Abstract
Objective
To assess the effect of combined laser-induced thermotherapy (LITT) and hepatic arterial embolization with degradable starch microspheres (DSM) on tumor response and intrahepatic temperature distribution in rats with liver tumors.
Summary Background Data
Laser-induced thermotherapy is a promising in situ ablation technique for malignant liver tumors. However, clinical use is still limited, mainly because of the small size of the inducible coagulation necroses. This results in insufficient tumor destruction.
Methods
Colon carcinoma CC531 was implanted in 60 WAG rat livers. Fourteen days later, a silicon catheter was implanted in the hepatic artery for DSM administration. Tumors were exposed to 1064 nm Nd:YAG laser light at 2 watts for 10 minutes from a diffuser tip applicator placed in the tumor. The animals were randomized into a sham-operated control (group I) and three test groups. Group II received DSM alone, group III received LITT alone, and group IV received DSM + LITT. Tumor control was examined 1, 7, and 14 days after treatment.
Results
A complete tumor remission was achieved in all rats treated with LITT + DSM (group IV). In contrast, tumor progression was seen in animals treated with LITT alone (group III) or DSM alone (group II), as well as in the sham-operated controls (group I).
Conclusions
The authors’ results suggest that the combination of LITT and DSM considerably increases the efficacy of LITT in the treatment of liver metastases in the rat.
Laser-induced thermotherapy (LITT) or interstitial laser thermotherapy is an in situ ablation technique for the treatment of malignant tumors of the liver. 1,2 The potential of LITT is that laser light is directly transmitted through flexible bare fibers into the tumor tissue with high precision. The biologic effect of the laser light is based on the transformation of laser photon energy into heat by the absorption of tissue-specific chromophores. The aim is to induce uniform coagulation necrosis of the treatment volume. 3 Of great importance to the method’s success is the size of the induced coagulation necrosis, which must contain the whole volume of the tumor tissue. This lesion size largely depends on the temperature distribution in the target volume, which is determined partly by the blood perfusion in the target organ. 4,5 In organs with a high blood perfusion, such as the liver, a cooling effect results because of the temperature difference between the hyperthermic area and blood. 6,7 Temporary interruption of hepatic blood flow by occlusion of hepatic inflow vessels (Pringle maneuver) during LITT has been proven to increase its efficacy. 8–11 The Pringle maneuver usually requires laparotomy 10,11 or at least laparoscopy 12 and may therefore be difficult to repeat in cases of insufficient therapy. LITT, however, can be applied percutaneously without laparotomy or laparoscopy and can thus be easily repeated. 1,2 An alternative to the temporary interruption of hepatic arterial blood flow to liver tumors is the application of degradable starch microspheres (DSM), as used for chemoembolization. 13,14
The purpose of this study was to assess the effect of combined LITT and DSM on intrahepatic temperature distribution and tumor response in rats with liver tumors.
MATERIALS AND METHODS
Animals, Tumor Model, and Catheter Implantation
Sixty inbred male WAG rats weighing 200 to 280 g were used in this study. Animals were kept separately during the experiment with 12 hours of light per day. They were fed a standard laboratory diet and tap water ad libitum. Maintenance and care of all experimental animals used in this study were carried out according to the guidelines of the responsible Animal Protection Commission and with the permission of the Senat für Gesundheit, Berlin (National Institute of Health for Use of Laboratory Animals). The tumor was a moderately differentiated adenocarcinoma of the rat colon (tumor cell line CC531) obtained from the DKFZ, Heidelberg, Germany. Intrahepatic tumor inoculation was performed with a tumor suspension produced in vitro. The tumor cell line was cultivated at 37°C and 8% CO2 in an incubator in 20 ml complete medium (RPMI 1640 [Gibco, Life Technologies, Eggenstein, Germany], 10% fetal calf serum [Seromed, Biochrom, Berlin, Germany], and 1% Pen/Strep [Seromed]). After 3 days, cells washed twice with phosphate-buffered saline (PBS) were detached with 3 ml trypsin. The trypsin was deactivated by adding the complete medium. After centrifugation and washing and resuspension with PBS, vitality was evaluated in a Bürker hematocytometer after the addition of trypan blue. After vital counting, the suspension was adjusted to 97% vitality with a density of 1 × 106 vital cells/100 μl suspension by recentrifugation and resuspension. For tumor implantation, the animals were laparotomized through a midline abdominal incision under general anesthesia (ketamine [Ketanest], Parke-Davis, Germany, 5 mg/100 g, and xylazine [Rompun], Bayer, Leverkusen, Germany, 0.3 mg/100 g), and 0.1 ml tumor cell suspension was injected under the capsule of the anterior surface of the left liver lobe. The puncture site was closed with acrylic glue to prevent the leakage of tumor cells into the peritoneal cavity.
Fourteen days after tumor inoculation, the animals were relaparotomized through a midline abdominal incision under the general anesthesia (ketamine and xylazine), and the liver was shifted forward onto sterile gauze compresses. The largest width and the maximum tumor diameter perpendicular to the width were macroscopically measured with a micrometer. Only tumors with a diameter ≥12 mm were included in the investigation.
