Abstract
Objective
To investigate the effects of the organ preservation solutions UW and Plegisol on endothelial permeability; occludin and vascular endothelial (VE)-cadherin content in human umbilical vein endothelial cells (HUVEC); and junctional localization of these proteins after exposure to these solutions.
Summary Background Data
Organ preservation for transplantation is limited by several challenges, including loss of tissue function, tissue injury, and tissue edema. Occludin and VE-cadherin are responsible for maintaining and regulating the endothelial solute barrier. Several studies have noted organ edema and dysfunction with preservation, as well as gaps between endothelial cells suggesting that disorganization of junctional proteins (e.g., occludin and VE-cadherin) is responsible for interstitial edema.
Methods
HUVEC monolayers were treated with 4°C UW and Plegisol for 3 and 6 hours and then reperfused with normal buffer. Permeability was examined using FITC-dextran tracer during the reperfusion phase. Occludin and VE-cadherin content at different time points was measured by Western blotting. Treated groups were also examined by immunofluorescence for occludin, VE-cadherin, and F-actin.
Results
Compared with untreated controls, cold preservation for 3 and 6 hours increased endothelial permeability after rewarming, which appears to depend on the duration of cold exposure. Monolayers exposed to 3 hours of cold preservation did not have increased permeability in the first hour after rewarming but had significantly increased permeability after the first hour and all subsequent time points. Monolayers exposed to 6 hours of cold preservation had increased permeability after the first hour and at all later time points. Western blotting demonstrated that occludin content was decreased to a similar extent with all solutions after 3 hours of cold preservation. Six hours of cold preservation in Plegisol reduced the occludin content significantly compared with UW and control. VE-cadherin content was unchanged after 3 hours of cold preservation but was dramatically reduced in all groups at 6 hours. Immunofluorescent staining demonstrated junctional gap formation and discontinuous staining of occludin and VE-cadherin with all cold preservation protocols; changes in F-actin organization were observed at 3 and 6 hours after cold preservation.
Conclusion
The changes in occludin, VE-cadherin, and F-actin content and organization and increased permeability associated with cold storage demonstrate that alterations of the tight and adherens junctions may underlie organ edema associated with cold organ preservation. These data also suggest that novel strategies to maintain the content and integrity of endothelial junctional proteins may provide an important therapeutic avenue for organ preservation.
The time during which organs remain viable for transplantation, especially for the heart and lungs, is limited by several factors, including loss of tissue function, tissue injury, and tissue edema. Currently, the time for heart and lung preservation in humans is limited to 4 and 8 hours, respectively. 1,2 Most investigators have examined the effects of cold and ischemia–reperfusion on organ metabolism and metabolic demands as this relates to cell swelling, free radical formation, and posttransplantation organ neutrophil accumulation. 2–8 An important finding has been that cardiac function after 4 hours of cold storage in UW solution is significantly better than tissue stored in Plegisol, and that the increased myocardial edema accompanying organ preservation in Plegisol may contribute to this injury. However, treatment with either solution increased tissue edema, which was associated with a deterioration in cardiac function. 3,9 The lungs are considered to be the organ most sensitive to preservation, and pulmonary edema is nearly universally observed within transplanted lungs. 10,11 Therefore, an important and novel direction in organ preservation research should be to address the specific cellular bases of interstitial edema. Because increased endothelial permeability is the apparent cause of tissue edema and dysfunction, endothelial junction dysregulation may represent the common link between all organ preservation limitations.
The junctional proteins responsible for the solute barrier are occludin (within the zonula occludens) and vascular endothelial (VE)-cadherin (within the zonula adherens), 12–14 both of which are associated with the actin cytoskeleton. Occludin has recently been described as the main functional component of the tight junction. 15,16 Using occludin antisense oligonucleotides, we have demonstrated that the decreased expression of occludin reduced barrier function in both human umbilical venous and arterial endothelial cells. 17
Cadherins (e.g., VE-cadherin), the principal transmembrane proteins in the adherens junction, require calcium for competent cell-to-cell adhesion. 18–20 VE-cadherin is the main endothelial cadherin, and we have previously described the roles of cadherinlike proteins in maintaining and regulating the endothelial solute barrier. 21 We have recently demonstrated that oxidants promote endothelial gaps through cadherin internalization. 22 Therefore, the content and organization of these proteins may be critical determinants of endothelial barrier function, and dysregulation of these proteins may occur in tissue edema.
