-1 levels have demonstrated anabolic effects, 3,9 and there are no reports of IGF-1–induced catabolism. Further analyses within and between groups were made by paired and unpaired two-tailed t tests. Significance was accepted at p < 0.05. Data are presented as means ± SEM.
RESULTS
Patient Demographics
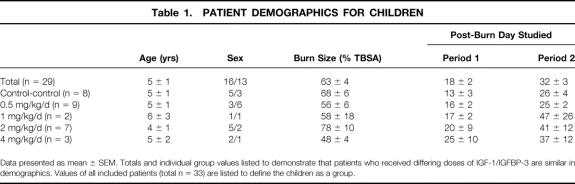
Patient demographics are depicted in Table 1. No differences between control or treatment groups could be shown for age, sex, burn size, or time of study after burn. The mean age of all enrolled children was 5 ± 1 years, with burn sizes of 61 ± 4% TBSA.
Table 1. Patient Demographics for Children
Serum Response to Different Doses of IGF-1/IGFBP-3
Serum IGF-1 levels increased significantly with the infusion of IGF-1/IGFBP-3 (p < 0.05 for 1, 2, and 4 mg/kg/day vs. control; Fig. 2), but not at 0.5 mg/kg/day. IGFBP-3 levels increased with the 4.0 mg/kg/day dose (p < 0.05). Doses >1.0 mg/kg induced no further increases in serum concentrations of IGF-1 or IGFBP-3. Growth hormone levels decreased in response to IGF-1/IGFBP-3 at doses of 0.5, 2, and 4 mg/kg/day (p < 0.05). No differences in insulin serum concentrations were detected at any dose. Because no differences were found in serum IGF-1 levels between doses of 1, 2, or 4 mg/kg/day, these patients were grouped for further analyses.
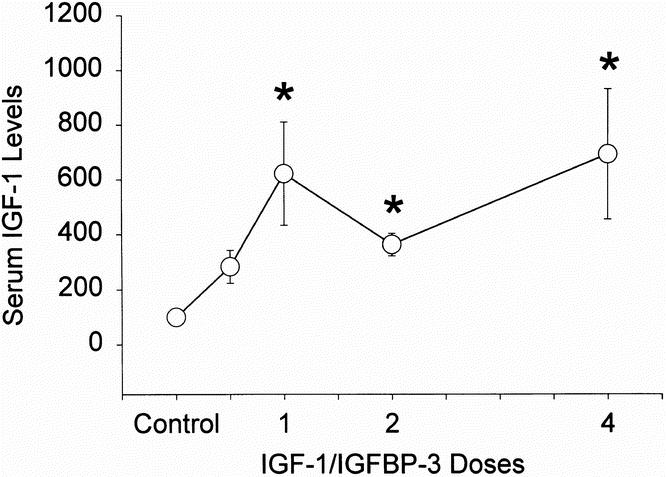
Figure 2. Serum IGF-1 levels with IGF-1/IGFBP-3 infusion. Circles represent means; bars represent SEM. Significant differences from control, p < 0.05.
Protein Metabolism in Response to, IGF-1/IGFBP-3
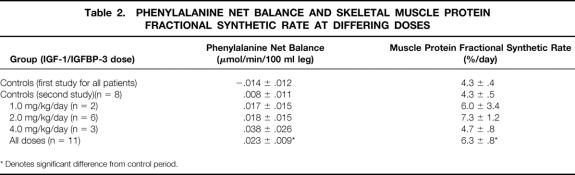
Children treated with IGF-1/IGFBP-3 at 1, 2, or 4 mg/kg/day had an increase in net balance of phenylalanine across the leg with treatment versus their control period (p = 0.043), which was associated with a significant increase in FSR (p = 0.031) (Table 2). No differences in phenylalanine net balance or FSR in a paired comparison could be found between periods in patients who received 0.5 mg/kg/day (0.016 ± 0.008 mmol/100 ml leg/min to 0.033 ± 0.038 mmol/100 ml leg/min for net balance; p = 0.33) (4.8 ± 0.7%/day to 6.4 ± 1.5; p = 0.21) (n = 9). This parallels the results in IGF-1 serum concentrations with infusion of IGF-1/IGFBP-3 at 0.5 mg/kg/day.
