Abstract
Objective
To assess the accuracy and clinical impact of 18fluorodeoxyglucose-positron emission tomography (18FDG-PET) on the management of patients with suspected primary or recurrent pancreatic adenocarcinoma, and to assess the utility of 18FDG-PET in grading tumor response to neoadjuvant chemoradiation.
Summary Background Data
The diagnosis, staging, and treatment of pancreatic cancer remain difficult. Small primary tumors and hepatic metastases are often not well visualized by computed tomographic scanning (CT), resulting in a high incidence of nontherapeutic celiotomy and the frequent need for “blind resection.” In addition, the distinction between local recurrence and nonspecific postoperative changes after resection can be difficult to ascertain on standard anatomic imaging. 18FDG-PET is a new imaging technique that takes advantage of increased glucose metabolism by tumor cells and may improve the diagnostic accuracy of preoperative studies for pancreatic adenocarcinoma.
Methods
Eighty-one 18FDG-PET scans were obtained in 70 patients undergoing evaluation for suspected primary or recurrent pancreatic adenocarcinoma. Of this group, 65 underwent evaluation for suspected primary pancreatic cancer. Nine patients underwent 18FDG-PET imaging before and after neoadjuvant chemoradiation, and in eight patients 18FDG-PET scans were performed for possible recurrent adenocarcinoma after resection. The 18FDG-PET images were analyzed visually and semiquantitatively using the standard uptake ratio (SUR). The sensitivity and specificity of 18FDG-PET and CT were determined for evaluation of the preoperative diagnosis of primary pancreatic carcinoma, and the impact of 18FDG-PET on patient management was retrospectively assessed.
Results
Among the 65 patients evaluated for primary tumor, 52 had proven pancreatic adenocarcinoma and 13 had benign lesions. 18FDG-PET had a higher sensitivity and specificity than CT in correctly diagnosing pancreatic carcinoma (92% and 85% vs. 65% and 62%). Eighteen patients (28%) had indeterminate or unrecognized pancreatic masses on CT clarified with 18FDG-PET. Seven patients (11%) had indeterminate or unrecognized metastatic disease clarified with 18FDG-PET. Overall, 18FDG-PET suggested potential alterations in clinical management in 28/65 patients (43%) with suspected primary pancreatic adenocarcinoma. Of the nine patients undergoing 18FDG-PET imaging before and after neoadjuvant chemoradiation, four had evidence of tumor regression by PET, three showed stable disease, and two showed tumor progression. CT was unable to detect any response to neoadjuvant therapy in this group. Eight patients had 18FDG-PET scans to evaluate suspected recurrent disease after resection. Four were noted to have new regions of 18FDG-uptake in the resection bed; four had evidence of new hepatic metastases. All proved to have metastatic pancreatic adenocarcinoma.
Conclusions
These data confirm that 18FDG-PET is useful in the evaluation of patients with suspected primary or recurrent pancreatic carcinoma. 18FDG-PET is more sensitive and specific than CT in the detection of small primary tumors and in the clarification of hepatic and distant metastases. 18FDG-PET was also of benefit in assessing response to neoadjuvant chemoradiation. Although 18FDG-PET cannot replace CT in defining local tumor resectability, the application of 18FDG-PET in addition to CT may alter clinical management in a significant fraction of patients with suspected pancreatic cancer.
The preoperative diagnosis of primary pancreatic adenocarcinoma remains challenging, even for experienced clinicians. This diagnostic uncertainty is associated with two types of adverse outcomes. First, less aggressive surgeons may abort attempted resection because of a lack of tissue diagnosis. This is borne out by the significant rate of “reoperative” pancreaticoduodenectomy performed at major referral centers. 1–3 In a recent review of the M.D. Anderson Cancer Center experience involving 29 patients undergoing successful pancreaticoduodenectomy after a failure to resect at the time of initial laparotomy, 31% did not undergo resection at the time of the initial procedure because of a lack of tissue confirmation of malignancy. 3
A second type of adverse outcome generated by failure to obtain a preoperative diagnosis occurs when more aggressive surgeons inadvertently resect benign disease. This is particularly notable in patients who have a suspected malignancy without an associated mass on computed tomography (CT) scan. Thompson et al 4 reported a series of 20 patients who underwent pancreaticoduodenectomy for suspected but unproven malignancy. On final pathologic examination, benign disease was noted in 55% of the patients. Similarly, Bouvet et al 5 reported a series of 22 patients with suspected periampullary carcinoma who had a normal CT scan and a normal ampulla on endoscopic examination. Of these 22 patients, 45% had a benign etiology for biliary obstruction.
To avoid these adverse outcomes, newer imaging modalities may improve the accuracy of preoperative diagnosis for pancreatic adenocarcinoma. In this regard, 18fluorodeoxyglucose-positron emission tomography (18FDG-PET) is a novel imaging modality that takes advantage of selective 18FDG-uptake and retention by malignant cells. The role of 18FDG-PET in the evaluation of suspected primary pancreatic adenocarcinoma is not yet well defined. The additional potential roles for 18FDG-PET in evaluating tumor response to neoadjuvant therapy or in the evaluation of disease recurrence after resection have not been previously examined. This study reviews the experience with 18FDG-PET at a single institution in the evaluation of suspected primary or recurrent pancreatic adenocarcinoma and in grading tumor response to neoadjuvant chemoradiation.
PATIENTS AND METHODS
Patient Population
Patients who were evaluated for suspected primary or recurrent pancreatic cancer at the Vanderbilt University Medical Center or the Nashville Veterans Affairs Medical Center between 1995 and 1998 were identified. Only patients undergoing both CT scanning and 18FDG-PET imaging were included in this study. In all cases, 18FDG-PET and CT imaging was accomplished within a 4-week interval. In the majority of patients, both studies were performed within a 2- to 3-day period. Also, patients undergoing evaluation with 18FDG-PET for possible recurrent pancreatic adenocarcinoma after resection or after neoadjuvant chemoradiation were identified. Demographic and clinical data were collected by retrospective evaluation of hospital and clinic records.
