Abstract
Objective
To determine the incidence and prognostic significance of documented eradication of breast cancer axillary lymph node (ALN) metastases after neoadjuvant chemotherapy.
Summary Background Data
Neoadjuvant chemotherapy is the standard of care for patients with locally advanced breast cancer and is being evaluated in patients with earlier-stage operable disease.
Methods
One hundred ninety-one patients with locally advanced breast cancer and cytologically documented ALN metastases were treated in two prospective trials of doxorubicin-based neoadjuvant chemotherapy. Patients had breast surgery with level I and II axillary dissection followed by additional chemotherapy and radiation treatment. Nodal sections from 43 patients who were originally identified as having negative ALNs at surgery were reevaluated and histologically confirmed to be without metastases. An additional 1112 sections from these lymph node blocks were obtained; half were stained with an anticytokeratin antibody cocktail and analyzed. Survival was calculated using the Kaplan–Meier method.
Results
Of 191 patients with positive ALNs at diagnosis, 23% (43 patients) were converted to a negative axillary nodal status on histologic examination (median number of nodes removed = 16). Of the 43 patients with complete axillary conversion, 26% (n = 11) had N1 disease and 74% (n = 32) had N2 disease. On univariate analysis, patients with complete versus incomplete histologic axillary conversion were more likely to have initial estrogen-receptor–negative tumors, smaller primary tumors, and a complete pathologic response in the primary tumor. The 5-year disease-free survival rates were 87% in patients with preoperative eradication of axillary metastases and 51% for patients with residual nodal disease after neoadjuvant chemotherapy. Of the 39 patients with complete histologic conversion for whom nodal blocks were available, occult nodal metastases were found in additional nodal sections in 4 patients (10%). At a median follow-up of 61 months, the 5-year disease-free survival rates were 87% in patients without occult nodal metastases and 75% in patients with occult nodal metastases.
Conclusions
Neoadjuvant chemotherapy can completely clear the axilla of microscopic disease before surgery, and occult metastases are found in only 10% of patients with a histologically negative axilla. The results of this study have implications for the potential use of sentinel lymph node biopsy as an alternative to axillary dissection in patients treated with neoadjuvant chemotherapy.
The use of neoadjuvant chemotherapy has assumed an increasing role in the management of several solid organ malignancies, including bone, head and neck, bladder, esophagus, and lung. 1–5 Neoadjuvant chemotherapy has become the standard of care for the treatment of patients with locally advanced breast cancer (LABC) and is being prospectively evaluated in patients with earlier-stage disease. 6–11 Carcinoma of the breast is a particularly suitable model for studying the direct effects of systemic therapy on lymphatic metastases. The axillary nodal basin is the most common site of lymphatic metastases in patients with breast cancer and can be accurately assessed with ease using fine-needle aspiration biopsy before beginning systemic therapy.
Although randomized studies such as the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 trial 12 have not shown a survival advantage for patients with operable breast cancer treated with preoperative chemotherapy compared with conventional postoperative chemotherapy, the use of neoadjuvant chemotherapy has several significant proven and theoretical advantages. Neoadjuvant chemotherapy allows for individual in vivo assessment of primary tumor and metastatic lymph node response to chemotherapy. In addition, although chemotherapy is primarily thought of as important in eradicating occult distant disease, it can have a significant effect on locoregional disease as well. Tumor downstaging with neoadjuvant chemotherapy can convert inoperable disease to operable disease and can allow breast-conserving surgery in patients for whom mastectomy is initially the only option for control of locoregional disease. 13–15
The absolute number of residual metastatic axillary lymph nodes (ALNs) after neoadjuvant chemotherapy has been established as an important prognostic factor for disease-free survival. 16–18 No previous reports have documented the incidence of complete eradication of cytologically confirmed ALN metastases after neoadjuvant chemotherapy. Further, information on the rate of occult ALN metastases in patients found to have negative ALNs after neoadjuvant chemotherapy is not only of academic interest, but also has clinical implications for the further management of patients with breast cancer treated with initial chemotherapy rather than initial surgery.
In the current study, we analyzed the incidence and prognostic significance of complete eradication of axillary disease, as well as the clinicopathologic factors associated with this finding in 191 patients with LABC and cytologically confirmed ALN metastases treated in two consecutive prospective neoadjuvant chemotherapy trials at The University of Texas M. D. Anderson Cancer Center.
