Abstract
Objective
To describe the safety and efficacy of radiofrequency ablation (RFA) to treat unresectable malignant hepatic tumors in 123 patients.
Background
The majority of patients with primary or metastatic malignancies confined to the liver are not candidates for resection because of tumor size, location, or multifocality or inadequate functional hepatic reserve. Local application of heat is tumoricidal; therefore, the authors investigated a novel RFA system to treat patients with unresectable hepatic cancer.
Patients and Methods
Patients with hepatic malignancies were entered into a prospective, nonrandomized trial. The liver tumors were treated percutaneously or during surgery under ultrasound guidance using a novel LeVeen monopolar array needle electrode and an RF 2000 generator. All patients were followed to assess complications, treatment response, and recurrence of malignant disease.
Results
RFA was used to treat 169 tumors (median diameter 3.4 cm, range 0.5 to 12 cm) in 123 patients. Primary liver cancer was treated in 48 patients (39.1%), and metastatic liver tumors were treated in 75 patients (60.9%). Percutaneous and intraoperative RFA was performed in 31 patients (35.2%) and 92 patients (74.8%), respectively. There were no treatment-related deaths, and the complication rate after RFA was 2.4%. All treated tumors were completely necrotic on imaging studies after completion of RFA treatments. With a median follow-up of 15 months, tumor has recurred in 3 of 169 treated lesions (1.8%), but metastatic disease has developed at other sites in 34 patients (27.6%).
Conclusions
RFA is a safe, well-tolerated, and effective treatment to achieve tumor destruction in patients with unresectable hepatic malignancies. Because patients are at risk for the development of new metastatic disease after RFA, multimodality treatment approaches that include RFA should be investigated.
Although relatively uncommon in Western countries, hepatocellular carcinoma (HCC) is probably the most common solid cancer in the world, with an annual incidence estimated to be at least 1 million new patients. 1–5 The optimal treatment for HCC is surgical excision with curative intent. Unfortunately, only 5% to 15% of newly diagnosed patients with HCC undergo a potentially curative resection. 6–11 Patients with disease confined to the liver may not be candidates for resection because of multifocal disease, proximity of tumor to key vascular or biliary structures that precludes a margin-negative resection, or inadequate functional hepatic reserve related to coexistent cirrhosis. Because there are few other curative treatment options in patients with unresectable liver-only disease, HCC is also one of the most lethal human malignancies, with 94% of patients dying as a result of their HCC. 12
The liver is second only to lymph nodes as a common site of metastasis from other solid cancers. 13 It is not uncommon, particularly in patients with colorectal adenocarcinoma, for the liver to be the only site of metastatic disease. Surgical resection of hepatic metastases in patients with colorectal cancer and carefully selected patients with other types of primary tumors with liver-only metastases can result in a significant long-term survival benefit in 20% to 35% of patients. 14–17 Further, prolongation of survival and significant palliation of symptoms may occur in many patients in whom cancer recurrence subsequently develops after resection of hepatic metastases. Once again, however, less than 10% to 15% of patients with liver-only solid tumor metastases are candidates for resection because of the presence of bilobar disease in which resection would sacrifice too great a volume of normal hepatic parenchyma; potentially unfavorable biology with the presence of more than four metastases; or tumor proximity to major vascular or biliary structures where at least a 1-cm tumor-free margin cannot be achieved. 18,19 Thus, for the majority of patients with primary or metastatic hepatic malignancies who are not candidates for surgical resection, novel treatment approaches to control and potentially to cure the liver disease must be explored.
Localized application of thermal energy produces destruction of tumor cells. When tumor cells are heated above 45° to 50°C, intracellular proteins are denatured and cell membranes are destroyed through dissolution and melting of lipid bilayers. 20–23 Thus, unlike cryoablation, thermal ablation produces direct cytodestruction in treated tissues. Radiofrequency ablation (RFA) is a localized thermal treatment technique designed to produce tumor destruction by heating tumor tissue to temperatures that exceed 50°C. In this study, we report our initial results in 123 patients with primary or metastatic hepatic malignancies treated with RFA of their liver tumors.
PATIENTS AND METHODS
We performed this prospective, nonrandomized study between January 1, 1996, and March 31, 1998. All patients with histologically confirmed primary or metastatic hepatic malignancies with no clinical, radiographic, or intraoperative evidence of extrahepatic disease were eligible for treatment on the RFA protocol, which was approved by the institutional review boards of both participating centers. All patients signed written informed consent as mandated by institutional policies. All patients were deemed to have unresectable hepatic disease based on number or bilobar location of tumors (although some patients did undergo resection of disease in one lobe and RFA of tumor in the remaining lobe), tumor proximity to major vascular structures precluding a margin-negative resection, and/or the presence of cirrhosis with a functional hepatic reserve inadequate to tolerate major hepatic resection. Patients were eligible for this study regardless of whether they had failed to respond to all other therapeutic modalities, and if they:
• Had not received chemotherapy or radiation therapy for at least 4 weeks before RFA
• Had a life expectancy of ≥3 months with a Zubrod performance status of ≤1
• Had Child class A or B cirrhosis (class C excluded)
• Had a serum total bilirubin level <3.0 mg/dl, a serum creatinine ≤2.0 mg/dl, a serum albumin level >3.0 g/dl, and a prothrombin time not >50% elevated above normal
• Had no history of hepatic encephalopathy
• Had no altered mental status
• Had minimal or no ascites
• Had no active infection
• Had a negative serum pregnancy test (for women of childbearing potential).