Table 2. Phenylalanine Net Balance and Skeletal Muscle Protein Fractional Synthetic Rate at Differing Doses
The protein-sparing effects observed in patients receiving 1, 2, and 4 mg/kg/day of IGF/IGFBP-3 were profound. The clinical relevance of the improvement in net balance is even more remarkable when we convert it into milligrams per hour of protein. The increase in net balance with treatment corresponds to an improvement of 5.9 mg of protein per hour. In a 60-kg patient with a large burn, for example, who may be treated for 10 days, this treatment would translate into 0.425 kg of additional muscle tissue. 11a
Nine children received one control study that was followed by another study without IGF-1/IGFBP-3 (control-control) in the next cycle to determine the effects of time on protein metabolism (Table 2). In the control-control group, no significant differences in phenylalanine net balance (−0.015 ± 0.012 mmol/100 ml leg/min to 0.008 ± 0.015 mmol/100 ml leg/min; p = 0.12) or FSR (4.4 ± 0.5%/day to 5.3 ± 0.6%/day; p = 0.13) could be found between periods in a paired one-tailed t test analysis (Table 2).
Analysis of Catabolic Versus Noncatabolic Patients
Children were divided into those who were catabolic at the control period, as evidenced by a negative net phenylalanine balance (n = 9), and those who were noncatabolic, shown by a zero or greater phenylalanine net balance (n = 3). The phenylalanine net balance did not change in the noncatabolic group of patients who received IGF-1/IGFBP-3 (0.055 ± 0.026 mmol/100 ml leg/min for the control period and 0.030 ± 0.018 mmol/100 ml leg/min for the IGF-1/IGFBP-3 treatment period). The change in response to IGF-1/IGFBP-3 in the catabolic group, however, was significant (−0.052 ± 0.019 mmol/100 ml leg/min for the control period and 0.021 ± 0.013 mmol/100 ml leg/min for the IGF-1/IGFBP-3 treatment period) (n = 8) (p = 0.03) (Fig. 3). In addition, catabolic children who were studied twice without IGF-1/IGFBP-3 as time controls did not differ in phenylalanine net balance between periods (−0.025 ± 0.006 mmol/100 ml leg/min in the first period and 0.008 ± 0.018 mmol/100 ml leg/min in the second period) (n = 7) (p = 0.11). A demographic analysis revealed no significant differences between catabolic and noncatabolic children in either group in terms of age, burn size, or time after burn. These results indicate that treatment with IGF-1/IGFBP-3 is more effective in those who are most catabolic.
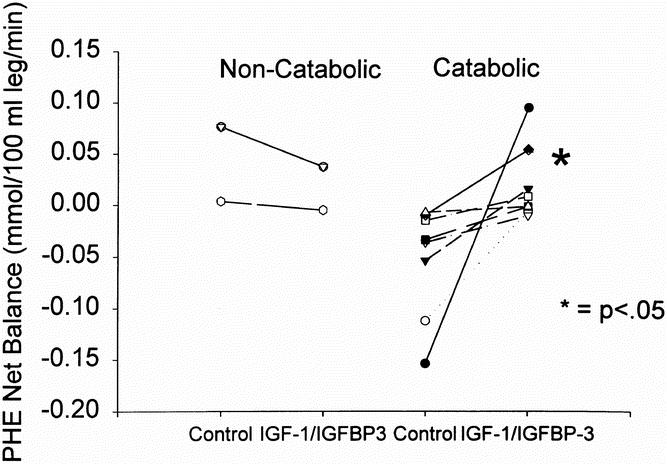
Figure 3. Phenylalanine net balance across the leg in noncatabolic and catabolic children as determined by net phenylalanine balance during the control period. Symbols represent individual patients. Significant differences were found in the change with treatment in the catabolic group only (p < 0.05).