PATIENTS AND METHODS
Between 1989 and 1996, 191 patients with locally advanced breast cancer and cytologically documented ALN metastases were treated in two prospective trials of neoadjuvant chemotherapy using 5-fluorouracil, doxorubicin (Adriamycin), and cyclophosphamide (FAC) at M. D. Anderson Cancer Center. LABC was defined as breast cancer histologically or cytologically documented as stage IIA (T2 ≥ 4 cm), IIB, IIIA, IIIB, or IV (with ipsilateral supraclavicular lymph node involvement only) using the 1998 American Joint Committee on Cancer classification system. 19 The diagnosis was usually established by fine-needle aspiration biopsy of the primary tumor and any involved ALNs. Patients with primary inflammatory carcinoma (development of erythema, peau d’orange, and breast mound ridging within 3 months before presentation) were excluded and offered enrollment in other treatment protocols.
Each patient was examined by a multidisciplinary team to confirm the diagnosis of LABC and to evaluate the clinical stage of disease at presentation and the response after four cycles of chemotherapy. The staging workup included a complete history and physical examination, complete blood count with differential and platelet count, blood chemistry analysis, electrocardiography, chest x-ray, abdominal computed tomography or abdominal ultrasonography, bone scan, and bilateral mammography at presentation and after four cycles of chemotherapy. Each patient was entered prospectively into the protocol database and followed longitudinally. Complete medical records of all patients were available for review at the time of this analysis.
Responding patients underwent either a segmental mastectomy with ALN dissection (n = 54) or a modified radical mastectomy (n = 118) after four cycles of chemotherapy. Nine patients had axillary dissection alone because of prior primary tumor excision with negative margins (n = 5) or because no primary tumor was detectable at diagnosis (n = 4). Patients with no change or progressive disease after neoadjuvant chemotherapy (n = 10) received preoperative radiation treatment (total dose of 50 to 60 Gy delivered to the breast, internal mammary lymph nodes, and supraclavicular/high ALNs) followed by modified radical mastectomy. No patients in this group were found to be without ALN metastases at dissection.
The histologic response to neoadjuvant chemotherapy was characterized as a complete pathologic response if there was no evidence of residual invasive tumor in the breast or ALNs. Patients with ≤1 cm3 residual tumor received four additional cycles of FAC. Patients with a partial response and a residual tumor >1 cm3, and those with four or more positive lymph nodes in the surgical specimen, were treated after surgery with four more cycles of FAC, followed by four cycles of methotrexate and vinblastine. Patients with no change or progression of disease received six cycles of methotrexate and vinblastine. Locoregional radiotherapy was instituted within 6 weeks of completion of chemotherapy. Postoperative radiotherapy was delivered to the chest wall, internal mammary lymph nodes, and supraclavicular/high ALNs.
Hematoxylin and eosin (H&E)-stained sections from patients who were identified from the protocol database as having no ALN metastases (n = 43) were reevaluated by light microscopy, and all were confirmed to be free of metastases. Negative ALN status had been determined on the basis of analysis of a single H&E-stained section from each block of serial sectioned lymph nodes removed during surgery. Paraffin-embedded ALN blocks from these patients (four unavailable) were obtained, and two additional consecutive 5-μm sections were cut. The first section was stained with H&E, and the second one was used for anticytokeratin immunohistochemical staining.
Immunohistochemical Staining With Anticytokeratin Monoclonal Antibodies
Sections were deparaffinized in three successive xylene baths and cleared in absolute ethanol. After being washed with tap water and deionized water, the slides were soaked in pH 7.2 to 7.4 buffer (Optimax Buffer, BioGenex, San Ramon, CA). Sections (n = 556) were then loaded consecutively into a standardized, automated tissue-staining unit and then washed three times with Optimax buffer (BioGenex). The sections were then subjected to protein blocking with normal goat serum. Primary monoclonal anticytokeratin cocktail (AE1/AE3, BioGenex) was applied to the sections, and the sections were incubated for 30 minutes and then washed three times with buffer. Subsequently, a Super Sensitive Multilink Detection Kit (BioGenex) was used for the link, label, substrate, and counterstain portion of the assay with times of 20 minutes, 20 minutes, 10 minutes, and 2 minutes, respectively. Between steps, the slides were washed with buffer. The slides were then removed from the autostainer and covered with coverslips for review. Human skin sections and human skin sections not incubated with the primary antibody were assayed at the same time and served as positive and negative controls, respectively. Sections stained with H&E and immunohistochemical methods were examined by a single pathologist (AS) without knowledge of the patient’s clinicopathologic history.