Patients were considered for RFA even if they had tumor abutting a major portal or hepatic vein branch or the inferior vena cava, but they were excluded if tumor involved the main right or left bile duct (or both) because of the probability of destruction of the major bile ducts by RFA.
All patients underwent a baseline evaluation, including a history and physical examination; serum laboratory tests consisting of a complete blood count, platelets, coagulation profile, renal panel, electrolytes, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), alkaline phosphatase, total bilirubin, and serum tumor markers (as appropriate) such as alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA); computed tomography (CT) or magnetic resonance imaging (MRI) scan of the abdomen and pelvis; and a chest radiograph. All patients had histologic confirmation of hepatic malignancy from prior intraoperative biopsy or from CT- or ultrasound-guided percutaneous fine-needle aspiration. The same battery of serum blood tests was obtained 1 and 7 days after the RFA procedure, then again 1 month after treatment. At 1 month and then every 3 months up to 2 years after treatment, a CT or MRI scan of the abdomen, a chest radiograph, and serum laboratory tests were obtained.
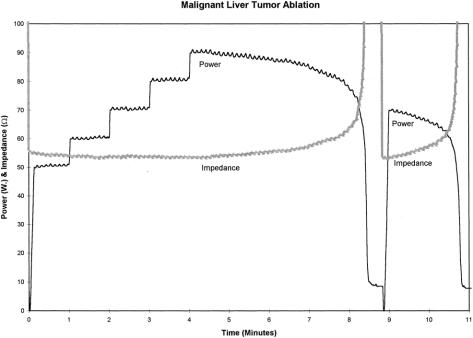
Patients with only one or two small (<3.0 cm in diameter) cancers located peripherally in the liver were considered for percutaneous ultrasound-guided RFA. All other patients were treated surgically during an open operative procedure. Intraoperative ultrasonography was used to place the RF needle into the lesions to be treated. The patients in this series were treated using the RF 2000 generator system produced by RadioTherapeutics Corp. (Mountain View, CA). The RF 2000 system consists of a generator that supplies up to 100 W of power, a LeVeen monopolar array needle electrode, and an indifferent dispersive electrode pad applied to the patient’s skin (much like the grounding pads used for hemostatic electrocautery during surgical procedures). The LeVeen needle electrode (Fig. 1) is a 15-gauge, 12- to 15-cm-long insulated cannula that contains ten individual hook-shaped electrode arms that are deployed in situ after ultrasound-guided placement of the needle electrode into the liver tumor. For tumors <3.0 cm in diameter, the multiple array is deployed into the center of the tumor. For larger lesions, the array is first deployed at the most posterior interface (ultrasonographically) between tumor and normal liver parenchyma and subsequently withdrawn and redeployed at 2.5- to 3.0-cm intervals within the tumor. Optimal positioning of the electrode permits complete destruction of tumor and at least a 1-cm zone of normal liver parenchyma. After deployment of the multiple array, the initial power is applied at 50 W and then increased in 10-W increments at 1, 2, 3, and 4 minutes to a maximum power of 90 W. Treatment continues until power “roll-off” occurs, indicating a precipitous drop in power output as tissue impedance increases markedly from coagulative necrosis. After a 20-second pause, power is reapplied at 75% of the maximum power achieved until power roll-off again occurs (Fig. 2). Thus, each tumor or area within a large tumor is treated with a two-phase application of RF power before retracting the multiple array and repositioning or removing the needle electrode. During treatment, the area of tissue ablation is monitored with ultrasonography to measure the zone of increased echogenicity corresponding to coagulation of the tissue.

Figure 1. LeVeen needle electrodes showing the multiple array retracted into the needle sheath (left) and fully deployed (right) from the needle tip. The ten individual tines of the multiple array are clearly seen with the array deployed to the full 3.5-cm diameter.
Figure 2. A graphic display of power (in watts) and tissue impedance (in ohms) during RFA of a malignant liver tumor. Using ultrasound guidance, the LeVeen needle electrode is placed into the tumor and the multiple array is deployed. Treatment is then initiated at 50 W of power and increased by 10 W at 1-minute intervals up to a maximum power of 90 W. At 8 minutes, RFA-induced coagulative necrosis of the tumor is occurring; the tissue impedance at this point rapidly rises to more than 200 ohms and the power output precipitously falls (roll-off). After waiting 20 seconds to allow heat in the tumor to dissipate, RF energy is again applied at approximately 75% (70 W) of the maximum power achieved, until tissue impedance again rises and power rolls off. This two-phase application of RF energy is performed in each area treated with the LeVeen multiple array needle electrode.