To our knowledge, no group has investigated the effect of cold preservation on the content and organization of occludin and VE-cadherin in endothelial cells. Given the significance of interstitial edema and organ dysfunction associated with cold preservation, in this study we examined how UW and Plegisol affected endothelial permeability, occludin and VE-cadherin content, and junctional organization of these proteins.
MATERIALS AND METHODS
Tissue Culture and Antibodies
Human umbilical venous endothelial cells (HUVEC) were cultured using standard procedures. 19 Cells were maintained in EGM media (Clonetics, San Diego, CA) and used at passage 2 for all experiments. Rabbit anti-occludin antibody was purchased from Zymed (San Francisco, CA) and mouse anti–VE-cadherin antibody was purchased from Hemeris (Sassenage, France).
Immunohistochemistry
HUVEC were cultured on glass coverslips. On confluency, coverslips were fixed at 4°C by graded ethanol dehydration for 5 minutes using 50%, 75%, 95%, 75%, and back down to 50%. TRITC-Phalloidin (Sigma, St. Louis, MO) was incubated with the coverslips at 25° C in the dark for 30 minutes and washed with phosphate-buffered solution (PBS) three times for 5 minutes. Subsequently, primary antibodies for occludin (1:250) and VE-cadherin (1:250) were added for 1 hour at 37°C. Coverslips were then washed with 0.1% milk/PBS solution for 5 minutes three times each. Secondary antibodies of donkey anti-mouse Cy5 (1:250) and fluorescein (DTAF) donkey anti-rabbit (1:250) (both Jackson Immunoresearch Laboratories Inc., West Grove, PA) were incubated at 37°C for 1 hour. Coverslips were again washed with 0.1% milk/PBS solution for 5 minutes three times each. Coverslips were mounted with Vectashield (Vector Laboratories, Inc., Burlingame, CA).
Preservation Solutions
ViaSpan (Belzer UW, DuPont Pharma, Wilmington, DE) was used at 1× concentration. Plegisol (Abbott’s Carioplegic Solution, Abbott Laboratories, North Chicago, IL) was pH-adjusted to 7.4 according to the manufacturer’s instructions.
Permeability
We have previously described the use of this model with a 10-kD dextran tracer to evaluate permeability in HUVEC monolayers. 17 In brief, cell culture inserts with 0.4-μm pore size (Becton Dickinson Labware, Lincoln Park, NJ) were seeded at confluency with HUVEC passage 2 and incubated for 4 to 6 days, with feedings every other day until use. Five hundred-microliter aliquots of UW, Plegisol, and normal perfusate (normal perfusate represents media that lacks only phenol red, a nonnutritive pH indicator, which interferes with absorbance measurements) were placed into the cell insert, and 1-ml aliquots of each solution were placed into the lower well. Fifty microliters Organ Preservation Solutions of fluorescein isothiocyanate (FITC)-Dextran (MW 10,000 kD) (50 μg/ml) was added to the insert and incubated at 4°C for 3 or 6 hours. The plates were then transferred to a 37°C incubator, and the solutions were replaced with normal perfusate to simulate the organ reperfusion phase.
FITC-dextran tracer was added, as above, to measure the permeability of a small-molecular-weight particle across the HUVEC monolayer. The number and size of endothelial gaps determine how much dye passes through the monolayer: the more dye that passes, the greater the permeability of the monolayer, and thus a potential change in the interstitial oncotic pressure of an organ.
After this transfer to the incubator, every hour for 5 hours a 50-μl sample was taken as described above. Absorbance of FITC-dextran was determined at 450 nm with a plate reader (Titer-tek MCC/340, ICN Pharmaceuticals, Inc., Costa Mesa, CA). Data are expressed as absorbance at 450 nm.