Effects of IGF-1/IGFBP-3 on Glucose Metabolism
Infusion of IGF-1 has previously been found to affect glucose uptake. 9 We assessed glucose metabolism in response to IGF-1/IGFBP-3 administration by examining differences in arterial and venous glucose concentrations. We found that arterial glucose concentrations were significantly decreased from control values in patients given IGF-1/IGFBP-3 at 1, 2, or 4 mg/kg/day (123 ± 7 mg/dl during control period and 112 ± 3 mg/dl; p < 0.05). However, no episodes of clinical hypoglycemia were encountered at any time point throughout the study in any patient. Glucose uptake across the leg, however, was not different between test periods (0.9 ± 0.5 mg/100 ml leg during control period and 1.1 ± 0.3 mg/100 ml leg during treatment) (Fig. 4). Oxygen consumption (1635 ± 323 kcal/day during control and 1645 ± 267 kcal/day during treatment) and respiratory quotient (0.95 ± 0.03 during control and 1.01 ± 0.03) were not different between periods, indicating no significant changes in substrate utilization.
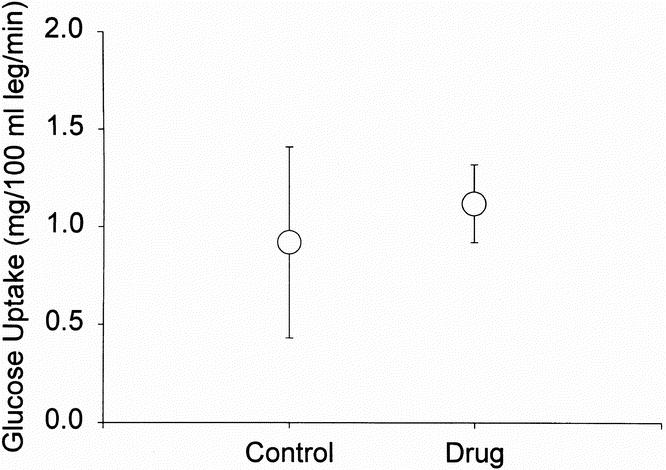
Figure 4. Glucose uptake across the leg in severely burned children after IGF-1/IGFBP-3 treatment. Circles represent means; bars represent SEM. No significant differences could be shown between control and treatment.
DISCUSSION
Patients with severe burns are highly catabolic with increased protein breakdown, primarily from active muscle tissue. In this study, we found that most of the severely burned children were catabolic, as evidenced by a negative net balance of phenylalanine across the leg at the time of the control study. IGF-1/IGFBP-3 treatment at 1 mg/kg/day increased serum IGF-1 and IGFBP-3 levels, with no further increases with higher doses. Treatment with IGF-1/IGFBP-3 improved protein metabolic measures, and these effects were most dramatic in those who were most catabolic. Previously, IGF-1 infused without IGFBP-3 in severely burned patients has been shown to increase glucose uptake. 9 In this study, we found that glucose uptake by the leg was not increased by IGF-1 infused with IGFBP-3, nor were there any changes in substrate utilization determined by indirect calorimetry. These results indicate relatively specific effects of IGF-1/IGFBP-3 on protein metabolism.
Sir David Cuthbertson first used pituitary extracts to increase nitrogen retention and decrease weight loss in an animal model of traumatic injury, 12 and then Wilmore in 1974 showed that human growth hormone had similar anabolic effects in patients after burn. 13 With the advent of a recombinant human growth hormone, the study of this anabolic agent has increased in an effort to modulate the postburn hypermetabolic response. Growth hormone treatment has been shown to improve leg protein metabolism, 3 accelerate donor site healing, 14 increase expression of dermal proteins in healing wounds, 15 and decrease hospital stays 16 in severely burned children. Unfortunately, treatment with growth hormone was shown to increase the mortality rate in critically ill nonburned adults. 8 On review of our population of severely burned children who received growth hormone, no increases in the mortality rate could be shown from blinded controls. Further, improvements in serum albumin concentrations and calcium metabolism were shown that were not previously detected. 6 However, given the concerns raised by the studies in critically ill adults, other agents with similar effects are being investigated.
