Abstract
Objective
To determine the outcome of orthotopic liver transplantation (OLT) for end-stage liver disease caused by hepatitis C virus (HCV).
Summary Background Data
HCV has become the leading cause of cirrhosis and hepatic failure leading to OLT. Recurrent HCV after OLT is associated with significant complications and may lead to graft loss that requires retransplantation (re-OLT). The authors studied the outcome of transplantation for HCV, the effect of primary immunotherapy, and causes of retransplantation.
Methods
The authors conducted a retrospective review of their experience during an 8-year period (1990–1997), during which 374 patients underwent transplants for HCV (298 [79.6%] received one OLT; 76 [20.4%] required re-OLT). Median follow-up was 2 years (range 0 to 8.3). Immunosuppression was based on cyclosporine in 190 patients and tacrolimus in 132 patients. In a third group of patients, therapy was switched from cyclosporine to tacrolimus or from tacrolimus to cyclosporine (cyclosporine/tacrolimus group).
Results
Overall, 1-, 2-, and 5-year actuarial patient survival rates were 86%, 82%, and 76%, respectively. The 2-year patient survival rate was 81% in the cyclosporine group, 85% in the tacrolimus group, and 82% in the cyclosporine/tacrolimus group. In patients receiving one OLT, overall 1-, 2-, and 5-year patient survival rates were 85%, 81%, and 75%, respectively. The 2-year patient survival rate was 79% in the cyclosporine group, 84% in the tacrolimus group, and 80% in the cyclosporine/tacrolimus group. The overall graft survival rates were 70%, 65%, and 60% at 1, 2, and 5 years, respectively. The graft survival rate at 2 years was similar under cyclosporine (68.5%), tacrolimus (64%), or cyclosporine/tacrolimus (60%) therapy., Re-OLT was required in 42 (11.2%) patients for graft dysfunction in the initial 30 days after OLT. Other causes for re-OLT included hepatic artery thrombosis in 10 (2.6%), chronic rejection in 8 (2.1%), and recurrent HCV in 13 (3.4%) patients. The overall survival rates after re-OLT were 63% and 58% at 1 and 2 years. The 1-year survival rate after re-OLT was 61% for graft dysfunction, 50% for chronic rejection, 60% for hepatic artery thrombosis, and 60% for recurrent HCV. At re-OLT, 85.3% of the patients were critically ill (United Network for Organ Sharing [UNOS] status 1); only 14.7% of the patients were UNOS status 2 and 3. In re-OLT for chronic rejection and recurrent HCV, the 1-year survival rate of UNOS 1 patients was 38.4%, compared with 87.5% for UNOS 2 and 3 patients. In patients requiring re-OLT, there was no difference in the 1-year patient survival rate after re-OLT when cyclosporine (60%), tacrolimus (63%), or cyclosporine/tacrolimus (56%) was used for primary therapy. With cyclosporine, three patients (1.5%) required re-OLT for chronic rejection versus one patient (0.7%) with tacrolimus. Re-OLT for recurrent HCV was required in four (3%) and seven (3.6%) patients with tacrolimus and cyclosporine therapy, respectively.
Conclusions
Orthotopic liver transplantation for HCV is performed with excellent results. There are no distinct advantages to the use of cyclosporine versustacrolimus immunosuppression when patient and graft survival are considered. Re-OLT is an important option in the treatment of recurrent HCV and should be performed early in the course of recurrent disease. Survival after re-OLT is not distinctively affected by cyclosporine or tacrolimus primary immunotherapy. The incidence of re-OLT for recurrent HCV or chronic rejection is low after either tacrolimus or cyclosporine therapy.
Hepatitis C is a major cause of chronic liver disease worldwide: nearly 4 million Americans and 100 million people worldwide are infected with the hepatitis C virus (HCV). 1 Not surprisingly, end-stage liver disease (ESLD) caused by HCV has become an increasingly frequent indication for liver transplantation. 2 The availability of second-generation antibody testing for HCV, and more recently the advent of polymerase chain reaction amplification of viral RNA, 3 have greatly assisted the accurate diagnosis of HCV infection. Molecular analysis of transplant recipients has identified that postoperative viral strains are identical to isolates detected before transplantation. 4 In addition, posttransplant serum levels of HCV RNA exhibited significant increases compared with preoperative levels. 5 However, with improvement in diagnostic techniques, it has become increasingly evident that recurrence of HCV infection after orthotopic liver transplantation (OLT) is nearly universal 6 and may lead to progressive liver disease and allograft injury. 7
Because recurrence of hepatitis B virus infection after OLT was associated with decreased patient and graft survival rates 8 before the advent of novel antiviral therapy, an increasing concern has developed regarding long-term outcomes and factors that determine the severity of recurrent HCV disease after transplantation. 9,10 In contrast to OLT for hepatitis B virus infection, long-term outcomes for patient and graft survival after transplantation for HCV are not yet clearly defined. Further, although retransplantation for recurrent hepatitis B is clearly associated with rapid recurrence and poor prognosis, 11 little is known about the outcome of retransplantation for recurrent HCV.
The role of increased immunosuppression in the acceleration of posttransplant viral replication and graft damage is unclear. Initial reports suggested that OKT3 therapy, 12 tacrolimus immunosuppression, 13 and repeated rejection episodes requiring steroid or OKT3 treatment 14,15 were associated with decreased outcomes. However, other studies have not shown such an association. 16,17
This study retrospectively evaluated the long-term clinical outcome and the effects of immunosuppressive therapy in a large cohort of patients who underwent transplants for ESLD caused by HCV over an 8-year period. We also examined the causes and outcome of patients undergoing retransplantation. Based on the long-term follow-up of a large patient population, this study may provide useful insights for the management of patients undergoing transplants for HCV-related ESLD.
MATERIALS AND METHODS
Patients
From January 1990 to December 1997, 374 adult patients underwent 450 OLTs for ESLD secondary to HCV infection at our center. Two hundred ninety-eight patients received a single allograft and 76 patients required retransplantation (re-OLT). We performed a retrospective analysis of the patient records. Patient survival was compared with a contemporary cohort of 701 adult patients who underwent transplantation during the same period for multiple indications other than viral hepatitis. Candidates for OLT were assigned, according to their medical condition, to one of the following United Network for Organ Sharing (UNOS) categories:
• UNOS status 1: patients in intensive care with expected survival <7 days
• Status 2: continuously hospitalized
• Status 3: at home but requiring medical attention.