Data Analysis
Data were analyzed using Statistica software (StatSoft, Inc., Tulsa, OK). Univariate comparisons of group outcomes were assessed by chi square analysis or the Wilcoxon rank sum test for comparisons based on tumor stage. All comparisons were two-tailed. For comparisons based on age, estrogen receptor status, nuclear grade, ALN status, and residual breast tumor, odds ratios and their corresponding 95% confidence intervals are reported. Overall survival was calculated from the date of diagnosis, and disease-free survival was calculated from the date of surgery using the method of Kaplan and Meier. 20 The log-rank statistic was used for univariate comparisons of survival end points. 21 The statistical significance level (p) was taken as a measure of the strength of evidence against the null hypothesis, and p ≤ 0.05 was considered statistically significant.
RESULTS
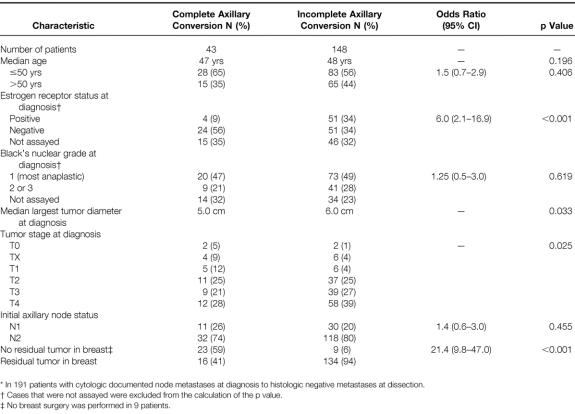
Table 1 summarizes the initial patient and tumor characteristics of 191 patients who had cytologically documented axillary metastases before initiation of neoadjuvant chemotherapy. Twenty-three percent of patients (n = 43) had complete axillary conversion from cytologically documented axillary metastases to histologically negative ALN status at dissection. The median number of lymph nodes removed and sectioned was 16 (range 7 to 36). The median age of the entire group of patients was 47 years, and there was no statistically significant difference found in the distribution of younger versus older patients (50 years or younger vs. older than 50 years) with respect to axillary conversion (p = 0.406). When initial estrogen receptor status was assayed, 85% of the patients with complete axillary conversion (n = 24) had negative estrogen receptor status tumors versus 50% of the patients with incomplete axillary conversion (n = 51, p < 0.001). No differences in the distribution of patients with complete versus incomplete axillary conversion were found with respect to initial tumor nuclear grade (p = 0.619). Patients with complete axillary conversion had smaller initial tumors than did patients with incomplete axillary conversion (median largest diameter 5 cm vs. 6 cm, respectively; p = 0.033). Patients with a more advanced initial tumor stage were less likely to have complete axillary conversion than patients with a less advanced initial tumor stage (p = 0.025). No statistical differences were found with respect to the distribution of patients stratified by initial ALN status. Patients with complete axillary conversion were more likely than patients with incomplete axillary conversion to have a complete pathologic response in the primary tumor (59% vs. 6%; p < 0.001).
Table 1. Patient and Tumor Characteristics by Metastatic Axillary Lymph Node Response to Neoadjuvant Chemotherapy
ALN tissue blocks were available from 39 of the 43 patients with complete axillary conversion. Occult metastatic deposits were detected in four lymph nodes from four patients (10%). One metastatic deposit was detected on serial H&E sectioning, and three deposits were detectable only by anticytokeratin staining and then by confirmation with H&E staining.
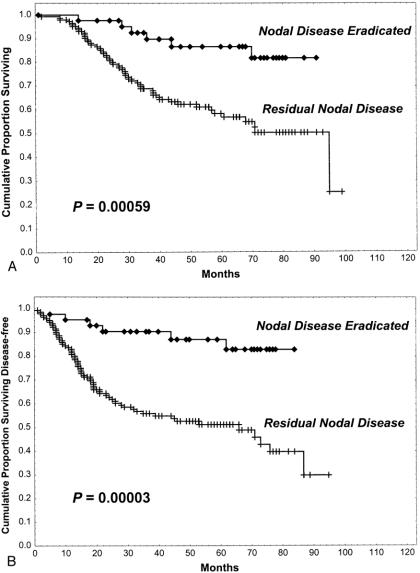
The clinical course of patients was analyzed with respect to histologic ALN response after neoadjuvant chemotherapy. At a median follow-up of 61 months (range 14 to 91 months), there were 2 local recurrences (1 chest wall and 1 breast) in patients with complete axillary conversion as assessed by H&E (5%) compared with 21 local recurrences (16 chest wall, 3 breast, and 3 axillary) in patients with residual nodal disease as assessed by H&E (14%, p = 0.091). There were significantly more distant recurrences in patients with residual nodal disease: distant recurrences occurred in 6 patients (15%) with complete axillary conversion compared with 60 patients (41%) with residual nodal disease as assessed by H&E (41%, p = 0.001). Distant recurrences were associated with significantly more deaths in patients who had residual nodal disease (n = 57, 39%) than in patients who had complete axillary conversion as assessed by H&E after neoadjuvant chemotherapy (n = 6, 15%, p = 0.003). Five-year overall and disease-free survival rates were significantly higher in patients with complete axillary conversion (87% and 87%, respectively) than in patients with residual nodal disease as assessed by H&E (58% and 51%, respectively, p < 0.001; Fig. 1).