For RFA treatments performed during an open surgical procedure, once 90 W of power is reached, 2 to 3 minutes of vascular inflow occlusion is performed by clamping the hepatic artery and portal vein in the porta hepatis (Pringle maneuver). 24 This facilitates attaining roll-off and increases the size of the zone of coagulative necrosis. 25
All patients were followed after treatment to observe any acute or long-term complications related to RFA. CT or MRI scans and chest radiographs were used to detect evidence of residual or recurrent tumor in treated lesions and to monitor for the development of new hepatic or extrahepatic metastatic disease.
Analysis of the statistical significance of differences between groups of data was performed using a Wilcoxon signed rank test, with a confidence interval of 0.95 deemed significant.
RESULTS
A total of 123 patients have been treated on the RFA protocol during the study period; no patients have been lost to follow-up. There were 71 men (57.7%) and 52 women (42.3%), with a median age of 57 years (range 24 to 80 years). RFA treatment was performed in 59 patients at The University of Texas M. D. Anderson Cancer Center and in 64 patients at the G. Pascale National Cancer Institute. The primary cancer diagnoses in these patients are shown in Table 1. RFA was used to treat primary liver cancer in 48 patients (39.1%); 75 patients (60.9%) were treated for metastatic lesions. All 48 of the patients with HCC were cirrhotic; 24 were Child class A and 24 were class B. Only 8 of the 48 patients with HCC (16.7%) had received chemotherapy before RFA, but 70 of the 75 patients with liver metastases (93.3%) had been previously treated with chemotherapy specifically for the metastatic disease.
Table 1. Primary Cancer Diagnosis in 123 Patients Treated with Radiofrequency Ablation of Malignant Hepatic Tumors
A total of 169 malignant liver tumors were treated with RFA in these 123 patients. RFA was used to treat a single tumor in 95 patients (77.2%), two tumors in 14 patients (11.4%), three tumors in 11 patients (9.0%), four tumors in 2 patients (1.6%), and five tumors in a single patient (0.8%). In 18 patients, 17 who underwent RFA of one lesion and 1 who underwent RFA of two lesions, there were actually more lesions in the liver than were treated with RFA. These 18 patients were treated with resection of tumor in one lobe of the liver and RFA of tumor in the opposite lobe (Fig. 3).
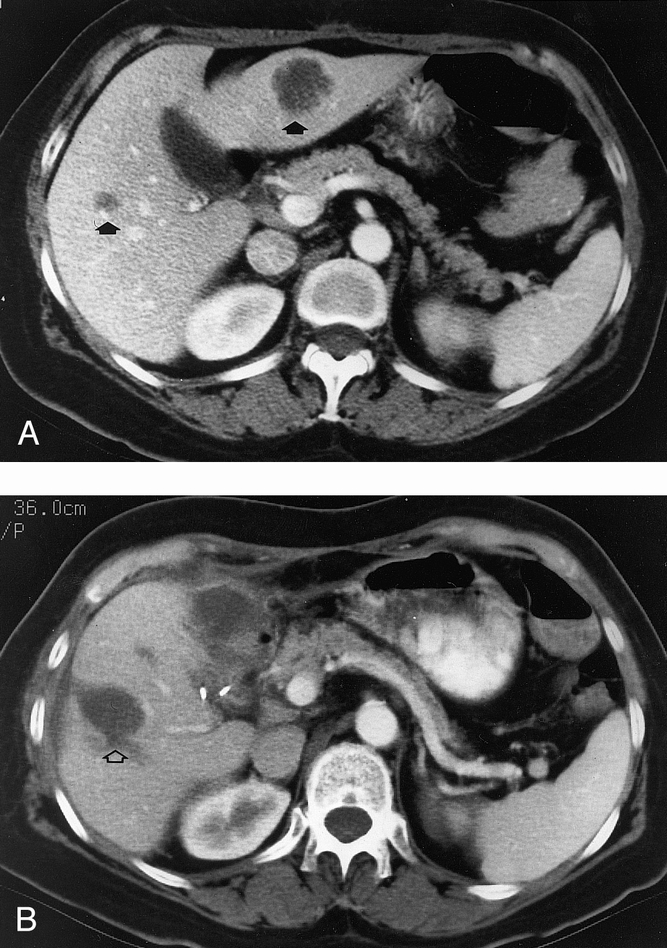
Figure 3. (A) Pretreatment CT scan in a patient with two colorectal cancer liver metastases (arrows). (B) CT scan 6 months after resection of the left lobe tumor and RFA of the right lobe tumor. The RFA lesion (open arrow) is larger than the original treated tumor; RFA treatment is planned in each tumor to coagulate the tumor and an approximately 1.0-cm rim of surrounding hepatic parenchyma. The intrahepatic cystic area at the site of RFA remains unchanged or involutes slightly on CT or MRI scans performed as long as 2 years after treatment.