Diagnosis and Definitions
Hepatitis C was diagnosed before OLT by anti-HCV seropositivity by enzyme-linked immunoadsorbent assay (ELISA 2.0) and/or polymerase chain reaction for detection of HCV RNA, as previously described. 18 Primary nonfunction was defined as immediate failure within 7 days after transplantation; delayed nonfunction was defined as graft failure in the initial 30 days, but not in the first 7 days, after transplantation. Graft nonfunction included both primary and delayed nonfunction and was diagnosed by increased liver function test results, encephalopathy, no or little bile production, and coagulopathy requiring transplantation. Recurrent HCV was diagnosed by biochemical graft dysfunction with the presence on liver biopsy of features consistent with recurrent HCV, including portal or lobular infiltration by mononuclear cells with piecemeal necrosis in the absence of any other specific causes. Chronic rejection was defined by the disappearance of >50% of bile ducts. The preoperative histologic diagnosis was correlated with the preoperative HCV RNA levels and confirmed by pathologic examination of the explanted livers after surgery.
Immunosuppression
Maintenance immunosuppression regimens consisted of either a triple cyclosporine-based drug regimen (Sandimmune or Neoral, azathioprine, and prednisone) or dual tacrolimus-based immunosuppression (tacrolimus and prednisone). Cyclosporine was administered orally to achieve a whole blood trough concentration of 250 to 350 ng/ml during the first month after transplantation. During the second month, the cyclosporine dose was gradually tapered to a maintenance level of 200 ng/ml. In 1996, Neoral was routinely substituted for Sandimmune. Intravenous azathioprine (2 mg/kg/day) was started on the first day after surgery and was subsequently converted to oral administration at 1 mg/kg/day. Routine use of tacrolimus was initiated at our institution in 1994. Tacrolimus was administered orally to achieve a whole blood trough concentration of 10 to 15 ng/ml during the first month after transplantation, 8 to 10 ng/ml during the second month, and 5 to 6 ng/ml thereafter. On the day of transplantation, patients were started on a rapid steroid taper according to our standard protocol. One gram of methylprednisolone (Solu-Medrol) was administered intravenously for the first day and was rapidly tapered to 20 mg/day over 1 week. Oral prednisone was started on day 8 (20 mg/day) and was tapered over 2 months to 5 mg/day.
Statistical Analysis
Survival analysis was performed using the Kaplan–Meier method. Group survival curves were compared using the log-rank test for nonparametric data. The nonparametric Wilcoxon rank sum test was used to compare median values. A probability value <0.05 was considered significant.
RESULTS
Incidence of Transplantation for Hepatitis C
From 1990 to 1997, increasing numbers of OLTs for ESLD caused by HCV were performed at our center. From 1990 to 1997, the 374 patients who were entered in the study underwent 450 OLTs. One OLT was performed in 298 (79.6%) patients; 76 (20.4%) patients required re-OLT. In the first 4 years (1990 to 1993), a mean of 40.75 ± 2.7 OLTs, representing 18.4% of the total transplants performed per year, were for HCV (Fig. 1). In contrast, between 1994 and 1997, 71 ± 4.7 (28.9%) OLTs were performed per year for HCV.
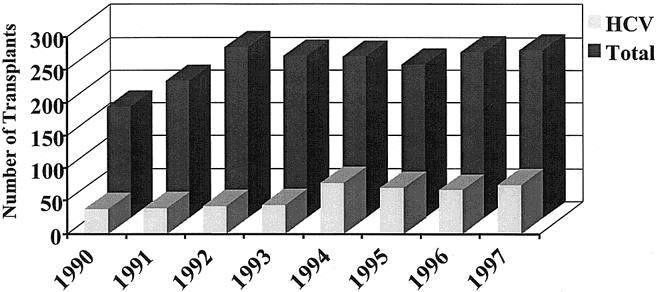
Figure 1. Number of liver transplants performed for HCV vs. the total number of transplants per year.
Overall Patient Survival
Kaplan–Meier patient survival estimates for the entire 374 patients included in the study period are shown in Figure 2. Median follow-up was 22.7 (range 0 to 96) months. Overall patient survival rates from the date of the first transplant at 1, 2, and 5 years were 86%, 82%, and 76%, respectively (Fig. 2A). There was no significant difference in survival of patients transplanted for HCV (n = 374) and a control cohort of patients (n = 701) who underwent transplantation for causes other than hepatitis C or B during the same time period (p = NS; Fig. 2A).
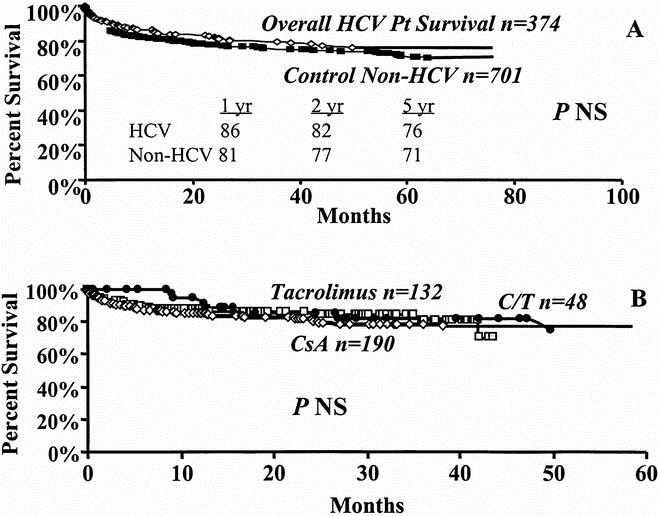
Figure 2. Kaplan-Meier patient survival curves. A. Overall patient survival (diamond) vs. a contemporary cohort (box). B. Effect of cyclosporine (diamond), tacrolimus (box), or cyclosporine/tacrolimus (circle) immunotherapy on overall patient survival.
Of the 374 patients, 190 received cyclosporine and 132 patients tacrolimus as their primary immunosuppression. A third group included 48 patients who were switched from cyclosporine to tacrolimus or from tacrolimus to cyclosporine (cyclosporine/tacrolimus, Table 1 ). Four patients died in the immediate postoperative period and did not receive immunosuppressive agents. As shown in Figure 2B, overall 1-, 2-, and 5-year patient survival rates of 85%, 81%, and 78% under cyclosporine were not significantly different from the rates with tacrolimus therapy (89%, 85%, and 71%). Similarly, survival rates in the cyclosporine/tacrolimus group (91%, 82%, and 74%) was not significantly different from either cyclosporine or tacrolimus therapy alone (see Fig. 2B).