Many of the effects of growth hormone are mediated through IGF-1. 17 Infusion of IGF-1 for 3 days produced a net anabolic effect on protein metabolism in burned patients 9 ; however, these effects were associated with episodic hypoglycemia and may be short-lived. Prolonged infusions of IGF-1 have been shown to improve muscle metabolism in other catabolic states, but the effect diminished after 9 days of treatment, possibly as a result of increased IGF-1 clearance. 18 It was postulated that other proteins required to maintain serum levels of IGF-1 were downregulated by infused IGF-1, and thus the effectiveness of IGF-1 in improving protein metabolism was diminished.
Growth hormone has been shown to stimulate both IGF-1 and the production of IGFBP-3. 19 This may explain why growth hormone is effective in protein metabolism over prolonged periods, whereas IGF-1 alone is not. Recently, recombinant IGF-1 combined with IGFBP-3 became available for clinical study. This combination has been shown to maintain serum levels of IGF-1 and biologic effectiveness during prolonged infusions in normal volunteers (written communication with Celtrix Pharmaceuticals). Preliminary studies in severely burned adults have corroborated these findings. 20 The combination of IGF-1 and its principal binding protein may improve regulation of the bioavailability of IGF-1 and thus enhance modulation of its metabolic activity. In this study, IGFBP-3 levels did not decrease even though growth hormone levels were markedly diminished. With infusion of IGF-1 alone, IGFBP-3 levels would have decreased in proportion to the fall in growth hormone. 18 IGF-1 binds to both the IGF-1 receptor and the insulin receptor, which may explain the development of hypoglycemia with its use. The combination of IGF-1 and IGFBP-3 did not elicit any evidence of hypoglycemia in either normal subjects or in burned adults, perhaps through improved specificity for the IGF-1 receptor. 20 Our results indicate that the combination of IGF-1 and IGFBP-3 holds promise for improving net protein synthesis in lean body mass in severely catabolic patients, with minimal side effects.
The current study demonstrates several findings that have implications for further understanding the hypermetabolic response and the effects of IGF-1/IGFBP-3. First, we showed that most children after a severe burn of >40% TBSA are catabolic in skeletal muscle in the first weeks after injury. This has been repeatedly shown elsewhere in severely burned patients, 3,5,13 and demonstrates again that pharmacologic intervention during this period to attenuate catabolism is likely to show the greatest effects.
Second, we showed a critical threshold dose for anabolic therapy with IGF-1/IGFBP-3. We showed that the pharmacokinetics of IGF-1, when given with IGFBP-3, may be governed by a ceiling effect. Specifically, there was a significant difference in the serum response between the control group and the groups given 1, 2, and 4 mg/kg/day. Interestingly, there were no incremental increases in serum IGF-1 levels between groups treated with 1, 2, and 4 mg/kg/day of IGF-1/IGFBP-3. This provides the rationale for examining the effects of IGF-1/IGFBP-3 on net balance and FSR in these groups as a whole. It also indicates that a minimum dose of 1 mg/kg/day is necessary to achieve the desired effect, with no additional benefit from higher doses. As expected, growth hormone levels decreased with IGF-1/IGFBP-3 treatment, which is consistent with the negative feedback loop between serum IGF-1 and release of growth hormone-releasing hormone. With respect to serum levels of insulin, there was no observed difference between IGF-1/IGFBP-3-treated patients and placebo-controls. This is consistent with the finding of no documented episodes of hyperglycemia or hypoglycemia with treatment. This also eliminates the possibility that increased circulating levels of insulin were responsible for the observed anabolic effects on leg amino acid kinetics.
A third finding is that IGF-1/IGFBP-3 at a threshold dose of ≥1 mg/kg/day significantly improves protein net balance through an acceleration of muscle protein synthesis in children with severe burn injury. No improvements were seen with a dose of 0.5 mg/kg/day in children in a paired analysis, although a biologic effect of decreasing serum growth hormone concentrations was seen. Many of these patients were anabolic by our criteria, and this may be the reason for no observed effect. Further study of this dose in catabolic patients may be necessary to elucidate its effects.