A silicone catheter (outer diameter 0.8 mm) was placed into the gastroduodenal artery for intraarterial DSM administration. For this purpose, the common hepatic artery and the gastroduodenal artery and its bifurcation into the right and left gastroduodenal artery were visualized under the operating microscope (Zeiss OPMI 6-S, Aalen, Germany). Through an arteriotomy of the gastroduodenal artery, the catheter was advanced into the proper hepatic artery and temporarily fixed with a ligature (8-0 Dexon). After injecting DSM, the catheter was removed and the gastroduodenal artery was ligated.
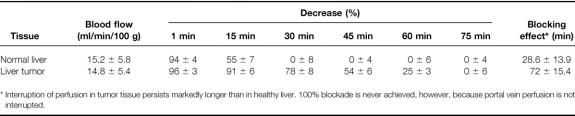
For measurements of the duration and degree of blockade of hepatic blood flow by the intraarterial administration of DSM, ten additional tumor-bearing rats were used in a separate experiment performed before the experiments proper. Tissue blood flow of tumor and normal liver was measured simultaneously by laser blood flowmeter (785 nm) (LMTB, Berlin, Germany). Measurements were performed before and for 90 minutes (10-second intervals) after the administration of 12 mg DSM (Spherex, Pharmacia & Upjohn, Erlangen, Germany) using the present technique. These measurements are shown in Table 1.
Table 1. Blocking Effect of DSM
Laser, Laser Application, and Temperature Measurement
A Nd:YAG laser (Medilas 2, MBB-Medizintechnik, Munich, Germany) emitting laser light at a wavelength of 1064 nm was used for laser applications. The laser light was delivered in the continuous wave mode through a 400-μm fiber, which had a diffuser tip applicator (outer diameter 0.8 mm, active length 5.0 mm) at its distal end. The technical details of this applicator have been described elsewhere. 15 For laser application, the applicator was inserted along the longest diameter of the tumor in its center at a depth of 0.5 cm. Laser application was performed at a power setting of 2 W. Laser power was measured before application at the distal end of the applicator with a power meter (Hüttinger, Umkirch, Germany). Application time was 600 seconds, corresponding to an energy of 1200 J. Because the purpose of this study was to examine the additional effect of DSM in combination with LITT on tumor response, the laser output power was limited to a level so that complete tumor destruction could not be achieved by LITT alone. This so-called suboptimal laser power for the tumor size was verified in separate experiments performed earlier.
For temperature measurement during laser application, a nickel-chrome-nickel thermocouple 0.6 mm in diameter (Standard Integrated Thermocouple Thermocoax, Phillips, Hamburg, Germany) was placed intrahepatically at the macroscopic tumor margin parallel to the previously placed applicator. The thermocouple was calibrated before use in a high-precision water bath. The geometric relation of the temperature probe and the applicator was maintained with an external holding device through which the probe and the applicator were inserted. Baseline temperature was recorded for 5 minutes before treatment. In group III (LITT alone) and group IV (LITT + DSM), temperature was continuously measured during application and for 5 minutes after laser application. In group I (control) and group II (DSM alone), temperature measurement was not performed.
Experimental Protocol and Procedures
The animals were randomized into a control and three test groups of 15 animals each.
In group I (sham operation), 0.4 ml of physiologic saline heated to 27°C was intraarterially administered using the previously placed silicone catheter in the hepatic artery. The laser applicator was inserted as described above and left in place for 10 minutes but was not activated.
In group II (DSM alone), a mixture of 0.2 ml DSM (corresponds to 12 mg) and 0.2 ml physiologic saline was intraarterially administered using the silicone catheter previously placed in the hepatic artery. The laser applicator was inserted as described above and left in place for 10 minutes but was not activated.
In group III (LITT alone), 0.4 ml of physiologic saline heated to 27°C was intraarterially administered using the silicone catheter previously placed in the hepatic artery. The laser applicator and the thermoelement were inserted as described above and left in place for 10 minutes. LITT was then performed with the above-mentioned parameters.
In group IV (LITT + DSM), a mixture of 0.2 ml DSM (corresponds to 12 mg) and 0.2 ml physiologic saline was intraarterially administered using the previously placed silicone catheter in the hepatic artery. The laser applicator and the thermoelement were inserted as described above and left in place for 10 minutes. LITT was subsequently performed with the above-mentioned parameters.
