Abstract
Objective
To determine the impact of clinical presentation variables on the management and survival of patients with gastrointestinal (GI) tract carcinoid tumors.
Methods
A 20-year (1975–1995) retrospective analysis of 150 patients with GI tract carcinoid tumors at the Massachusetts General Hospital was conducted. Median follow-up was 66 months (range 1–378). Survival estimates for prognostic factors were calculated using Kaplan-Meier product limit estimators, with death from carcinoid as the outcome. Univariate analyses for each factor were obtained using a log-rank test, and multivariate survival analysis was performed.
Results
All but two patients underwent surgical intervention with the intent to cure (90%) or debulk the tumor (9%). Mean age at presentation was 55 ± 18 years (range 11–90). There was a slight female/male predominance (80:70). Symptoms were nonspecific; the most common were abdominal pain (40%), nausea and vomiting (29%), weight loss (19%), and GI blood loss (15%). Incidental carcinoids, discovered at the time of another procedure, occurred in 40% of patients and were noted at multiple sites throughout the GI tract. The distribution of tumors was ileojejunum (37%), appendix (31%), colon (13%), rectum (12%), stomach (4%), duodenum (1.3%), and Meckel’s diverticulum (1.3%). Of the 27 patients with documented liver metastases, carcinoid syndrome developed in only 13 patients (48%), manifested by watery diarrhea (100%), upper body flushing (70%), asthma (38%), and tricuspid regurgitation (23%). All 13 patients with carcinoid syndrome had elevated levels of 5-HIAA, but the absolute levels did not correlate with the severity of symptoms. An additional 11 patients, 3 without liver metastases, had elevated levels of 5-HIAA without any evidence of carcinoid syndrome. Multicentric carcinoid tumors occurred in 15 patients (10%), and all but one of these tumors were centered around the ileocecal valve. There was no difference in the incidence of liver metastases between solitary (18%) and multicentric carcinoids (20%). Synchronous noncarcinoid tumors were present in 33 patients (22%), and metachronous tumors developed in an additional 14 patients (10%) in follow-up. Age and tumor size, depth, and location were significant predictors of metastases. By multivariate analysis, age ≥50 years, metastases, and male gender were statistically significant predictors of death.
Conclusions
Gastrointestinal tract carcinoid tumors have a nonspecific clinical presentation, except in the case of the carcinoid syndrome. Surgical resection is the treatment of choice for improving survival. Surgically treated patients with carcinoid tumor have an overall favorable 83% 5-year survival rate.
Carcinoid tumors are neuroendocrine tumors and, as such, are part of the APUD (amine precursor uptake and decarboxylation) system. Oeberndorfer 1 was the first to use the term carcinoid to denote a less-aggressive behavior in carcinomalike tumors. In 1914, Gosset and Masson 2 demonstrated that the cells of these tumors contained silver-salt reducing (argentaffin) granules; thus, the concept of argentaffinomas arising from the Kultchitsky cells of the crypts of Lieberkuhn was established. In 1963, Williams and Sandler 3 classified carcinoid tumors according to their embryonic site of origin into foregut (respiratory tract, stomach, proximal duodenum, biliary system, and pancreas), midgut (distal duodenum, ileojejunum, proximal colon), and hindgut (distal colon and rectum). Numerous studies documenting the clinical and pathologic characteristics of these tumors and their management have been conducted in a retrospective manner. Limited by the small numbers of cases available at a single institution for analysis, the results between institutions have often been variable and somewhat conflicting. However, large epidemiologic studies based on statistical data from tumor registry surveys have yielded information on patient demographics, incidence, and survival but lack the clinical correlation. 4,5 To address both these issues, we present our experience in a large series of patients with carcinoid tumors at the Massachusetts General Hospital over a recent 20-year period.
METHODS
We reviewed the medical records of 150 patients identified through the tumor registry and admitted to Massachusetts General Hospital between 1975 and 1995 who were diagnosed with carcinoid tumors of the GI tract. Clinical variables for analysis included age, gender, presenting signs and symptoms, mode of presentation (incidental vs. nonincidental), and diagnostic workup. Overall median follow-up was 66 months (range 1 to 378 months). Particular attention was given to the development of the carcinoid syndrome, with emphasis on the temporal relation to the initial diagnosis, size, and multicentricity (presence of multiple carcinoid tumors). Synchronicity (presence of a concurrent noncarcinoid neoplasm) and metachronicity (occurrence of a different neoplasm at a later time) were also evaluated. Depth of bowel wall invasion was defined as follows: 6 T1, tumor invading the submucosa; T2, tumor invading the muscularis propria; T3, tumor invading through the muscularis propria into the subserosa; and T4, tumor perforating the visceral peritoneum or directly invading other organs or structures.
Statistical Analysis
Survival estimates for each prognostic factor, namely age, gender, site (except for stomach and duodenum, which were excluded because of small numbers), tumor size, tumor depth of penetration, and liver and lymph node metastasis, were calculated using the Kaplan-Meier product limit estimator, with death from carcinoid as the outcome. Patients who died from other causes or were alive at the most recent follow-up were treated as censored in this analysis. Categorical variables were compared using Fisher’s or Kruskal-Wallis exact tests (StatXact, Cambridge, MA). Univariate analyses of each factor were made using the log-rank test. A multivariate Cox model was fit using stepwise forward and backward procedures in SAS (SAS Institute, Cary, NC). Continuous outcomes are presented as the mean ± standard deviation.