The diameter of each tumor was measured in three dimensions by ultrasonography before RFA. The median greatest diameter of the tumors treated by RFA was 3.4 cm, with a range of 0.5 to 12.0 cm (Fig. 4). The goal of RFA treatment is to destroy the entire gross tumor and a margin of normal hepatic parenchyma—thus, the area of necrosis seen on the postablation CT scan is larger than the original tumor (see Fig. 3). Percutaneous RFA was used in 31 patients (25.2%), but general anesthesia was required during treatment in more than one third because of severe pain when the RF current was applied. The 31 patients treated with percutaneous RFA included 26 patients with a solitary, small (<3 cm in diameter), peripherally located HCC and cirrhosis (Child class B in 23 and class A in 3) who were high-risk surgical candidates because of their cirrhosis, and 5 patients with a solitary liver metastasis who preferred percutaneous treatment. Intraoperative RFA was performed in 92 patients (74.8%). Open surgical treatment was used in patients with multiple tumors, bilobar tumors in which the disease in one lobe was resected and tumor in the opposite was treated with RFA, large tumors, or tumors near major intrahepatic blood vessels. The median greatest diameter of tumors treated percutaneously (2.4 cm) was significantly less than that of tumors treated during a surgical procedure (3.8 cm, p < 0.01). The tumor treated by RFA was on or within 5 mm of a major portal and/or hepatic vein branch (defined as the primary vessel or one of its first large bifurcation vessels) or the inferior vena cava in 121 of the 169 tumors (71.6%, Fig. 5).
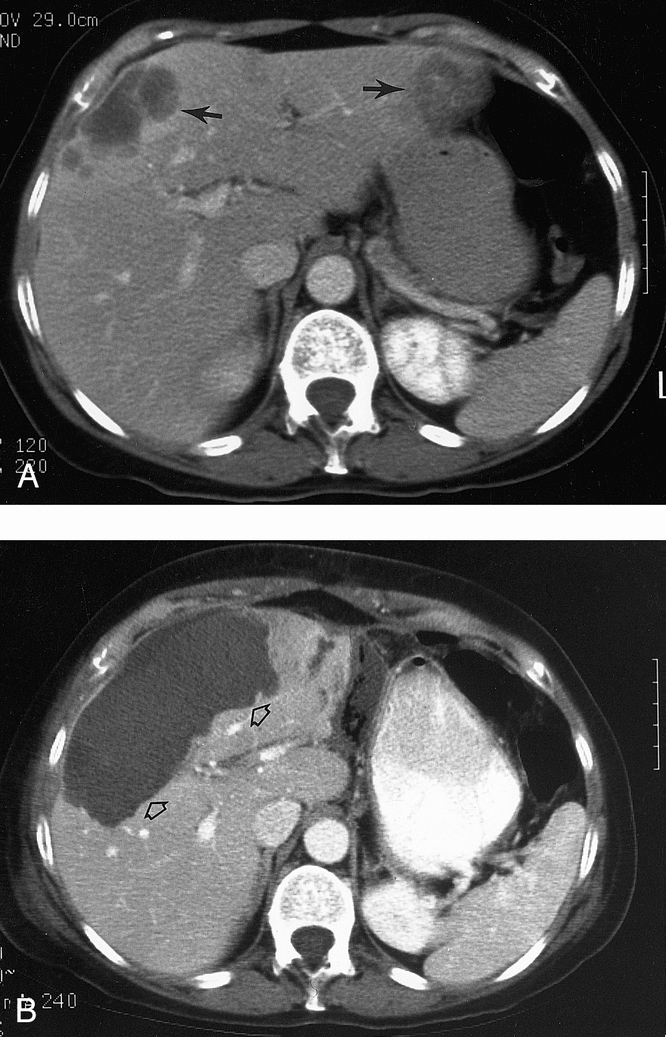
Figure 4. (A) Pretreatment CT scan in a patient with two large liver metastases (arrows) from colorectal cancer. (B) CT scan 1 month after resection of the left lobe lesion and RFA of the right lobe tumor that measured 11.0 × 8.0 × 6.5 cm. There is complete necrosis of the large right lobe lesion (open arrows) that was treated with multiple intraoperative applications of RF energy to all areas of the tumor.
