Abstract
Objective
To review the features of patients with benign and malignant cystadenomas of the pancreas, focusing on preoperative diagnostic accuracy and long-term outcome, especially for nonoperated serous cystadenomas and resected cystadenocarcinomas.
Summary Background Data
Serous cystadenomas (SCAs) are benign tumors. Mucinous cystic neoplasms should be resected because of the risk of malignant progression. A correct preoperative diagnosis of tumor type is based on morphologic criteria. Despite the high quality of recent imaging procedures, the diagnosis frequently remains uncertain. Invasive investigations such as endosonography and diagnostic aspiration of cystic fluid may be helpful, but their assessment is limited to small series. The management of typical SCA may require resection or observation. Survival after pancreatic resection seems better for cystadenocarcinomas (MCACs) than for ductal adenocarcinomas of the pancreas.
Methods
Three hundred ninety-eight cases of cystadenomas of the pancreas were collected between 1984 and 1996 in 73 institutions of the French Surgical Association. Clinical presentation, radiologic evaluation, and surgical procedures were analyzed for 144 operated SCAs, 150 mucinous cystadenomas (MCAs), and 78 MCACs. The outcome of 372 operated patients and 26 nonoperated patients with SCA was analyzed.
Results
Cystadenomas represented 76% of all primary pancreatic cystic tumors (398/522). An asymptomatic tumor was discovered in 32% of patients with SCA, 26% of those with MCA, and 13% of those with MCAC. The tumor was located in the head or uncinate process of the pancreas in 38% of those with SCA, 27% of those with MCA, and 49% of those with MCAC. A communication between the cyst and pancreatic duct was discovered in 0.6% of those with SCA, 6% of those with MCA, and 10% of those with MCAC. The main investigations were ultrasonography and computed tomography (94% for SCA, MCA, and MCAC), endosonography (34%, 28%, and 22% for SCA, MCA, and MCAC respectively), endoscopic retrograde cholangiopancreatography (16%, 14%, 22%), and cyst fluid analysis (22%, 31%, 35%). An accurate preoperative diagnosis of tumor type was proposed for 20% of those with SCA (144 cases), 30% of those with MCA, and 29% of those with MCAC. An atypical unilocular macrocyst was observed in 10% of SCA cases. The most common misdiagnosis for mucinous cystic tumors was pseudocyst (9% of MCAs, 15% of MCACs). Intraoperative frozen sections (126 cases) allowed a diagnosis according to definitive histologic examination in 50% of those with SCA and MCA and 62% of those with MCAC. For management, 93% of patients underwent surgery. Nonoperated patients (7%) had exclusively typical SCA. A complete cyst excision was performed in 94% of benign cystadenomas, with an operative mortality rate of 2% for SCA and 1.4% for MCA. Resection was possible in 74% of cases of MCAC. Mean follow-up of 26 patients with nonresected SCAs was 38 months, and no patients required surgery. For resected MCACs, the actuarial 5-year survival rate was 63%.
Conclusions
Spiral computed tomography is the examination of choice for a correct prediction of tumor type. Endosonography may be useful to detect the morphologic criteria of small tumors. Diagnostic aspiration of the cyst allows differentiation of the macrocystic form of SCA (10% of cases) and the unilocular type of mucinous cystic neoplasm from a pseudocyst. Surgical resection should be performed for symptomatic SCAs, all mucinous cystic neoplasms, and cystic tumors that are not clearly defined. Conservative management is wholly justified for a well-documented SCA with no symptoms. An extensive resection is warranted for MCAC because the 5-year survival rate may exceed 60%.
Cystic tumors of the pancreas, a pathologically heterogeneous group currently diagnosed more frequently because of the wider availability of imaging procedures, account for approximately 10% to 15% of cystic lesions of the pancreas. 1 Benign cystadenomas (serous and mucinous) and mucinous cystadenocarcinomas (MCACs) represent >75% of all cystic tumors of the pancreas. 1 Serous cystadenomas (SCAs) are benign tumors, and serous cystadenocarcinomas are extremely rare. 2 Mucinous cystadenomas (MCAs) should be resected because of the risk of progression to MCAC.