RESULTS
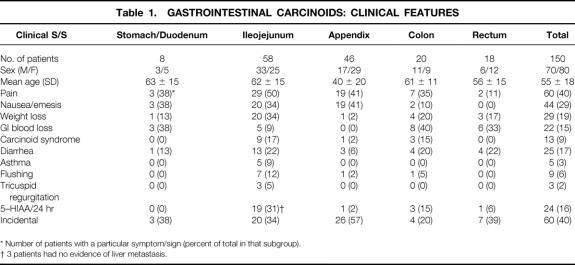
Table 1 shows the relation between tumor site and patient demographics with presenting symptoms. Ileojejunal (58 patients) and appendiceal (46 patients) were the most common sites of presentation. The mean age of the female patient (51 years) at presentation was less than that of the male patients (58 years). The presence of carcinoid syndrome was the only clinical characteristic that was diagnostic of a carcinoid tumor. Otherwise, the signs and symptoms attributable to carcinoid tumors were vague and varied according to the anatomic subsite. Abdominal pain was the most common presenting complaint and occurred in 60 patients (40%). The nature of the pain was variable: ileojejunal tumors caused intermittent colic associated with small bowel obstruction from a primary tumor size effect (14 patients), bowel involvement in dense fibrotic reaction (10 patients), and peritoneal carcinomatosis (2 patients). Pain presenting as acute appendicitis was seen in 19 patients. Three patients with gastric carcinoids had pain mimicking that of peptic ulcer disease.
Table 1. Gastrointestinal Carcinoids: Clinical Features
Nausea and vomiting were prominent clinical features in 44 patients (29%) and were often associated with pain and abdominal distention indicating a bowel obstruction. Twenty-nine patients (19%) had documented weight loss. This was most commonly secondary to metastatic liver disease or a locally extensive tumor burden. GI blood loss occurred in 22 patients (15%) and ranged in severity from occult blood loss with or without anemia to frank upper or lower GI bleeding. GI blood loss was an uncommon clinical feature for small bowel carcinoid (9%) but was the main reason for investigation of colonic (40%), rectal (33%), and gastric (38%) carcinoids.
Sixty patients (40%) had their carcinoid tumors discovered incidentally, most commonly during a gynecologic or urologic procedure. An incidental carcinoid tumor is defined as one where no clinical manifestation could have been attributed to the tumor, or one discovered incidentally that contributed minimally to the clinical picture, but was not the reason for exploration. This is distinct from the usual situation, where the clinical presentation was clearly the result of the carcinoid tumor and was the rationale for surgical intervention. Incidental discovery was most common with the appendix subsite (57%). The biologic behavior of these incidental carcinoids was clearly less aggressive: only 2 patients (3.3%) had liver metastases and 12 patients (20%) had lymph node metastases. There were significantly fewer metastases among the patients with incidental carcinoids (21%) versus the patients with nonincidental carcinoids (53%, p = 0.002).
In 13 patients (9% of total, 48% of those with liver metastasis), the carcinoid syndrome developed. Carcinoid syndrome was present at initial diagnosis in 10 patients. In the other three patients (two ileal and one appendiceal), the carcinoid syndrome developed 36, 144, and 192 months, respectively, after initial diagnosis and treatment of the primary tumor. There was no significant predilection for either sex (seven male patients, six female patients). Ileum was the most common site, with nine patients (69% of the total and 17% of all ileal cases). We encountered carcinoid syndrome with only one case of each of the following sites: appendix, sigmoid, ascending colon, and ileocecal valve. None of the stomach or rectal site tumors developed carcinoid syndrome, regardless of the metastatic status of the liver. The frequency of the specific carcinoid syndrome-associated symptoms was diarrhea (100%), facial flushing (70%), asthma and bronchospasm (38%), and tricuspid valve regurgitation (23%). All patients with documented carcinoid syndrome had elevated levels of the serotonin degradation product 5-hydroxyindoleacetic acid (5-HIAA) (p < 0.0001), but an additional 11 patients without the carcinoid syndrome also had elevated 5-HIAA levels.
Of interest, there were three patients with increased urinary levels of 5-HIAA without evidence of liver metastasis or of the carcinoid syndrome, either at diagnosis or follow-up. One patient with an ileal carcinoid had lost 20 pounds and had diarrhea, steatorrhea, malabsorption, and chylous ascites. This patient died from disease, but without liver metastases, 5 months after diagnosis. The second case was a 46-year-old white man with an ileal carcinoid discovered incidentally during cholecystectomy. He underwent a right hemicolectomy and was found to have extensive regional lymph node disease. He was lost to follow-up after 51 months but had no evidence of liver metastases at that time. The third case was a patient with an ileal carcinoid discovered incidentally during a vascular procedure. This patient also had extensive regional lymph node metastases. No evidence of liver metastases was seen at 166 months of follow-up. These three patients did not have invasion into the retroperitoneum.
Fifteen patients (10%) had multicentric carcinoid tumors. In all but one of these patients, the tumors were centered around the ileocecal valve. Within this subgroup, liver metastasis developed in three patients (20%), which was not statistically different from the 18% rate with solitary carcinoids. Thirty-three patients (22%) had synchronous noncarcinoid tumors, and metachronous tumors developed in 14 patients (10%) (Table 2). The most common carcinoid sites in our series with associated tumors were ileum (19 patients), appendix (12 patients), and colon (10 patients). The metachronous tumors usually developed within 4 years of the original carcinoid tumor and represented a diffuse array of histologic types. The most common associated tumors (benign or malignant) were adenomatous colonic polyps (26%), GI carcinomas (21%), urinary tract malignancy (19%), and female reproductive organ malignancies (17%).