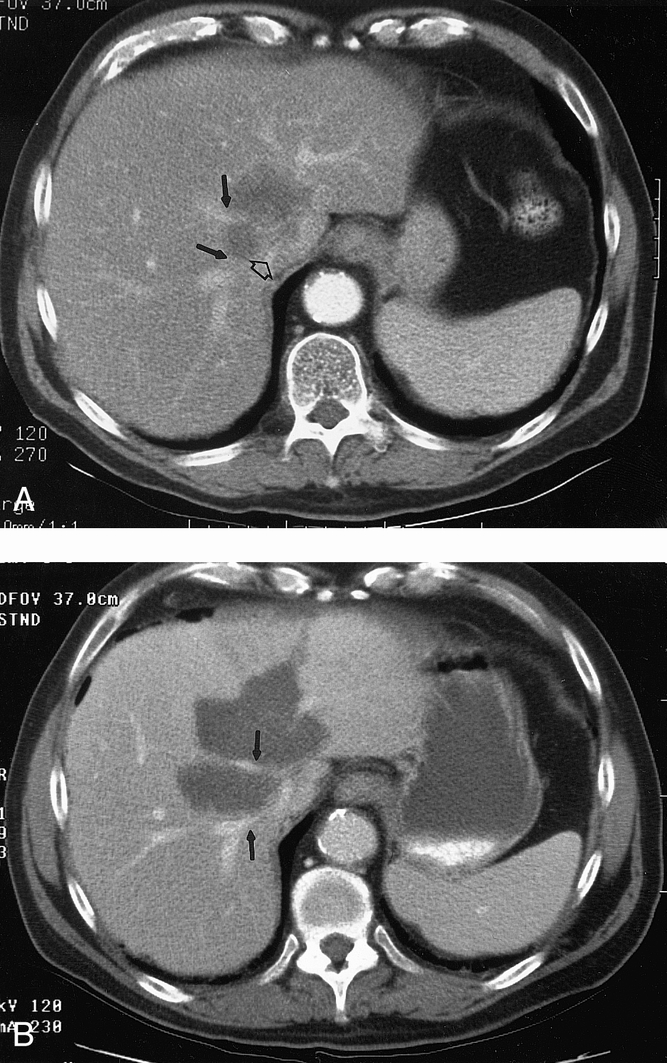
Figure 5. (A) Pretreatment CT scan in a patient with HCC near the inferior vena cava (open arrow) and the right and middle hepatic veins (solid arrows). (B) CT scan 3 months after intraoperative RFA of the liver tumor. Again, the RFA lesion is larger than the original treated tumor. Complete necrosis of tumor is noted, but the right and middle hepatic veins (solid arrows) are patent.
There have been no deaths after RFA treatment in these 123 patients. Hepatic insufficiency, renal insufficiency, or a coagulopathy has not developed in any patient after RFA treatment. The serum liver function tests (ALT, AST, GGT) that were elevated 24 hours after RFA returned to baseline values in most patients by day 7 and in all patients by day 30. Serum bilirubin levels rose transiently to 2.0 to 3.0 mg/dl in four patients (3.3%) but returned to normal within 2 weeks of the RFA treatment. Two patients who underwent resection of tumor in one lobe and RFA of tumor in the opposite lobe required percutaneous drainage of a perihepatic abscess at the site of the hepatic resection. There have been no hepatic abscesses in the tumors treated with RFA.
The only other complication after RFA was hemorrhage into the treated tumor 5 days after surgery in a patient with Child class B cirrhosis and HCC. The patient was treated with transarterial embolization of the right hepatic artery and required transfusion with two units of packed red blood cells. In general, bleeding from the needle track occurs infrequently because of the complete tissue coagulation with RFA. Intraoperative bleeding from the RFA needle track was noted in 29.8% of the needle electrode withdrawals; however, in all cases it was minimal (<5 cc) and controlled easily with cauterization of the liver capsule puncture site.
The median follow-up in these 123 patients is 15 months. Tumor has recurred at the site of RFA in 3 of the 169 treated tumors (1.8%); two of these tumors were >6 cm in diameter (one HCC and one colorectal cancer metastasis), and the third (a colorectal metastasis) was located near the inferior vena cava between the right and middle hepatic veins. In the first two patients, the recurrence at or near the periphery of the lesion treated with RFA was diagnosed at the same time new liver metastases were diagnosed. New hepatic or extrahepatic metastatic disease has developed in 34 patients (27.6%). The liver was the first site of failure in 27 of these 34 (79.4%). In two of these patients, the new disease was a solitary liver metastasis that was treated with RFA. The development of new metastatic disease is consistent with results noted from serum tumor marker studies. A serum tumor marker (AFP or CEA) was elevated before RFA treatment in 105 of the 123 patients (85.4%). After RFA, serum tumor markers returned to normal in 76 of the 105 (72.4%). Most of the patients in whom new metastatic lesions have developed are among the group whose serum tumor markers did not return to normal after RFA.