Table 1. Immunosuppression for Patients Who Received, Transplants for HCV
Overall Graft Survival
Graft survival analysis that included all causes of graft failure for the 374 patients demonstrated graft survival rates of 70%, 65%, and 60% at 1, 2, and 5 years, respectively (Fig. 3A). Median follow-up was 22.7 months. Figure 3B demonstrates that at 1, 2, and 5 years, overall graft survival rates under cyclosporine immunosuppression were 74%, 68%, and 64%, respectively. Such graft survival rates were not significantly different from the tacrolimus group (67%, 64%, and 54%) or the cyclosporine/tacrolimus group (67%, 60%, and 47%). However, overall graft survival analysis included graft nonfunction that occurred in the immediate postoperative period, which is unrelated to immunosuppressive therapy or to the cause of ESLD. Graft survival estimates were therefore performed after excluding graft nonfunction as a cause of graft failure to reflect more accurately the effects of immunosuppression and underlying disease on graft function. Such analysis is demonstrated in Figure 4. Graft survival rates, after excluding graft nonfunction (n = 332), were 79%, 74%, and 65% at 1, 2, and 5 years (Fig. 4A). After cyclosporine therapy in the 178 patients without graft nonfunction, graft survival rates increased to 79%, 73%, and 68% at 1, 2 and 5 years. Similarly, graft survival rates under tacrolimus in 109 patients increased to 81%, 78%, and 65%; these rates were not different from either cyclosporine alone or cyclosporine/tacrolimus treatment (78%, 70%, and 55%; Fig. 4B).
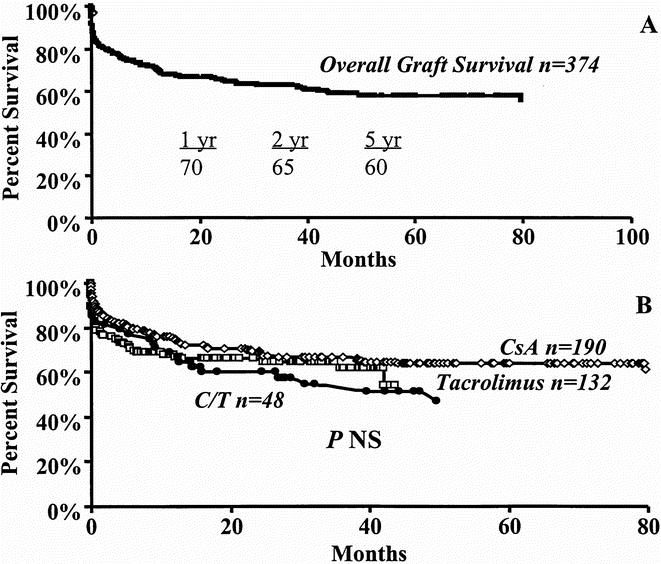
Figure 3. Kaplan-Meier graft survival estimates. A. Overall graft survival. B. Effect of cyclosporine (diamond), tacrolimus (box), or cyclosporine/tacrolimus (circle) immunosuppression on overall graft survival.
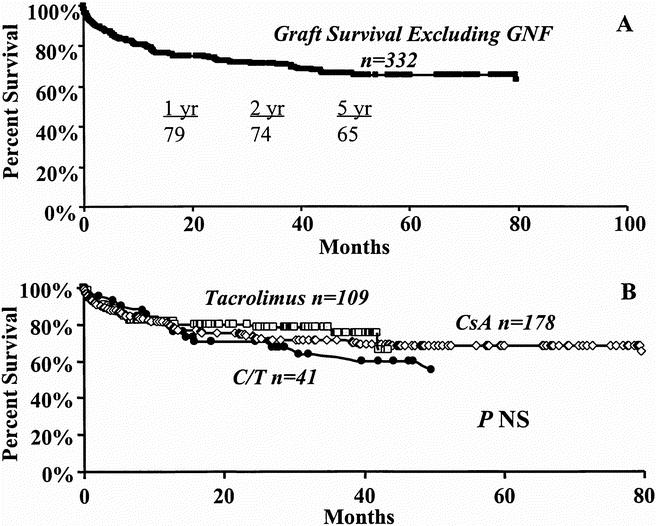
Figure 4. Graft survival estimates, excluding graft nonfunction. A. Overall graft survival. B. Effect of cyclosporine (diamond), tacrolimus (box), or cyclosporine/tacrolimus (circle).
Patients Receiving a Single Transplant
In the 298 (79.6%) patients who received a single OLT, Kaplan–Meier estimates demonstrated patient survival rates of 85%, 81%, and 75% at 1, 2, and 5 years, respectively. Median follow-up for this group was 30.4 (range 0 to 96) months (Fig. 5A). Cyclosporine therapy was used in 160, tacrolimus in 102, and cyclosporine/tacrolimus in 32 (see Table 1). As shown in Figure 5B, no significant differences in patient survival at 1, 2, and 5 years were demonstrated under cyclosporine (84%, 79%, and 76%), tacrolimus (88%, 84%, and 70%), or cyclosporine/tacrolimus (90%, 84%, and 73%).
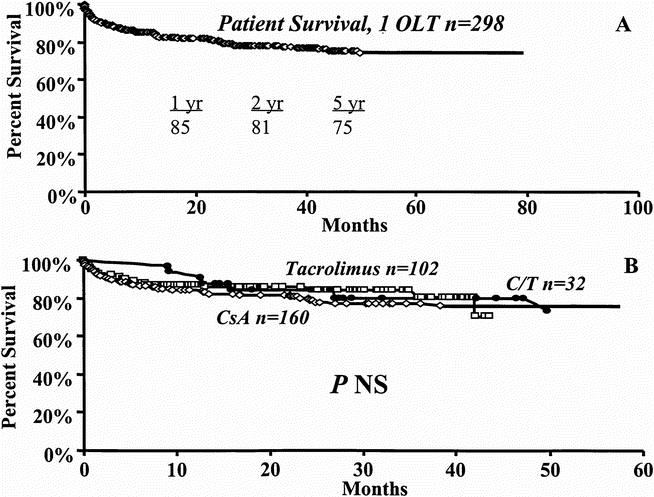
Figure 5. Kaplan-Meier survival curves for patients who received a single liver transplant. A. Overall patient survival. B. Effect of cyclosporine (diamond), tacrolimus (box), or cyclosporine/tacrolimus (circle).
Causes of Retransplantation
Graft loss requiring re-OLT occurred in 76 (20.4%) patients. As shown in Table 1, causes of re-OLT included graft nonfunction in 42 patients (11.2%), hepatic artery thrombosis in 10 (2.6%), recurrent HCV in 13 (3.4%), chronic rejection in 8 (2.1%), and biliary complications in 3. Differentiation between chronic rejection and recurrent HCV was based on pre-OLT liver biopsies and pathologic examination of the explanted livers. HCV RNA levels were significantly higher in patients who underwent re-OLT for recurrent HCV than in patients who underwent re-OLT for chronic rejection (48.6 ± 45 × 106 vs. 2.9 ± 4 × 106mEq/ml; p < 0.01).