Figure 1. Overall survival rate (A) and disease-free survival rate (B) of patients with complete eradication of cytologically confirmed ALN metastases (n = 43) compared with patients found to have residual ALN metastases (n = 148) after neoadjuvant chemotherapy.
The four patients initially found to be free of ALN metastases by histologic examination and later found to have occult metastases (by immunohistochemical examination and further sectioning) had stage IIB disease (one patient) or stage IIIA disease (three patients) and had a median of 13 lymph nodes (range 12 to 26) initially removed and examined. In patients with occult ALN metastases, there was one local breast recurrence, no distant recurrences, and no deaths. No significant survival difference was found between patients without occult ALN metastases and patients with occult metastases. The 5-year overall and disease-free survival rates were 87% and 87% in patients without occult metastases versus 100% and 75%, respectively, in patients with occult metastases (p = NS).
DISCUSSION
The results of our study indicate that neoadjuvant chemotherapy can completely clear ALN metastases as assessed by standard histologic examination in approximately 23% of patients with LABC. Further, occult micrometastases in ALNs are found in only 10% of these patients. This rate of occult metastases is consistent with previously reported rates of occult lymph node metastases (9% to 15%) identified in histologically “negative” nodes in patients not initially treated with chemotherapy. 22–26 Although the present report appears to be the first to document the eradication of cytologically proven ALN metastases by neoadjuvant chemotherapy, conversion of the clinically involved axilla to a pathologically negative status has been reported by both McCready et al 16 and Schwartz et al 14 to occur in about 25% of patients with LABC after neoadjuvant chemotherapy. Similar findings have been reported for women with less advanced disease at presentation: of 185 patients in the neoadjuvant chemotherapy arm of the NSABP B-18 trial who had clinical N1 axillary disease at diagnosis, 71 patients (38%) were found to have negative ALNs on histologic examination after neoadjuvant chemotherapy. 10
It has been established that the presence, degree, or absence of residual ALN metastases in patients with breast cancer after neoadjuvant chemotherapy correlates directly with the disease-free survival rate. 16–18 This finding was confirmed in the present study. Patients with negative ALNs on H&E or further analysis were found to have 5-year survival rates of 80% to 90%, whereas patients with residual nodal disease had 5-year survival rates of 50% to 60% (p < 0.001). However, in patients with negative nodes as assessed by traditional histologic examination who have received chemotherapy and will receive additional cycles of chemotherapy and potentially axillary irradiation, the clinical significance of occult micrometastases is questionable. It is unknown whether occult lymph node metastases will be eradicated with further chemotherapy or irradiation, are destined to initiate distant disease, or will undergo apoptotic arrest and therefore be of no clinical significance to the patient.
Because only four patients in this study were found to have occult ALN metastases after neoadjuvant chemotherapy, no conclusions may be drawn with respect to the effect of occult ALN metastases on the disease-free survival rate compared with patients who were confirmed to be free of nodal disease on histologic and immunohistochemical examination. Indeed, in one of the earliest reports on occult ALN metastases, Fisher et al 27 estimated that to detect a survival difference of 10% between patients with and those without occult metastases, 1400 cases would have to be studied. Notwithstanding, although there still remains controversy with respect to the clinical significance of occult nodal metastases, the largest study addressing the subject of occult metastases in patients not treated with neoadjuvant chemotherapy was performed in 921 patients with breast cancer the Ludwig Breast Cancer Group. 24 These investigators found a statistically significant difference in disease-free survival rates between patients with and those without occult micrometastases. In their series, serial lymph node sectioning revealed previously undetected metastases in 9% of patients. The Ludwig findings suggested that the subset of patients with occult nodal disease could derive a benefit from adjuvant chemotherapy. However, this concept is irrelevant in patients who received neoadjuvant chemotherapy and will continue to receive additional cycles of chemotherapy as part of the treatment protocol. Thus, finding occult metastases in one or more lymph nodes after neoadjuvant chemotherapy most likely should not affect further treatment decisions with respect to chemotherapy.