There is still considerable controversy about the treatment of cystadenomas of the pancreas. Most authors recommend resection whenever possible because of the difficulty in determining which tumors are malignant or potentially malignant, 3 whereas others consider that asymptomatic SCAs can be observed safely for years. 4,5 However, making the correct preoperative diagnosis of pancreatic cysts is often difficult despite modern imaging tests and, more recently, cyst fluid analysis. 6
The present study was designed to assess the clinical parameters, diagnostic tests, pathologic features, and long-term results of cyst excision in a survey of 398 cystadenomas and cystadenocarcinomas of the pancreas.
METHODS
Five hundred twenty-two cases of primary cystic pancreatic neoplasms seen during a 13-year period from January 1984 to December 1996 were collected in a multicenter retrospective study involving surgeon members of the French Surgical Association working in 73 adult surgical units. A median of 3.5 patients (range 1 to 66) was contributed by each unit (only one case each for 21 units). The clinical history, main investigations, tentative diagnosis, histologic findings, type and results of the surgical procedure (except for nonoperated SCAs), and follow-up were obtained for each case.
The diagnostic categories of the survey, according to Kloppel’s classification, 7 are shown in Table 1. We collected 398 cases of cystadenomas and cystadenocarcinomas, which represented 76% of all primary pancreatic cystic tumors.
Table 1. PRIMARY CYSTIC NEOPLASMS OF THE PANCREAS (522 CASES) ACCORDING TO KLOPPEL’S CLASSIFICATION
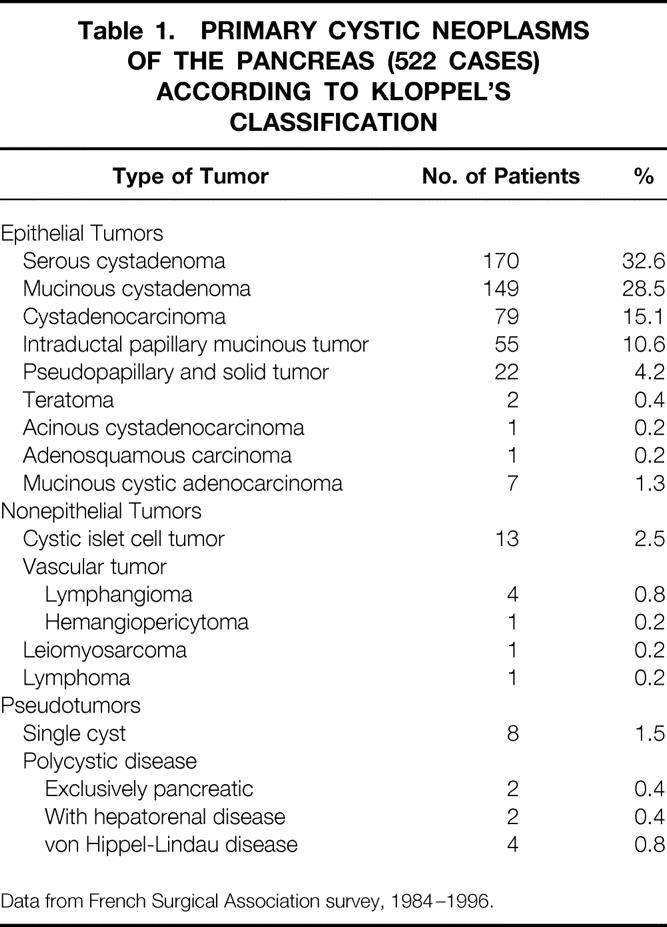
Data from French Surgical Association survey, 1984–1996.
Statistical analysis was performed to compare data between the MCA group and the MCAC group. Fisher’s exact test was used to analyze proportions between the two groups and Student’s t test for comparison of quantitative data. A probability value <0.05 was considered statistically significant. Survival for the MCAC group was calculated by the Kaplan–Meier method, and survival curves between resected carcinomas and nonresected carcinomas were obtained using the log-rank test.