The mean time to re-OLT for graft nonfunction was 2.7 ± 1.6 (range 1 to 7) days, which is consistent with primary nonfunction in 31 (8.2%) patients, and 17 ± 4.4 (range 9 to 24) days in 11 (2.9%) recipients with delayed nonfunction of the transplanted hepatic allografts. Re-OLT for hepatic artery thrombosis exhibited a time interval of 86 ± 91 (range 6 to 260) days. The mean time interval of 573 ± 643 (106 to 2420) days between the first and second OLT for recurrent HCV was not significantly different from the time interval for patients who underwent re-OLT for chronic rejection (385.7 ± 310; range 76 to 971).
Effect of Immunosuppression on Retransplantation
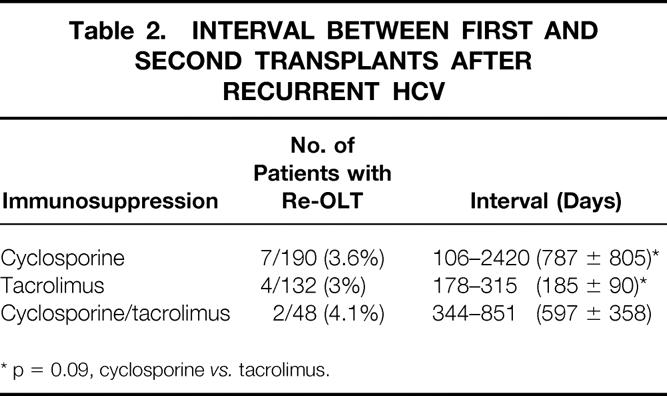
Retransplantation for chronic rejection occurred in 1.5% and 8.3% of patients under cyclosporine and cyclosporine/tacrolimus immunosuppression, respectively. Only 1 of 132 patients (0.7%) in the tacrolimus group had a re-OLT for chronic rejection (see Table 1). Re-OLT for HCV was required in 7 of 190 patients (3.6%) treated with cyclosporine, 4 of 132 (3%) with tacrolimus, and 2 of 48 (4.1%) with cyclosporine/tacrolimus (see Table 1). However, as shown in Table 2, the time interval between the first and second OLT in patients who underwent re-OLT for recurrent HCV was longer under cyclosporine therapy and approached statistical significance when compared with tacrolimus immunosuppression (787 ± 805 vs. 185 ± 90 days, p = 0.09).
Table 2. Interval between First and, Second Transplants After, Recurrent HCV
Primary immunosuppression did not affect patient survival after re-OLT. Figure 6 shows that transplant recipients undergoing re-OLT had similar patient survival rates after re-OLT whether cyclosporine (n = 30), tacrolimus (n = 30), or cyclosporine/tacrolimus (n = 16) was used between the first and second transplants.
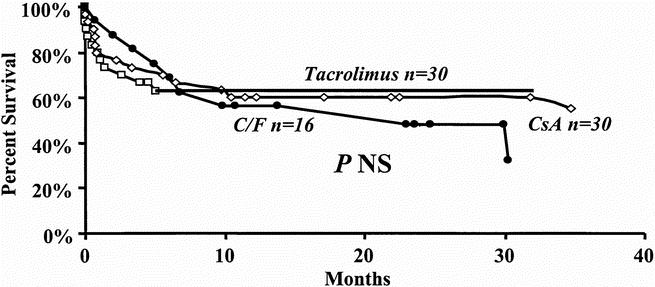
Figure 6. Effect of cyclosporine (diamond), tacrolimus (box), or cyclosporine/tacrolimus (circle) used after the first transplant on patient survival after retransplantation.
Patient Survival After Retransplantation
Kaplan–Meier patient survival analysis in the 76 patients who underwent re-OLT demonstrated patient survival rates of 63% and 58% at 1 and 2 years, respectively (Fig. 7A) from the date of the second transplant. Median follow-up was 12.7 months (range 0 to 95 months). The cause of re-OLT did not affect the patient survival rate, as shown in Figure 7B. The 1-year patient survival rate after the second OLT was 61% for graft nonfunction, 50% for chronic rejection, 60% for hepatic artery thrombosis, and 60% for recurrent HCV (p = NS). At re-OLT, 65 of 76 patients (85.3%) were assigned UNOS status 1 because of their critical condition. Only 11 of 76 patients (14.7%) who underwent re-OLT were assigned UNOS status 2 or 3. Comparison of overall patient survival rates after re-OLT for UNOS status 1 patients versus UNOS status 2 and 3 patients approached but did not achieve statistical significance (p = 0.25, Fig. 8A). When re-OLT for chronic rejection and recurrent HCV was considered, the 1-year survival rate of UNOS status 1 patients (n = 13) was 38.4% versus 87.5% for UNOS status 2 and 3 (n = 8) patients (p = 0.06, Fig. 8B).
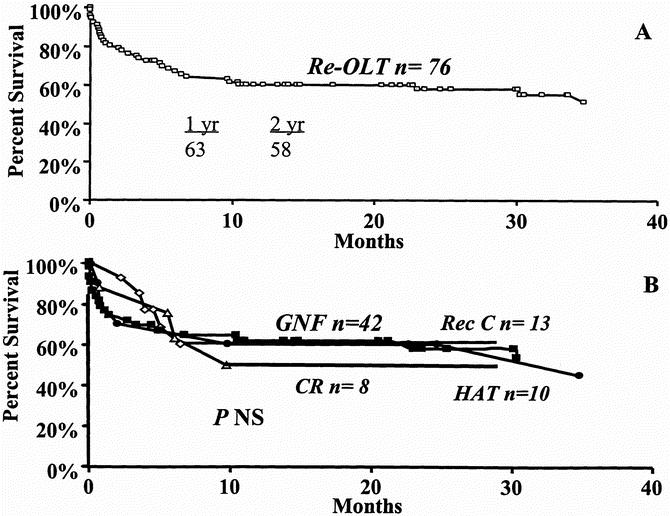
Figure 7. Patient survival after retransplantation from the date of the second transplant. A. Overall patient survival. B. Patient survival according to cause of retransplantation. Graft nonfunction (box), recurrent HCV (diamond), chronic rejection (triangle), hepatic artery thrombosis (circle).