After treatment, the diffuser tip applicator and the thermocouple were removed from all animals and the abdomen was closed in two layers. The animals were kept under standard conditions. Five animals from each group were killed by an overdose of anesthetic 24 hours, 7 days, and 14 days after treatment. The animals were injected with 2-bromodesoxyuridine (200 mg/kg) in the tail vein 6 hours before they were killed. The animals were relaparotomized through a midline incision, and the greatest lesion width and the maximum diameter perpendicular to the width were macroscopically measured with a micrometer. Basically, the size of the total lesion was measured regardless of whether it was undamaged/necrotic tumor or liver tissue or an inflammatory reaction, because differentiation was impossible with the naked eye. The question of whether tumor remission was obtained was answered by the histologic section samples. Lesion volume was calculated using the formula v = 4 × π × ab2/3, in which a and b are the radii of the measured axes. The liver was then completely sectioned. The specimens were snap-frozen in liquid nitrogen and precooled isopentane and stored at −80°C until further analysis. Adjacent sections were fixed in 4% formalin for 5 days, embedded in paraffin, and stained with hematoxylin and eosin (H&E). For immunohistochemical detection of the BrdU antibody, 16 5-μm cryostat sections were used. They were air-dried overnight at room temperature and subsequently fixed in acetone for 10 minutes. An indirect three-step immune peroxidase technique was used. The sections were washed with PBS buffer (pH 7.2) after each incubation step. Endogenous peroxidase was blocked by incubating the sections for 10 minutes in 0.3% H2O2 in methanol. PBS-rehydrated sections were incubated with the anti-BrdU-AB (mouse-IgG1) (Boehringer, Mannheim, Germany) for 30 minutes. They were counterstained with H&E. Negative staining control reaction was performed with an unspecific IgG1 anti-mouse AB. Tumor remission was determined when no vital tumor cells could be detected in the H&E staining by light microscopy and immunohistochemical detection of BrdU was negative after in vivo incorporation.
Statistical Analysis
The mean tumor and lesion volumes ± SEM were calculated for each group. The differences between the tumor and lesion volumes in the groups were determined using the Kruskal–Wallis test. The Mann–Whitney test was used to calculate the temperature differences in groups III and IV. A probability value <0.05 was considered significant.
RESULTS
Temperature Measurements
Mean changes in the intrahepatic temperature during LITT with or without DSM are shown in Figure 1. In group III (LITT alone), the mean temperature increased during application from 26.9° ± 0.3°C at baseline to 40.8° ± 0.7°C after application of 1200 J. In comparison, the initial mean temperature of 27.0° ± 0.5°C in group IV (LITT + DSM) increased to 43.3° ± 0.7°C after application of 1200 J. The temperature difference between groups III and IV after applying 1200 J was 2.6°C. The temperature in group IV after laser application was significantly higher than that in group III.

Figure 1. The temperature course in group III (LITT alone) and in group IV (LITT + DSM) during laser application (n = 30). There was a temperature difference of 2.6°C after the application of 1200 J (* p = 0.03, Mann-Whitney test).
Tumor and Lesion Volumes
The calculated mean tumor and lesion volumes at the different time points are shown in Figure 2. No significant differences in the tumor volumes were found before therapy or 24 hours thereafter between the four groups.
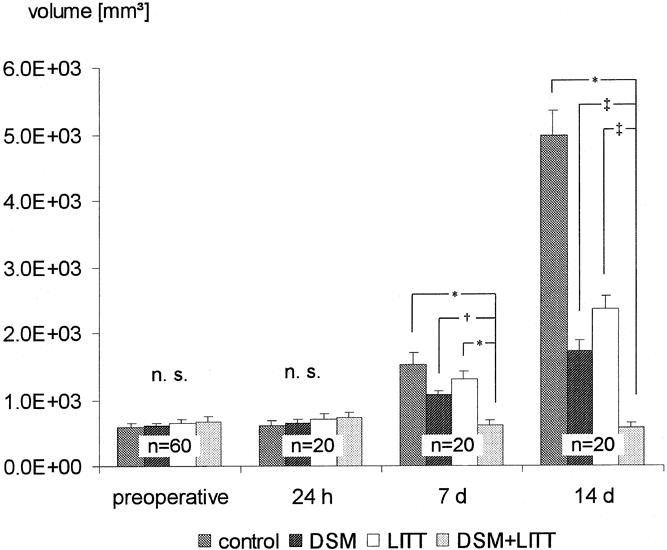
Figure 2. The mean lesion volumes ± SEM of groups I to IV (n = 60) before treatment and 24 hours, 7 days, and 14 days after therapy (* p < 0.005, § p < 0.01, # p < 0.05, Kruskal-Wallis test; n.s., not significant).
Tumor volumes in group I (control) increased from 595 ± 10 mm3 on the day of the sham operation to 1521 ± 11 mm3 on the 7th day and 4966 ± 77 mm3 on the 14th day thereafter.
In groups II and III, compared with pretherapeutic findings, the lesion volumes were larger on the 7th and 14th day after therapy (group II, 1063 ± 08 mm3 and 1720 ± 29 mm3; group III, 1311 ± 47 mm3 and 2366 ± 18 mm3). Concomitantly, the lesion volumes in groups II and III on the 7th and 14th day after therapy were significantly smaller than in group I (group II, p = 0.021 and p = 0.009; group III, p = 0.036 and p = 0.005). There were no significant differences in sizes between groups II and III on the 7th and 14th day.
In group IV, lesion volumes were 682 ± 75 mm3 before therapy and grew to 730 ± 50 mm3 24 hours after treatment, 615 ± 52 mm3 on the 7th day, and 580 ± 44 mm3 on the 14th day. The volumes were significantly smaller compared with both groups II and III (group II, p = 0.0009 and p = 0.026; group III, p = 0.004 and p = 0.018).