RESULTS
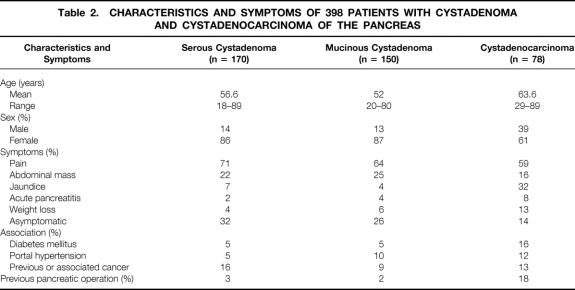
The characteristics and symptoms of the 398 patients (170 SCAs, 150 MCAs, 78 MCACs) are summarized in Table 2. The median age of patients was 58.5 years for those with SCA, 50.5 for those with MCA, and 65 for those with MCAC, with a statistically significant difference between MCA and MCAC (p < 0.001). Female predominance was the same for SCA and MCA (86% and 87%). Sex distribution was different between MCA and MCAC (87% vs.61% of women), and the difference was statistically significant (p < 0.001). Twenty-eight patients with SCA (16%) had a previous (n = 20) or concurrent (n = 8) extrapancreatic neoplasm. Another associated pancreatic neoplasm occurred in seven cases: adenocarcinoma (n = 2), intraductal papillary and mucinous tumor (n = 2) or islet cell tumor (n = 3). In three cases, SCA was noted in patients with renal cell carcinoma and von Hippel-Lindau disease. For MCA, a previous (n = 5) or concurrent (n = 9) extrapancreatic neoplasm was noted in 14 patients (9%). Another pancreatic neoplasm was associated in four cases: adenocarcinoma (n = 2) and islet cell tumor (n = 2). A case of MCAC was associated with polycystic disease of the kidney.
Table 2. CHARACTERISTICS AND SYMPTOMS OF 398 PATIENTS WITH CYSTADENOMA AND CYSTADENOCARCINOMA OF THE PANCREAS
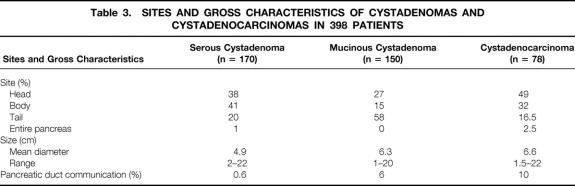
Serum CA19-9 levels were elevated (>37 IU/ml) in 12% of patients with SCA (10/81), 21% of those with MCA (15/72), and 70% of those with MCAC (33/47); there was a significant statistical difference (p < 0.001) between MCA and MCAC. The size and location of the cyst, as well as the communication between the pancreatic duct and the cyst cavity observed in resected specimens, are indicated in Table 3. Location in the head of the pancreas was noted for 38% of patients with SCA, 27% of those with MCA, and 49% of those with MCAC; there was a statistically significant difference between MCA and MCAC (p < 0.001).
