Abstract
Objective
The long-term outcomes of patients undergoing local excision with or without pelvic irradiation were examined to define the role of adjuvant irradiation after local excision of T1 and T2 rectal cancers.
Methods
Ninety-nine patients with T1 or T2 rectal cancers underwent local excision with or without adjuvant irradiation at Massachusetts General Hospital and Emory University Hospital between January 1966 and January 1997. Of these, 52 patients were treated by local excision alone and 47 patients by local excision plus adjuvant irradiation. Twenty-six of these 47 patients were treated by irradiation in combination with 5-fluorouracil chemotherapy. The outcomes of these groups were compared.
Results
The 5-year actuarial local control and recurrence-free survival rates were 72% and 66%, respectively, for the local excision alone group and 90% and 74%, respectively, for the adjuvant irradiation group. This improvement in outcome was evident despite the presence of a higher-risk patient population in the adjuvant irradiation group. Adverse pathologic features such as poorly differentiated histology and lymphatic or blood vessel invasion decreased local control and recurrence-free survival rates in the local excision only group. Adjuvant irradiation significantly improved 5-year outcomes in patients with high-risk pathologic features. Four cases of late local recurrence were seen at 64, 72, 86, and 91 months in the adjuvant irradiation group.
Conclusions
The authors recommend adjuvant chemoradiation for all patients undergoing local excision for T2 tumors, and for T1 tumors with high-risk pathologic features. The four cases of late local failures beyond 5 years in the adjuvant irradiation group underscores the need for careful long-term follow-up in these patients.
For selected rectal cancers, local excision with or without pelvic irradiation is an alternative to radical surgery such as low anterior resection and abdominoperineal resection. 1,2 Local excision may be carried out using a transanal approach, a posterior transsphincteric (York-Mason) approach, or a posterior proctotomy (Kraske procedure). Local excision alone, however, may not suffice for patients with tumors demonstrating features associated with higher rates of local failure or spread to regional lymph nodes. The treatment of tumors with lymphatic or blood vessel invasion, poorly differentiated histology, or positive surgical margins may be optimized by adjuvant irradiation. In this study, the long-term outcomes of patients undergoing local excision of T1 and T2 rectal cancers were examined to define the role of adjuvant irradiation.
METHODS AND MATERIALS
From January 1966 to January 1997, 99 patients with T1 and T2 rectal cancers underwent local excision at the Massachusetts General Hospital and Emory University Hospital. The mean and median ages of the patients were 67 and 68 years, respectively (range 38 to 91). There were 54 men and 45 women.
Fifty-two patients were treated by local excision (LE) alone and 47 patients by LE plus adjuvant irradiation (LE + EBRT). Of these 47, 21 patients were treated by LE + EBRT, and 26 patients were treated by LE + EBRT + 5-fluorouracil (5-FU) chemotherapy. Surgical procedures included excision using a transanal or transsphincteric approach (88 patients), excision through a midline posterior proctotomy (10 patients), and transanal fulguration (1 patient).
For the 47 patients treated by LE + EBRT, the mean dose was 53.6 Gy (range 45 to 64.8). Forty-five of the 47 patients received postoperative irradiation; the remaining two received preoperative irradiation. Thirty-eight of the 47 patients referred for adjuvant irradiation had T2 tumors or T1 tumors with high-risk pathologic features (poorly differentiated histology and/or lymphatic or blood vessel invasion). To the initial pelvic field, 45 Gy was delivered in 25 fractions using a four-field technique over 5 to 6 weeks. For the APA fields, the superior border of the field was placed between the L5/S1 vertebral bodies, with the field caudally extending 5 cm below the region excised and laterally 1.5 to 2.0 cm on the bony pelvis. For the lateral fields, the posterior border was placed 1.5 cm behind the sacrum; the anterior border was defined by placement of a vaginal probe in women and the location of the prostate in males.
The tumor volume was boosted with photons, protons, or interstitial implants. Boost doses >55 Gy were generally given for patients with tumor involvement of the surgical margins. The boost was delivered by interstitial implant or proton beam in an attempt to treat only involved rectum and adjacent tissues and spare contralateral and uninvolved rectal and pelvic tissues. Since 1986, it has been our policy to administer 5-FU chemotherapy with pelvic irradiation. Twenty-three patients received intravenous 5-FU (500 mg/m2) for 3 consecutive days during the first and last week of radiation treatment. These patients received continuous infusion 5-FU (225 mg/m2/24 hrs) during the entire course of irradiation.