Computed Tomography
For scans performed in our institution, helical CT images of the abdomen were obtained with 5 mm collimation and a table speed of 5 mm/sec (pitch 1 or 2), after administration of oral contrast and 30 seconds after intravenous contrast (150 ml at 3 ml/sec). The images were reconstructed with 5 mm thickness. Several patients had CT scans performed at outside institutions. CT imaging was not repeated when intravenous contrast was identified within the superior mesenteric vein and pancreatic parenchymal enhancement was noted. Patients whose scans did not meet these criteria underwent repeat imaging at our institution. The CT scans were considered positive for pancreatic adenocarcinoma when a discrete low-attenuation mass was identified within the pancreas. In the setting of metastatic disease, diffuse enlargement of the pancreatic head or uncinate process in the absence of a discrete low attenuation mass was also considered positive for pancreatic carcinoma.
Positron Emission Tomography
18FDG-PET images were obtained using a Siemens ECAT 933/08/16 tomograph, which has eight ring detectors that simultaneously collect images in 15 planes, each of 8 mm thickness. The axial field of view of this system is 12.8 cm with an intrinsic resolution at the center of the field of 4.8 mm and a reconstructed resolution of 6.5 × 6.5 × 8.0 mm (full width, half maximum). Patients were required to fast for ≥4 hours before the 18FDG-PET scan. The patients were scanned in as many sequential images as necessary to include the entire thorax, abdomen, and pelvis. Transmission images were obtained for 10 minutes per bed position to correct for photon attenuation using a germanium-68 ring source. After the intravenous administration of 370 MBq (10 mCi) of 18FDG, emission images were acquired for 15 minutes per bed position. The uptake period between 18FDG injection and the beginning of the emission scan was approximately 60 minutes. Accurate positioning of the patient between transmission and emission scanning was performed using laser marking.
Both attenuation-corrected and non-attenuation-corrected images were interpreted visually. Also, the attenuation-corrected images were analyzed semiquantitatively using the standard uptake ratio (SUR). Regions of interest measuring 1.0 ± 0.5 cm2 were drawn over the area of maximum activity in a lesion. The SUR was calculated as follows:
18FDG-PET images were considered positive for pancreatic adenocarcinoma when a focal area of 18FDG uptake was noted to be within the pancreas on correlation with anatomic imaging and the SUR was ≥2.8.
Statistical Analysis
Comparison of SUR values between patients with pancreatic ductal adenocarcinoma and those with benign disease was performed using the Mann-Whitney test for two independent samples, with significance defined as p < 0.05.
RESULTS
Evaluation of Suspected Primary Pancreatic Adenocarcinoma
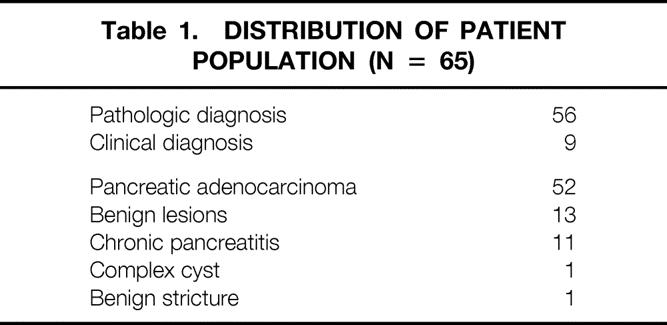
A total of 65 patients underwent both CT scanning and 18FDG-PET imaging for the diagnosis of suspected primary pancreatic adenocarcinoma during the study period. The distribution of patients is summarized in Table 1. Among these 65 patients, 52 with pancreatic adenocarcinoma and 13 with benign lesions were identified. Among the 52 patients with pancreatic adenocarcinoma, maximum tumor diameter was measurable by CT, endoscopic ultrasound, or pathology in 49 patients. Tumor size ranged from 1.0 to 6.7 cm (mean 3.1 ± 1.7). Fifty-six patients had pathologic confirmation of their diagnosis, and nine patients had diagnoses based on clinical and radiologic follow-up. Two of these nine patients were diagnosed with unresectable disease by CT scan and died within a few weeks of the imaging studies. Seven of these nine patients had no definite evidence of malignancy. These patients have remained clinically stable, with no evidence of malignant disease over a minimum 8-month follow-up period (range 8–23 months).
Table 1. Distribution of Patient Population (n = 65)
18FDG-PET SURs for the primary tumors ranged from 2.2 to 15. Among the 52 pancreatic cancers identified, none failed to uptake 18FDG. Among the 13 patients with benign lesions, 10 showed no uptake of 18FDG (SUR = 0.0), and 3 with chronic pancreatitis showed 18FDG uptake with SUR values of 2.4, 3.0, and 4.9. The overall mean SUR for the patients with pancreatic adenocarcinoma was 5.0 ± 1.2, compared with 0.85 ± 0.1 for patients with benign disease. This difference was statistically significant (p < 0.0001). Among the 13 patients with benign disease, a total of five false-positive CT scans were noted. Among the 52 patients with pancreatic adenocarcinoma, 18 had false-negative CT scans. In contrast, there were only two false-positive and four false-negative 18FDG-PET scans. All 18 patients with false-negative CT scans had clarification with true-positive 18FDG-PET imaging.
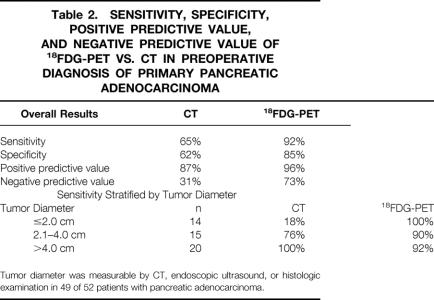
The relative sensitivity, specificity, positive predictive value, and negative predictive value of CT versus 18FDG-PET are presented in Table 2. For the preoperative diagnosis of pancreatic adenocarcinoma, 18FDG-PET imaging was superior to CT in all categories. The sensitivity of CT scanning was noted to improve with increasing lesion size; however, the sensitivity of 18FDG-PET imaging did not appear to be affected by size.