DISCUSSION
RF electrocautery devices have been used for more than 70 years to achieve direct tissue ablation, usually for hemostasis during surgical procedures. Advances in electrocautery technology led to the development of monopolar and bipolar tissue ablation devices designed to destroy larger areas of tissue, particularly malignant tumors. 20–22 Only tissue through which RF electrical current passes directly is heated above a cytotoxic temperature. The tissue temperature in the tumor to be ablated can be controlled between 50° and 100°C by increasing the RF power and current delivered. For monopolar tumor ablation, a needle electrode is placed in the tumor using a percutaneous or intraoperative approach, and an indifferent dispersive electrode pad is applied to the patient’s outer skin surface. The geometry of the RF current pathway around the ablation electrode creates a relatively uniform zone of radiant/conductive heat within the first few millimeters of electrode-tissue interface. The conductive heat emitted from the tissue uniformly radiates out from the electrode, and if the tissue impedance is relatively low, a dynamic expanding sphere of ablated tissue is created. The final size of the sphere of heat-ablated tissue is proportional to the square of the RF current, also known as the RF power density. The RF power/current delivered using a monopolar electrode decreases in proportion to the square of the distance from the electrode. Therefore, the tissue temperature falls rapidly with increasing distance away from the electrode.
The decrease in tissue heating with increasing distance away from the electrode results in only 1.0- to 1.5-cm spheres of tumor tissue ablation when using monopolar simple needle electrodes. 21,22 New needles (15 to 18 gauge in diameter) have been developed with multiple array hook electrodes, like the LeVeen needle we used. The needle electrode shaft is placed into the tumor with the array retracted. Using real-time ultrasound guidance, the array is then deployed from the needle tip into the tumor. These deployed multiple array hooks create a series of electrodes with a diameter up to 4 cm across which the RF current can be passed. Using an RF current generator with a 50- to 90-W power output for 5 to 15 minutes, a 4.0- to 5.0-cm-diameter tumor can be ablated with the hook electrodes fully deployed.
The multiple array electrode is one technologic innovation that permits ablation of much larger zones of tissue than simple needle electrodes. Other RFA techniques to achieve the same goal are being investigated. One strategy to enlarge the zone of tissue ablation is to infuse standard 0.9% saline or hypertonic 5% saline solution through the needle electrode during RFA. 26–29 The infused saline solution acts as a liquid electrode to increase the area of RF current conduction around the needle tip. Another technique involves the use of cooled electrodes. 30–34 If RFA is performed with immediate application of high power, the tissue heats and desiccates too rapidly. The resultant tissue coagulum markedly reduces the propagation of RF current and heat through the tissue, thus yielding a smaller zone of coagulative necrosis. The cooled electrodes slow the heating of tissue in the immediate area around the electrode tip, which ultimately results in a 3- to 4-cm zone of distribution of RF current and heat. We have not encountered problems with limited heat distribution because our RF protocol initiates treatment at 50 W of power with 10-W advances up to 90 W; starting at a lower power with incremental increases produces gradual heating of tissue around the multiple array and does not impede propagation of RF current and heat.
The majority of patients in our study underwent RFA of hepatic tumors during an open surgical procedure. This is our preferred approach in patients with large tumors (>4 to 5 cm in diameter), multiple tumors, or tumor that abuts a major intrahepatic blood vessel. In contrast to percutaneous RFA treatments, it is possible to perform temporary occlusion of hepatic inflow during the intraoperative RFA procedure. Hepatic inflow occlusion facilitates RFA of large or hypervascular tumors and tumors near blood vessels. The amount of blood flow to a tumor is known to be a critical determinant of temperature response to a given increment of heat. 35,36 Because heat loss or cooling effect is principally dependent on blood circulation in a given area, temperature response and blood flow are inversely related. By temporarily occluding hepatic inflow during RFA, the cooling effect of blood flow on perivascular tumor cells is minimized. 37 The inflow occlusion increases the size of the zone of coagulative necrosis and enhances the likelihood of complete tumor cell kill, even if tumor abuts a major intrahepatic blood vessel. Our previous preclinical work demonstrated that RFA treatment combined with vascular inflow occlusion can produce complete circumferential necrosis of tissue around major portal or hepatic vein branches without damaging the integrity of the vessel wall. 25
Cryoablation has been used to treat otherwise unresectable primary and metastatic liver cancers. Studies have demonstrated that liver tumors must be cooled to at least −35°C throughout the entire tumor to achieve a reliable tumor cell kill. 38,39 Tumor cell death is not a direct consequence of lowering tissue temperature, but rather is caused by ice crystal formation during rapid freezing, with resultant destruction of normal cellular structures. The low temperature necessary for tumor cell destruction with cryoablation is difficult to achieve at the periphery of tumors >5 cm in diameter, when the tumor abuts a major intrahepatic branch of the portal or hepatic veins, or if it lies near the inferior vena cava. Also, the complication rates associated with cryoablation of hepatic tumors are high.