Patients were evaluated for local failure, distant metastases, and survival after treatment. The mean and median follow-up times for both groups were 51 months from surgery (range 4 to 162). Actuarial recurrence-free survival (RFS) and local control (LC) rates were analyzed by the Kaplan–Meier method. These outcome parameters were assessed according to treatment, tumor stage, and pathologic features of tumor grade, lymphatic or blood vessel involvement, and margin status.
RESULTS
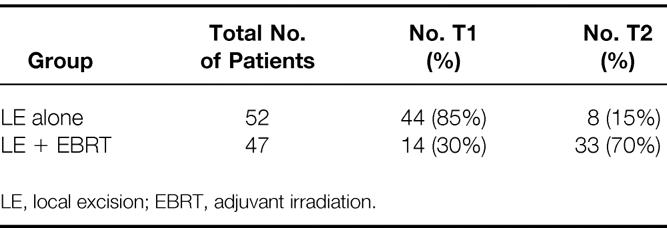
The 99 patients were analyzed according to treatment (i.e., patients undergoing LE with or without EBRT). The outcomes of patients receiving adjuvant treatment were further assessed based on whether they received 5-FU chemotherapy. The results are interpreted in view of the higher T-stage distribution and high-risk pathologic features of the patients in the irradiated group (Table 1). Seventy percent of the group receiving adjuvant irradiation were stage T2 versus only 15% of patients in the LE group. Seven of the 14 patients with T1 disease (50%) undergoing adjuvant irradiation had the high-risk pathologic features of lymphatic or blood vessel involvement or poorly differentiated histology, or positive or uncertain surgical margins.
Table 1. Stage Distribution
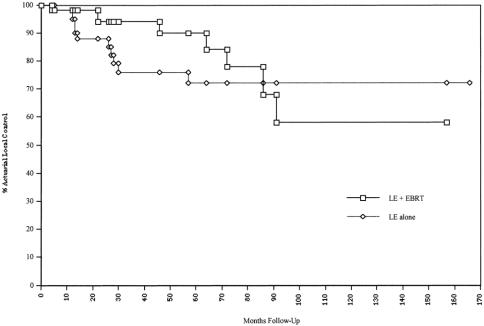
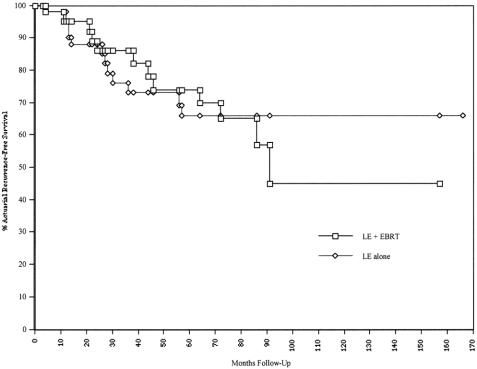
The 5-year actuarial LC and RFS rates by treatment group are shown in Table 2. The 5-year actuarial LC and RFS for T1/T2 patients in the LE group were 72% and 66%, respectively. The corresponding values for all patients undergoing LE + EBRT were 90% and 74%, respectively. Figures 1 and 2 show the actuarial LC and RFS rates for the LE group versus the LE + EBRT group.
Table 2. 5-Year Actuarial Treatment Outcomes
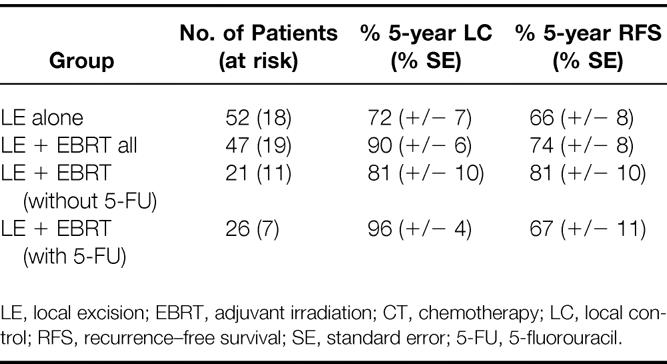
Figure 1. Actuarial local control rate.
Figure 2. Actuarial recurrence-free survival rate.
The differences in LC (p = 0.18) and RFS (p = 0.8) were not statistically significant. The results, however, are best interpreted in light of the higher T-stage distribution in the irradiated group. In the LE group, only 8 of the 52 patients had stage T2 tumors; in the LE + EBRT group, 33 of the 47 patients had stage T2 tumors.