The finding in the present study that 20% of patients (n = 39) with ALN metastases at diagnosis were free of axillary disease after neoadjuvant chemotherapy as assessed by standard and additional analyses suggests that axillary dissection might not be necessary in some patients with LABC treated with neoadjuvant chemotherapy. However, at present, our ability to identify which patients have axillary disease after neoadjuvant chemotherapy is suboptimal. In fact, although we have recently shown that the sensitivity of axillary ultrasonography is significantly better than that of physical examination in detecting residual nodal disease after neoadjuvant chemotherapy, the overall sensitivity and specificity of axillary ultrasound were only 62% and 70%, respectively. 28 One alternative to axillary dissection in patients found to have a clinically negative axilla after neoadjuvant chemotherapy and who are appropriate candidates for breast conservation would be to add an additional radiation field to control subclinical axillary disease. 29 Randomized data from the NSABP B-04 trial established that simple mastectomy plus axillary irradiation is equivalent to radical mastectomy with respect to survival in patients with a clinically negative axilla. 30 Further, these findings suggest that patients who have a clinically negative axilla after neoadjuvant chemotherapy might be candidates for nonsurgical management of the axilla. However, this can be established only by prospective randomized evaluation in patients with LABC treated with neoadjuvant chemotherapy.
Another alternative to complete axillary dissection in all patients whose disease is downstaged with neoadjuvant chemotherapy is intraoperative lymphatic mapping with sentinel lymph node (SLN) biopsy. 31,32 The findings in the present study have clinical implications for the use of this technique in patients with breast cancer treated with neoadjuvant chemotherapy. Before this study, the rate of occult ALN metastases in patients treated with neoadjuvant chemotherapy who had “negative” ALNs on traditional histologic examination was unknown. If we had found that 80% to 100% of patients (rather than 10%) with complete histologic axillary conversion had occult metastases, then SLN biopsy would potentially benefit only an extremely small number of patients, and the procedure would not be cost-effective because the majority of patients would later require complete axillary dissection. However, our finding that only 10% of patients with complete axillary conversion have occult nodal metastases suggests that SLN biopsy may be appropriate in patients whose disease is downstaged with neoadjuvant chemotherapy. A note of caution is in order, though: SLN biopsy in patients treated with neoadjuvant chemotherapy will prove accurate only if the metastatic deposits within each ALN respond identically to the effects of chemotherapy. SLN biopsy in patients with LABC treated with neoadjuvant chemotherapy has not been reported. To address the feasibility of SLN biopsy in patients with breast cancer treated with neoadjuvant chemotherapy, we are prospectively evaluating SLN biopsy in patients with LABC treated in our current neoadjuvant chemotherapy protocols.
Acknowledgments
The authors thank J. Martinez, E. Werrlein, and B. Kerns of BioGenex Corporation for technical assistance with the immunohistochemical staining portion of this project.
Footnotes
Correspondence: S. Eva Singletary, MD, Dept. of Surgical Oncology, Box 106, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030.
Dr. Kuerer is currently affiliated with the University of California San Francisco, Department of Surgery, UCSF Cancer Center, San Francisco, CA.
Accepted for publication January 28, 1999.