Table 2. Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of 18FDG-PET vs. CT in Preoperative Diagnosis of Primary Pancreatic Adenocarcinoma
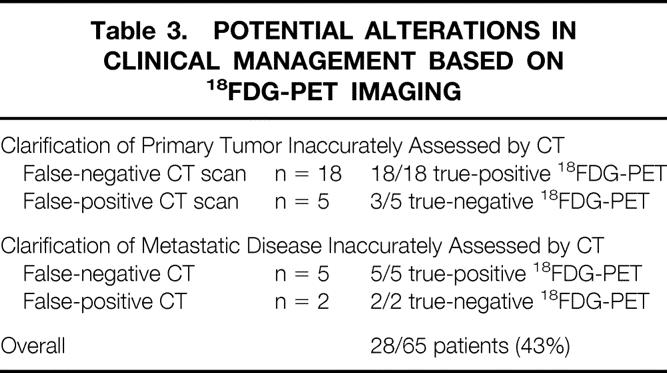
An example of 18FDG-PET imaging for CT-occult pancreatic cancer is presented in Figure 1. A 63-year-old woman with obstructive jaundice underwent helical CT scanning, which failed to reveal a low-attenuation mass within the head of her pancreas. Endoscopy showed no ampullary mass. On whole-body 18FDG-PET imaging, however, an isolated focus of uptake was noted within the head of the pancreas. The patient underwent pancreaticoduodenectomy and was found to have a 1.2 cm T1N0M0, stage I pancreatic adenocarcinoma.
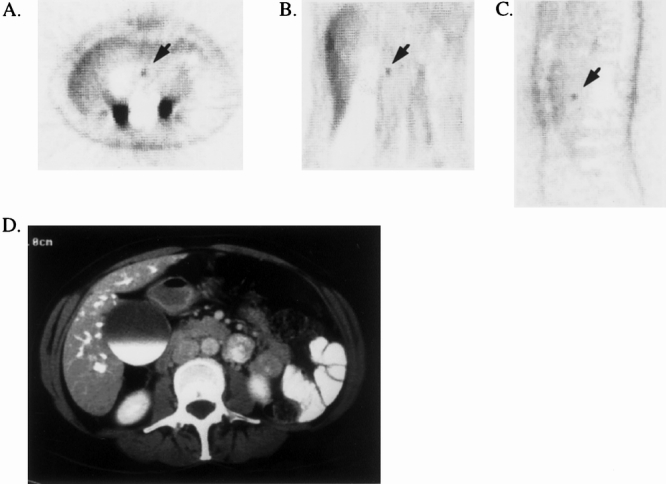
Figure 1. 18FDG-PET documentation of CT-occult primary pancreatic ductal adenocarcinoma. The patient had obstructive jaundice and distal common bile duct stricture on endoscopic retrograde cholangiopancreatography. Helical CT failed to demonstrate a low-attenuation mass in the pancreatic head. 18FDG-PET demonstrated a focus of increased uptake in the region of the pancreatic head (SUR = 4.3). Pancreaticoduodenectomy was performed with histologic confirmation of a 1.2-cm pancreatic ductal adenocarcinoma. (A) Transverse reconstruction of 18FDG-PET demonstrating pancreatic head lesion (arrow). (B) Coronal view. (C) Sagittal view. (D) CT scan at the level of the tumor, demonstrating failure to detect the primary lesion.
Among the 65 patients undergoing primary evaluation of suspected pancreatic cancer, 18FDG-PET imaging frequently led to potential alterations in clinical management. These alterations are presented in Table 3. 18FDG-PET scanning clarified the presence of a primary pancreatic adenocarcinoma in 18 of 18 patients who had a false-negative CT scan. All 18 patients with CT-occult tumors underwent subsequent exploration, and 16/18 underwent successful resection. In addition, three of five patients with false-positive CT scans for pancreatic malignancy had subsequent clarification with true-negative 18FDG-PET scanning. Five patients had CT-occult metastatic disease that was discovered on 18FDG-PET imaging, and an additional two patients had clarification of false-positive metastatic disease on CT scan. Overall, potential alterations in management were suggested by 18FDG-PET in 28/65 patients (43%).
Table 3. Potential Alterations in Clinical Management Based on 18FDG-PET Imaging
An example of the utility of 18FDG-PET scanning in the clarification of metastatic disease is presented in Figure 2. A 59-year-old woman with obstructive jaundice underwent a helical CT scan. This demonstrated a low-attenuation pancreatic head mass as well as a subcentimeter abnormality in the liver that was considered too small to characterize and too small for a biopsy. Whole-body 18FDG-PET imaging revealed multiple foci of increased uptake within the liver, as well as bony metastases in rib and pelvis. She was subsequently found to have biopsy-documented metastatic pancreatic adenocarcinoma.
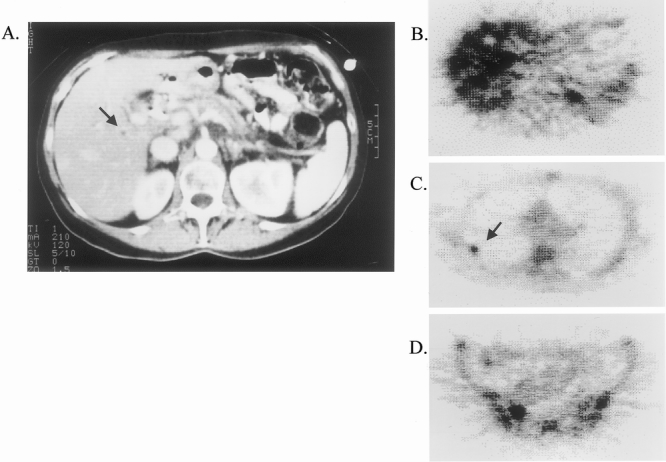
Figure 2. 18FDG-PET documentation of CT-occult metastatic disease. The patient had obstructive jaundice. CT demonstrated a potentially resectable primary lesion in the pancreatic head, with an indeterminate subcentimeter lesion in the right lobe of the liver (A, arrow). 18FDG-PET demonstrated multiple CT-occult hepatic metastases (B), as well as unsuspected skeletal metastases in rib (C, arrow) and pelvis (D).