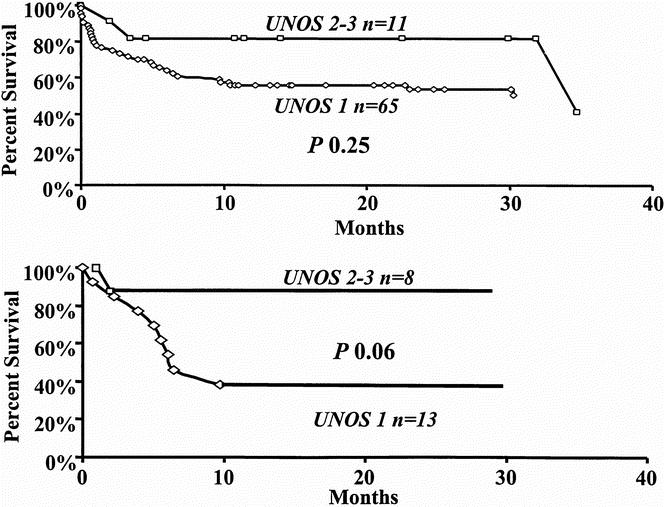
Figure 8. Patient survival according to UNOS status. A. All patients undergoing retransplantation. B. Patients undergoing retransplantation for chronic rejection and HCV. UNOS status 1 (diamond), UNOS status 2 and 3 (box).
Causes of Death in Transplantation for Hepatitis C
In patients undergoing a single OLT, sepsis was the major cause of death after transplantation (Table 3). Fatal infections occurred in 25 of the 66 patients (37.8%) who died after a single transplant. Only 7 of 66 recipients (10.6%) died from graft loss caused by recurrent HCV, where no other causes were identified. Deaths unrelated to sepsis or recurrent hepatitis occurred in 34 of 66 patients (51.5%). The majority of deaths—46 of 66 patients (69.6%)—occurred within the first year after transplantation (92 ± 107 days). Deaths from graft failure caused by recurrent HCV occurred between 120 and 810 days (median 482 ± 266).
Table 3. Causes of Death in Patients Who Received Transplants for HCV
In those who died after re-OLT, sepsis was the leading cause of death (21/33 [63%]; see Table 3). Graft failure from recurrent HCV was the cause of death in 3/10 and 1/18 patients who died after re-OLT for recurrent HCV and graft nonfunction, respectively. Thirty of the 33 patients died within the first year after re-OLT.
DISCUSSION
This study represents one of the largest series reported on transplantation for ESLD from HCV. At our institution, transplantation of 374 patients during an 8-year period achieved overall patient survival rates of 86%, 82%, and 76% at 1, 2, and 5 years. Comparison with a contemporary cohort of 701 patients who underwent transplantation for causes other than viral infections did not reveal any difference in patient survival rates. Overall graft survival rates in our series were 70%, 65%, and 60% at 1, 2, and 5 years, respectively. In contrast to our results, Boker et al 19 reported 2- and 5- year patient survival rates of 67% and 62% in 61 patients; these were not significantly different from the survival rates of control non-HCV transplant recipients. Recently, two larger series demonstrated results similar to ours. Casavilla et al 20 reported patient survival rates of 80% and 75% at 1 and 5 years in a cohort of 183 patients. In another study that included 149 transplant recipients, patient and graft survival rates were not significantly different from those of HCV-negative patients at 1 and 5 years after the transplant. 21 Thus, despite the risk of recurrent HCV after transplantation, OLT is performed with good results.
The effects of immunosuppression on viral replication that may increase the rate and severity of HCV recurrence after transplantation have caused increasing concern. 10,12–16,22,23 OKT3 treatment for steroid-resistant infection has been convincingly shown to be associated with early and severe HCV recurrence in allografts. 12,23 Cirrhosis was documented in 26.3% of allografts in patients who received OKT3 during their posttransplant course, compared with 6% of patients who did not. 12 Similarly, severe or multiple rejection episodes were associated with early recurrence. 14 Higher levels of viral replication were documented with excessive steroid use for treatment of rejection episodes. 23 Tacrolimus was thought to increase recurrence rates in one study 13 and was associated with a poor clinical outcome in another. 24 However, in the first study, patients received excessive doses of steroids for treatment of rejection, and in the second, excessively high doses of tacrolimus were used. More recently, the same group demonstrated that the use of lower doses of tacrolimus resulted in patient and graft survival rates similar to those in non-HCV transplant patients. 20 Interestingly, low mean levels of cyclosporine correlated with early HCV recurrence. 15,25
In our study, tacrolimus demonstrated excellent patient and graft survival rates that were not significantly different from those of cyclosporine. Further, survival of patients who received a single OLT was equivalent in both groups, and survival after re-OLT was not distinctively affected by cyclosporine or tacrolimus use after the first OLT. The only difference observed in our study between tacrolimus and cyclosporine was in re-OLT for recurrent HCV. The time interval between the first and second transplant was shorter with tacrolimus than with cyclosporine. However, the incidence of re-OLT for recurrent HCV, under either cyclosporine or tacrolimus therapy, was low, and more patients would be required for adequate evaluation. Tacrolimus immunosuppression may be particularly useful in patients with HCV because of the lower incidence of acute rejection when compared with cyclosporine. 26 Thus, tacrolimus may prevent the occurrence of rejection episodes in HCV transplant recipients that demand the use of steroids and/or OKT3, which are associated with viral activation.
Initial reports of poor outcomes associated with re-OLT for recurrent HCV 25,27 and the suggestion that viral reinfection may worsen the prognosis of retransplanted livers 28 raised questions about the wisdom of the procedure. However, re-OLT carries a worse prognosis than primary OLT, for all indications. Evaluation of 250 patients who underwent re-OLT at UCLA for a variety of indications revealed 1-, 5-, and 10-year survival rates of 55%, 47%, and 44% from the date of the second transplant. 29 In the current study, patient survival rates from the date of the second transplant for all causes of re-OLT were 63% and 58% at 1 and 2 years; the rate for recurrent HCV was 59% at 1 and 2 years. Thus, there appears to be no difference in the survival rate for patients undergoing re-OLT for recurrent HCV when compared with other causes of re-OLT.
Several independent variables were found to predict a poor outcome for re-OLT. These factors included UNOS status 29,30 and the poor preoperative condition of the patients 30,31 but not the recipient’s primary diagnosis. 28 In our series, 85.5% of patients undergoing re-OLT were UNOS status 1. When patients undergoing re-OLT for chronic rejection and recurrent HCV were stratified according to UNOS status, an 85% survival rate at 1 and 2 years was achieved in healthy recipients, compared with only 38% at 1 and 2 years (p = 0.06) in critically ill patients. Recently, Sheiner at al 31 demonstrated a patient survival rate of 64% at 1 and 2 years after re-OLT for HCV and observed that poor outcomes were associated with critically ill recipients. Thus, re-OLT early in the course of recurrent disease, before the deterioration of the patient, may exhibit a better outcome.