Western Blotting
HUVEC were cultured in 12-well plates. Three to 5 days after confluency, the monolayers were incubated in UW, Plegisol, or media for 3 or 6 hours at 4°C. At the end of each incubation period, the perfusate was removed and 50 μl of Lammeli sample buffer (0.125 M Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.025% bromophenol blue) was directly applied to each well. The cell lysate was removed and heated to 90°C for 5 minutes. Twenty microliters (35 μg of protein) for occludin samples and 5 μl (10 μg of protein) for VE-cadherin samples were then run on 10% SDS-PAGE gels, transferred to nitrocellulose membranes (Sigma), and blocked with blotto (5% milk powder in PBS) for 2 hours. (It was found that different quantities of protein were required to be loaded on the gels for occludin and VE-cadherin because the overall occludin content is significantly less then VE-cadherin in the monolayers. Therefore, if equal amounts of protein were run, the VE-cadherin blot would demonstrate a thick, smeared-out band that could not be quantitated.) Membranes were incubated in rabbit anti-occludin antibody (1:1000) for 2 hours. Membranes were then washed in 0.1% milk and PBS for 5 minutes three times each. Secondary antibodies of goat anti-rabbit IgG alkaline phosphatase (Sigma) were incubated with the membranes for 2 hours at a 1:1000 dilution. Membranes were again washed in 0.1% milk with PBS for 5 minutes each and developed using nitroblue tetrazolium/bromochloroindoyl phosphate (Sigma) colorimetric reagents. The membranes were then densitometrically analyzed and then rehydrated in 0.1% milk PBS and reprobed with mouse anti–VE-cadherin as described above for anti-occludin. Secondary sheep anti-mouse IgG alkaline phosphatase (Sigma) was incubated and developed and densitometry was performed as previously mentioned. All values were normalized to control.
Image and Statistical Analysis
Western densitometric analysis was performed using a HP ScanJet licx flat-bed scanner and Image Pro Plus software (Media Cybernetics, Silver Spring, MD). Data analysis were performed with Graphpad Instat (Graphpad Software, San Diego, CA). Statistical analysis used was one-way analysis of variance with Bonferroni’s posttesting. All error bars for values are recorded as standard error of the mean.
RESULTS
Permeability
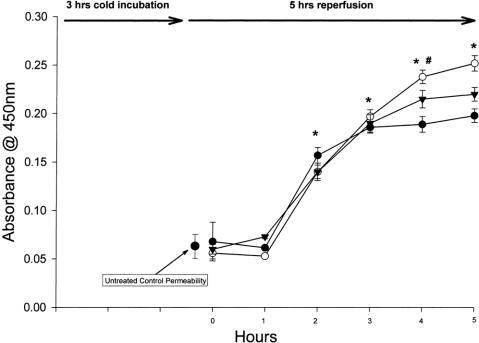
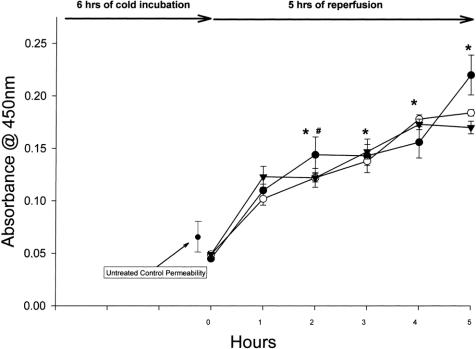
Compared with previously determined untreated controls at 37°C, cold preservation for 3 hours (Fig. 1) and 6 hours (Fig. 2) increased endothelial permeability after rewarming, which appears to depend on the duration of cold exposure. 17 Monolayers exposed to 3 hours of cold preservation did not have increased permeability in the first hour after rewarming but had significantly increased permeability after the first hour and all subsequent time points. Monolayers exposed to 6 hours of cold preservation had increased permeability within the first hour and significantly at all later time points. No difference was observed between the different types of preservation solutions.
Figure 1. HUVEC solute flux after 3 hours of cold preservation with UW (clear circle, n = 8), Plegisol (solid triangle, n = 7), and media (solid circle, n = 8). After 1 hour of reperfusion with normal perfusate, all treatments demonstrated a significant increase from time 0. From 2 to 5 hours of reperfusion, all values were significantly increased from control (* p < 0.001). In addition, UW permeability was significantly increased over media at 4 and 5 hours (# p < 0.01 and * p < 0.001, respectively).
Figure 2. HUVEC solute flux after 6 hours of cold preservation with UW (clear circle, n = 8), Plegisol (solid triangle, n = 8), and media (solid circle, n = 5). Permeability increased immediately after reperfusion for all treatments and was significantly different from time 0 and control at 2 hours reperfusion (# p < 0.01 for UW and Plegisol, * p < 0.001 for media). For 3 to 5 hours reperfusion, all points showed a significant difference from control (* p < 0.001).