Table 2. Gastrointestinal Carcinoids: Second Neoplasms
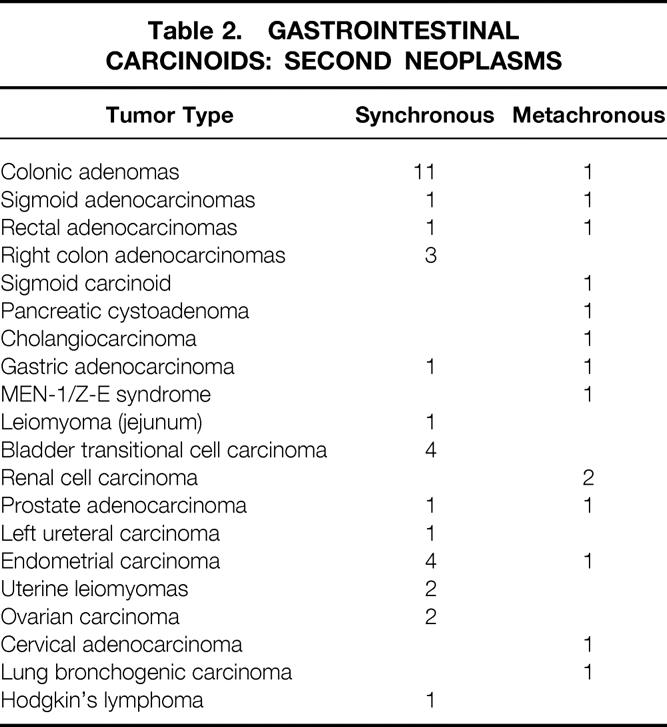
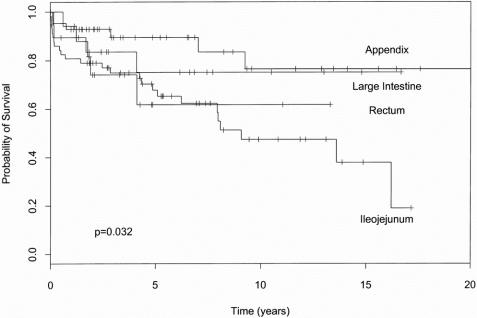
Table 3 presents the relation between tumor site, size, depth of penetration, gender, and age to metastatic disease and survival. One can see from these data that increasing size correlated with metastatic disease (either liver or lymph node, p < 0.001). The size of the primary tumor clearly had an impact on aggressive behavior: when the primary tumor was ≤1 cm, no liver metastases were observed and the rate of lymph node metastases was only 10%. By contrast, primary tumors >2 cm had a 28% rate of liver metastases and a 72% rate of lymph node metastases. The effect of size on survival trended toward significance (p = 0.1) but was more closely related to metastatic status. The site of the primary tumor also influenced the metastatic potential of the tumor. For tumors in the size range 1.1 to 2.0 cm, 13% developed liver metastases, and all were of the ileojejunal type. Similarly, of the 30 patients with tumors in this size range, 17 had lymph node metastases, and 15 of these patients had ileojejunal carcinoids. Nine of the 13 patients with carcinoid syndrome had ileal tumors. Because of this more aggressive behavior with more frequent metastases, the ileojejunal carcinoids have the worst prognosis of any subsite (Fig. 1). Patients with rectal carcinoids have an early drop in survival and do worse than those with ileojejunal carcinoid in the first 4 years, but ultimately the tumors stabilize and behave similarly to the nonileojejunal sites.
Table 3. Metastatic and Survival Prognostic Variables
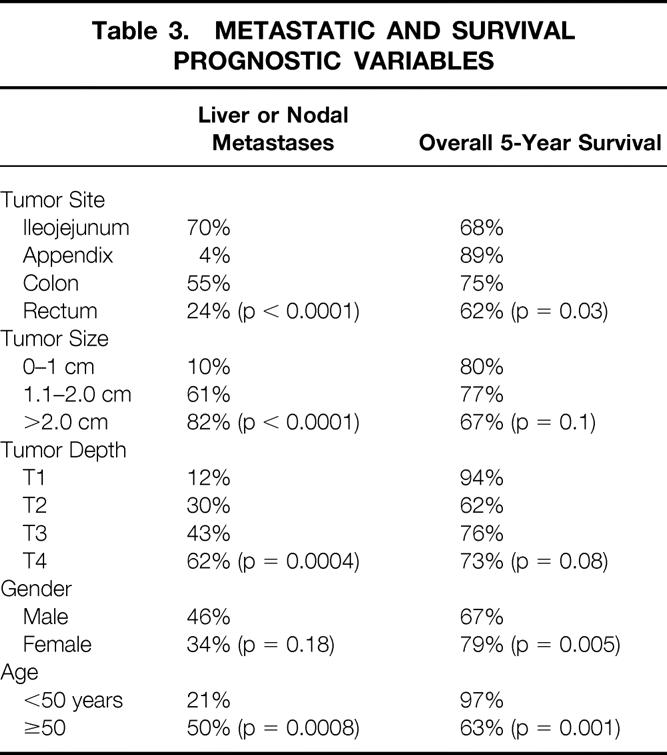
Figure 1. The effect of tumor site on cumulative survival.
Tumor depth had a linear correlation with metastatic disease (p = 0.0004, Kruskal-Wallis test). The differences were most pronounced when comparing T1 (where there were no liver metastases in 18 cases) to T2, T3 (9% rate of liver metastases) and T4 (25% rate of liver metastasis). There was no noticeable difference between T2 and T3. Similar to the effect seen with size and survival, the impact of tumor depth on survival trended toward significance (p = 0.08) but was more closely related to metastatic status.