An analysis of 5-year LC by T stage and treatment gives a better illustration of outcome in these two groups (Table 3). In the LE group, the 5-year actuarial LC rates were 89% for patients with T1 tumors and 33% for those with T2 tumors. In comparison, for the LE + EBRT group, the LC rates were 100% and 85%, respectively, for patients with T1 and T2 tumors. For patients with T2 tumors, adjuvant irradiation significantly improved the 5-year actuarial LC rate (p = 0.004).
Table 3. 5-Year Actuarial Local Control by Treatment and T Stage
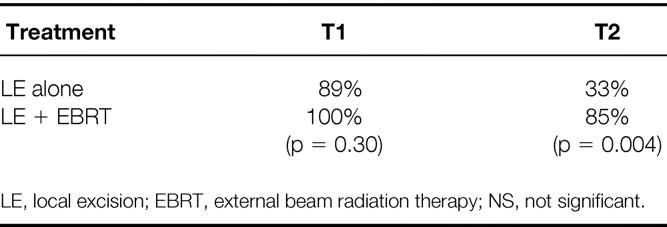
For T1 and T2 tumors, the 5-year actuarial RFS rates were 80% and 33%, respectively, for patients in the LE group. The corresponding values for the LE + EBRT group were 65% and 76%, respectively, for patients with T1 and T2 tumors. Adjuvant irradiation significantly improved the 5-year RFS rate for patients with T2 tumors (p = 0.02) compared with LE alone.
Subgroup analysis was performed on patients who received LE + EBRT versus LE + EBRT + 5-FU chemotherapy. Of the 21 patients treated by LE + EBRT, the LC and RFS rates were 81% and 81%, respectively. In the LE + EBRT + 5-FU group, the rates were 96% and 67%, respectively. The differences in LC (p = 0.15) and RFS (p = 0.35) by chemotherapy administration were not significantly different.
Of the 18 local recurrences in this series, 17 were mucosal and 1 was a one-nodal recurrence. Seven of the 18 patients with local failures had associated distant metastasis. Salvage information was available on 14 of the 18 patients with local failures. Nine of the 14 patients underwent abdominoperineal resection for salvage. Of these nine patients, four died of disease, three died of unrelated causes and were free of disease at their last follow-up, and two were alive and free of disease at 5 and 6 years after surgical salvage. One of the 14 patients underwent pelvic exenteration and later died of metastases. Three of the 14 patients had no further surgery and ultimately died of their cancer.
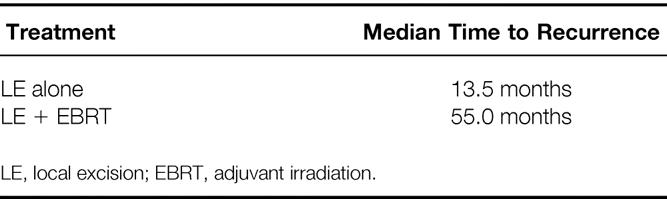
Table 4 shows the times to recurrence for the patients undergoing LE alone and those undergoing LE + EBRT with or without chemotherapy. The median time to recurrence was 13.5 months (range 4 to 57) in the LE group versus 55.0 months (range 26 to 91) in the LE + EBRT group. This difference demonstrates that patients with local failures in the LE + EBRT tended to fail later than their cohorts treated by LE alone.
Table 4. Recurrence Patterns
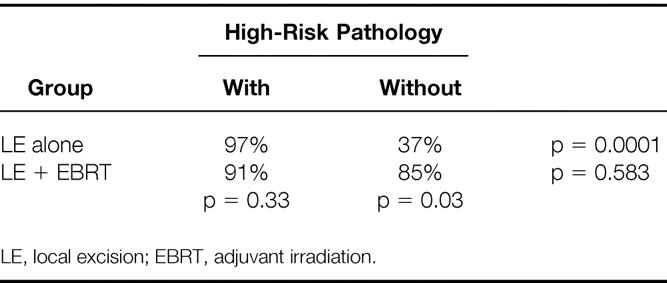
Subgroup analysis was performed on patient outcomes stratified by treatment and the presence of high-risk pathologic features. The high-risk group comprised all patients with T1/T2 tumors with lymphatic or blood vessel invasion and/or poorly differentiated histology. The low-risk group had tumors with none of these adverse pathologic features. The 5-year actuarial LC rate for the LE-alone group (Table 5) was 97% for those with low-risk pathology versus 37% for those with high-risk pathologic features (p = 0.0001). Interestingly, there was no significant difference in the 5-year LC rate within the LE + EBRT group stratified by the presence of high-risk pathologic features. For patients with tumors demonstrating high-risk pathology, the benefit of adjuvant irradiation becomes clear: the 5-year actuarial LC rate improved from 37% for patients treated by LE alone to 85% for patients treated by LE + EBRT (p = 0.03).