Western Analysis
After 3 hours of cold incubation, the occludin content was decreased with all treatments versus untreated control monolayers. The occludin content for UW-treated cells at 3 hours was 67% ± 9% of control (p < 0.05). The occludin content for cells treated with 4°C culture medium for 3 hours was 62% ± 9% (p < 0.05, Fig. 3). At 3 hours, Plegisol-treated cells contained 73% ± 7% of the control level of occludin (p > 0.05). After 6 hours of cold incubation with UW, Plegisol, or media, occludin concentration was significantly decreased versus control (UW, 54% ± 7%, p < 0.01; Plegisol, 20% ± 3%, p < 0.001; media, 37% ± 7%, p < 0.001). We observed a significant difference in occludin content between Plegisol at 3 and 6 hours (73% ± 7% vs. 20% ± 3%, p < 0.001), but no difference was noted between either UW or media at 3 or 6 hours. Moreover, occludin expression at 6 hours with Plegisol was significantly decreased compared with UW at 6 hours (54% vs. 20%, p < 0.01).
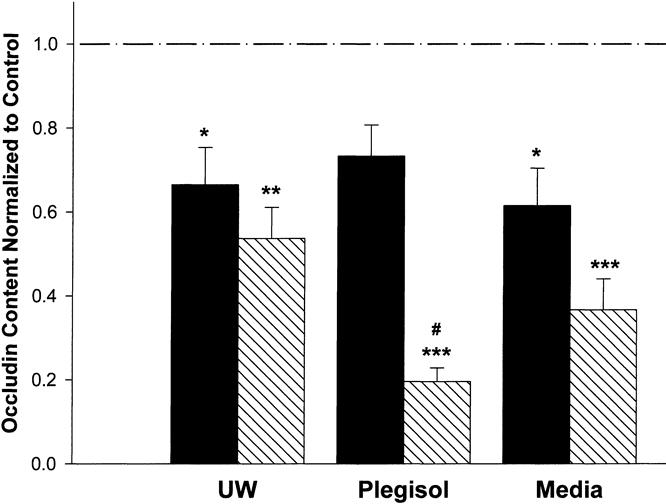
Figure 3. Occludin content after 3 and 6 hours of cold preservation with UW, Plegisol, and media. After 3 hours of preservation (solid column), occludin content was significantly decreased (* p < 0.05) for UW (n = 6) and media (n = 6) from control (n = 5); Plegisol (n = 7) trended toward a significant decrease. After 6 hours (hatched column), occludin content was significantly decreased for Plegisol (n = 10) compared with control (*** p < 0.001), Plegisol at 3 hours, and UW at 6 hours (n = 6) (p < 0.01). UW and media at 6 hours were significantly decreased from control (** p < 0.01 [n = 6] and *** p < 0.001 [n = 6], respectively).
VE-cadherin content was also altered by our treatments. After 3 hours of cold incubation, none of the solutions were significantly different from control (Fig. 4). This stands in contrast to the 6-hour treatment groups, where VE-cadherin content was dramatically decreased to 8% ± 1% with UW, 1% ± 0.3% with Plegisol, and 17% ± 1.7% with media. These values were not significantly different from each other; however, all were dramatically and significantly different from untreated controls (p < 0.001).
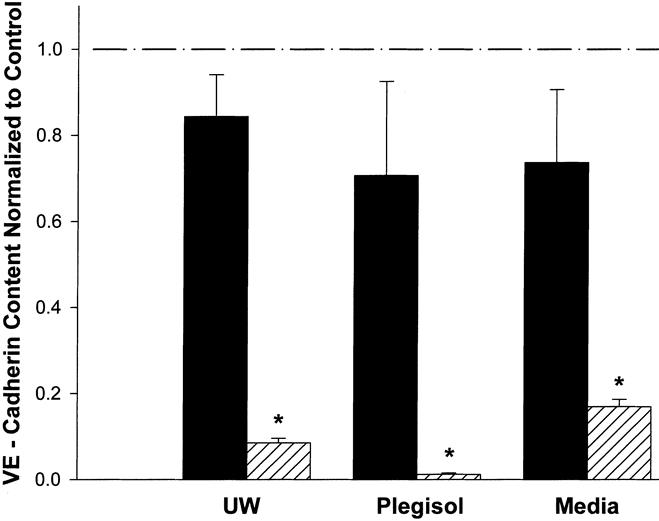
Figure 4. VE-cadherin content after 3 and 6 hours of cold preservation with UW, Plegisol, and media. After 3 hours of treatment (solid column), there was no significant difference from control. Six hours of treatment (hatched column) saw the VE-cadherin content significantly decrease from control and the corresponding content at 3 hours (* p < 0.001; n = 3 for each sample expect Plegisol at 6 hours, n = 5).