Both age and gender significantly predicted survival (see Table 3). Female patients had less metastatic disease and significantly greater survival. Patients younger than 50 years likewise had significantly fewer metastases, and there were almost no deaths in this group.
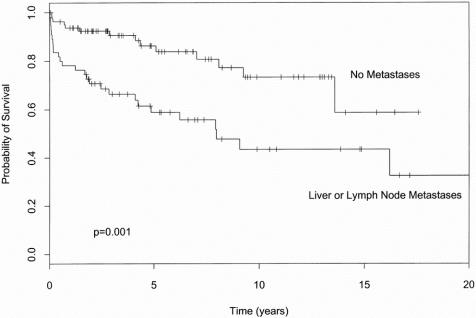
The presence of metastatic disease significantly decreased survival, as depicted in Figure 2. There were 31 patients with lymphatic nodal metastases, 20 patients with additional liver metastases either at presentation or subsequent follow-up, and 6 patients with liver metastases but no lymph node metastases. The effect of either lymphatic or liver metastases on survival was highly correlated, and thus they were combined for statistical analysis in Figure 2 and Table 3. Even patients with metastatic carcinoid tumors could have prolonged survival because of the often indolent nature of this tumor. The overall median survival of patients with metastatic disease was still 8 years. We have one patient who has survived 31.5 years with metastatic liver carcinoid; to the best of our knowledge this patient is the longest reported survivor of this type.
FIGURE 2. The effect of metastatic disease on cumulative survival.
Seventeen patients who died from other causes were censored from this survival analysis. The overall 1-year survival rate was 88%, and the overall 5-year survival rate was 83%. A multivariate survival model including all variables selected age 50 years or older (p = 0.005, risk ratio = 7.8), liver or lymph node metastases (p = 0.027, risk ratio = 2.1), and male gender (p = 0.064, risk ratio = 1.8) as statistically significant independent predictors of death.
DISCUSSION
The current study highlights the paradox encountered in treating GI tract carcinoid tumors. Patients with metastatic disease may live for years with indolent disease, but this same indolent nature makes these tumors difficult to diagnose at an early stage, when they are more likely to be cured by surgical resection. Unless a patient displays the carcinoid syndrome, which is generally indicative of metastatic disease, there is no clinical feature that leads the clinician to suspect a carcinoid tumor specifically. In particular, carcinoid tumors of the small bowel—and especially the ileum—have been difficult to diagnose. 7 The typical misdiagnoses associated with carcinoid tumor of the ileum include Crohn’s disease, adenocarcinoma of the cecum and ascending colon, and adhesive small bowel obstruction. Prominent features of the small bowel carcinoids were pain, nausea, and vomiting characteristic of bowel obstruction. Obstruction of the intestine generally resulted from the size of the primary tumor or the involvement of a segment of bowel in a dense fibrotic reaction (desmoplastic reaction), causing shortening of the mesentery with resulting bowel kinking, angulation, and obstruction. This radiologic appearance was often misinterpreted as Crohn’s disease. This desmoplastic reaction occurred in 10 of our patients (6%) and had no specific effect on outcome. Carcinoids are also capable of producing mesenteric angiopathy and vascular fibroelastosis, which can lead to bowel ischemia. The alleged mechanism for that is the elaboration of various secretagogues. 8–11
During an investigation for GI blood loss, the carcinoid was frequently not the cause of the bleeding but was rather an incidental finding. This is in contrast to GI adenocarcinomas, where blood loss directly from the tumor is characteristic. When the bleeding is caused by the carcinoid tumor, it is often through atypical mechanisms; mucosal ulceration from this submucosal tumor is the most common. Another cause was severe carcinoid-induced retroperitoneal fibrosis with evident gross venous engorgement leading to GI bleeding. A third method was ischemic bowel with resultant bleeding secondary to encroachment of mesentery by a dense fibrosis and arterial occlusion.
As a result of this vagueness of presentation, 40% of the tumors in our series, and 5% to 82.5% of tumors in other series, were found incidentally during the treatment or workup of other conditions. 12–18 The most important point about the incidental carcinoid tumors is that lymph node and liver metastases were distinctly less common than in patients with symptomatic carcinoids. This is similar to the series by Thompson et al, 15 who reported a metastatic rate of 4.3% in incidental disease. The incidentally resected carcinoids were detected at an earlier stage than in the symptomatic patients. This supports the concept that surgical resection of early-stage carcinoid tumors is an excellent means of obtaining control of disease.