Table 3. SITES AND GROSS CHARACTERISTICS OF CYSTADENOMAS AND CYSTADENOCARCINOMAS IN 398 PATIENTS
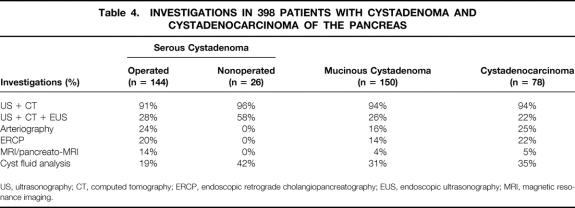
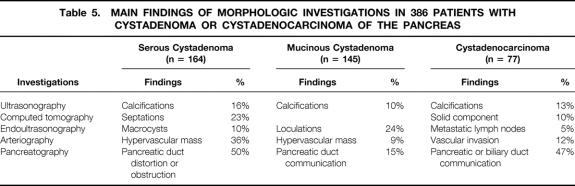
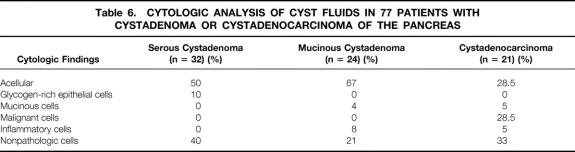
The main investigations and findings are shown in Tables 4 and 5. No preoperative exploration was performed for 12 tumors found incidentally during laparotomy (6 SCA, 5 MCA, 1 MCAC). For nonoperated patients with SCA (n = 26), ultrasonography, computed tomography (CT), and endoscopic ultrasonography were performed in 58% of patients (15/26) and cyst fluid analysis in 42% (11/26). Septations (23%), central calcifications (16%), clusters of small cysts (16%), and single macrocysts (10%) were the main morphologic findings in patients with SCA. The most typical features of MCA were loculations (24%) and peripheral calcifications (10%). The major signs for MCAC were peripheral calcifications (13%), vascular involvement (12%), and a solid eccentric component (10%). Pancreatography, performed in 14% of patients with MCA (20/145) and 22% of those with MCAC (17/77), revealed pancreatic duct communication in 15% of those with MCA and 47% of those with MCAC. Results of the cytologic examination of cyst fluids obtained by fine-needle aspiration biopsy in 77 patients are summarized in Table 6. Typical cells—glycogen-rich cells for SCA, mucin-secreting columnar cells for MCA, and malignant cells for MCAC—were observed in 10% of patients with SCA, 4% of those with MCA, and 29% of those with MCAC.
Table 4. INVESTIGATIONS IN 398 PATIENTS WITH CYSTADENOMA AND CYSTADENOCARCINOMA OF THE PANCREAS
US, ultrasonography; CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography; MRI, magnetic resonance imaging.
Table 5. MAIN FINDINGS OF MORPHOLOGIC INVESTIGATIONS IN 386 PATIENTS WITH CYSTADENOMA OR CYSTADENOCARCINOMA OF THE PANCREAS
Table 6. CYTOLOGIC ANALYSIS OF CYST FLUIDS IN 77 PATIENTS WITH CYSTADENOMA OR CYSTADENOCARCINOMA OF THE PANCREAS
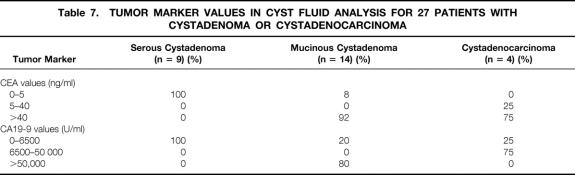
Tumor marker determinations in cyst fluids (carcinoembryonic antigen [CEA], CA19-9) were interpretable in 27 cases (Table 7). Carcinoembryonic antigen values were low (<4 ng/ml) in all cases of SCA (n = 9), high (>5 ng/ml) in 92% of cases of MCA (n = 14), and very high (>40 ng/ml) for 75% of cases of MCAC (n = 4). The CA19-9 level was less than 6500 U/ml for all SCAs.
Table 7. TUMOR MARKER VALUES IN CYST FLUID ANALYSIS FOR 27 PATIENTS WITH CYSTADENOMA OR CYSTADENOCARCINOMA
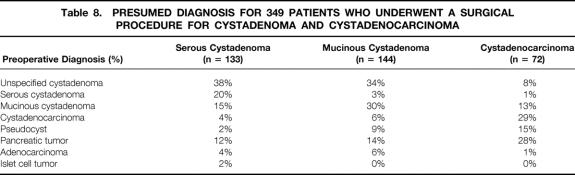
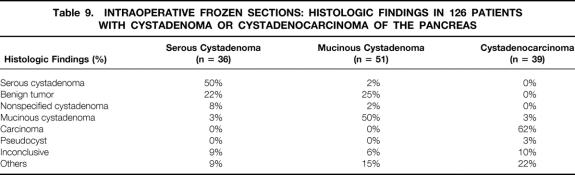
Table 8 lists the presumed preoperative diagnosis for 349 patients (133 CSA, 144 MCA, 72 MCAC) who underwent a surgical procedure. In 26 nonoperated patients, the diagnosis of SCA was based on typical morphologic features and cyst fluid analysis. An accurate diagnosis was noted for 20% of operated patients with SCA, 30% of those with MCA, and 29% of those with MCAC. The most frequently proposed diagnosis for benign cystadenomas was unspecified cystadenoma (38% for SCA, 34% for MCA). For MCAC, a diagnosis of mucinous cystic neoplasm was proposed in 42% of cases. The most common misdiagnosis for mucinous cystic tumors was pseudocyst (9% for MCA, 15% for MCAC). Intraoperative frozen sections were obtained from 126 patients (histologic findings are shown in Table 9 ). The intraoperative diagnosis was concordant with the definitive diagnosis in 50% of cases of SCA, 50% of cases of MCA, and 62% of cases of MCAC.