Histology
The light microscopic examination of the histologic section samples of groups III and IV demonstrated four separate zones. The samples from groups I and II showed no zonal arrangement. The zones have already been described elsewhere: 17
• Application zone: This zone was directly adjacent to the laser applicator and showed regular signs of hyperthermic cell damage.
• Central zone: The majority of the hyperthermically damaged cells surrounded by the transition zone were located here.
• Transition zone: This zone was between the hyperthermic tissue of the central zone and the undamaged tissue of the reference zone.
• Reference zone: This zone corresponded to the undamaged tissue.
In group I, H&E staining revealed a completely intrahepatic unilocular adenocarcinoma of moderate differentiation. The tumor showed mainly tubular structures with vacuolelike lumen formation and was infiltrated with fibrous septa. On the tumor–liver border, growth of the tumor with its broad homogeneous invasion front caused displacement of hepatic tissue. The spontaneous necrosis rate was estimated to be <20%. The large basophilic tumor cells evidenced pronounced cellular and nuclear polymorphism with nucleus-plasma shifting in favor of the nucleus. Numerous mitoses were detected. The immunohistochemical examinations with anti-BrdU after in vivo BrdU incorporation revealed a positive signal in tumor cells and in only some hepatocytes.
In group II, the tumor showed irregularly distributed, indirect signs of irreversible cell damage 24 hours after treatment. 18 Tumor cells displayed clear shrinkage and partial loss of cell contact. The cell nuclei were pyknotic and basophilic and the cytoplasm was eosinophilic. Marked hemorrhages and thromboses of the larger adjacent vessels and central veins were found on the tumor–liver border (Fig. 3). Tumor progression was seen in all but one of the samples on the 7th day after treatment. In the tumor, there were central, large and partially confluent necrotic areas composed of completely homogenized, eosinophilic tumor cells. The tumor architecture of these necrotic formations was extensively preserved. Hemorrhages and thromboses were only partially detectable on the tumor–liver border. There was a similar histologic picture 14 days after treatment. In addition, there was considerable tumor progression in all but one sample. BrdU incorporation was focally detected in the peripheral tumor 24 hours after therapy. Seven and 14 days after treatment, there was strong BrdU incorporation of the tumor cells in all but two samples, which had shown no tumor progression in the conventional histology. The eosinophilic tumor necroses never demonstrated BrdU incorporation.
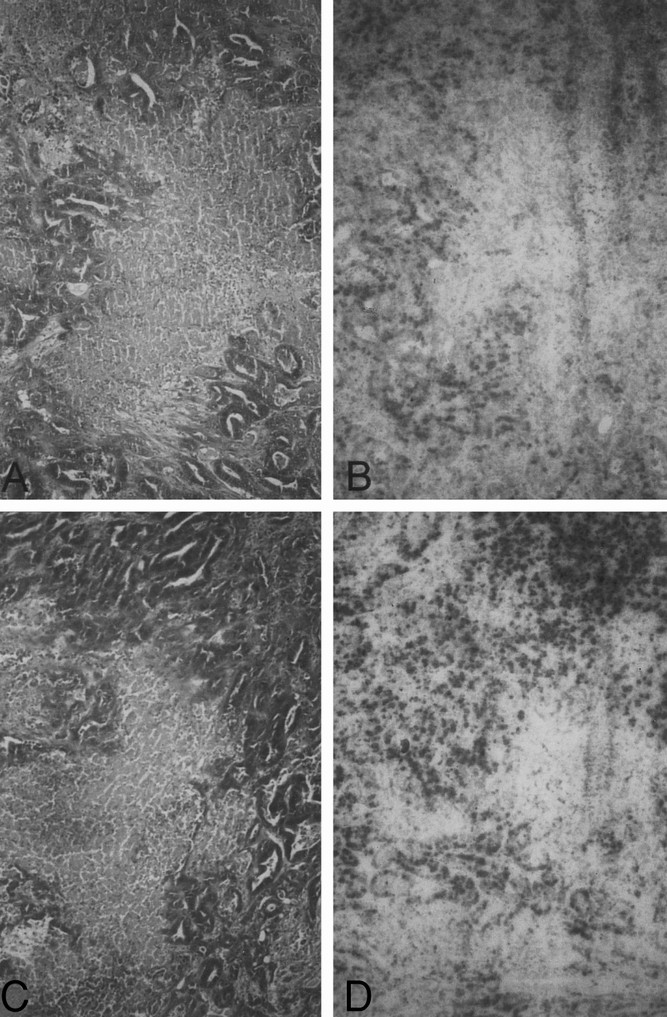
Figure 3. Histologic findings in group II (DSM alone). (A) Microscopic section of tumor tissue showing irregularly distributed, indirect signs of irreversible cell damage 24 hours after treatment. Tumor cells display clear shrinkage and partial loss of cell contact. The cell nuclei are pyknotic and basophilic and the cytoplasm is eosinophilic. There were central, large and partially confluent necrotic areas comprising completely homogenized, eosinophilic tumor cells (bar = 2 μm, H&E). (B) BrdU incorporation was focally detected in the peripheral tumor 24 hours after therapy (bar = 5 μm, BrdU histochemistry). (C) Histologic picture 14 days after treatment is similar to that 24 hours after treatment (bar = 2 μm, H&E). (D) Microscopic section 14 days after treatment shows strong BrdU incorporation of the tumor cells. The eosinophilic tumor necrosis never demonstrated BrdU incorporation (bar = 5 μm, BrdU histochemistry).