Perhaps the most significant finding in this study was the difference in effect of IGF-1/IGFBP-3 in those who were catabolic and those who were not. This finding makes intuitive sense, because an anabolic agent would not be expected to induce further anabolism in a person at bed rest who is already at a zero net balance or greater. However, a person who is highly catabolic, with an efflux of amino acids from the muscle, might respond to a signal to increase net protein synthesis. IGF-1/IGFBP-3 was effective for the entire group of severely burned patients, and it was only with post hoc analysis that this difference in patients who were catabolic and those who were not was found. Therefore, IGF-1/IGFBP-3 is effective at improving protein metabolism in the population of severely burned patients, and consideration should be given for the use of this agent in all severely burned children. However, it was most effective in those who were most catabolic. In a few instances, patients in a positive net balance actually experienced a decrease in phenylalanine net balance across the leg with treatment. However, given the standard deviation, these differences were not statistically significant.
This raises the question as to whether anabolic agents might be better suited for those deemed to be catabolic by some measure other than mechanism of injury and clinical assessment. Simple methods to determine those who are truly catabolic should be explored. Perhaps the method of measuring protein balance across the leg by measuring concentrations of phenylalanine in femoral arterial and venous blood should be more routine to determine those who are catabolic and therefore would benefit most from anabolic treatment.
The effect of IGF-1/IGFBP-3 on protein net balance was significantly different in severely burned children (p = 0.048), whereas no difference in protein net balance was found in the children who did not receive IGF-1/IGFBP-3 (p = 0.12). Therefore, IGF-1/IGFBP-3 improved protein metabolism by our measures that were independent of time. However, net protein balance appeared to be improving even without IGF-1/IGFBP-3. It has been postulated that the catabolic response to severe burn injury is relatively constant during the wound-healing phase of treatment. 1 A power analysis revealed that 20 patients meeting the criteria for this study would be required to show a significant difference in protein net balance without IGF-1/IGFBP-3. This indicates that improved protein metabolism in severely burned children is minimal but likely during the acute hospital stay.
In conclusion, we have found that IGF-1/IGFBP-3 is effective in improving protein metabolism in severely burned children, particularly in those who are most catabolic. Studies to determine outcome measures of enhanced retention of lean body mass and improved rehabilitation should follow to determine if this treatment has clinical utility.
Discussion
Dr. William C. Cioffi, Jr. (Providence, Rhode Island): In this study the authors have reported on the effects of a complex of IGF-1 and its primary binding protein on the catabolic response in a large series of pediatric and adult patients.
They have provided additional data concerning differences in the hypermetabolic response between adults and children. And they have concluded that children have a lesser hypermetabolic response than adults and, second, that IGF-1 decreases net protein flux across the leg and increases skeletal muscular protein synthesis. I have several questions:
First, why the difference between the children and the adults? Is this data indexed to lean body mass or some other index?
Second, as you have indicated, review of your data shows a rather marked and mixed response, with some responders and nonresponders. For the most part, the responders started with a negative flux and the nonresponders with a positive flux across the leg. I am curious, though, why the nonresponders then reacted with a more negative flux when they received the drug. Can you explain this net negative effect of getting IGF-1 in the patients who start with a negative protein flux? You have reported on extremity protein data and balance, but what are the systemic and hepatic effects of IGF-1? I am quite surprised by the lack of a significant insulin effect, given the propensity of IGF-1 to bind to both its receptor and the insulin receptor.
Finally, comparison of two study points in children indicate phenylalanine flux of 0.02 versus 0.01 in treated and control patients. Are these really different, or is there more of a time effect than you have indicated?
I would hope that you would put this in some clinical perspective for us and give us some idea of just how much of an effect IGF-1 is really having in salvaging the autocatabolism which occurs following thermal injury.
Dr. David W. Mozingo (Gainesville, Florida): This experiment required time-intensive data sampling and a number of invasive procedures, and I applaud your ability to accomplish this study during the first 10 days postburn when these patients with extensive burns require intensive clinical intervention and surgical therapy. I have several questions:
In the European growth hormone trial, we were all dismayed to find a higher mortality rate in growth-hormone–treated patients. Since the anabolic effects of growth hormone administration act through IGF-1, and we incompletely understand the reasons for increased mortality in the European study, what differences in the mechanisms of these two hormones lead you to believe that IGF treatment will be safer?