Table 5. 5-Year Actuarial Local Control by Treatment and Presence of High-Risk Pathology
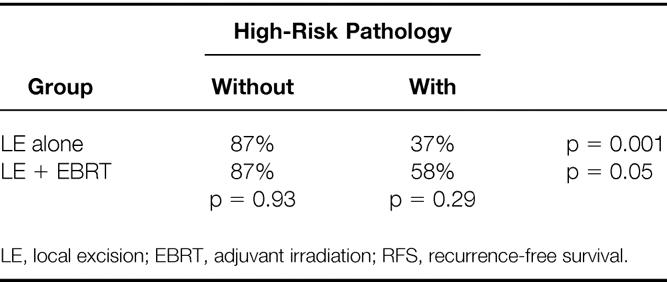
An analysis of 5-year actuarial RFS rates by treatment and the presence of high-risk pathology demonstrates similar trends (Table 6). Within the LE group, the 5-year actuarial RFS rate was only 37% in the high-risk group versus 87% in the low-risk group (p = 0.001). The difference in the 5-year RFS rate within the LE + EBRT group stratified by the presence of high-risk pathologic features was significant (p = 0.05). Adjuvant irradiation results in a trend toward improved 5-year RFS rates in tumors with high-risk pathologic features. The 5-year RFS rate improved from 37% for patients treated by LE alone to 58% for those treated by LE + EBRT (p = 0.29). It also becomes evident that distant metastasis is an important cause for failure in patients with high-risk pathology treated by LE + EBRT: the 5-year RFS rate was only 58% in this subset, whereas the LC rate was 85%.
Table 6. 5-Year Actuarial RFS by Treatment and Presence of High-Risk Pathologic Features
By margin status, 4 of the 52 patients in the LE alone group had positive margins. The actuarial 5-year LC rate was 38% versus 75% for those with negative margins (48 patients) in the LE alone group (p = 0.11). All six patients with positive margins had local control at 5 years in the LE + EBRT group; however, one patient had local failure after 5 years. Five of these six patients were treated by radiation doses >60 Gy. The 5-year LC rate for the 41 patients with negative margins in the LE + EBRT group was 88% (p = 0.76).
Four late local failures in the LE + EBRT group occurred at 64, 72, 86, and 91 months. The pathologic characteristics of the primary tumors of the late local failures were as follows:
• All tumors were stage T2.
• One tumor was poorly differentiated.
• Two tumors demonstrated lymphatic or blood vessel invasion.
• Two tumors exhibited no high-risk pathologic features.
Two of the four patients had local failure in association with distant metastases. As shown in Figure 1, the actuarial LC rate in the adjuvant irradiation group fell from 90% at 5 years to 57% at 8 years. In contrast, all the local failures in the LE alone group occurred within 5 years after treatment. Likewise, in the LE + EBRT group, the actuarial RFS rate fell from 74% at 5 years to 46% at 8 years (see Fig. 2). There were no serious treatment-related late effects (e.g., small bowel obstruction).
DISCUSSION
Choosing local excision instead of low anterior resection or abdominoperineal resection for rectal cancer treatment can be a difficult decision. Several issues emerge when considering this more conservative approach. The treatment outcomes must be compared with those of more standard approaches. To date, there have been no published randomized studies comparing local excision with abdominoperineal resection. In a retrospective study, the outcomes of patients treated by this conservative approach have been found to be comparable to those of abdominoperineal resection for patients with T1 and T2 tumors having favorable histologies. 3 The data from recent retrospective studies on patients undergoing LE procedures have been encouraging, with local control rates of 76% to 86% 4–8 (Table 7). A prospective randomized study comparing transanal excision with anterior resection for patients with ultrasound-staged T1N0 rectal cancers was recently reported. 9 Survival was equivalent in the two groups, although the patients who underwent the transanal procedure had less intraoperative blood loss and fewer complications.