Assessment of Response to Neoadjuvant Chemoradiation
Pre- and posttreatment 18FDG-PET scans have further been used to assess the response to neoadjuvant chemoradiation in patients with potentially resectable pancreatic cancer. A reduction in tumor 18FDG uptake of ≥50% was considered evidence of a response to therapy, whereas an increase of ≥50% was considered a sign of tumor progression. Among the nine patients had 18FDG-PET scanning before and after chemoradiation, four had evidence of tumor response by PET, three showed stable disease, and two showed tumor progression. Among patients in whom 18FDG-PET suggested a tumor response, CT failed to document a measurable reduction in tumor size in any of the four. Among the two patients with progressive disease documented by 18FDG-PET, one showed tumor progression on CT and the other demonstrated stable disease. Notably, all four patients who had 18FDG-PET evidence of tumor response went on to successful resection, all with histologic evidence of grade 2 treatment effect in the resected specimen (range of estimated tumor necrosis 20% to 80%). Among the five patients who showed no response by 18FDG-PET, the disease could subsequently be resected in only two, and only one patient who underwent resection showed evidence of chemoradiation effect in the resected specimen. Examples of pre- and postchemoradiation 18FDG-PET images are provided in Figure 3.
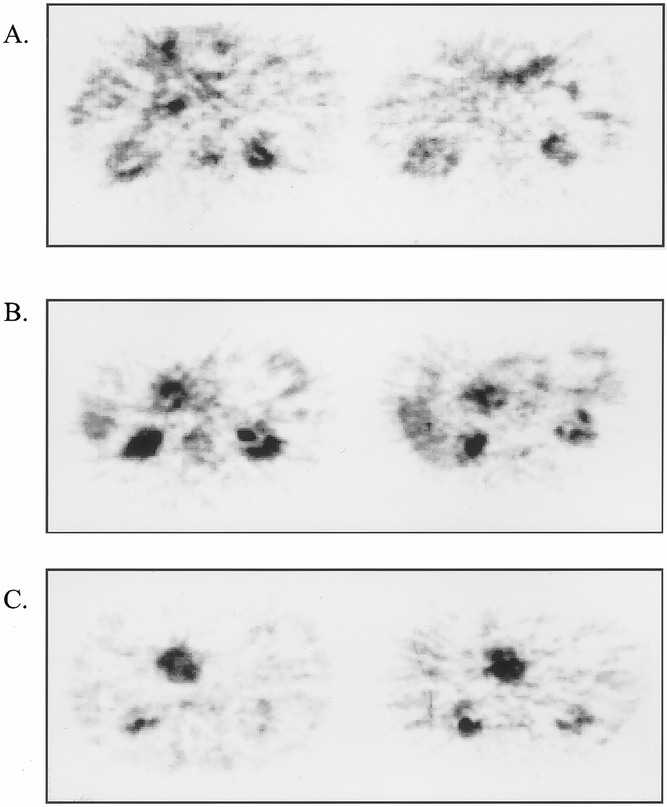
Figure 3. Assessment of response to chemoradiation using 18FDG-PET. Three representative examples of patients with biopsy-documented pancreatic ductal adenocarcinoma assessed by 18FDG-PET before (left panels) and after (right panels) neoadjuvant chemoradiation. In all three patients, CT showed no change in measurable tumor diameter. (A) Patient with treatment response documented by 18FDG-PET (pretreatment SUR = 3.0, posttreatment SUR = 0.0); note physiologic uptake of 18FDG in stomach anteriorly and in kidneys bilaterally. The resected specimen demonstrated grade 2A treatment effect (30% necrosis). (B) Patient with stable disease (<50% reduction in SUR) documented by 18FDG-PET (pretreatment SUR = 3.8, posttreatment SUR = 3.2). Resected specimen showed grade 1 treatment effect (<10% tumor necrosis). (C) Patient with progressive disease documented by 18FDG-PET (pretreatment SUR = 3.5, posttreatment SUR = 5.6). Disease was unresectable at laparotomy because of local tumor extension.
Evaluation of Suspected Disease Recurrence After Resection
Eight patients undergoing 18FDG-PET scanning for the evaluation of possible recurrent pancreatic adenocarcinoma were identified. All patients had previously undergone apparently curative resection, and follow-up ranged from 6 to 39 months. All eight patients had either indeterminate CT findings suggestive of recurrence or a rise in serum CA 19–9 levels. All patients had abnormal regions of 18FDG uptake identified, four in the surgical resection bed (mean SUR = 4.7) and four in new hepatic metastases (mean SUR = 5.8). In all patients, recurrent and/or metastatic adenocarcinoma was pathologically or clinically confirmed.
DISCUSSION
During the process of malignant transformation, the majority of cells become avid glucose scavengers, with increased glucose transport and utilization. This fact has been recognized since the early observations of Warburg in 1931. 6 18FDG-PET) takes advantage of this enhanced glucose uptake to functionally identify malignant tissue. 18FDG is a glucose analog labeled by the positron-emitting radioisotope fluorine-18 at the C2 position. This agent is actively taken up into the cell and phosphorylated by hexokinase during the first step in the glycolytic pathway. Unlike normal glucose, however, phosphorylated 18FDG cannot continue glycolysis and becomes trapped within the cell. Studies comparing the uptake of 18FDG by malignant versus nonmalignant cells have revealed differences in both the uptake and metabolism of glucose. Of note, several authors have reported overexpression of glucose transporter 1 (Glut-1) in human pancreatic adenocarcinoma. 7–10 Reske et al 8 showed a significant increase in Glut-1 mRNA in 12 patients with pancreatic adenocarcinoma when compared with 15 patients with mass-forming pancreatitis. In addition to increased transport, the metabolism of glucose has also been noted to be altered in malignancy. Hexokinase and other glycolytic enzymes have been shown to be overexpressed in multiple cancers, including pancreatic adenocarcinoma. 10–15 These malignancy-associated alterations in glucose metabolism provide the basis for the application of physiologic imaging using 18FDG.
18FDG-PET imaging has been increasingly used in the last 5 years to identify and stage multiple tumor types, including mammary carcinoma, melanoma, head and neck cancer, and colorectal carcinoma. 16–21 The majority of these studies show 18FDG-PET to be superior to traditional CT imaging in the diagnosis of malignancy. This has been particularly notable in the evaluation of recurrent or metastatic disease, including both local/regional and distant tumor spread. Of note, 18FDG-PET has proven extremely accurate in our institution for the detection of metastatic colorectal carcinoma and in the differentiation between benign and malignant lesions of the liver. 22–24 However, the role of 18FDG-PET imaging in the preoperative diagnosis and management of patients with suspected pancreatic adenocarcinoma remains less well established.