In group III, there was no carbonization in the application zone 24 hours after treatment; this could not be morphologically differentiated from the central zone. The central zone evidenced the typical indirect signs of hyperthermic cell damage, as described elsewhere. 17,18 The tumor cells were partially loosened from the cell unit, but the tumor structure was mostly preserved. Compared with the samples in group II at the same time point (24 hours after treatment), basophilia was clearly stronger in the cell nuclei (Fig. 4). There was a pronounced hemorrhagic border with no round-cell infiltrates in the transition zone. The reference zone showed hyperthermically undamaged tumor tissue. There was a clear increase of eosinophilia and homogenization in the central zone 7 days after therapy. Neither round-cell infiltrates nor mesenchymal proliferation were detected in the transition zone. Fourteen days after treatment, tumor progression was detected in the reference zone of all but one sample, which showed, in contrast to all other samples in the group, a wide, collagen-fiber-rich connective tissue border in the transition zone. In the same sample, there was undamaged liver tissue in the reference zone. BrdU incorporation of the tumor cells was negative in the application and central zones of all samples. All but one sample showed strong BrdU incorporation of the tumor cells in the reference zone.

Figure 4. Histologic findings in group III (LITT alone). (A) 24 hours after treatment, the tumor cells partially loosened from the cell unit but the tumor structure is mostly preserved (bar = 2 μm, H&E). (B, D) BrdU incorporation of the tumor cells was negative in the application zone and the central zone of the hyperthermically damaged area of all samples. Samples show BrdU incorporation of the tumor cells in the reference zone 24 hours (B) and 14 days (D) after treatment (bar = 5 μm, BrdU histochemistry). (C) Microscopic section 14 days after interstitial laser application showing tumor progression in the reference zone (bar = 2 μm, H&E).
In contrast to the samples in group III, in group IV the tumor architecture in the application and central zones clearly lost its structure 24 hours after treatment. The central zone spread over the entire tumor in all samples. The transition zone was located exactly on the liver–tumor border and, in addition to the changes in group III, showed an extensive infiltration border comprising immunocompetent round cells such as granulocytes, lymphocytes, and macrophages. In addition to the already described changes in all samples in the transition zone, a cellular and collagen-rich circular connective tissue border with many vessel and bile duct proliferates was found 7 and 14 days after treatment. At all times, the reference zone in all samples comprised undamaged liver tissue. Tumor tissue was not detected in the reference zone in any of the samples. BrdU incorporation of tumor cells was not found in any of the samples in the application, central, or transition zone. Comparable to group I, the reference zone showed positive BrdU incorporation of some hepatocytes (Fig. 5).
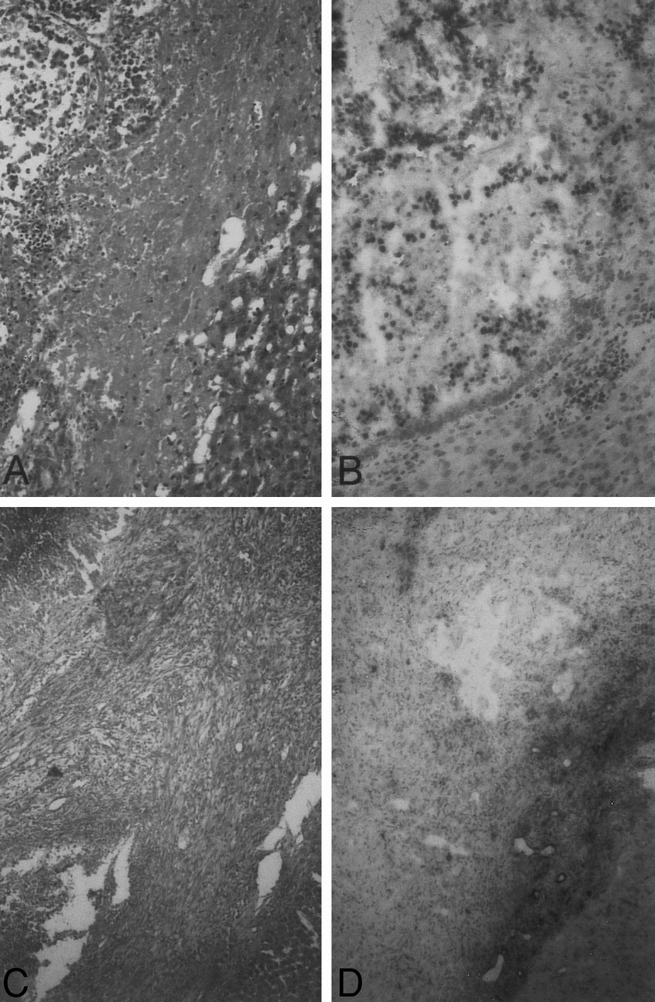
Figure 5. Histologic findings in group IV (LITT + DSM). (A) The tumor architecture has clearly lost its structure 24 hours after treatment (bar = 2 μm, H&E). (B, D) Microscopic section showing no BrdU incorporation of tumor cells in the application, central, or transition zones 24 hours (B) and 14 days (D) after treatment. The reference zone showed positive BrdU incorporation of some hepatocytes (bar = 5 μm, BrdU histochemistry). (C) 14 days after treatment, all samples showed in the transition zone a cellular and collagen-rich circular connective tissue border with many vessel and bile duct proliferates (bar = 2 μm, H&E).