Immunofluorescent Staining
Control HUVEC immunofluorescent images illustrate three important features. First, in control (Fig. 5) , occludin and VE-cadherin staining is continuous, circumscribing the cell, and is localized to intercellular junctions, a characteristic that is altered by preservation. Second, the junctional proteins appear to colocalize in the color image (Fig. 5A), where the green and blue of occludin and VE-cadherin overlap and appear as a turquoise color. This characteristic turquoise appearance is reduced when junctions are disorganized and occludin and VE-cadherin content is decreased after cold storage. Further, the actin image (Fig. 5B) shows dense peripheral bands (DPB) of F-actin and some central staining.

Figure 5. Control triple-labeled HUVEC monolayers. (A) Color projection of (B) actin (red), (C) occludin (green), and (D) VE-cadherin (blue). White arrows in B indicate DPB.
At 3 and 6 hours, UW (Fig. 6) shows loss of occludin and VE-cadherin staining where gaps are located. Compared with control (see Fig. 5C), occludin now appears as fingerlike projections at the junction after 3 hours; by 6 hours it has become interrupted and discontinuous. VE-cadherin, which was continuous in untreated monolayers, gradually looses its junctional association at 3 and 6 hours. Actin organization also changes from a junctional appearance to central stress fibers over 3 to 6 hours.
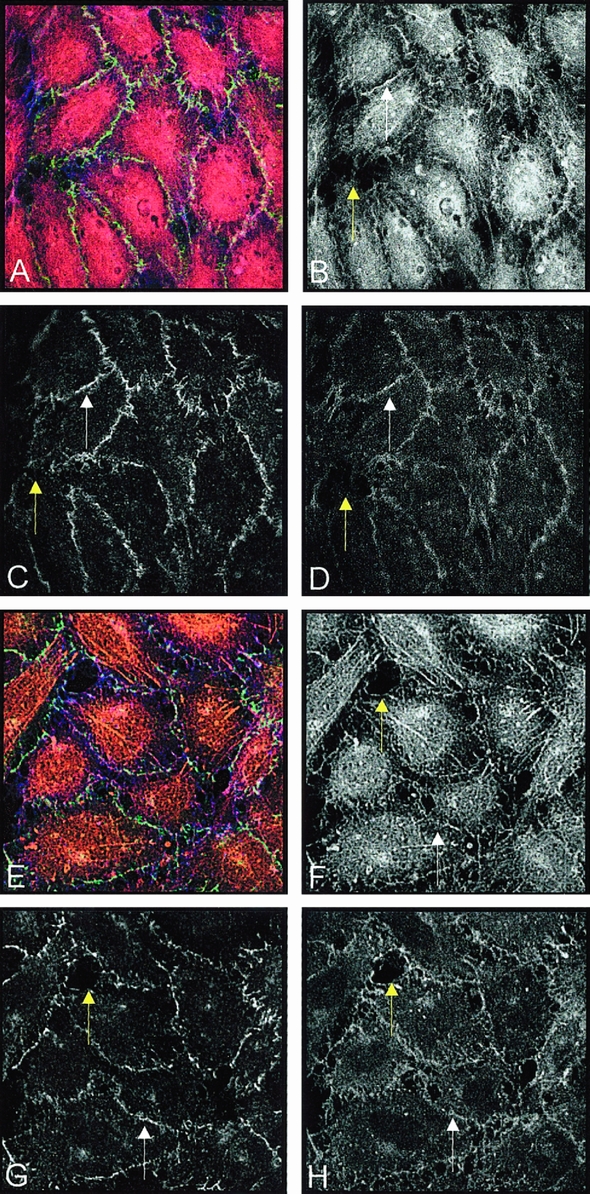
Figure 6. (A) After 3 hours of UW treatment; color projection of (B) actin, (C) occludin, and (D) VE-cadherin. White arrows indicate DPB in B and same area in C and D. (E) After 6 hours of UW treatment; color projection of (F) actin, (G) occludin, and (H) VE-cadherin. White arrows indicate DPB in F and same areas in G and H. Yellow arrows indicate one of several gaps in the monolayer B–D, F–H.
At 3 and 6 hours, Plegisol (Fig. 7) also shows rearrangement of occludin and VE-cadherin staining where gaps have formed. Occludin staining becomes progressively discontinuous over the course of 6 hours. A similar and more dramatic loss of junctional staining is noted for VE-cadherin over this time course. As with UW, junctional actin staining became more central at 3 hours and was completely nonjunctional at 6 hours.