18FDG-PET images are evaluated both visually and semiquantitatively using an objective value based on local 18FDG concentration corrected for injected dosage per body weight (the SUR). In the reported series, the cutoff SUR for identification of malignancy ranged from 1.5 to 4.0, with widely variant mean values for benign and malignant lesions. The utility of semiquantitative interpretation of 18FDG-PET in the diagnosis of pancreatic cancer has been debated in the literature. Zimny et al 31 reported that visual interpretation of the images was superior to the use of the SUR. Conversely, Inokuma et al 29 and Stollfuss et al 26 found the two techniques of interpretation to be comparable. Clearly, different sensitivities and specificities can be achieved by choosing various cutoff levels for the SUR. In the current series, dropping the cutoff SUR from 2.8 to 2.0 would increase the sensitivity of 18FDG-PET imaging from 92% to 100% while decreasing the specificity from 85% to 77%. Of interest, one analysis involving a small number of patients suggested that SUR values may have prognostic utility. Nakata et al 34 noted a correlation between SUR and survival in 14 patients with pancreatic adenocarcinoma. Patients with an SUR ≥3.0 had a mean survival of 5 months, compared with 14 months in those with an SUR <3.0. Because both visual interpretation and placement of the region of interest are partially subjective, we believe that each institution must analyze its own experience to ensure optimal interpretation of 18FDG-PET imaging.
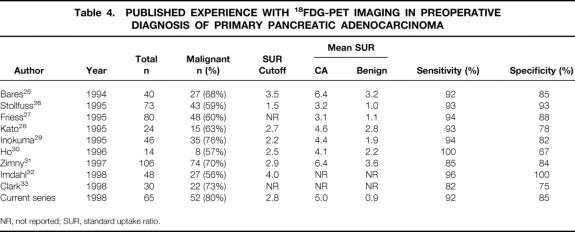
A summary of previously published series involving 18FDG-PET in the evaluation of pancreatic adenocarcinoma is presented in Table 4. All series report relatively high rates of sensitivity (82% to 100%) and specificity (67% to 100%) for 18FDG-PET, and the majority suggest improved accuracy compared with anatomic imaging with CT scanning. This experience is confirmed by the current series, in which we observed a sensitivity of 92% and a specificity of 85% for 18FDG-PET versus 65% and 62% for CT scanning. Clark et al 33 reported the only series that failed to show an improved accuracy in the diagnosis of pancreatic adenocarcinoma; they concluded there was no advantage to this imaging modality. As a group, however, these series support our conclusion that 18FDG-PET imaging may represent a useful adjunctive study in the evaluation of patients with suspected pancreatic cancer.
Table 4. Published Experience with 18FDG-PET Imaging in Preoperative Diagnosis of Primary Pancreatic Adenocarcinoma
The rate with which 18FDG-PET results may lead to alterations in clinical management clearly depends on the specific therapeutic philosophy employed by the evaluating surgeon. In our center, we advocate pancreaticoduodenectomy only for patients with potentially curable pancreatic cancer, and take an aggressive approach to resection, including en bloc retroperitoneal lymphadenectomy with selective resection of the superior mesenteric-portal vein confluence when necessary to obtain a negative margin. 35 This type of approach is obviously inappropriate in patients with benign disease, and intraoperative biopsy cannot be relied on to make this distinction. Although certain patients with chronic pancreatitis may also benefit from pancreaticoduodenectomy, the majority of patients with nonmalignant biliary strictures are optimally managed without resection. By providing reliable preoperative distinction between benign and malignant pancreatic lesions, 18FDG-PET may facilitate selection of the optimal surgical approach.
For patients with no discrete mass on CT but a positive signal on 18FDG-PET (SUR ≥2.8), the surgeon may confidently undertake resection with a minimal risk of inadvertently resecting benign disease. In this situation, our data suggest an extremely high risk of malignancy. Conversely, no adenocarcinoma in this series failed to demonstrate at least some abnormal uptake of 18FDG, with a minimum SUR of 2.2 among the 52 malignant lesions. When 18FDG-PET imaging is entirely normal (SUR = 0.0), the likelihood of an occult adenocarcinoma appears to be negligible, and pancreaticoduodenectomy should be performed only in the presence of additional nononcologic indications.
In addition to the evaluation of suspected primary pancreatic adenocarcinoma, we have examined the utility of this imaging technique in the assessment of tumor response to neoadjuvant therapy and the evaluation of possible recurrent disease after resection. In this series, 18FDG-PET successfully predicted histologic evidence of chemoradiation-induced tumor necrosis in all four patients who demonstrated ≥50% reduction in tumor SUR after chemoradiation. Among these patients, none showed measurable change in tumor diameter as assessed by CT. Definitive conclusions regarding the utility of 18FDG-PET in this regard will obviously require evaluation in a larger group of patients. However, given the poor track record of CT in assessing histologic response to neoadjuvant chemoradiation, the potential utility of 18FDG-PET in this capacity deserves further investigation.
The majority of prior reports concerning the clinical use of 18FDG-PET scanning for nonpancreatic malignancy have emphasized the identification of recurrent nodal or distant metastatic disease. 16,18,20,22–24 The use of 18FDG-PET in the evaluation of suspected recurrent pancreatic adenocarcinoma has not yet been reported. In the eight patients evaluated for possible recurrence in this series, all were noted to have significant new regions of 18FDG uptake, and all proved to have metastatic pancreatic adenocarcinoma. This technique may be particularly useful when CT identifies an indistinct region of change in the bed of the resected pancreas that is difficult to differentiate from postoperative or postradiation fibrosis. In addition, we have found 18FDG-PET to be useful in the evaluation of new hepatic lesions that are often too small for a biopsy. In this setting, we have relied on 18FDG-PET documentation of recurrence instead of biopsy as the basis for additional tumor-directed therapy.