Tumor Remission
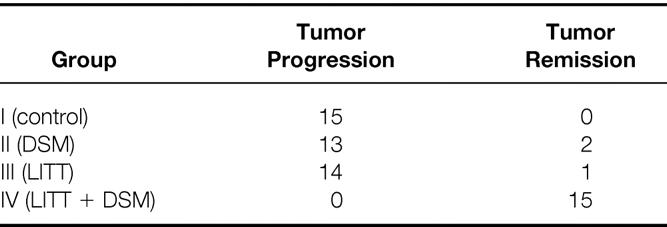
In group I, all animals demonstrated tumor progression. In group II, 13 animals had tumor progression and 2 had tumor remission (1 animal 7 days and 1 animal 14 days after treatment). In group III, 14 animals had tumor progression and 1 had tumor remission. All animals in group IV showed tumor remission (Table 2).
Table 2. Histologically Detected Tumor Progression or Remission
Discussion
LITT is an in situ ablation technique for the treatment of malignant tumors. Pilot clinical studies show that LITT is effective in achieving local control of liver tumors. 2,10 Despite these promising results, the clinical application of LITT is still limited. One major problem of LITT is insufficient tumor destruction because of the limited size of the inducible coagulation necroses. Different approaches regarding the application system and the application mode have been undertaken to increase the efficacy of LITT. 15,19,20 One of these approaches is the temporary interruption of hepatic blood flow by clamping the hepatic inflow vessels during LITT to prevent the so-called cooling effect of blood flow. 7,11 However, this usually requires laparotomy 10 or at least laparoscopy, 12 whereas LITT can be applied percutaneously. 1,2 To our knowledge, this is the first experimental study to assess the effect of combined LITT and DSM on tumor response and intrahepatic temperature distribution in liver tumors of rats. Our results show that this combined approach considerably increases the efficacy of LITT in the treatment of liver metastases in rats.
The prognostic gain of surgical resection of colorectal liver metastases has been repeatedly substantiated. 21,22 Because of the histopathologic growth pattern of liver metastases of colorectal carcinomas, a safety margin of at least 1 cm into healthy tissue should be maintained in surgical R0 resection. 21 Thus, if curative treatment is the goal, maintaining a corresponding safety margin is also required for LITT as an in situ ablation technique. Therefore, hyperthermic lesions inducible with LITT must be at least 20 to 25 mm, because the limit for reliable detection of hepatic metastases with modern imaging procedures is a minimum of approximately 1 cm. 23,24 This minimum can be met when using thermostable diffusing tip applicators. 15,19,20 However, in the majority of cases under clinical conditions, larger lesions are necessary, given the size of human liver metastases at the time of diagnosis. 22
To simulate this clinical situation in the selected rat tumor model, the application parameters in the trials were set in such a way that no complete tumor remission could be obtained with LITT alone. Correspondingly, histologic tumor necroses developed in the animals after LITT alone (group III), but a tumor volume increase and vital tumor cells in the reference zone were seen in all but one animal (see Fig. 2 and Table 1). Intraarterial DSM administration alone (group II) also caused tumor necroses, but tumor progression could be prevented in only two animals (see Fig. 3). In contrast, the combination of LITT and intraarterial DSM administration (group IV) in the selected tumor model led to complete tumor remission with no detection of vital tumor cells in the reference zone (see Fig. 5).
The local heating of biologic tissue with laser light is decisively influenced by the temperature distribution in the target volume. The time course of temperature distribution is determined by local heat generation and by the simultaneous conduction of heat energy. 5 The first process is determined by light distribution and the optical properties of the target tissue, as well as by the wave length of the laser light used; the second process depends on heat conduction, metabolic changes in the target volume, and especially organ-specific blood perfusion. 3 Thus, the temperature rises in the tissue with an increase in the laser energy applied, up to a threshold at which the amount of heat dissipated by heat transport is the same as the applied laser energy. 5,25
Besides temperature distribution, the configuration of the laser-thermoinduced hepatic lesions is also influenced by perfusion. Thus, Matthewson et al 26 have observed viable hepatocytes or tumor cells growing close to patent vessels after LITT. They explained these phenomena by the thermoprotective effect of blood vessels on the surrounding liver tissue. Sturesson et al 8 showed in rats with liver tumor that a temporary interruption of perfusion by occlusion of the portal vein and hepatic artery (Pringle maneuver) led to a 47% increase in the diameter of laser-thermoinduced lesions.