Severe allograft injury that prompts re-OLT appears to develop in only a small subset of patients. 32,33 In 49 patients who underwent re-OLT after a successful first transplant for HCV, only 14 were performed for recurrent HCV. 32 In 166 HCV-infected transplant recipients, only 5 required re-OLT for recurrent HCV. 34 In our study, only 13 of 76 retransplants were for recurrent HCV. The major indication for re-OLT in our study was graft nonfunction, similar to what was reported in two other series. 27,32,33 Other indications for re-OLT included hepatic artery thrombosis and chronic rejection. Under tacrolimus therapy, only one patient required re-OLT for chronic rejection.
Much attention is currently directed toward factors that influence the rate and severity of recurrent HCV. Pretransplant HCV RNA levels 34 and infection with HCV genotype 1b 21 were associated with poor outcome and increased rate of graft damage. Although graft reinfection with HCV is universal, graft loss and death from hepatitis C appear to be less common. In one study that included 149 patients who underwent transplants for HCV, graft loss occurred in 27, but only 8 patients suffered graft loss secondary to HCV. 21 Recurrent HCV with ensuing graft failure, in a series of 166 HCV-infected transplant recipients, was the cause of death in 11 of 39 patients who died during the follow-up period. 34 Sepsis was the single most common cause of death in our series. In patients who underwent a single OLT, sepsis resulted in 37.8% of deaths. Deaths related to graft failure caused by HCV, where no other causes were observed, occurred in only 10.6%. Further, the majority of deaths (69.6%) occurred within the first year, whereas deaths related to HCV occurred at a mean of 482 days. In re-OLT, the major cause of death was also sepsis (63%). Death from recurrent HCV, without concurrent infections, occurred in only 4 of 76 patients undergoing re-OLT. Thus, sepsis, not recurrent HCV, accounted for the majority of deaths in HCV transplant recipients. It is possible, however, that recurrence of HCV may predispose recipients to infectious complications. Singh et al 35 recently demonstrated that the incidence of viral and fungal, but not bacterial, infections was significantly higher in patients with recurrent HCV.
In summary, transplantation for HCV is performed with excellent results. Despite the risk of recurrence, the clinical outcome of transplantation for HCV is equivalent to that performed for other causes of ESLD. To date, HCV recurrence has not resulted in decreased patient and graft survival rates. However, longer periods of follow-up may demonstrate different results. There appears to be no distinct advantages for the use of tacrolimus or cyclosporine when patient and graft survival rates are considered. Re-OLT for recurrent HCV achieves good results in healthy patients and should be considered an important option for the treatment of recurrent disease.
Acknowledgment
The authors thank J. Gornbein, DrPH, for his expert statistical analyses of the data.
Discussion
Dr. John McDonald (Shreveport, Louisiana): This paper establishes the good results obtained transplanting livers into patients with hepatitis C. This might not be surprising, since for years patients with hepatitis C were transplanted with a diagnosis of chronic active hepatitis and known to do well. Nevertheless, this scientifically establishes that fact.
Since they are all reinfected, the question is why do they do so well. Theoretically, the stronger the immune response to hepatitis viruses, the more severe the acute disease but the smaller the incidence of chronic disease. Thus, with hepatitis C, this paradigm seems to hold. Immunosuppressed patients have the virus but have mild disease.
We have been led to believe this is not the case with hepatitis B. And while it is not the subject of this paper, I wonder if the authors would comment upon why this is true. Or is it true, since we commonly spend enormous amounts of money trying to passively immunize patients with hepatitis B?
There are several other points made by this paper that bear discussion. Among them is the evidence that tacrolimus does not appear to be superior to cyclosporine as an immunosuppressant. But I would like to hear some discussion about the advisability of giving top priority for transplantation to UNOS status 1 patients.
As many of you know, this has been the subject of vigorous debate and political activity over the past 2 to 3 years. Some feel that a national “sickest first” policy should be implemented. That is, all patients who are status 1 should be given top priority before others are considered. In this series, patient survival at 2 years, of 298 primary transplants, was 81%. On the other hand, the 2-year survival of retransplanted status 1 patients was 38%—half as good.
How have these data influenced your thoughts about a national allocation policy, Dr. Busuttil?
Dr. A. Osama Gaber (Memphis, Tennessee): This large single-center experience documents the excellent results and outcomes in hepatitis C-positive patients and, finally, I think, lays to rest the debate regarding the role of various immunosuppressants affecting the rate of hepatitis C recurrence. This also confirms my personal bias that there is really not a difference between the two immunosuppressants in terms of determining the recurrence.
What Dr. Busuttil has so eloquently showed us today is that it is not the type of drug regimen that you use, but if you read his manuscript carefully, he seems to indicate that it is the amount of total immunosuppression used that determines the recurrence. What he has taught us, I think, in his usual fashion, is that you should use just enough immunosuppression, because extremes at both ends of the spectrum are associated with problems.
Most intriguing to me in this manuscript is the description of the population requiring retransplantation following primary treatment for hepatitis C by transplantation. The authors clearly define the causes of early and late retransplantation in hepatitis C-positive patients. Interestingly, they document the poor outcomes of patients retransplanted in urgent status for both chronic rejection and recurrent hepatitis C. And, as Dr. McDonald has discussed, this is almost one half of the survival rate of the primary transplantations and also compares very unfavorably to those retransplantations that transplanted in elective status.
The paper, however, stops somewhat short in answering some of the important questions that I would like to ask Dr. Busuttil and give him a chance to elaborate on first.
I did not see any analysis to try to determine which patients are going to require retransplantation. Considering that reinfection with hepatitis C is almost universal amongst the patients, could they identify—because they have such a large number of patients—some determinants? I know you showed the viral serology, but could they predict, slightly before the patients get into this pretty extreme status, which patients will require retransplantation?
I was somewhat puzzled by the fact that almost two thirds of the patients requiring retransplantation in the—not in the immediate posttransplant period—required urgent retransplantation.
And the other question that I have is could they define for us the role then of the new drug therapies used in adjuvant therapy with a patient with hepatitis C in their program?
What is the current protocol today in treating patients with hepatitis C? And what do they do particularly for the infected patient that starts showing deterioration of function? Is there something specific that they do in terms of the drug regimens?