Table 8. PRESUMED DIAGNOSIS FOR 349 PATIENTS WHO UNDERWENT A SURGICAL PROCEDURE FOR CYSTADENOMA AND CYSTADENOCARCINOMA
Table 9. INTRAOPERATIVE FROZEN SECTIONS: HISTOLOGIC FINDINGS IN 126 PATIENTS WITH CYSTADENOMA OR CYSTADENOCARCINOMA OF THE PANCREAS
Ninety-three percent of patients underwent surgery (372/398). Those who did not (7%; 26/398) had typical paucisymptomatic SCA. All patients with MCA (n = 150) and MCAC (n = 78) underwent surgery. Surgery involved complete cyst excision in 94% of benign cystadenomas (135/144 SCA, 140/150 MCA). The other procedures were partial resection (six MCAs, two SCAs), biopsy (five SCAs, one MCA), biliary bypass (one SCA, two MCAs), and cystoenterostomy (one MCA). A resection was technically possible in 74% of MCACs (58/78) and was extended for local visceral or vascular involvement in 26% of resected MCACs (15/58). Aggressive resections were extended to colectomy (n = 4), gastrectomy (n = 4), nephrectomy (n = 1), hepatic resection (n = 2), and mesentericoportal venous resection (n = 4). A positive resection margin was found in 9% of resected MCACs (5/58). Lymph node status was clearly defined for 44 resected MCACs, and lymph node metastases were observed in 25% (11/44) of the cases. Histopathologic examination of resected specimens (n = 58) showed the coexistence of benign-appearing and malignant epithelia in 55% of cases (32/58). In 45% of cases, there was no area of benign-appearing epithelium. Adjuvant chemotherapy and/or radiation therapy was performed after resection in 34% of cases (20/58). In two cases, MCAC became resectable after neoadjuvant therapy. A palliative procedure was performed in patients with unresectable MCAC (20/78): biliary bypass (n = 12), partial resection (n = 2), neurectomy (n = 2), external drainage (n = 1), and biopsy alone (n = 3).
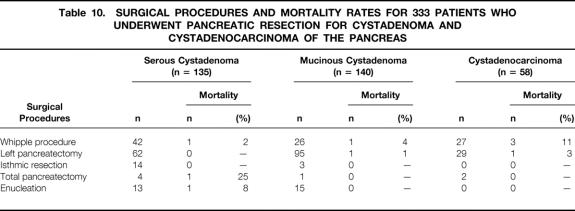
Surgical procedures and mortality rates for the 333 patients who underwent pancreatic resection are shown in Table 10. The postoperative mortality rates after pancreatic resection were 2.2%, 1.4%, and 7% respectively for patients with SCA (3/135), MCA (2/140), and MCAC (4/58). For pancreatoduodenectomy, postoperative mortality rates were 2% for patients with SCA (1/42), 4% for MCA (1/26), and 11% for MCAC (3/27). With left pancreatectomy, there were no postoperative deaths for patients with SCA (n=62); the death rate was 1% for patients with MCA (1/95) and 3% for those with MCAC (1/29). There were no postoperative deaths after isthmic resection for SCA (n = 14) and MCA (n = 3). After enucleation, the postoperative mortality rate was 8% for patients with SCA (1/13) and 0% for MCA (n = 15). The cause of postoperative death after enucleation for SCA was a necrotizing pancreatitis.