As with any imaging modality, 18FDG-PET has identifiable limitations in the evaluation of pancreatic cancer. First, this functional imaging modality obviously cannot replace anatomic imaging in the assessment of local tumor resectability. Second, theoretical concerns have been raised regarding the limitations of this modality in a population of patients with a significant rate of glucose intolerance. Low SUR values and false-negative 18FDG-PET scans have been noted in hyperglycemic patients and patients with diabetes, presumptively because of increased competition for glucose uptake. 36–38 The true impact of serum glucose levels on the accuracy of 18FDG-PET in pancreatic cancer remains controversial; Friess et al 27 and Ho et al 30 noted no variation in the accuracy of 18FDG-PET imaging based on serum glucose levels. Conversely, Zimny et al 31 noted significant difficulties in the interpretation of 18FDG-PET in patients with diabetes. Of the four false-negative 18FDG-PET studies in the current series, one patient had diabetes, with a glucose level of 111 mg/dl at the time of imaging, and one patient had a serum glucose level of 176 mg/dl. Overall, no influence of serum glucose on the accuracy of 18FDG-PET imaging was observed in the current study.
Both glucose and 18FDG are avidly taken up by inflammatory cells. 39 False-positive 18FDG-PET scans have been noted in the presence of acute and chronic inflammatory reactions, including granulomatous disease, osteomyelitis, and abdominal abscesses. 40,41 Inokuma et al 29 and Ho et al 30 have previously reported false-positive imaging in the face of inflammatory changes in the pancreas. Of the two patients with false-positive 18FDG-PET studies in this series, one was noted to have microabscesses in a focus of mass-forming pancreatitis on final examination of the resected specimen. Inflammation as a source of false-positive 18FDG-PET studies should therefore always be considered when interpreting these images.
Overall, 18FDG-PET imaging appears to a sensitive and specific adjunct to CT when applied to the preoperative diagnosis of pancreatic adenocarcinoma. We have found this imaging modality to be of particular use in patients with suspected pancreatic cancer in whom CT fails to identify a discrete tumor mass. By providing preoperative documentation of pancreatic malignancy in these patients, laparotomy may be undertaken with purely therapeutic intent, and the risk of aborting resection because of diagnostic uncertainty is minimized. 18FDG-PET imaging would also appear to be useful in the clarification of CT-occult metastatic disease, allowing nontherapeutic resection to be avoided altogether in this group of patients.
The field of functional tumor imaging is expanding rapidly, with significant recent improvements in imaging strategies and techniques. 42 With ongoing refinement in functional and anatomic imaging, the goal of accurately diagnosing and staging pancreatic cancer before the delivery of multimodality therapy may become a reality. In this regard, the application of 18FDG-PET appears to represent a significant advance.
Acknowledgments
The authors thank Mr. Joel Vaughn for assistance with graphic images and Ms. Kimberly Pickett for assistance with manuscript preparation.
Discussion
Dr. Murray F. Brennan (New York, New York): The authors examined retrospectively 18FDG-PET, and 65 patients with suspected or proven cancer. By the mere fact that it is a retrospective study, we must conclude that there was selectivity in doing a PET scan. For example, only 36% were considered to have a negative CT scan, despite 3-mm cuts. A very high false-negative value, I would have thought, for pancreatic adenocarcinoma.
They examined three groups—a general group undergoing evaluation, a pre- and postchemotherapy group, and a group being evaluated for recurrent disease—and claim that PET scan is better at making the diagnosis than CT. I would suggest that CT has never claimed to make a diagnosis, only to indicate the presence of a mass and, in addition, provide evidence for resectability—something PET cannot possibly do.
The important observation, however, is that 11% had unrecognized metastasis. That is actually surprisingly similar to the 20% rate we find with laparoscopy. Alternatively, if the PET shows metastasis, do the authors do laparoscopy to prove that by biopsy?
So my first question is, have they considered comparing the PET scanning to laparoscopy in the most rewarding group, i.e., those that have unrecognized metastasis? It is very important to recognize that they have essentially only changed the management of 11% of patients by this analysis. Pre- and postchemotherapy is in part irrelevant and the detection of recurrence in pancreatic cancer is, unfortunately, a diagnosis looking for a treatment, as we have little or nothing significant to offer these patients.
So, in summary, I agree we must do such studies. They are important. But we need to do them with the intent of actually evaluating new studies against other studies that do or can substitute for them. We have, unfortunately, in my own institution and others, the approach to develop a new test rather than evaluating the test against other studies in the hope of discarding one or the other; we just add it to the armamentarium, and we are appropriately criticized.
I would hope to see us take a leadership role in defining the minimum number of tests that influence management decisions. So I do congratulate the authors on the study, but I would urge all of you to have some hesitation about the broad interpretation that this is an essential investigation for most patients.
Dr. Keith D. Lillemoe (Baltimore, Maryland): The authors have provided a clear demonstration that fluorodeoxyglucose PET scanning is useful in the preoperative identification of pancreatic cancers, quantitating response to neoadjuvant therapy, and in their manuscript described the detection of tumor recurrence following resection.
A PET scan is just one of a number of newer technologic advances that the pancreatic surgeon can now choose to employ in the preoperative assessment in staging of pancreatic cancer. Other techniques include spiral CT scan, ERCP, endoscopic ultrasound, MRI and, more specifically, MRCP and, more invasively, staging laparoscopy. The real question is not if any or all of these techniques are accurate or useful, but which is the one or perhaps two of these tests that are most useful in providing as much information as possible with respect to both the primary tumor and its potential for both local invasion and distant metastasis.
The significance of this answer reflects both cost containment as well as the expedient work-up of the patient with suspected pancreatic cancer.
I would, therefore, first like to ask the authors which studies has PET scanning eliminated in their routine work-up of a patient with suspected pancreatic cancer? And do they suggest that the PET scan be used routinely in all patients?
Clearly, the value of the PET scan is in identification of primary tumors, specifically with the functional component of the scan with enhanced glucose uptake in the cancer, particularly when there is no mass seen on CT scan. This scenario particularly describes some of the other periampullary carcinomas—distal bile duct, ampullary, and duodenal. Do the authors have any experience with this technique with nonpancreatic periampullary tumors?