References
- 1.Bacci G, Picci P, Ferrari S, et al. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer 1993; 15: 3358–3366. [DOI] [PubMed] [Google Scholar]
- 2.Spaulding MB, Fischer SG, Wolf GT. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. J Clin Oncol 1994; 12: 1592–1599. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Bajorin DF, Scher HI. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: ten-year outcome. J Clin Oncol 1998; 16: 1298–1301. [DOI] [PubMed] [Google Scholar]
- 4.Swisher SG, Holmes EC, Hunt KK, et al. The role of neoadjuvant therapy in surgically resectable esophageal cancer. Arch Surg 1996; 8: 819–824. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker DJ, Herndon J, Kohman LJ, et al. Results of cancer and leukemia group B protocol 8935. A multiinstitutional phase II trimodality trial for stage IIIA (N2) non-small-cell lung cancer. Cancer and Leukemia Group B Thoracic Surgery Group. J Thorac Cardiovasc Surg 1995; 109: 473–483. [DOI] [PubMed] [Google Scholar]
- 6.Delena M, Zucali R, Viganotti G, Valagussa P, Bonadonna G. Combined chemotherapy–radiotherapy approach in locally advanced (T3b-T4) breast cancer. Cancer Chemother Pharmacol 1978; 1: 53–59. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Blumenschein GR, Spanos W, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer 1983; 51: 763–768. [DOI] [PubMed] [Google Scholar]
- 8.Lippman ME, Sorace RA, Bagley CS, et al. Treatment of locally advanced breast cancer using primary induction chemotherapy with hormonal synchronization followed by radiation therapy with or without debulking surgery. NCI Monogr 1986; 1: 156–159. [PubMed] [Google Scholar]
- 9.Scholl SM, Fourquet A, Asselain B, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 1994; 5: 645–652. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Brown A, Mamounas E, Wieand S, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15: 2483–2493. [DOI] [PubMed] [Google Scholar]
- 11.Mamounas EP. Overview of National Surgical Adjuvant Breast Project neoadjuvant chemotherapy studies. Semin Oncol 1998; 25: 31–35. [PubMed] [Google Scholar]
- 12.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672–2685. [DOI] [PubMed] [Google Scholar]
- 13.Singletary SE, McNeese MD, Hortobagyi GN. Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer 1992; 69: 2849–2852. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GF, Birchansky CA, Komarnicky LT, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer 1994; 73: 362–369. [DOI] [PubMed] [Google Scholar]
- 15.Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998; 16: 93–100. [DOI] [PubMed] [Google Scholar]
- 16.McCready DR, Hortobagyi GN, Kau SW, et al. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg 1989; 124: 21–25. [DOI] [PubMed] [Google Scholar]
- 17.Botti C, Vici P, Lopez M, et al. Prognostic value of lymph node metastases after neoadjuvant chemotherapy for large-sized operable carcinoma of the breast. J Am Coll Surg 1995; 181: 202–208. [PubMed] [Google Scholar]
- 18.Kuerer HM, Newman LA, Buzdar AU, et al. Residual metastatic ALNs following neoadjuvant chemotherapy predicts disease-free survival in locally advanced breast cancer patients. Am J Surg 1998; 176: 502–509. [DOI] [PubMed] [Google Scholar]
- 19.American Joint Committee on Cancer. Manual for staging of cancer, 6th ed. Philadelphia: Lippincott-Raven; 1998.
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 185: 1457–1481. [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two row rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170. [PubMed] [Google Scholar]
- 22.Wells CA, Heryet J, Brochier J, et al. The immunohistochemical detection of axillary micrometastases in breast cancer. Br J Cancer 1984; 50: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trojani M, de Mascarel I, Bonichon F, et al. Micrometastases to ALNs from carcinoma of breast: detection by immunohistochemistry and prognostic significance. Br J Cancer 1987; 55: 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International (Ludwig) Breast Cancer Study Group. Prognostic importance of occult ALN micrometastases from breast cancers. Lancet 1990; 335: 1565–1568. [PubMed] [Google Scholar]
- 25.Hainsworth PJ, Tjandra JJ, Stillwell RG, et al. Detection and significance of occult metastases in node-negative breast cancer. Br J Surg 1993; 80: 459–463. [DOI] [PubMed] [Google Scholar]
- 26.Clare SE, Sener SF, Wilkens W, et al. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol 1997; 4: 447–451. [DOI] [PubMed] [Google Scholar]
- 27.Fisher ER, Swamidoss S, Lee CH, et al. Detection and significance of occult axillary node metastases in patients with invasive breast cancer. Cancer 1978; 42: 2025–2031. [DOI] [PubMed] [Google Scholar]
- 28.Kuerer HM, Newman LA, Fornage BD, et al. Role of ALN dissection after tumor downstaging with induction chemotherapy for locally advanced breast cancer. Ann Surg Oncol 1998; 5: 673–680. [DOI] [PubMed] [Google Scholar]
- 29.Kuerer HM, Newman LA, Buzdar AU, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts ALN status. Cancer J Sci Am 1998; 4: 230–236. [PubMed] [Google Scholar]
- 30.Fisher B, Redmond, C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med 1985; 312: 674–681. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg 1995; 222: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997; 349: 1864–1867. [DOI] [PubMed] [Google Scholar]