In contrast to patients with incidental carcinoid tumors who are asymptomatic and are generally cured by surgical resection, patients with the carcinoid syndrome are often distressingly symptomatic and rarely cured of their disease. The classical carcinoid syndrome involves intermittent secretory diarrhea and flushing of the face and the upper part of the chest. Less common symptoms include asthma secondary to bronchospasm and tricuspid and pulmonary valvular heart disease from a peculiar fibrosis of the valve leaflets with resulting incompetence and/or stenosis leading to right-sided heart failure. 19 This complex is produced through the elaboration of several vasoactive substances, including serotonin, histamine, and 5-hydoxytrypyophan, and probably others, depending on the site of origin. 20,21 For the carcinoid syndrome to be manifest, the vasoactive secretory substance(s) must have direct access to the systemic circulation, escaping hepatic degradation. Liver metastases generally precede the rise in 5-HIAA, but this is not universally true, as evidenced by three patients in our series. Tumors may directly drain into the paravertebral (systemic) veins either by direct invasion into the retroperitoneum or some other anomalous venous route in the absence of liver metastasis. Non-GI tract carcinoids (bronchial and gonadal) can also produce the carcinoid syndrome by direct systemic drainage. 22 As was seen in our series, small intestinal carcinoids are the most common cause of the carcinoid syndrome. 22,23
The incidence of carcinoid syndrome is said to be 10%. 21 In our series, 9% of the patients developed the carcinoid syndrome. Diarrhea was the most common symptom, followed by flushing. Although two types of flushing have been described, 20,21 we encountered only the type that occurs with midgut carcinoids. The patients have transient attacks of a faint-pink discoloration of the face and upper trunk, usually provoked by drinking alcohol or eating or drinking tyramine-containing foods (e.g., blue cheese, chocolate, red wines). Asthma and tricuspid regurgitation were relatively uncommon. All patients with the carcinoid syndrome had elevated levels of 5-HIAA, but nearly half of the patients with elevated 5-HIAA levels did not have the carcinoid syndrome. Likewise, the level of 5-HIAA did not correlate well with the severity of symptoms or the extent of liver metastases. This supports the concept that serotonin is not the only mediator of carcinoid syndrome symptoms.
Carcinoid tumors have a propensity for multicentricity; this was first described by Lubarsch. 24 Our incidence rates by site of origin are similar to those of Saha et al, 25 who reported a 10% multicentricity rate, with 82% of the ileojejunal type. Surprisingly, we observed no difference in the rate of liver metastasis between solitary (18%) and multicentric carcinoids (20%). Thus, multicentricity did not appear to carry an adverse prognosis.
Forty-seven patients (31%) were diagnosed with a noncarcinoid tumor during their clinical course, either synchronous (22%) or metachronous (9%). This is higher than the 13% rate of second neoplasms found in a recent SEER study but mirrored a similar distribution of carcinoid sites. 5 Although the association of carcinoid with other neoplasms is well established, the cause is unclear. The fact that carcinoid tumors can be multicentric and are often associated with a second primary indicates that any patient found to have a carcinoid tumor needs a thorough exploration and evaluation for other tumors, both carcinoid and noncarcinoid. They should be followed on a long-term basis both for delayed liver metastases and for a metachronous second tumor.
Whether a carcinoid tumor will follow an aggressive or benign course cannot be determined by histopathologic examination. For this reason, other parameters have been investigated to predict survival and to direct treatment strategies. These parameters include size of the primary tumor, site of origin, depth of bowel wall invasion, and presence of lymph node and liver metastasis. As demonstrated in our study and others, 8,14,15,26 size has been the standard for predicting metastatic potential. However, we did not find that size was the best predictor of overall survival: depth of bowel wall penetration, site of tumor, and especially age, gender, and metastatic status were significantly more accurate in determining outcome.
Depth of bowel wall invasion is another parameter that can be used to determine the appropriate extent of surgical resection. In the case of small gastric or rectal carcinoids resected endoscopically, depth of bowel wall invasion can be an added variable in determining whether an endoscopic excision is sufficient or a segmental excision and mesenteric lymphadenectomy is needed. T1 lesions <1 cm can be adequately resected through the endoscope if negative margins are obtained. However, the majority of these tumors are best treated with a more extensive surgical resection, including the draining mesenteric lymph nodes.
Tumors arising in the small bowel have a more aggressive behavior and a greater risk of ultimate death from tumor than any other site. This information should be used along with the other prognostic factors to determine the appropriate surgical treatment. Generally speaking, this implies that these patients should undergo a wide mesenteric resection, both to ascertain the nodal status and to reduce the risk of subsequent mesenteric desmoplastic reaction and bowel obstruction. Similarly, factoring in a patient’s age and gender adds to the ability to predict the risk of metastatic disease and risk of death. This can likewise be used to determine the appropriate extent of surgical resection and subsequent follow-up.
Although carcinoids are the second most common neoplasm of small bowel and the most common neoplasm of the appendix, they are still rare, occurring at a rate of 2.5/100,000 people. Carcinoid tumors are difficult to diagnose before surgery, and the standard armamentarium of tests often cannot make the diagnosis. Because of this, surgeons must be familiar with the clinical behavior of carcinoids and perform the appropriate surgical procedure. The surgeon needs to understand that many factors go into determining the patient’s subsequent risk of metastatic disease and outcome. The goal is to resect the primary tumor with negative margins and at the same time to determine and potentially treat metastatic disease, with the goal of improving outcome and/or quality of life.
The surgical procedure itself should be appropriate for the disease-specific risk. For example, standard recommendations have been that appendiceal carcinoids <1 cm are adequately treated with simple appendectomy because of low metastatic potential and excellent survival. Patients with appendiceal carcinoids >2 cm should undergo a formal right hemicolectomy because of greater metastatic potential. However, the treatment of carcinoids 1 to 2 cm has been the subject of debate. The additional prognostic factors (age, gender, and depth of penetration) we have identified can be used in choosing between appendectomy and hemicolectomy.
When a carcinoid tumor is diagnosed by frozen section, the surgeon already knows many of the factors that will determine metastatic risk and outcome: gender, age, and tumor size and location. The depth of penetration is not specifically known, but whether it has reached the serosa is usually evident on gross inspection. Essentially, the risk of metastasis can be determined with reasonable accuracy. Patients with metastases are at risk of dying from their disease and/or developing the carcinoid syndrome, with its associated problems. Liver metastases are generally multicentric and not amenable to a curative hepatic resection—only one patient with hepatic involvement in our series was considered a candidate for a curative surgical resection. However, even with metastatic disease and the carcinoid syndrome, patients can still enjoy longevity. Octreotide has been efficacious in controlling symptoms, and the addition of hepatic chemoembolization has been able to induce tumor regression. 27,28 Fortunately, surgically treated patients with carcinoid tumors have an overall favorable 83% 5-year survival rate. Because the diagnosis of carcinoid tumor is often an intraoperative finding, the surgeon must be able to assess patient risk factors and perform an appropriate surgical resection.