Table 10. SURGICAL PROCEDURES AND MORTALITY RATES FOR 333 PATIENTS WHO UNDERWENT PANCREATIC RESECTION FOR CYSTADENOMA AND CYSTADENOCARCINOMA OF THE PANCREAS
For SCA, mean follow-up after surgery was 44 months for 141 patients (three postoperative deaths), including 28% (40/141) for >5 years. There were no SCA-related deaths, but recurrence of SCA was observed in two cases. Mean follow-up was 38 months for the 26 nonoperated patients, none of whom required surgery. For MCA, mean follow-up was 47 months for 147 surviving patients (three postoperative deaths), including 32% (47/147) for >5 years. One hundred thirty-six were alive with no evidence of disease, whereas six died of an unrelated cause and one of pancreatic adenocarcinoma associated with MCA. In four cases, a new pancreatic cystic lesion was observed: two MCAs after partial resection, a second MCA and a pseudocyst. There were no cases of MCAC. For the subset of borderline tumors (n = 13), mean follow-up was 55 months, with no recurrences. For patients with resected MCAC, the actuarial 5-year survival rate was 63%. Two patients with late local recurrence underwent a second resection 5 and 10 years after the initial pancreatectomy. The second procedure was an extended pancreatectomy with colectomy after an initial limited distal resection and a total pancreatectomy after an initial Whipple’s resection. The nonresected group had a 2-year survival rate of 12% (Fig. 1).
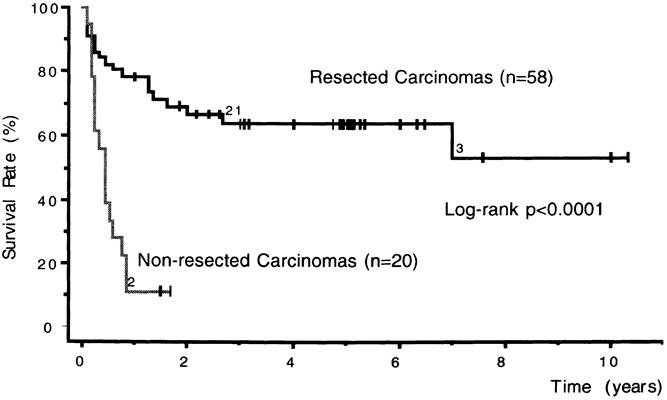
Figure 1. Survival rate for resected (n = 58) and nonresected (n = 20) cystadenocarcinomas (Kaplan–Meier).
DISCUSSION
Serous Cystadenoma
Serous cystadenomas are benign cystic tumors composed of cuboidal epithelium producing serous fluid. 7 This most common single type of cystic tumor is largely predominant in women and is asymptomatic in a third of cases. 1 When symptoms occur, they usually involve mild upper abdominal pain and concern the abdominal mass. Although up to 50% of SCAs are located in the head of the pancreas, 1 a related biliary obstruction is infrequent. 8 Acute pancreatitis is rare and related to an obstruction or rarely to communication with the pancreatic duct. 9 Portal hypertension without bleeding was noted in 5% of operated patients in our series. Serous cystadenomas are associated with another extrapancreatic neoplasm in up to 22% of cases 10,11 or a second pancreatic tumor, or are detected during assessment of von Hippel-Lindau disease. 4,12
Typically, SCAs have a microcystic appearance, with numerous small (<2 cm), well-defined cystic loculations, central calcifications, enhancement around microcysts after injection, and larger cysts on the periphery of the mass. The presence of such CT scan signs is conclusive for diagnosis. 13–15 In practice, except for centers experienced in pancreatic imaging, 13,16 preoperative diagnostic accuracy is about 40% for SCA, 4,17 whereas the rate was only 20% in this survey (see Table 8). There are several possible explanations for these poor results. First, this was a multicenter study of an unusual disease in which only one cystic tumor was contributed by 21 of the 73 collaborating centers. Second, only preoperative diagnoses were considered; in other words, nonoperated patients with a correct diagnosis of SCA were excluded. Third, the preoperative diagnosis of unspecified cystadenoma in 38% of cases was imprecise but not inaccurate. Fourth, there has been an improvement in diagnostic accuracy from 11% to 30% since 1990 because of better knowledge of these tumors and of the contribution of endoscopic ultrasound to the detection of the microcystic component of small tumors. Fifth, imaging was inaccurate for preoperative diagnosis in atypical cases such as the unilocular macrocystic forms described by Lewandrowski et al, 18 which represented 10% of the cases in our series. In this situation, preoperative cyst fluid analysis and intraoperative biopsy for frozen section can be helpful. Cytologic and biochemical analysis of pancreatic cyst fluid can reveal glycogen-rich cells and very low CEA levels. 6,19,20 However, cytologic analysis is acellular in 50% of cases, as in our series, and does not provide a reliable means of distinguishing SCA from mucinous cystic tumor.