Table 7. Multiinstitutional Data on Local Control and Survival Rates After Local Excision for Rectal Cancers
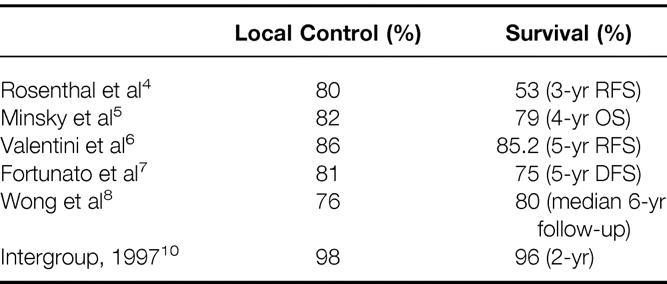
The preliminary results of a recent intergroup study on 113 patients with T1/T2 rectal cancers were recently reported. 10 Sixty of these patients had stage T1 disease and received no further treatment. Fifty-three patients had stage T2 disease and were treated by external beam irradiation to 54 Gy combined with 5-FU (500 mg/m2 intravenous bolus on days 1 to 3 and 29 to 31). After a median follow-up of 24 months, 2 of the 113 patients had isolated local recurrences; both patients are alive after undergoing subsequent resections. Four of the 113 patients analyzed had died of disease at this follow-up interval.
Of necessity, the present study is a retrospective analysis. It contains all the potential pitfalls of subgroup analysis. Also, the wide time interval in which patients were accrued, from 1966 to 1997, certainly introduces the possible perturbing influence of evolving surgical and radiotherapeutic techniques during that interval. In general, however, this study presents some of the longest follow-up available in a sizable group. Our data are consistent with previously reported studies with regard to 5-year LC and RFS rates for patients with T1/T2 tumors. The 5-year LC and RFS rates were 72% and 66%, respectively, for patients undergoing LE alone and 90% and 74%, respectively, for patients undergoing LE and adjuvant irradiation. This trend toward improved LC and RCS rates was evident in the patients treated with adjuvant irradiation, despite the presence of a higher T-stage distribution. Adjuvant irradiation significantly improved 5-year outcomes for patients with T2 tumors versus LE alone.
Another issue is identifying patients with risk factors for perirectal lymph node metastasis high enough to justify adjuvant treatment. Brodsky et al analyzed the pathologic features associated with increased risk of lymph node involvement in patients treated by radical resection. 11 The incidence of lymph node metastasis increased with T stage and exceeded 20% in patients with T2 lesions. Patients with lymphatic or blood vessel invasion were found to have a significantly higher incidence of lymph node metastasis. None of the patients with T1 tumors with an absence of lymphatic or blood vessel invasion had lymph node metastases. Patients with well-differentiated tumors were also found to have significantly lower rates of lymph node metastases.
In the current series, the presence of lymphatic or blood vessel invasion and/or poorly differentiated histology significantly lowered the 5-year actuarial LC and RFS rates in the LE alone group. In the LE alone group, the 5-year actuarial LC rates were 97% and 37%, respectively, for the low- and high-risk groups (p = 0.0001). Likewise, in the LE alone group, the 5-year RFS rates were 87% and 37%, respectively, for the low- and high-risk groups (p = 0.001). Adjuvant irradiation improved the 5-year actuarial LC rate from 37% in the LE alone group to 85% in the LE + EBRT group (p = 0.03). Because distant metastases were an important cause for failure in patients with high-risk pathology treated by LE + EBRT, it remains to be seen whether continuous-infusion administration of 5-FU produces superior results to bolus administration for this group of high-risk patients. Maintenance chemotherapy may also help to reduce the frequency of distant metastases in the high-risk patient population.
We analyzed the time interval between treatment and diagnosis of recurrence in patients treated by surgery alone versus those who received adjuvant irradiation. The median time to recurrence in the adjuvant irradiation group was 55.0 months, substantially longer than the 13.5 months to recurrence in the LE alone group. With four cases of late recurrences occurring at 64, 72, 86, and 91 months after adjuvant irradiation, this underscores the need for longer-term follow-up to assess treatment outcomes in these patients. As a point of reference, most of the published retrospective series have average follow-up times of 3 to 5 years. Likewise, the recent intergroup study 10 provides promising preliminary data on local excision with or without radiation, but with a median follow-up of only 2 years, more time is needed for the data to mature.
It is difficult to explain these late local recurrences from a radiobiologic perspective. In principle, to achieve local control of a tumor, all clonogens must be destroyed. A clonogen is defined as a surviving tumor cell that has the ability to proliferate indefinitely to produce colonies of cells. If any clonogens are left viable, they can cause tumor repopulation. Often this repopulation is accelerated rather than delayed. In head and neck tumors, delayed recurrences are often attributed to second primary cancers; in cases of rectal cancer recurrences, this remains less clear. Because median follow-ups in most retrospective series are <5 years, data regarding late relapses with LE and adjuvant irradiation are limited at present.