Discussion
Dr. John S. Bolton (New Orleans, Louisiana): It’s hard to get one’s arms around this problem of carcinoid tumors of the GI tract because of the wide spectrum of clinical behavior among patients with this disease, varying from the incidentally discovered 1-cm appendiceal carcinoid to the patient with advanced liver metastases and the carcinoid syndrome.
Dr. Ott and colleagues have given us a very nice study confirming several previously identified prognostic factors, such as size, site, depth of penetration, and presence of metastatic disease. This study also confirms once again that even patients with metastatic disease may survive a long time—a median of 8 years in this study, despite the metastatic disease.
This study also identifies a new prognostic factor—I am not aware of it having been presented before—and that is the patient’s age. And I thought it was interesting that this came through so strongly as a prognostic factor in a multivariate analysis.
I have three questions for Dr. Ott.
One, was the increased mortality in the older-than-50-years age group disease-specific in every case? I assume it probably was since they did have a higher rate of metastatic disease. But in some cases, was it related to noncarcinoid causes such as the increased incidence of other neoplasms, competing medical illnesses? Finally, were elderly patients more susceptible to carcinoid heart disease perhaps, and that perhaps explaining the high mortality?
Two, for a patient with a jejunal-ileal carcinoid, completely resected, how do you recommend following the patient from that point on, or do you recommend any specific follow-up for the patient?
Number three, a patient with the carcinoid syndrome and an intact primary, what is your management algorithm? We struggle with this; it’s an occasional problem, but when it does present, we have several things in our armamentarium, including surgical debulking, chemoembolization, and octreotide-based therapy. And I’d like to know your thoughts about the management algorithm for a patient who still has an intact primary and also for the patient whose primary has been previously removed.
Dr. Charles J. Yeo (Baltimore, Maryland): Drs. Souba and Ott and their colleagues have applied the TNM staging criteria to predict metastatic potential, and they have found that age, gender, tumor size, tumor depth, and location of primary tumors are all important predictors.
I would say the caveat that Dr. Bolton pointed out, that is, disease-specific survival, will be important in looking at the age issue. I have four questions.
In the manuscript, you talked about the really fascinating desmoplastic reaction, that is, the sclerosing mesenteritis that you sometimes see in these patients. Does the presence of this phenomenon in any way correlate with the metastatic potential with the aggressive disease or with mortality?
Number two, in the 13 patients with the carcinoid syndrome, you noted that all had elevated levels of 5-HIAA in the urine. Did you look at any other mediators—serotonin, bradykinin, histamine—in the serum to see if they correlated with severity of syndrome, et cetera?
Number three, you have nicely demonstrated the very often indolent nature of these tumors, as you have a very long 5-year survival, and, in fact, in the manuscript talks about the patient who survived 30 years. Is there a role for debulking of hepatic metastases, as Dr. Bolton went over, or do you selectively apply hepatochemoembolization therapy preferentially?
And, lastly, in the survival curves which are very nicely presented and drawn out for us, you have lumped together lymph node and hepatic metastases in one group and showed that that combination of adverse prognostic features has a bad survival. What happens when you separate out simply nodal disease from nodal plus hepatic metastases? Are hepatic metastases more ominous?
Dr. Bernard M. Jaffe (New Orleans, Louisiana): This paper provides evidence for one of the pieces of information that I think has been most seriously misdiscussed in the surgical literature. Each of us were taught, and medical students continue to be taught, that carcinoids of less than 2 cm have a very small likelihood of metastasis. I have been bothered by that statement for years and have really gone out of my way to try to point out that there is no direct step less than 1 cm less than 20%, more than 2 cm 80 or 90%. There, obviously, is an important gradation. And I think the literature continues to be inappropriate in not recognizing the fact that between 1 and 2 cm there is a relatively high index of metastatic disease.
The 60% that is described in this paper, I think, is appropriate, but I think it needs to be highlighted in further studies and needs to be picked up in textbooks because our students and young residents are being taught misinformation. I think this is an important point.
On the other hand, I am slightly disappointed with the fact that the endpoint was used as a 5-year survival. There is an enormous amount of interest now, particularly in Scandinavia and to a lesser degree in this country, about the appropriate treatment for metastatic disease in patients with carcinoid. There is, obviously, even a huge French study now involved in looking at liver transplantation for patients who have metastases to the liver. So the concept of survival becomes even more important now in a day when such new modalities as chemoembolization have really become very important and are used more frequently.
A 5-year survival is obviously not the answer. If 83% of the patients survive 5 years, that is not the critical point. The paper obviously recognizes the fact that there are patients who survive a long time. I myself have taken care of patients who had liver metastases who lived for more than 30 years symbiotically with their tumor. So we really do need appropriate statistics for survival. I don’t know if it is 10 years or 15 years, and I wonder if the authors can extrapolate from their data to a longer survival period so we can really begin to make sense of whether or not our modalities are improving prognosis.
We all think that if we do debulking or resection of liver metastases or chemoembolization, we are prolonging survival. The data to document that that is the case are nonexistent. Perhaps this information that we have heard presented today could be extrapolated to provide the information we need to be able to talk ourselves into or out of aggressive therapy for nodal metastases or liver metastases in carcinoid disease.