A less invasive alternative for temporary interruption of arterial perfusion of hepatic tumors is the intraarterial administration of DSM. 13,14 In this study, the combination of DSM and LITT resulted in a rapid temperature increase and a higher intrahepatic temperature at the end of laser application than with LITT alone (see Fig. 1). However, the temperature increase was much less pronounced than with the complete interruption of hepatic perfusion performed by Sturesson et al. 8 In a comparable tumor model in rats with complete blood flow occlusion during LITT, they achieved intrahepatic temperatures of >45°C with a distal laser power of 1.5 W after 10 minutes of laser application with a 6-mm distance from the applicator. In our investigations, the temperature measured after laser application directly on the tumor border in group IV (LITT + DSM) was 43.3° ± 0.7°C after 10 minutes; this was lower than reported by Sturesson et al although greater laser energy was applied at this time point (1200 vs. 900 J).
Thus, the increased efficiency of LITT with the immediately preceding intraarterial administration of DSM cannot be explained solely by a prevention of the cooling effect of blood flow. A possible explanation for the observed efficacy increase may be the greater thermosensitivity of the cells caused by temporary hypoxia of longer duration. Möller et al 27 showed in a comparable tumor model that a temperature of 54° to 61°C on the tumor border must be maintained for 30 minutes to achieve complete tumor remission. Although in our study a lower temperature (43.3° ± 0.7°C) was measured in group IV at the end of laser application directly on the tumor border, complete tumor remission was obtained in all animals.
This observation provides evidence that the thermosensitivity of tumor cells is increased after the administration of DSM. It is well known that hypoxia, with a decrease in pH and energy state of the cell, leads to greater thermosensitivity. 28,29 Selective DSM thermosensitization of tumor cells versus hepatocytes is also substantiated in our study by the observation that the necrotic zones did not extend into healthy liver tissue of any animals in group IV (LITT + DSM); rather, they stopped directly at the peripheral tumor border (see Fig. 5). It was demonstrated in in vivo and in vitro experiments that tumor versus healthy cells show greater time-dependent temperature sensitivity in the range of 42° to 44°C. 30
However, other mechanisms may also be responsible for the synergism between arteriolar embolization and LITT observed in this model. One of those mechanisms may be the vascular occlusion produced by hyperthermia itself, as already demonstrated. 31
In conclusion, our results suggest that the combination of LITT and hepatic arterial embolization with DSM considerably increases the efficacy of LITT, and that LITT should be combined with hepatic arterial embolization with DSM in the treatment of liver metastases.
Footnotes
Correspondence: Christoph-Thomas Germer, MD, Dept. of Visceral, Vascular and Thoracic Surgery, University Medical Center Benjamin Franklin, Freie Universität Berlin, Hindenburgdamm 30, 12200 Berlin, Germany.
Supported by Deutsche Forschungsgemeinschaft grant Nr. Al 143/1-1.
Accepted for publication November 30, 1999.
References
- 1.Masters A, Steger AC, Lees WR, et al. Interstitial laser hyperthermia: a new approach for treating liver metastases. Br J Cancer 1992; 66: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogl TJ, Mack MG, Straub R, et al. Percutaneous MRI-guided laser-induced thermotherapy for hepatic metastases for colorectal cancer. Lancet 1997; 350: 29. [DOI] [PubMed] [Google Scholar]
- 3.Roggan A, Müller G. Dosimetrie and computer-based irradiation planning for laser-induced interstitial thermotherapy (LITT). In: Müller G, Roggan A, eds. Laser-induced interstitial thermotherapy. Bellingham: SPIE Press; 1995:114–156.
- 4.Sturesson C, Andersson-Engels S. A mathematical model for predicting the temperature distribution in laser-induced hyperthermia: experimental evaluation and applications. Phys Med Biol 1995; 40: 2037. [DOI] [PubMed] [Google Scholar]
- 5.Svaasand LO, Gomer CJ, Morinelli E. On the physical rationale of laser-induced hyperthermia. Lasers Med Sci 1990; 8: 99–105. [Google Scholar]
- 6.Arkin H, Xu L, Holmes KR. Recent developments in modeling heat transfer in blood-perfused tissue. IEEE Trans Biomed Eng 1994; 41: 97. [DOI] [PubMed] [Google Scholar]
- 7.Whelan WM, Wyman DR. Investigations of large vessels cooling during interstitial laser heating. Med Phys 1995; 22: 105. [DOI] [PubMed] [Google Scholar]