In relation to your glucose uptake measurements, how do you explain the fact that glucose levels were lower in treated patients, yet no increase in glucose uptake was observed? Also, substrate delivery and uptake may be influenced by the presence of burns on the affected extremity. What was the distribution of burn injury on the limbs cannulated for the uptake measurements? Also, is indirect colorimetry sensitive enough to detect changes in substrate utilization in this setting?
In a recent publication from the Institute of Surgical Research, Kellerman reported that for burn sizes of 20% of the body surface area, metabolic rate plateaued at 1.5 times the predicted basal rate when studies were performed at thermoneutrality. Were the ambient conditions of these studies comparable for the adults and children? It seems technically easier to provide a warm environment for a small child than a large adult. Could these differences explain the discrepancy between the degree of catabolism measured in adults and children?
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): The use of IGF-1/IGFBP-3 improved muscle protein fractional synthetic rate without associated net glucose uptake and no change in substrate utilization, meaning that it is relatively free from hypoglycemia seen with IGF-1 and hyperglycemia seen with human growth hormone. The answers to several questions may help us to assess the authors’ findings. In earlier studies, the increase in nutritional efficiency associated with human growth hormone therapy was observed only when carbohydrate intake equalled measured metabolic rate. How well did the carbohydrate intake in these patients match REE in each study period in all patients? Was that verified by indirect calorimetry at each study time?
Your finding that the effects of IGF-1/IGFBP-3 were independent of time postinjury is rather surprising, since the magnitude of postburn hypermetabolism decreases with time. What accounts for the time insensitivity of the treatment?
There was no statistical significance of the difference in glucose uptake across the leg with and without treatment, but there was a three-fold decrease during treatment. I wonder whether that represents a Type II error and whether the IGF-1/IGFBP-3 complex eliminates IGF-associated hypoglycemia but increases the risk of hyperglycemia?
Is the increase in catabolism in the adults as compared to children related to the higher preentry level of growth hormone and IGF-1 levels associated with normal growth in children, which minimized postinjury muscle wasting?
The graphs of metphenylalanine balance in children show that the IGF-1/IGFBP-3 balance increased in nine, decreased in four, and remained the same in one. Similarly, in regard to fractional protein synthetic rate, that rate increased in seven, decreased in six, and remained the same in one with treatment. What differentiated the responders from the nonresponders and how can the number of responders be increased?
In adults, it appears as if the increase in phenylalanine balance and fractional protein synthetic rate of two patients with respect to each variable may account for the significance of the differences. If those two potential outliers are excluded, David, is the beneficial effect of IGF-1/IGFBP-3 still observed?
Lastly, IGF-1 is known to improve renal function and morphology after ischemia in animal models and improve intestinal mucosal structure and function in other animal models. Did you observe improved renal function and improved intestinal absorption of nutrients during the IGF-1/IGFBP-3 treatment period?
Dr. Murray F. Brennan (New York, New York): Dr. Herndon and his colleagues continue their very long-term commitment to improving clinical burn care by improving what we all might call metabolic recovery. The long-standing interest in nutritional support and anabolic hormones to actually improve muscle mass by inhibiting both catabolism and promoting synthesis is well known, both in the burn patient and, in our experience, in the cancer patient. The present study advances on the prior studies with—and I emphasize—continuous nutritional support plus growth hormone and insulin-like growth factor by examination of a new agent, IGF-1/IGFBP-3, which introduces my first question:
Just what exactly is this new agent? What prompted the modification of IGF-1? And do we know how it acts? How does giving an anabolic hormone along with its principal binding protein improve or indeed alter efficacy? Specifically, do we know that this agent alters amino acid transport in vitro, which would help explain the results?
In any event, the authors show that treatment with this agent improves metabolic measurements in both children and adults, and the biological effect is demonstrated by inhibition of nitroformant concentration. And in the present study, the authors show that in contradistinction to IGF-1 alone, IGF-1/IGFBP-3 does not show an increase in glucose uptake. Conversely, arterial glucose concentration is diminished. How do the authors explain this?