President Griffen: Dr. Copeland would like to ask a question.
Dr. Edward M. Copeland, III (Gainesville, Florida): Doctor, let me ask you a specific question. One of your favorite friends has done an incidental appendectomy on a 25-year-old male who has a 1.5-cm carcinoid that invades into the muscular wall of the appendix. He wants to know whether or not to send the patient to you for a right colectomy. Maybe you might answer that question for me.
Dr. Mark J. Ott (Closing Discussion): Specifically, Dr. Bolton’s comments about the increased mortality, was it in the greater than 50-year age group, is that disease-specific or due to other causes related to other diseases and other tumors. It is a disease-specific survival, with the caveat that yes, these patients, when they get a bowel obstruction from a carcinoid tumor are more likely to have an adverse complication affecting their prognosis, but that was secondary to the carcinoid tumor.
Yes, if they do get tricuspid disease and have that at the time of presentation, they are more likely to suffer other cardiac anomalies and have death secondary to that, as a secondary manifestation of their disease.
In terms of the management of the syndrome with an intact primary or a patient who has the primary removed, I think that there are several options for managing the carcinoid syndrome. Certainly, I think that most people would feel that the appropriate treatment for an intact primary is to remove the primary. These patients, as you saw, particularly the ones with small-bowel primaries, do have a very high incidence of bowel obstruction. And even if they are manifesting with syndrome at that time point without bowel obstruction, they are likely, given the longevity of the disease, to present with a bowel obstruction at some point. So I think that an intact primary in the bowel almost certainly should be resected.
If the primary has been resected and all they have is metastatic disease as the basis of their carcinoid syndrome, there are several options for managing that. Perhaps the easiest, which will usually work for the first 1 to 2 years in terms of treating the diarrhea symptoms, which are by far the ones that bother the patients the most, can be simply the combination of cholestyramine and Metamucil, which will manage most of those patients for the first year or two. After that period, that control generally lapses with that management.
If the patient then goes beyond that, certainly octreotide therapy has been very efficacious in controlling the carcinoid syndrome symptoms. However, many of these patients then go on to develop progressive replacement of hepatic parenchyma with hepatomegaly, which becomes symptomatic and painful. And assuming that these patients were not resectable initially, the management of that has generally devolved to the chemoembolization. And that, in both the original study in Surgery ’94 as well as more recent data from M. D. Anderson, has shown a very good 70 to 80% control of symptomatic pain in particular that these patients have by chemoembolization.
Certainly, in answer to that question as well as to Dr. Yeo’s question, I think that possible surgical debulking is preferable for these patients. There has been no study that has shown that the palliative treatment of liver metastases has increased survival. But certainly, the quality of life for these patients is substantially better.
In terms of Dr. Yeo’s question about the desmoplastic reaction, does the patient with that have an increased propensity for metastases or mortality, these desmoplastic reactions are almost exclusively related to the small-bowel carcinoid tumors. And they constitute about 20% of the patients who have small-bowel carcinoids. It’s very unusual to see that desmoplastic reaction at the other sites.
These patients have, to start with, a worse prognosis based on their inherent behavior. And since they only constitute 20% of that, it was not possible in a multivariate analysis to cull out whether that had an adverse prognosis independent of the site, which was the overriding factor for those patients.
The survival curves—do the lymph node and liver metastases separate out? The data we presented grouped those two together, and we did that for several reasons. One, it is possible to die of carcinoid disease without metastatic disease. Secondly, it is possible to die of carcinoid tumors with just lymph node disease. And, third, it is possible to die with just isolated liver metastases from the carcinoid tumor.
If you separate those out—and we have done that—every patient who gets carcinoid tumors, of those who develop lymph node metastases, approximately a third go on to then develop hepatic metastases. And the survival curves for those two are different, with hepatic metastases having about a 20 to 30% greater mortality than the isolated lymph node metastases.
Dr. Jaffe’s point about the 5-year survival statistics not being appropriate for this disease, I agree entirely with that. We present the 5-year survival statistics purely as a point of reference for comparison to other articles in the literature. The Kaplan-Meier curves that you see drawn out extend out to 20 years. And I think that that makes that data much more meaningful. And certainly, each of those time points can be obtained from those curves. And certainly, there is a continual degradation in survival in a steady fashion that goes on beyond 5 years.
Finally, Dr. Copeland’s specific question about a 25-year-old who has an appendiceal carcinoid with a T2 lesion involving the muscularis that is 1.5 cm in size, what would be the appropriate management for that patient? I think that this patient has, on size criteria, a lesion that is of intermediate metastatic risk, and certainly, that is one thing that makes you uncomfortable. A T2 lesion behaves, in our analysis, essentially the same as a T3 lesion for any specific area. And so based on the fact that he has an intermediate size lesion, he has one that is also intermediate in terms of its risk for metastatic potential, and the fact that he is male, I think the best treatment for that patient certainly would be a completion hemicolectomy.
Footnotes
Correspondence: Mark J. Ott, MD, Department of Surgery, Division of Surgical Oncology, Massachusetts General Hospital, 626 Cox Building, 100 Blossom St., Boston, MA 02114.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Accepted for publication December 1998.