- 8.Sturesson C, Liu DL, Stenram U, Andersson-Engels S. Hepatic inflow occlusion increases the efficacy of interstitial laser-induced thermotherapy in rat. J Surg Res 1997; 71: 67–72. [DOI] [PubMed] [Google Scholar]
- 9.Möller PH, Hannesson PH, Ivarsson K, et al. Interstitial laser thermotherapy in pig liver: effect of inflow occlusion on extent of necrosis and ultrasound image. Hepato-Gastroenterol 1997; 44: 1302–13011. [PubMed] [Google Scholar]
- 10.Tranberg KG, Möller PH, Hannesson P, Stenram U. Interstitial laser treatment of malignant tumours: initial experience. Eur J Surg Oncol 1996; 22: 47–54. [DOI] [PubMed] [Google Scholar]
- 11.Albrecht D, Germer CT, Isbert C, et al. Interstitial laser coagulation (ILC): evaluation of the effect of normal liver perfusion and the application mode on lesion size. Lasers Surg Med 1998; 23: 40–47. [DOI] [PubMed] [Google Scholar]
- 12.Germer CT, Albrecht D, Roggan A, et al. An experimental study of laparoscopic laser-induced thermotherapy treatment for liver tumours. Br J Surg 1997; 84: 317–320. [PubMed] [Google Scholar]
- 13.Taguchi T. Chemo-occlusion for the treatment of liver cancer. Clin Pharmacokinet 1994; 26: 275–291. [DOI] [PubMed] [Google Scholar]
- 14.Hakansson L, Hakansson A, Morales O, et al. Spherex (degradable starch microspheres) chemo-occlusion—enhancement of tumor drug concentration and therapeutic efficacy: an overview. Sem Oncol 1997; 24(Suppl 6): 100–109. [PubMed] [Google Scholar]
- 15.Germer CT, Albrecht D, Isbert C, et al. Diffusing fiber tip for the minimally invasive treatment of liver tumors by interstitial laser coagulation (ILC): an experimental ex vivo study. Lasers Med Sci 1999; 14: 32–39. [DOI] [PubMed] [Google Scholar]
- 16.Schutte B, Reyders MMJ, Bosman FT, Blijham GH. Effect of tissue fixation on antibromodesoxyuridine immunohistochemistry. J Histochem Cytochem 1987; 35: 1343–1345. [DOI] [PubMed] [Google Scholar]
- 17.Germer CT, Isbert C, Albrecht D, et al. Laser-induced thermotherapy for the treatment of liver metastasis: correlation of gadolinium-DTPA-enhanced MRI with histomorphological findings to determine criteria for follow-up monitoring. Surg Endosc 1998; 12: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen S. Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem Photobiol 1991; 53: 825–835. [DOI] [PubMed] [Google Scholar]
- 19.Van Hillegersberg R, Van Staveren HJ, Kort WJ, et al. Interstitial Nd:YAG laser coagulation with a cylindrical diffusing fiber tip in experimental liver metastases. Lasers Surg Med 1994; 14: 124–138. [DOI] [PubMed] [Google Scholar]
- 20.Orth K, Russ D, Duerr J, et al. Thermo-controlled device for inducing deep coagulation in the liver with the Nd:YAG laser. Lasers Surg Med 1997; 20: 149–156. [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1996; 15: 938–946. [DOI] [PubMed] [Google Scholar]
- 22.Scheele J, Stangl R, Altendorf-Hofmann A, Paul M. Resection of colorectal metastases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 23.Barakos JA, Goldberg HI, Brown JJ, Gilbert TJ. Comparison of computed tomography and magnetic resonance imaging in the evaluation of focal hepatic lesions. Gastrointest Radiol 1990; 15: 93–101. [DOI] [PubMed] [Google Scholar]
- 24.Chezmar JL, Rumancik WM, Megibow AJ, et al. Liver and abdominal screening in patient with cancer: CT versus MR imaging. Radiology 1993; 168: 43–47. [DOI] [PubMed] [Google Scholar]
- 25.Crezee J, Mooibroek J, Lagendijk JJW, van Leeuwen GMJ. The theoretical and experimental evaluation of the heat balance in perfused tissue. Phys Med Biol 1994; 39: 813–832. [DOI] [PubMed] [Google Scholar]
- 26.Matthewson K, Coleridge-Smith P, O’Sullivan JP, et al. Biological effects of intrahepatic Nd:YAG laser photocoagulation in rats. Gastroenterology 1987; 93: 550–557. [DOI] [PubMed] [Google Scholar]
- 27.Möller PH, Ivarsson K, Stenram U, et al. Interstitial laser thermotherapy of adenocarcinoma transplanted into rat liver. Eur J Surg 1997; 163: 861–870. [PubMed] [Google Scholar]
- 28.Gerweck LE, Urano M, Koutcher J, et al. Relationship between energy status, hypoxic cell fraction, and hyperthermic sensitivity in a murine fibrosarcoma. Radiat Res 1989; 117: 448–458. [PubMed] [Google Scholar]
- 29.Vaupel P, Kallinowski F, Okunieff D. Blood flow, oxygen and nutrient supply and microenviroment of human tumors: a review. Cancer Res 1989; 49: 6449–6465. [PubMed] [Google Scholar]
- 30.Maehara Y, Kusumoto T, Kusumoto H, et al. Excised human neoplastic tissues are more sensitive to heat than the adjacent normal tissue. Eur Surg Res 1988; 20: 254–259. [DOI] [PubMed] [Google Scholar]
- 31.Boddie A, Wright K, Frazer JW, et al. Mechanism of synergism between arteriolar embolization and hyperthermia in a rabbit V-2 model of solitary hepatic metastasis. Cancer Res 1986; 46: 4576–4581. [PubMed] [Google Scholar]