The authors tell us that a positive PET scan altered their management. But I would caution in this interpretation, however, with respect to the 18 patients with a negative CT scan. This fact must be tempered with the fact that other characteristics such as that were not provided in the text, but in the two clear examples that were shown here, were the age of the patient, the clinical presentation with pain or jaundice, an abnormal ERCP, and a dilated pancreatic or biliary ductal system would clearly lead to the right action, that is, pancreaticoduodenectomy, with a high level of confidence of malignancy.
The real value of this test may be in those patients with suspected chronic pancreatitis and the suspicion of pancreatic cancer. Unfortunately, all of your patients with false-positives due to increased glucose uptake and no cancer had chronic pancreatitis, therefore leading to questions concerning the value in this setting. Could you again expand on the role of PET scan in patients with chronic pancreatitis?
PET scanning can detect liver metastases. As Dr. Brennan mentioned, laparoscopy is often an alternative. Do you have any evidence of identification of peritoneal implants using PET scanning?
Finally, for any study or test to make a difference, it must make a difference. In two of your indications, I really question what difference these results make. First, in judging the response to neoadjuvant therapy, are you going to choose your quality of management in those patients who do not respond and, therefore, choose not to explore those patients?
And how does the early detection of recurrent disease influence your management? Have you protocols underway to try to treat this dreaded disease in its recurrent stage?
Finally and, again, most importantly, short of detecting liver mets not seen on CT scan, I see little value from the PET scanning in the staging of pancreatic cancer, especially with respect to major vessel invasion. In my mind, the lack of this information will clearly limit the value of PET scanning as compared to spiral CT scan and perhaps new MR techniques. Would you, finally, please comment on the role of PET scanning in the further staging of these patients?
Dr. Steven D. Leach (Closing Discussion): Let me first say from the outset that we don’t advocate the nonselective use of FDG-PET scanning for all patients with pancreatic cancer.
Both of the discussants asked, rather than continuing to add an ever-increasing panoply of tests in the evaluation of these patients, what can we now discard, and I might just address that joint question initially. I think for patients who have a well-demarcated, low attenuation mass on a well-done dynamic helical contrast CT scan, no further evaluation is necessary, and those patients should proceed directly to pancreaticoduodenectomy as long as they are predicted to be resectable by CT scan.
As in other centers, we have essentially eliminated angiography in the preoperative evaluation of these patients but, rather, rely exclusively on helical CT to delineate the relationship between tumor and the superior mesenteric vasculature.
I think the role of laparoscopy in the evaluation of patients with pancreatic head cancer continues to be somewhat controversial. And in spite of early publications that suggest that a high rate of CT occult metastatic disease detection, that in patients who are truly predicted to be resectable by helical CT, the yield of peritoneal metastases or capsullary liver mets on laparoscopy for head lesions is small. And we have also eliminated laparoscopy in the evaluation of patients with suspected pancreatic head cancer and reserved that for patients with lesions of the body and tail.
With respect to Dr. Brennan’s comments on whether PET detects the same types of metastases that are seen on laparoscopy, the answer is no, that we have seen purely visceral and osseous metastases detected by PET, and I believe that the subcentimeter peritoneal implants are below the level of detection of PET.
With respect to Dr. Brennan’s question about selection, I think there certainly was selection in this series. It was our intention, with the help of NIH funding, to study all patients consecutively with both CT and PET. But there was some selection toward difficult-to-clarify lesions, and 30% of our patients had tumors less than 2 cm, confirming this issue of selection.
With respect to Dr. Lillemoe’s questions, regarding other nonpancreatic ductal periampullary malignancies, we did not have a big experience in the evaluation of these lesions. Certainly, simple endoscopy diagnosis of ampullary and duodenal cancers. And we don’t have a big experience with cholangiocarcinoma, although other centers have suggested that this may be a useful modality in that regard.
With respect to chronic pancreatitis, this is, obviously, the major conundrum that we often face in evaluating patients in terms of cancer versus pancreatitis, the specificity of PET in our series was 85%. What we can say is among the 52 patients with pancreatic cancer, that none had an absolutely normal FDG-PET with an SUR of 0.0.
So when the surgeon is faced with a patient who has obstructive jaundice in the setting of pancreatitis, if the PET scan is entirely normal, the chances for malignancy are negligible, and those patients are likely best managed by means other than resection, unless there is a pressing nononcologic indication for pancreaticoduodenectomy.
With respect to assessing response after neoadjuvant therapy, we don’t base treatment decisions in terms of subsequent resection on FDG-PET assessment of response. Nevertheless, we have seen a clear correlation between FDG-PET evidence of response and subsequent resectability. And I think that this application is going to be optimally applied to unresectable patients in the protocol-based evaluation of new chemoradiation regimens and other novel therapeutic options.
Footnotes
Correspondence: Steven D. Leach, MD, Division of Surgical Oncology, Vanderbilt University Medical Center, T-2104 Medical Center North, Nashville, TN 37232-2736.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Supported by NIH grant CA74773 from the National Cancer Institute (SDL).
Accepted for publication December 1998.
References
- 1.McGuire GE, Pitt HA, Lillemoe KD, et al. Reoperative surgery for periampullary adenocarcinoma. Arch Surg 1991; 126: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 2.Tyler DS, Evans DB. Reoperative pancreaticoduodenectomy. Ann Surg 1994; 219: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson EK, Lee JE, Lowy AM, et al. Reoperative pancreaticoduodenectomy for periampullary carcinoma. Am J Surg 1996; 172: 432–438. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JS, Murayama KM, Edney JA, Rikkers LF. Pancreaticoduodenectomy for suspected but unproven malignancy. Am J Surg 1994; 169: 571–575. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet M, Bold R, Lee J, et al. Presumed malignant biliary obstruction despite a normal CT: data to support pancreaticoduodenectomy. Gastroenterology 1998; 114: S0033. [Google Scholar]
- 6.Warburg O. The metabolism of tumors. New York: Richard R. Smith Inc.; 1931: 129–169.