References
- 1.Oberndorfer S. Uber die “Kleinen Dunndarmcarcinome.” Verhandl Deutsch Pathol 1907; 11: 113–116. [Google Scholar]
- 2.Gosset A, Masson P. Tumerus endocrines de l’appendize. Presse Med 1914; 22: 237–240. [Google Scholar]
- 3.William ED, Sandler M. The classification of carcinoid tumors. Lancet 1963; 1: 238–239. [DOI] [PubMed] [Google Scholar]
- 4.Godwin JD. Carcinoid tumors. An analysis of 2,837 cases. Cancer 1975; 36 (2): 560–569. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997; 79 (4): 813–829. [DOI] [PubMed] [Google Scholar]
- 6.Hermanek P, Sobin LH. TNM classification of malignant tumors. Berlin: Springer; 1987. [DOI] [PubMed]
- 7.Loftus JP, Heerden JAV. Small bowel carcinoids. In Cameron JL, ed. Current surgical therapy. St. Louis: Mosby; 1995: 139–141.
- 8.Moertel CG, Sauer WG, Dockerty MB, Baggenstoss AH. Life history of the carcinoid tumor of small intestine. Cancer 1961; 14: 901–912. [DOI] [PubMed] [Google Scholar]
- 9.Eckhauser FE, Argenta LC, Strodel WE, et al. Mesenteric angiopathy, intestinal gangrene, and midgut carcinoids. Surgery 1981; 90 (4): 720–728. [PubMed] [Google Scholar]
- 10.Warner TF, O’Reilly G, Lee GA. Mesenteric occlusive lesion and ileal carcinoids. Cancer 1979; 44 (2): 758–762. [DOI] [PubMed] [Google Scholar]
- 11.Kowlessar OD, Law DH, Sleisenger MH. Malabsorption syndrome associated with metastatic carcinoid tumor. Am J Med 1959: 673–677. [DOI] [PubMed] [Google Scholar]
- 12.Roggo A, Wood WC, Ottinger LW. Carcinoid tumors of the appendix. Ann Surg 1993; 217 (4): 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald SD, Meagher AP, Moniz-Pereira P, et al. Carcinoid tumor of the rectum. DNA ploidy is not a prognostic factor. Dis Colon Rectum 1996; 39 (6): 643–648. [DOI] [PubMed] [Google Scholar]
- 14.Naunheim KS, Zeitels J, Kaplan EL, et al. Rectal carcinoid tumors—treatment and prognosis. Surgery 1983; 94 (4): 670–676. [PubMed] [Google Scholar]
- 15.Thompson GB, van Heerden JA, Martin JK Jr, et al. Carcinoid tumors of the gastrointestinal tract: presentation, management, and prognosis. Surgery 1985; 98 (6): 1054–1063. [PubMed] [Google Scholar]
- 16.Eller R, Frazee R, Roberts J. Gastrointestinal carcinoid tumors. Am Surgeon 1991; 57 (7): 434–437. [PubMed] [Google Scholar]
- 17.Zeitels J, Naunheim K, Kaplan EL, Straus FD. Carcinoid tumors: a 37-year experience. Arch Surg 1982; 117 (5): 732–737. [DOI] [PubMed] [Google Scholar]
- 18.MacGillivray DC, Synder DA, Drucker W, ReMine SG. Carcinoid tumors: the relationship between clinical presentation and the extent of disease. Surgery 1991; 110 (1): 68–72. [PubMed] [Google Scholar]
- 19.Thorson A, Biorck G, Bjorkman G, Waldenstorm J. Malignant carcinoid of the small intestine with metastasis to liver, valvular disease of right side of heart (pulmonary stenosis and tricuspid regurgitation without septal defect), peripheral vasomotor symptoms, brochoconstriction, and unusual type of cyanosis; clinical and pathologic syndrome. Am Heart J 1954; 48: 795–817. [DOI] [PubMed] [Google Scholar]
- 20.Pernow B, Waldenstorm J. Paroxysmal flushing and other symptoms caused by 5-hydroxytryptamine and histamine in patients with malignant tumors. Lancet 1954; 2: 951–956. [DOI] [PubMed] [Google Scholar]
- 21.Vinik AI, Thompson N, Eckhauser F, Moattari AR. Clinical features of carcinoid syndrome and the use of somatostatin analogue in its management. Acta Oncol 1989; 28 (3): 389–402. [DOI] [PubMed] [Google Scholar]
- 22.Moertel CG. Treatment of carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol 1983; 1: 727–740. [DOI] [PubMed] [Google Scholar]
- 23.Kvols LK, Reubi JC. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Acta Oncol 1993; 32 (2): 197–201. [DOI] [PubMed] [Google Scholar]
- 24.Lubarsch O. Uber den primren krebs des ileum nebst Bemerkungen òber das gleichzeitige vorkommen von krebs und tuberculse. Virchows Arch Path Anat 1888; 3: 281–317. [Google Scholar]
- 25.Saha S, Hoda S, Godfrey R, et al. Carcinoid tumors of the gastrointestinal tract: a 44-year experience. South Med J 1989; 82 (12): 1501–1505. [DOI] [PubMed] [Google Scholar]
- 26.Burke AP, Thomas RM, Elsayed AM, Sobin LH. Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic study of 167 cases. Cancer 1997; 79 (6): 1086–1093. [PubMed] [Google Scholar]
- 27.Perry LJ, Staurt K, Stokes KR, Clouse ME. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Surgery 1994; 116: 1111–1116. [PubMed] [Google Scholar]
- 28.Diamandidou E, Ajani JA, Yang DJ, et al. Two-phase study of hepatic artery vascular occlusion with microencapsulated cisplatin in patients with liver metastases from neuroendocrine tumors. Am J Roentgenol 1998; 170: 339–344. [DOI] [PubMed] [Google Scholar]