Figure 7. (A) After 3 hours of Plegisol treatment; color projection of (B) actin, (C) occludin, and (D) VE-cadherin. White arrows indicate DPB in B and same area in C and D. (E) After 6 hours of Plegisol treatment; color projection of (F) actin, (G) occludin, and (H) VE-cadherin. White arrows indicate DPB in F and same areas in G and H. Yellow arrows indicate one of several gaps in the monolayer B–D, F–H.
At 3 and 6 hours, media (Fig. 8) demonstrated the most disruption of the junction of the three treatments. Whereas 3 hours of cold preservation with media demonstrated levels of disorganization similar to those with UW and Plegisol, by 6 hours occludin, VE-cadherin, and actin had become completely disrupted.

Figure 8. (A) After 3 hours of media treatment; color projection of (B) actin, (C) occludin, and (D) VE-cadherin. (E) After 6 hours of media treatment; color projection of (F) actin, (G) occludin, and (H) VE-cadherin. Yellow arrows indicate one of several gaps in the monolayer B–D, F–H.
DISCUSSION
Organ preservation is associated with tissue edema after transplantation in a time-dependent fashion; the longer an organ is stored before transplantation, the greater the degree of edema observed. It is widely recognized that tissue edema results in organ dysfunction, 3,23,24 but it remains controversial as to how interstitial edema occurs after cold storage. Several mechanisms have been advanced to explain tissue edema under these conditions, including neutrophil-mediated injury, oxidant injury, and disorganization of endothelial junctions. To our knowledge, no studies have investigated the effects of preservation solutions on junctional proteins and how cold storage in these solutions affects endothelial solute barrier. Here we demonstrate that cold storage in preservation solutions significantly alters endothelial permeability and is correlated with dramatic changes in endothelial occludin and VE-cadherin content and organization. In vivo, endothelial permeability changes are manifested as tissue edema. In this regard, preservation solutions have been shown to cause perivascular edema in heart models and alveolar edema and congestion in lung models. 3,23,25 In the heart, an increase in edema can reduce coronary flow after reperfusion, promoting graft dysfunction. In the rat, it has been reported that UW maintains better coronary blood flow during reperfusion than Plegisol; this was suggested to be a result of myocardial edema, although this was not demonstrated histologically. 9 In another study, dog hearts were stored at 4°C for 24 hours in a modified Krebs/KCl solution. That study demonstrated that organ preservation was associated with marked pericapillary edema and further documented that there was a significant relation between decreased cardiac compliance and increased coronary vascular resistance, which appeared to be related to the observed increase in pericapillary interstitial edema.
Based on these studies in vivo, we expected UW to preserve the solute barrier compared with Plegisol or media. However, we did not observe this in our model; in fact, permeability was significantly increased on reperfusion with all solutions. Clearly, the reason for the increased solute flux is related to alterations in junctional proteins and endothelial gap formation (see Figs. 6, 7, and 8). Hidalgo et al 23 demonstrated numerous gaps in rat lung endothelium ultrastructurally and believed that these gaps represented weakened intercellular connections between endothelial cells. Our immunofluorescent staining also demonstrated numerous gaps between cells after exposure to UW, Plegisol, and media, as well as a decrease in occludin and VE-cadherin staining at cell junctions. The decreased staining for these junctional proteins at gaps was also confirmed by our Western blotting data. We found significantly less occludin in cells treated for 3 hours with UW and media. Although Plegisol also reduced occludin content, its effect at 3 hours was variable and not significant. At 6 hours, there was a further reduction in occludin content in cells treated with UW and media. Importantly, at this time point, Plegisol reduced the occludin content more than any other treatment. Therefore, the effect of Plegisol on barrier integrity appears to be time-dependent.
Conversely, VE-cadherin content was unchanged at 3 hours but also dropped dramatically after 6 hours of cold preservation in all solutions. It has been shown that endothelial cells that are deprived of growth factors will shed VE-cadherin, 26 an event that is dependent on the action of proteases (e.g., caspases and metalloproteinases). We have also observed that during episodes of acute respiratory distress syndrome, VE-cadherin is shed from endothelial cells, an event that may be mediated by elastase. 27 This mechanism for the loss of junctional molecules may provide some insight into the increased permeability observed in this model. However, we did not find a correlation between permeability and VE-cadherin content at 3 hours. At 3 hours, occludin content was significantly reduced in these cells, suggesting that it may play a more important role in barrier. The early loss of occludin (at 3 hours) was in fact correlated with increased permeability.