Based on our experience with RFA of liver tumors, an apparent advantage of RFA over cryoablation is the much lower treatment-related complication rate. Complications described after hepatic cryoablation include up to a 4% death rate, significant intraoperative hemorrhage, cold injury in adjacent organs, biliary fistulas, coagulopathies, acute renal failure, intrahepatic abscess in the cryolesion, and symptomatic pleural effusions. 38,39 The overall complication rate after cryoablation ranges from 15% to 50%. 38,39 In contrast, we had no posttreatment deaths in our 123 patients. The only treatment-related complications were two perihepatic abscesses associated with concomitant hepatic resection, and hemorrhage into a treated lesion in a single patient with Child class B cirrhosis and HCC (overall complication rate 2.4%). There were no episodes of heat injuries to adjacent organs, renal failure, coagulopathy, intrahepatic abscess, symptomatic pleural effusion, or intraoperative bleeding. We avoided RFA-induced biliary fistulas by excluding patients with tumors involving the perihilar region.
Our group of 123 patients treated with RFA of malignant liver tumors is the largest series reported to date. The other recent reports contain ≤30 patients and generally describe percutaneous ultrasound- or CT-guided RFA of small, peripheral liver metastases or HCC. 40–43 We also use a percutaneous, ultrasound-guided approach to treat patients with small, readily accessible primary or metastatic liver cancers. However, these selected patients treated percutaneously represent the minority of patients who may receive a survival and/or palliative benefit from operative RFA of large, multiple, perivascular, or otherwise inaccessible liver malignancies.
Obviously, we are evaluating RFA of hepatic malignancies in the hope that a significant proportion of the treated patients will achieve long-term survival. However, as with other local techniques, including liver resection and cryoablation, the majority of patients are not likely to be cured by RFA alone. At present, our 1.8% local recurrence rate in the tumors treated by RFA is lower than the 10% to 15% local recurrence rates reported after cryoablation. 38,39 Longer follow-up in our patients is needed before we can validate this difference. More importantly, in 27.6% of our patients new sites of clinically detectable metastatic disease have already developed, and that number is certain to rise with extended follow-up. Resection, RFA, or cryoablation of liver tumors will not cure most patients with primary or metastatic malignant disease, although long-term disease-free survival rates of 20% to 40% justify such an aggressive, invasive approach in patients with liver-only disease.
We are very encouraged by our initial experience with RFA as a treatment for malignant liver tumors because it is safe, well tolerated, associated with few complications, and usually effective in controlling grossly or ultrasonographically evident liver tumors. We are initiating a trial combining RFA of hepatic malignancies with regional and systemic chemotherapy in hopes that such a multimodality treatment approach will reduce hepatic and extrahepatic recurrence rates and thus enhance long-term survival rates.
Footnotes
Correspondence: Steven A. Curley, MD, Dept. of Surgical Oncology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Box 106, Houston, TX 77030.
Accepted for publication October 8, 1998.
References
- 1.Beasley R. Hepatitis B virus: the major etiology of hepatocellular carcinoma. Cancer 1988; 61: 1942–1956. [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie A. Hepatocellular carcinoma: molecular biology of its growth and relationship to hepatitis B virus infection. Med Clin North Am 1989; 73: 985–995. [DOI] [PubMed] [Google Scholar]
- 3.Arya S, Ashraf S, Parande C, et al. Hepatitis B and delta markers in primary hepatocellular carcinoma patients in the Gizan area of Saudi Arabia. APMIS Suppl 1988; 3: 30–34. [PubMed] [Google Scholar]
- 4.Bridbord K. Pathogenesis and prevention of hepatocellular carcinoma. Cancer Detect Prev 1989; 14: 191–192. [PubMed] [Google Scholar]
- 5.Di Bisceglie A, Rustgi V, Hoofnagle J, et al. NIH conference on hepatocellular carcinoma. Ann Intern Med 1988; 108: 390–401. [DOI] [PubMed] [Google Scholar]
- 6.Tsuzuki T, Sugioka A, Ueda M. Hepatic resection for hepatocellular carcinoma. Surgery 1990; 107: 511–520. [PubMed] [Google Scholar]
- 7.Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg 1990; 211: 277–284. [PMC free article] [PubMed] [Google Scholar]
- 8.Choi TK, Lai Edward CS, Fan ST, et al. Results of surgical resection for hepatocellular carcinoma. Hepatogastroenterology 1990; 37: 172–175. [PubMed] [Google Scholar]
- 9.Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery 1989; 106: 740–748. [PubMed] [Google Scholar]
- 10.Paquet K-J, Koussouris P, Mercado MA, et al. Limited hepatic resection for selected cirrhotic patients with hepatocellular or cholangiocellular carcinoma: a prospective study. Br J Surg 1991; 78: 459–462. [DOI] [PubMed] [Google Scholar]
- 11.Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinoma: results in 72 European patients with cirrhosis. Gastroenterology 1990; 98: 733–738. [PubMed] [Google Scholar]
- 12.Rustgi V. Epidemiology of hepatocellular cancer. Gastroenterol Clin North Am 1987; 16: 545–551. [PubMed] [Google Scholar]
- 13.Weiss L, Grundmann E, Torhorst J, et al. Hematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol 1986; 150: 195–203. [DOI] [PubMed] [Google Scholar]
- 14.Hughes KS, Simon R, Songhorabadi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery 1988; 103: 278–284. [PubMed] [Google Scholar]
- 15.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997; 15: 938–946. [DOI] [PubMed] [Google Scholar]
- 16.Blumgart LH, Fong Y. Surgical options in the treatment of hepatic metastasis from colorectal cancer. Curr Probl Surg 1995; 32: 333–421. [DOI] [PubMed] [Google Scholar]
- 17.Tuttle T. Hepatectomy for noncolorectal liver metastases. In: Curley SA (ed). Liver cancer. New York: Springer-Verlag; 1998: 201–211.