Surely protein synthesis is an energy-consuming process. Is that energy not derived from glucose? Have they measured other substrates to account for the energy consumption? Indirect calorimetry did not show increased energy consumption.
Insulin concentrations were not changed, but arterial blood sugar was lower, and yet, net uptake was not increased. An explanation: is it possible that IGF-1 along with the binding protein acts in a manner to diminish insulin resistance—a common event in the severely injured—thereby promoting amino acid transport at very low energy cost?
Finally, I believe it would be important, David, to comment on the serious morbidity seen in the European studies of human growth hormone, not seen in your studies in burns and our studies in patients with malignancy. We have assumed that this has been due to failure to appropriately control blood sugar. Is that indeed correct?
Dr. David N. Herndon (Closing Discussion): Responders versus nonresponders: why did the nonresponders act differently than the responders? We believe it is because they were in a positive nitrogen balance before receiving the drug. Those who were in a negative nitrogen balance across leg responded. Those who were in a positive nitrogen balance did not respond. I can’t define why the nonresponders were anabolic, however, at the time of study. That could not be differentiated on the basis of size of burn. It was differentiated on the age of the patients.
Time effect: the perspective of the whole body response, I can only say that this time effect probably does exist. There probably is a Type II error. We studied 8 patients over time and showed a slight improvement, but not statistically significant in protein synthesis in net balance across leg over time. I think if more patients were studied, that that would be a real effect. But the effect of the drug appears to be greater than the effect of time alone.
The glucose lower in the treated patients, I believe that clearly there is an effect of IGF-1/IGFBP-3 on glucose metabolism. There is a decrease in arterial blood glucose levels. The sensitivity of the examination of glucose uptake across leg did not allow us to see that difference.
I believe clearly that IGF-1/IGFBP-3 has an effect on the insulin receptor and causes an improvement in glucose absorption, and as Dr. Brennan suggests, that may improve amino acid synthesis in the periphery. It is not as rabidly bound to the insulin receptor as IGF-1 alone. IGF-1 alone causes gross hypoglycemia under those circumstances, and the presence of the binding protein appears to decrease the avidity of that response. We therefore think the efficacy of the system may be somewhat better.
The lack of change in glucose oxidation then across the limb may be due to the sensitivity of the study and not absolutely true.
We did not see any effects on renal function or gut function in this study. The amount of food that was given to each individual was the same throughout all groups, and resting energy expenditure determinations were made, so that the amount of infused food was in excess of resting energy expenditure.
Dr. Mozingo makes a very interesting comment that perhaps the adults were more catabolic because they are harder to warm. That indeed is true, though the attempt to maintain thermoneutral conditions was the same for both groups of patients. There may indeed be a mass effect.
Dr. Cioffi, the difference between children and adults may be a difficulty to warm. These results were corrected to volume. But the amount of injury for an adult is proportionately much greater than it is for a child, as burn is body surface area function, which is two thirds times weight. So any given burn for an adult is harder to operate on. It takes longer to operate on. They have more blood transfusions. The degree of ancillary trauma is greater than it is in children, and that may explain the difference between children and adults.
In regard to the two outliers being excluded in the adult population, we did do that, and there still was a significant increase, but it is very slight. The adult patient population was only 12 patients. Given the variability of these studies, the pediatric results are more robust than the adult studies, and I thank you for pointing that out, Dr. Pruitt.
Dr. Brennan and, I believe, Dr. Cioffi asked about the European results which indeed were quite sobering. A very large number of patients died in a multicenter study in which growth hormone was given to critically injured individuals. This was not the case in pediatric burn studies that have been analyzed in detail by ourselves where there was no mortality effect. And, in fact, an anabolic response to growth hormone was demonstrated.
The data from the European study has not been analyzed by anybody, or it has not been publicly circulated. The small chances I have had to look at it show institutional differences from institution to institution, and perhaps, as you allude to, inability to control hyperglycemia may have been the primary contributor to mortality.