- 7.Higashi T, Tamaki N, Honda T, et al. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study. J Nucl Med 1997; 38: 1337–1344. [PubMed] [Google Scholar]
- 8.Reske SN, Grillenberger KG, Glatting G, et al. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med 1997; 38: 1344–1348. [PubMed] [Google Scholar]
- 9.Yamamoto T, Seino Y, Fukomoto H, et al. Overexpression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 1990; 170: 223–230. [DOI] [PubMed] [Google Scholar]
- 10.Schek N, Hall BL, Finn OJ. Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res 1988; 48: 6354–6359. [PubMed] [Google Scholar]
- 11.Wahl RL. Targeting glucose transporters for tumor imaging: sweet idea, sour result. J Nucl Med 1996; 37: 1038–1041. [PubMed] [Google Scholar]
- 12.Ahn YS, Rempel A, Zerban H, Bannasch P. Overexpression of glucose transporter isoform glut-1 and hexokinase I in rat renal oncocytic tubules and oncocytomas. Virchows Arch 1994; 452: 63–68. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara Y, Yamamoto K, Kogure K, et al. Steady-state transcript levels of the type II hexokinase and type 1 glucose transporter in human tumor cell lines. Cancer Lett 1994; 82: 27–32. [DOI] [PubMed] [Google Scholar]
- 14.Rempel A, Mathupala SP, Griffin CA, et al. Glucose metabolism in cancer cells: amplification of the gene encoding type II hexokinase. Cancer Res 1996; 56: 2468–2471. [PubMed] [Google Scholar]
- 15.Weber G. Enzymology of cancer cells. N Engl J Med 1977; 296: 541–551. [DOI] [PubMed] [Google Scholar]
- 16.Moon DH, Maddahi J, Silverman DH et al. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med 1998; 39: 431–435. [PubMed] [Google Scholar]
- 17.Noh DY, Yun IJ, Kim JS, et al. Diagnostic value of positron emission tomography for detecting breast cancer. World J Surg 1998; 22: 223–227. [DOI] [PubMed] [Google Scholar]
- 18.Holder WD, White RL, Zuger JH, et al. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg 1998; 227: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kole AC, Nieweg OE, Pruim J, et al. Detection of unknown occult primary tumors using positron emission tomography. Cancer 1998; 82: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan FL, Dehdashti F, Ogunbiyi OA, et al. Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg 1998; 227: 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Nabi H, Doerr RJ, Lamonica DM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology 1998; 206: 755–760. [DOI] [PubMed] [Google Scholar]
- 22.Vitola JV, Delbeke D, Sandler MP, et al. Positron emission tomography to stage suspected metastatic colorectal carcinoma to the liver. Am J Surg 1996; 171: 21–26. [DOI] [PubMed] [Google Scholar]
- 23.Delbeke D, Vitola JV, Sandler MP, et al. Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med 1997; 38: 1196–1201. [PubMed] [Google Scholar]
- 24.Delbeke D, Martin WH, Sandler MP, et al. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg 1998; 133: 510–516. [DOI] [PubMed] [Google Scholar]
- 25.Bares R, Klever P, Haupmann S, et al. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology 1994; 192: 79–86. [DOI] [PubMed] [Google Scholar]
- 26.Stollfuss JC, Glatting G, Friess H, et al. 2-(fluorine-18)-fluoro-2-deoxy-D-glucose PET in detection of pancreatic cancer: value of quantitative image interpretation. Radiology 1995; 195: 339–344. [DOI] [PubMed] [Google Scholar]
- 27.Friess H, Langhans J, Ebert M, et al. Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut 1995; 36: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Fukatsu H, Ito K, et al. Fluorodeoxyglucose positron emission tomography in pancreatic cancer: an unsolved problem. Eur J Nucl Med 1995; 22: 32–39. [DOI] [PubMed] [Google Scholar]
- 29.Inokuma T, Tamaki N, Torizuka T, et al. Evaluation of pancreatic tumors with positron emission tomography and F-18 fluorodeoxyglucose: comparison with CT and US. Radiology 1995; 195: 345–352. [DOI] [PubMed] [Google Scholar]
- 30.Ho C-L, Dehdashti F, Griffeth LK, et al. FDG-PET evaluation of indeterminate pancreatic masses. J Comput Assist Tomogr 1996; 20: 363–369. [DOI] [PubMed] [Google Scholar]
- 31.Zimny M, Bares R, Fass J, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med 1997; 24: 678–682. [DOI] [PubMed] [Google Scholar]
- 32.Imdahl A, Nitzschel E, Krautmann F, et al. Evaluation of the FDG-PET for the differentiation of chronic pancreatitis and pancreatic cancer: a benefit for the surgeon? Gastroenterology 1998; 114: G1911. [Google Scholar]
- 33.Clark L, Perez-Tamayo RA, Hurwitz H, et al. The role of positron emission tomography in the diagnosis and staging of pancreatic cancer. Gastroenterology 1998; 114: S0044. [Google Scholar]
- 34.Nakata B, Chung YS, Nishimura S, et al. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer 1997; 79: 695–699. [PubMed] [Google Scholar]
- 35.Leach SD, Lee JE, Charnsanavej C, et al. Patient survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg 1998; 85: 611–617. [DOI] [PubMed] [Google Scholar]
- 36.Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology 1992; 183: 643–647. [DOI] [PubMed] [Google Scholar]
- 37.Bares R, Klever P, Hellwig D, et al. Pancreatic cancer detected by positron emission tomography with 18F-labelled deoxyglucose: method and first results. Nucl Med Commun 1993; 14: 596–601. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm P, Minn H, Leskinen-Kallio S, et al. Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med 1993; 34: 1–6. [PubMed] [Google Scholar]
- 39.Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo : high accumulation in macrophages and granulocytes studied by microautoradiography. J Nucl Med 1992; 33: 1972–1980. [PubMed] [Google Scholar]
- 40.Tahara T, Ichita Y, Kuwabara T, et al. High [18F]-fluorodeoxyglucose uptake in abdominal abscesses: a PET study. J Comput Assist Tomogr 1989; 13: 829–831. [DOI] [PubMed] [Google Scholar]
- 41.Guhlmann A, Brecht-Krauss D, Suger G, et al. Chronic osteomyelitis: detection with FDG-PET and correlation with histopathological findings. Radiology 1998; 206: 749–754. [DOI] [PubMed] [Google Scholar]
- 42.Niederhuber JE. Future of positron-emission tomography in oncology. Ann Surg 1998; 227: 324–325. [DOI] [PMC free article] [PubMed] [Google Scholar]