And do they use these viral load measurements to sort of adjust their immunosuppression? Because clearly, as he indicated, enough immunosuppression without excesses is very important for these patients.
Dr. Richard J. Howard (Gainesville, Florida): Hepatitis C is currently present in an estimated four million people in this country and will undoubtedly grow in prevalence in the coming years. There is no vaccine on the horizon. Antibody isn’t protective, unlike hepatitis B. And so therefore, like HIV, it’s going to be very difficult to construct a vaccine for virus infection.
What I’d like to ask Dr. Busuttil is whether or not he has done any genotyping of hepatitis C and looked at which strains are responsible for recurrence. We have been interested in this. There are currently numerous different strains of hepatitis C and, depending which hepatitis C virologist you talk to, you can get a varied number. But there are at least in the 20s to 30s. And we have done that in some of our transplant recipients with hepatitis C. It seems that only a couple of strains account for the most common presentation of patients who end up needing liver transplantation.
We have had problems with recurrence in a larger fraction of our patients who then go on to needing retransplantation. Are there certain strains that seem to lead to an earlier need for retransplantation?
We have also asked a question whether patients who recur rapidly should get a second and even in some cases a third transplant, because of the rapidity of recurrence and the extremely poor prognosis in a group who is now consuming valuable resources, especially a liver that could benefit other patients to a greater degree.
Dr. J. Michael Henderson (Cleveland, Ohio): You presented us data on the HCV RNA titers, I think, for the retransplants, for those with recurrent disease against those with chronic rejection. Do you have data on the HCV RNA titers for your good groups, the excellent outcome patients who were neither chronic rejection or recurrent disease? I think that would be interesting to know, because I think those of us in the field know either they don’t come back or they come back real bad. And I wondered if you had data that actually quantitated that.
The second point that struck me was your fairly high primary nonfunction rate. You’re running a 10% primary nonfunction rate. Although this is probably not directly related to the hepatitis C population, I am curious if you could give us a brief comment as to what factors you believe led to that 1-in-10 retransplant earlier. Were these the borderline fatty livers or were there other factors there with that relatively high early retransplant rate?
Dr. Ronald W. Busuttil (Closing Discussion): Let me try to group these questions in classes.
First of all, the questions that came up about retransplantation. Retransplantation for this disease is a very difficult issue, and the allocation of organs to patients who are UNOS status 1 with poor results is, obviously, something which the entire transplant community is grappling with.
We are very interested in retransplantation. In fact, we recently published a series of 299 patients in the Annals, and tried to determine which patients would benefit from retransplantation. That also included the hepatitis C patients. And we have five variables that, on multivariate analysis, that was also validated by UNOS data and validated by another large liver transplant center that came to be predictive of how the patients would do. And these included the one donor variable with whether you had an organ that had a cold ischemia time of greater than 12 hours. And that’s why, Dr. McDonald, I have stated repeatedly that we shouldn’t be shipping livers all around the country because, invariably, when you do that, you are going to have a long period of ischemia time. And that is the basis of my position for not having a national list.
The four recipient variables were whether the patient was on a ventilator, whether the patient had a bilirubin that was greater than 13, whether the creatinine was greater than 1.6, and whether the patient was an adult. If you have fulfilled three of those criteria, your survival is going to be less than 45% in 1 year, and we don’t believe that that is a proper utilization of a limited donor supply. And that is what we are now doing and have been doing for the last couple of years. This series stopped in 1997, and so we were a lot more liberal at that time.
In regards to the difference between hepatitis B and hepatitis C, I think there is a distinct difference. I think that the hepatitis B virus under immunosuppression is much more cytopathic than is the hepatitis C virus. And that is the reason that when we transplant patients for hepatitis B, unless we use an aggressive antiviral prophylactic regimen with both hyperimmune gammaglobulin and lamivudine, that the results are really abysmal.
Nowadays, however, with this prophylactic regimen of HBIG and lamivudine, the results with hepatitis B are what we see now with hepatitis C and all other indications for liver transplantation, with the exception of hepatocellular carcinoma.
Dr. Gaber, I am not quite sure that my study really is the bottom line on whether tacrolimus is better or inferior to cyclosporine. You have to recall that our study was not a randomized study. It was a retrospective study, with all the pitfalls that we see with retrospective studies. We now are a part of a multicenter study which is looking at, in a randomized fashion, tacrolimus versus cyclosporine for patients who have hepatitis C and, hopefully, we will be able to provide you with that information in the next year or so.
You asked about how we predict when we should retransplant patients. Well, I would use the same criteria that I enumerated a moment ago. You can’t wait until the patient develops this cholestatic syndrome akin to what we see with hepatitis B. Once they develop that, it’s too late. At the first sign of portal hypertension in somebody who has hepatitis C, we would opt for retransplantation.
The role of interferon and ribavirin is, again, not at all decided upon. As you know, there is data in naive patients who have not undergone transplantation—and clearly the combination of ribavirin and alpha interferon is quite efficacious if they are treated for 48 weeks.
There is now a multicenter trial which is looking at the efficacy, or lack thereof, of ribavirin and alpha interferon in the posttransplant setting. We don’t have any data. The overwhelming majority of the patients in this series were not treated with any type of antiviral therapy.
Dr. Howard wanted to know about genotyping. I don’t have any systematic data looking at genotyping. As you know, 80% of hepatitis C patients in this country have genotype 1B, and I can’t tell you what the genotypes were in the patients that underwent retransplantation.
And, finally, Dr. Henderson, we don’t have, again, systematic data of pretransplant HCV RNA levels. As you know, the NIDDK paper suggested that this might be the most important variable in determining how these patients would do. We are now doing that systematically, but I don’t have any information regarding this series of patients.
And, finally, the issue about PNF is an important one. I might just add, not trying to be glib, the reason our PNF rate is what it is, is because we take livers that nobody else wants. We are very, very aggressive in the organs that we take. We take marginal donors, and we believe that our results justify the use of those organs because, otherwise, those organs are going to be trashed and not used.
Footnotes
Correspondence: Ronald W. Busuttil, MD, PhD, Dumont-UCLA Transplant Center, UCLA School of Medicine, 10833 LeConte Ave., 77-132 CHS, Los Angeles, CA 90095.
Presented at the 110th Annual Meeting of the Southern Surgical Association, December 6–9, 1998, The Breakers, West Palm Beach, Florida.
Accepted for publication December 1998.