IGF-1/IGFBP-3 was thought of because of the studies of Cioffi that showed that BP-3 levels were down and the effect of IGF-1 seemed to tachyphylax over time. He and others have shown that. The presence of the two together should allow delivery of this substance to accrue its positive effects over time. And I think that is the advantage of it.
Footnotes
Correspondence: David N. Herndon, MD, Shriners Burns Hospital, 815 Market, Galveston, TX 77550.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Supported by SHC grants 8660 and 8490, NIH grants 1 RO1-GM56687–01 and 5 T32 GM08256–07, and Celtrix Pharmaceuticals.
Accepted for publication December 1998.
References
- 1.Wilmore DW, Long JM, Mason AD. Catecholamines: mediators of the hypermetabolic response to thermal burn patients. Ann Surg 1974; 180: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildreth MA, Herndon DN, Desai MH, Broemeling LD. Current treatment reduces calories required to maintain weight in pediatric patients with burns. J Burn Care Rehab 1990; 11: 405–409. [DOI] [PubMed] [Google Scholar]
- 3.Gore DC, Honeycutt D, Jahoor F, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on whole-body and isolated limb protein kinetics in burned patients. Arch Surg 1991; 126: 38–43. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai Y, Aarsland AA, Chinkes DL, et al. Anabolic effects of insulin in burned patients. Ann Surg 1996; 222: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez RJ, Wolf SE, Barrow RE, Herndon DN. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg 1998; 228: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knox J, Demling R, Wilmore D, Sarraf P, Santos A. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma 1995; 39: 526–532. [DOI] [PubMed] [Google Scholar]
- 8.Public Communications from Pharmacia & Upjohn Pharmaceuticals and Rolf Gunnarsson, MD, to all industry and medical community involved with the use or potential use of recombinant human growth hormone, Oct. 31, 1997.
- 9.Cioffi WG, Gore DC, Rue LW, et al. Insulin-like growth factor-I lowers protein oxidation in patients with thermal injury. Ann Surg 1994; 220: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biolo G, Fleming RYD, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol 1995; 268: E75–84. [DOI] [PubMed] [Google Scholar]
- 11.Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol 1998; 275: E864–E871. [DOI] [PubMed] [Google Scholar]
- Dempster WT, Gaughan GRL. Properties of body segment based on size and weight. Am J Anat 1965; 120: 33–54. [Google Scholar]
- 12.Cuthbertson DP, Shaw GB, Young FG. The anterior pituitary gland and protein metabolism: the nitrogen retaining action of anterior lobe extracts. J Clin Endocrinol Metab 1941; 2: 459–467. [Google Scholar]
- 13.Wilmore DW, Moylan JA, Bristow BF, Mason AD Jr, Pruitt BA Jr. Anabolic effects of human growth hormone and high caloric feedings following thermal injury. Surg Gynecol Obstet 1974; 138: 875–884. [PubMed] [Google Scholar]
- 14.Gilpin DA, Barrow RE, Rutan RL, Broemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg 1994; 220: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Hawkins HK, Nguyen TT, Pierre E, Cox R, Barrow RE. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg 1995; 221: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor site healing in severely burned children. Ann Surg 1990; 212: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmid CM, Ernst K, Binz JZ. The endocrine/paracrine actions of IGFs on bone. In Spencer EM, ed. Modern concepts of insulin-like growth factors. New York: Elsevier; 1991: 129–141.
- 18.Lieberman SA, Butterfield, GE, Harrison D, Hoffman AR. Anabolic effects of recombinant human insulin like growth factor-I in cachectic patients with acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1994; 78: 404–410. [DOI] [PubMed] [Google Scholar]
- 19.Klein GL, Wolf SE, Langman CB, et al. Effect of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab 1998; 83: 21–24. [DOI] [PubMed] [Google Scholar]
- 20.Debroy MA, Zhang X-J, Wolf SE, Wolfe RR, Herndon DN. Anabolic effects of administration of recombinant human insulin-like growth factor-I/insulin-like growth factor binding protein-3 on protein metabolism in adult burn patients. Surg Forum 1998; 49: 56–57. [Google Scholar]