Some other possibilities exist to explain the alterations in barrier that were observed. We have found that cadherins are transiently internalized in endothelial cells in response to stressful conditions. 28 Perhaps the stress of cold storage promotes the internalization of cadherins to prevent them from maintaining barrier while not significantly affecting the VE-cadherin content. Several groups have documented that tight and adherens junctions are reciprocally regulated through cytoplasmic linker proteins, especially ZO-1. 29 Based on the significant decrease in VE-cadherin at 6 hours, it is reasonable to assume that tight junction integrity is diminished in this setting and is the cause of gap formation and increased solute flux.
With regard to the actin cytoskeleton, another component of the junctional complex, Hall et al 30 showed that different preservation solutions reduced F-actin content to different extents, and that the normal level of actin was not restored until >20 hours. Importantly, the restoration of F-actin content after 1 hour of rewarming did not correlate with permeability. In that study, UW-treated monolayers exhibited high permeability compared with media, despite the same actin content. Our confocal images showed similar results. Actin staining was significantly decreased by 3 hours and further reduced by 6 hours. Several authors suggest that the association of actin microfilaments with the tight junction and changes in their distribution result in increased permeability. 10,31,32 Because cadherins associate with the actin cytoskeleton, their function may reflect these changes. 33,34 Permeability is regulated not only by the loss of junctional proteins but also by actin reorganization after reperfusion, which governs junction protein organization and barrier.
In summary, the dysregulation of junctional proteins and actin results in acute changes in permeability after cold storage. These changes are probably reversible after reperfusion, given the fact that hearts and lungs remain viable for up to 8 hours of preservation. However, organ function is not always optimal during the acute postoperative period.35 A contributing factor to this dysfunction may be that interstitial edema develops during reperfusion as well, and consequently neutrophils may be able to cross a compromised endothelial barrier with greater ease.
Organ preservation has many limitations, including edema and organ dysfunction, which remain poorly understood. Although some studies suggest that UW solution may be better than Plegisol, our studies indicate that both solutions promote some forms of endothelial stress that are associated with the loss or shedding of both VE-cadherin and occludin. Future studies in this area may extend the time for organ preservation and enhance tissue recovery by designing strategies to maintain components of the endothelial barrier that are responsible for normal barrier properties.
Footnotes
Correspondence: J. Steven Alexander, PhD, Dept. of Molecular and Cellular Physiology, LSU Medical Center-Shreveport, 1501 Kings Highway, Shreveport, LA 71130.
Supported by NIH grants HL47615 and DK43785.
Accepted for publication September 16, 1998.
References
- 1.Lampugnani MG, Corada M, Caveda L, et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 1995; 129 (1): 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Alessandro AM, Southard JH, Love RB, Belzer FO. Organ preservation. Surg Clin North Am 1994; 74 (5): 1083–1095. [PubMed] [Google Scholar]
- 3.Bethencourt DM, Laks H. Importance of edema and compliance changes during 24 hours of preservation of the dog heart. J Thorac Cardiovasc Surg 1981; 81 (3): 440–449. [PubMed] [Google Scholar]
- 4.Southard JH, Belzer FO. Principles of organ preservation part II. Surgical Rounds 1993: 443–450. [Google Scholar]
- 5.Southard JH, Belzer FO. Principles of organ preservation part 1. Surgical Rounds 1993: 353–360. [Google Scholar]
- 6.Chiang C-H, Hsu K, Yan H-C, et al. PGE1, Dexamethasone, U-74389G, or Bt2-cAMP as an additive to promote protection by UW solution in I/R injury. Am J Physiol 1997: 583–590. [DOI] [PubMed] [Google Scholar]
- 7.Fischer JH, Jeschkeit S, Klein P. Adding a new principle to hypothermic storage preservation—reduction of edema formation by hyaluronidase. Transplantation 1994; 58: 748–753. [DOI] [PubMed] [Google Scholar]
- 8.Parks DA, Bulkley GB, Granger DN. Role of oxygen free radicals in shock, ischemia, and organ preservation. Surgery 1983; 94: 428–32. [PubMed] [Google Scholar]