References
- 1.Alter MJ. Epidemiology of hepatitis C. Hepatology 1997; 26 62S–65S. [DOI] [PubMed] [Google Scholar]
- 2.Wright TL. Liver transplantation in patients with chronic hepatitis B and C. In Maddrey WC, Sorrell MF, eds. Transplantation of the liver, 2d ed. Norwalk, CT: Appleton and Lange; 1995: 477.
- 3.Gretch DR. Diagnostic tests for hepatitis C. Hepatology 1997; 26: 43S. [DOI] [PubMed] [Google Scholar]
- 4.Feray C, Samuel D, Thiers V, et al. Reinfection of liver grafts by hepatitis C virus after liver transplantation. J Clin Invest 1992; 89: 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chazouilleres O, Kim M, Combs C, et al. Quantitation of hepatitis C virus RNA in liver transplant recipients. Gastroenterology 1994; 106: 994–999. [DOI] [PubMed] [Google Scholar]
- 6.Wright TL, Donegan E, Hsu HH, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology 1992; 103: 317–322. [DOI] [PubMed] [Google Scholar]
- 7.Rosen H, Gretch DR, Oehlke M, et al. Timing and severity of initial hepatitis C recurrence as predictors of long-term liver allograft injury. Transplantation 1998; 65: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 8.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzal TE. Orthotopic liver transplantation for hepatitis B virus-related liver disease. Hepatology 1991; 13: 619–626. [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman JA, Rubin RH, Koziel MJ, Periera BJ. Hepatitis C virus and organ transplantation. Transplantation 1996; 62: 147–154. [DOI] [PubMed] [Google Scholar]
- 10.Sorrell MF. Hepatitis C recurrence after liver transplantation: is there cause for concern? Am J Gastroenterol 1997; 92: 1416–1417. [PubMed] [Google Scholar]
- 11.Crippin J, Foster B, Carlen S, Borcich A, Bodenheimer H. Retransplantation in hepatitis B: a multicenter experience. Transplantation 1994; 57: 823–826. [DOI] [PubMed] [Google Scholar]
- 12.Rosen HR, Shackleton CR, Higa L, et al. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol 1997; 92: 1453–1457. [PubMed] [Google Scholar]
- 13.Singh N, Gayowski T, Ndimbie OK, Nedjar S, Wagener MM, Yu VL. Recurrent hepatitis C virus in liver transplant recipients receiving tacrolimus: association with rejection and increased immunosuppression after transplantation. Surgery 1996; 119: 452–456. [DOI] [PubMed] [Google Scholar]
- 14.Sheiner PA, Schwartz ME, Mor E, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology 1995; 21: 30–34. [PubMed] [Google Scholar]
- 15.Herrero JI, De La Pena A, Quiroga J, et al. Risk factors for recurrence of hepatitis C after liver transplantation. Liver Transpl Surg 1998; 4: 265–270. [DOI] [PubMed] [Google Scholar]
- 16.Freeman RB, Tran S, Lee YM, Rohrer RJ, Kaplan MM. Serum hepatitis C RNA titer after liver transplantation are not correlated with immunosuppression or hepatitis. Transplantation 1996; 61: 542–546. [DOI] [PubMed] [Google Scholar]
- 17.Ghobrial RM, Colquhoun S, Rosen H, et al. Retransplantation for recurrent hepatitis C following tacrolimus or cyclosporine immunosuppression. Transplant Proc 1998; 30: 1470–1471. [DOI] [PubMed] [Google Scholar]
- 18.Fabrizi F, Martin P, Dixit V, et al. Acquisition of hepatitis C virus in hemodialysis patients: a prospective study by branched DNA signal amplification assay. Am J Kidney Dis 1998; 31: 647–654. [DOI] [PubMed] [Google Scholar]
- 19.Boker KHW, Dalley G, Bahr MJ, et al. Long-term outcome of hepatitis C virus infection after liver transplantation. Hepatology 1997; 25: 203–210. [DOI] [PubMed] [Google Scholar]
- 20.Casavilla FA, Rakela J, Kapur S, et al. Clinical outcome of patients infected with hepatitis C virus infection on survival after primary liver transplantation under tacrolimus. Liver Transplant Surg 1998; 4: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Eng J Med 1996; 334: 815–820. [DOI] [PubMed] [Google Scholar]
- 22.Gane EJ, Naoumov NV, Qian KP, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology 1996; 110: 167–177. [DOI] [PubMed] [Google Scholar]
- 23.Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 1996; 23: 971–976. [DOI] [PubMed] [Google Scholar]
- 24.Casavilla A, Mateo R, Rakela J, Irish W, Demetris AJ, Starzal TE. Impact of hepatitis C viral infection on survival following primary liver transplantation under FK [abstract]. Hepatology 1994; 20: 133A. [Google Scholar]
- 25.Feray C, Habsanne A, Samuel D, Farges O, Reynes M, Bismuth H. Poor prognosis of patients retransplanted for hepatitis C virus. Hepatology 1995; 22: 135A. [Google Scholar]
- 26.The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med 1994; 331: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 27.Casavilla FA, Lee R, Lim J, et al. Outcome of liver retransplantation for recurrent hepatitis C infection. Hepatology 1995; 22: 153A.7601408 [Google Scholar]
- 28.Rosen H, Martin P. Hepatitis C infection in patients undergoing liver transplantation. Transplantation 1998; 66: 1612. [DOI] [PubMed] [Google Scholar]
- 29.Markmann JF, Markowitz JS, Yersiz H, et al. Long-term survival after retransplantation of the liver. Ann Surg 1997; 226: 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong T, Devlin J, Roland N, et al. Clinical characteristics affecting the outcome of liver retransplantation. Transplantation 1997; 64: 878–882. [DOI] [PubMed] [Google Scholar]
- 31.Sheiner PA, Schluger LK, Emre S, et al. Retransplantation for hepatitis C. Liver Transpl Surg 1997; 3: 130–136. [DOI] [PubMed] [Google Scholar]
- 32.Doyle HR, Morelli F, McMichael J, et al. Hepatic retransplantation—an analysis of risk factors associated with outcome. Transplantation 1996; 61: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen HR, O’Reilly PM, Shackleton CR, et al. Graft loss following liver transplantation in patients with chronic hepatitis C. Transplantation 1996; 62: 1773–1776. [DOI] [PubMed] [Google Scholar]
- 34.Charlton M, Seaberg E, Wiesner R, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology 1998; 28: 823–830. [DOI] [PubMed] [Google Scholar]
- 35.Singh N, Gayowski T, Wagner MM, Marino IR. Increase infections in liver transplant recipients with recurrent hepatitis C virus hepatitis. Transplantation 1996; 61: 402–406. [DOI] [PubMed] [Google Scholar]