CONCLUSIONS
Our data indicate a trend toward improved 5-year actuarial LC and RFS rates in patients with T1/T2 rectal cancers managed by LE in combination with radiation and concurrent chemotherapy versus LE alone. By T stage, adjuvant irradiation significantly improved 5-year actuarial LC and RFS rates for patients with T2 tumors. The presence of high-risk pathologic features of lymphatic or blood vessel involvement and/or poorly differentiated histology significantly lowered 5-year actuarial LC and RFS rates in the LE alone group. Adjuvant irradiation significantly improved outcomes for this high-risk patient population. Nevertheless, distant metastases remain an important cause for failure in this subset of high-risk patients, and investigations should be undertaken to optimize systemic therapy for these patients.
Long-term follow-up studies are needed to assess the long-term benefits of adjuvant irradiation because the median time to recurrence was 55.0 months in this group, with recurrences evident as late as 91 months. In comparison, the median time to recurrence was substantially shorter at 13.5 months in the LE alone group. The four cases of late local failures in the LE + EBRT group are of concern and underscore the need for careful long-term follow-up in these patients. A delay in recurrence could certainly be considered beneficial, particularly in older patients, but the role of this conservative approach in younger patients needs to be explored with further follow-up.
Therefore, we recommend adjuvant chemoradiation for all patients undergoing LE for T2 tumors and for T1 tumors with high-risk pathologic features. This approach appears to improve outcome up to 5 years after treatment. Whether adjuvant treatment in patients with poor pathologic features lengthens the time to recurrence beyond 5 years or ultimately results in lasting RFS remains unclear.
Footnotes
Correspondence: Christopher G. Willett, MD, Dept. of Radiation Oncology, Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114.
Accepted for publication December 11, 1999.
References
- 1.Wood WC, Willett CG. Update of the Massachusetts General Hospital experience of combined local excision and radiotherapy for rectal cancer. Surg Oncol Clin North Am 1992; 1: 131–136. [Google Scholar]
- 2.Willett CG, Teppler JE, Donnelly S, et al. Patterns of failure following local excision and local excision and postoperative radiation therapy for invasive rectal adenocarcinoma. J Clin Oncol 1989; 8: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 3.Willett CG, Compton CC, Shellito PC, Efird JT. Selection factors for local excision or abdominoperineal resection of early stage rectal cancer. Cancer 1994; 73: 2716–2720. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal SA, Yeung RS, Weese JL, et al. Conservative management of extensive low-lying rectal carcinomas with transanal local excision and combined preoperative and postoperative radiation therapy. A report of a phase I-II trial. Cancer 1992; 69: 335–341. [DOI] [PubMed] [Google Scholar]
- 5.Minsky BD, Enker WE, Cohen AM, Lauwers G. Local excision and postoperative radiation therapy for rectal cancer. Am J Clin Oncol 1994; 17: 411–416. [DOI] [PubMed] [Google Scholar]
- 6.Valentini V, Morganti AG, De Santis M, et al. Local excision and external beam radiotherapy in early rectal cancer. Int J Radiat Oncol Biol Phys 1996; 35: 759–764. [DOI] [PubMed] [Google Scholar]
- 7.Fortunato L, Ahmad NR, Yeung RS, et al. Long-term follow-up of local excision and radiotherapy for invasive rectal cancer. Dis Colon Rectum 1995; 38: 1193–1199. [DOI] [PubMed] [Google Scholar]
- 8.Wong CS, Stern H, Cummings BJ. Local excision and post-operative radiation therapy for rectal carcinoma. Int J Radiat Oncol Biol Phys 1993; 25: 669–675. [DOI] [PubMed] [Google Scholar]
- 9.Winde G, Nottberg H, Keller R, et al. Surgical cure for early rectal carcinomas (T1). Dis Colon Rectum 1996; 39: 969–976. [DOI] [PubMed] [Google Scholar]
- 10.Steele GD, et al. CALGB, RTOG, ECOG, SWOG: sphincter-sparing treatment for distal rectal adenocarcinoma: a phase II intergroup study. Proceedings of the American Society of Clinical Oncology, May 1997; 16:256.
- 11.Brodsky JT, Richard GK, Cohen AM, Minsky BD. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer 1992; 69: 322–326. [DOI] [PubMed] [Google Scholar]