- 9.Ledingham SJ, Katayama O, Lachno DR, Yacoub M. Prolonged cardiac preservation. Evaluation of the University of Wisconsin preservation solution by comparison with the St. Thomas’ Hospital cardioplegic solutions in the rat. Circulation 1990; 82(Suppl 5): IV351–358. [PubMed] [Google Scholar]
- 10.Meza I, Ibarra G, Sabanero M, et al. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol 1980; 87 (3 Pt 1): 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledo-Pereyra LH, Rodriguez FJ. Scientific basis and current status of organ preservation. Transplant Proc 1994; 26 (1): 309–311. [PubMed] [Google Scholar]
- 12.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol 1995; 269 (4 Pt 1): G467–475. [DOI] [PubMed] [Google Scholar]
- 13.Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. Faseb J 1995; 9 (10): 910–918. [PubMed] [Google Scholar]
- 14.Tsukita S, Furuse M, Itoh M. Molecular dissection of tight junctions. Cell Struct Funct 1996; 21: 381–385. [DOI] [PubMed] [Google Scholar]
- 15.Hirase T, Staddon JM, Saitou M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 1997; 110 (Pt 14): 1603–1613. [DOI] [PubMed] [Google Scholar]
- 16.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 1997; 136: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kevil CG, Okayama N, Trocha SD, et al. Expression of zonula occludens and adherens junctional proteins in human venous and arterial endothelial cells: role of occludin in endothelial solute barriers. Microcirculation 1998; 5: 197–210. [PubMed] [Google Scholar]
- 18.Lampugnani MG, Resnati M, Raiteri M, et al. A novel endothelial-specific membrane protein is a marker of cell–cell contacts. J Cell Biol 1992; 118: 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lampugnani MG, Caveda L, Breviario F, et al. Endothelial cell-to-cell junctions. Structural characteristics and functional role in the regulation of vascular permeability and leukocyte extravasation. Baillieres Clin Haematol 1993; 6: 539–558. [DOI] [PubMed] [Google Scholar]
- 20.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol 1994; 267 (3 Pt 1): L223–241. [DOI] [PubMed] [Google Scholar]
- 21.Alexander JS, Blaschuk OW, Haselton FR. An N-cadherin-like protein contributes to solute barrier maintenance in cultured endothelium. J Cell Physiol 1993; 156: 610–618. [DOI] [PubMed] [Google Scholar]
- 22.Kevil CG, Ohno N, Gute DC, et al. Role of cadhernin internalization in hydrogen peroxide-mediated endothelial permeability. Free Radical Biology and Medicine 1998; 24: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 23.Hidalgo MA, Shah KA, Fuller BJ, Green CJ. Cold ischemia-induced damage to vascular endothelium results in permeability alterations in transplanted lungs. J Thorac Cardiovasc Surg 1996; 112: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 24.Limas C, Spector D, Wright JR. Histologic changes in preserved cadaveric renal transplants. Am J Pathol 1977; 88: 403–428. [PMC free article] [PubMed] [Google Scholar]
- 25.Copeland JG, Jones M, Spragg R, Stinson EB.In vitro preservation of canine hearts for 24 to 28 hours followed by successful orthotopic transplantation. Ann Surg 1973; 178 (6): 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herren B, Levkau B, Raines EW, Ross R. Cleavage of β-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role of caspases and metalloproteinases. Mol Biol Cell 1998; 9: 1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carden D, Xiao F, Moak C, Robinson-Jackson S, Alexander JS. Neutrophils promote lung microvascular injury via elastase-mediated damage to endothelial junctions. Am J Physiol 1998; 275 (2 Pt Z): H385–392. [DOI] [PubMed] [Google Scholar]
- 28.Kevil CG, Ohno N, Gute D, et al. Hydrogen peroxide induces cadherin endocytosis in vitro: role in barrier regulation. Free Radical Biol Med 1998; 24: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 29.Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol 1986; 102 (2): 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall SM, Komai H, Reader J, Haworth SG. Donor lung preservation: effect of cold preservation fluids on cultured pulmonary endothelial cells. Am J Physiol 1994; 267 (5 Pt 1): L508–517. [DOI] [PubMed] [Google Scholar]
- 31.Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 1987; 253 (1 Pt 1): C171–175. [DOI] [PubMed] [Google Scholar]
- 32.Madara JL, Moore R, Carlson S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol 1987; 253 (6 Pt 1): C854–861. [DOI] [PubMed] [Google Scholar]
- 33.Luna EJ, Hitt AL. Cytoskeleton-plasma membrane interactions. Science 1992; 258 (5084): 955–964. [DOI] [PubMed] [Google Scholar]
- 34.Takeichi M. Cadherins: a molecular family important in selective cell–cell adhesion. Ann Rev Biochem 1990; 59: 237–252. [DOI] [PubMed] [Google Scholar]