- 18.Adson MA, Van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119: 647–651. [DOI] [PubMed] [Google Scholar]
- 19.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Ann Surg 1989; 210: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi S, Fornari F, Paties C, Buscarini L. Thermal lesions induced by 480-KHz localized current field in guinea pig and in pig livers. Tumori 1990; 76: 54–57. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez H, vanSonnenberg E, D’agostine H, et al. Percutaneous tissue ablation by radiofrequency thermal energy as a preliminary to tumor ablation. Minim Invasive Ther 1993; 2: 299–305. [Google Scholar]
- 22.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990; 25: 267–270. [DOI] [PubMed] [Google Scholar]
- 23.Lounsberry W, Goldschmidt V, Linke C. The early histologic changes following electrocoagulation. Gastrointest Endosc 1995; 41: 68–70. [DOI] [PubMed] [Google Scholar]
- 24.Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections. Ann Surg 1989; 209: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curley SA, Davidson BS, Fleming RYD, et al. Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endoscopy 1997; 11: 729–733. [DOI] [PubMed] [Google Scholar]
- 26.Miao Y, Ni Y, Mulier S, et al. Ex vivo experiment on radio-frequency liver ablation with saline infusion through a screw-tip cannulated electrode. J Surg Res 1997; 71: 19–24. [DOI] [PubMed] [Google Scholar]
- 27.Hoey MF, Mulier PM, Shake JG. Intratumoral ablation using radiofrequency energy via screw-tip catheter and saline electrode. PACE 1995; 18 (II): 917. [Google Scholar]
- 28.Leveillee RJ, Hoey MF, Hulbert JH, et al. Enhanced radiofrequency ablation of canine prostate utilizing a liquid conductor: the virtual electrode. J Endourol 1996; 10 (1): 5–11. [DOI] [PubMed] [Google Scholar]
- 29.Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 1997; 202: 205–210. [DOI] [PubMed] [Google Scholar]
- 30.Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol 1996; 3: 556–563. [DOI] [PubMed] [Google Scholar]
- 31.Lorentzen T, Christensen NE, Nolsle CP, et al. Radiofrequency tissue ablation with a cooled needle in vitro: ultrasonography, dose response, and lesion temperature. Acad Radiol 1997; 4: 292–297. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol 1996; 3: 636–644. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, McLoud TC. Radio-frequency tissue ablation of VX2 tumor nodules in the rabbit lung. Acad Radiol 1996; 3: 929–935. [DOI] [PubMed] [Google Scholar]
- 34.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology 1997; 205: 367–373. [DOI] [PubMed] [Google Scholar]
- 35.Patterson J, Strang R. The role of blood flow in hyperthermia. Int J Radiat Oncol Biol Phys 1979; 5: 235–242. [DOI] [PubMed] [Google Scholar]
- 36.Kolios MC, Sherar MD, Hunt JW. Large blood vessel cooling in heated tissues: a numerical study. Phys Med Biol 1995; 40: 477–494. [DOI] [PubMed] [Google Scholar]
- 37.Sturesson C, Liu DL, Stenram U, et al. Hepatic inflow occlusion increases the efficacy of interstitial laser-induced thermotherapy in rats. J Surg Res 1997; 71: 67–72. [DOI] [PubMed] [Google Scholar]
- 38.Quebbeman EJ, Wallace JR. Cryosurgery for hepatic metastases. In: Condon RE (ed). Current techniques in general surgery. New York: Mosby; 1997: 1–75.
- 39.Gagne DJ, Roh MS. Cryosurgery for hepatic malignancies. In: Curley SA (ed). Liver cancer. New York: Springer-Verlag; 1998: 173–200.
- 40.Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J Intervent Radiol 1993; 8: 97–103. [Google Scholar]
- 41.Rossi S, Di Stasi M, Buscarini E. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am 1995; 1: 72–81. [PubMed] [Google Scholar]
- 42.Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am J Roentgenol 1996; 167: 759–768. [DOI] [PubMed] [Google Scholar]
- 43.Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology 1997; 202: 195–203. [DOI] [PubMed] [Google Scholar]