A CEA cyst fluid value <5 ng/ml is very indicative of SCA. A value above this threshold was not found for any SCA in this series. Intraoperative biopsy may allow a definitive diagnosis but fails to classify a pancreatic cystic lesion in 20% to 42% of cases because of frequent extensive denudation of cyst epithelial lining. 19,21,22
Most patients with SCA undergo pancreatic resection for a symptomatic tumor or when the nature of the cystic lesion cannot be definitively established. In these cases, a conservative procedure is suitable to limit late sequelae, as in preservation of the pylorus in proximal pancreatectomy, segmental resection for isthmic/body tumors, and preservation of the spleen in distal pancreatectomy. Simple cystic enucleation seems suitable for peripheral lesions, but very high rates of mortality and morbidity were reported in a previous series. 4 The routine use of prophylactic octreotide might be considered for this indication to decrease the high rate of pancreatic fistula. The decision to perform pancreatic resection is easier for body/tail tumors, which are not subject to surgical mortality (as in our series), than for proximal tumors, which have an operative mortality rate of up to 2%. Some authors have suggested that cystic lesions with a presumptive diagnosis of SCA should simply be observed. 4,5 Thus, conservative management seems warranted for a well-documented symptom-free SCA without duct or vascular obstruction, although this strategy has not been clearly evaluated.
With a mucinous cystic tumor, there is a potential risk of misdiagnosis, particularly for macrocystic forms or islet cell tumor. Doubtful tumors should be resected. Another lesser risk is incorrect treatment of a serous cystadenocarcinoma, a low-grade carcinoma representing an extremely rare malignant form of SCA (only five cases have been reported 2,23–26 ). Finally, in some nonoperated patients with proximal SCA, complications unpredictably develop, such as obstructive jaundice, pancreatic duct dilatation, or portal encasement, and require surgery. Conservative management implies monitoring by at least annual ultrasound. In our series, 26 patients were observed, and none required surgery after a mean follow-up of 38 months.
Mucinous Cystadenoma
Mucinous cystadenomas are benign cystic tumors composed of columnar mucin-producing epithelium, 7 including borderline tumors defined by the existence of moderately dysplastic changes. They are detected in nearly 90% of cases in women, whose mean age is lower than that for SCA. 8,22,27 The clinical presentation is identical for both benign MCA and SCA, although recurrent pancreatitis is more frequent in MCA; this suggests a possible communication between the cyst and the pancreatic duct. Almost 75% of MCAs are located in the body/tail region. Therefore, left-sided portal hypertension can be observed on surgical exploration (10% in our series), whereas bleeding is exceptional. An asymptomatic or minimally symptomatic MCA is detected in 25% of cases by ultrasound or CT scan.
Typically, the CT scan shows large cysts with septa, peripheral calcifications, and sometimes a solid intracystic component. A large multilocular cyst should be resected without further investigation because of the risk of malignant progression, 27 which is probably high but is difficult to determine. 28 A large unilocular cyst, in the absence of any history of pancreatitis or of pathologic features in the pancreas, is probably a mucinous neoplasm and should be resected. 15 If there is a history of pancreatitis or any uncertainty about macrocystic SCA, examination of cyst fluid can discriminate between a pseudocyst and SCA. Biochemical examination of the aspirates in MCA has shown low levels of pancreatic enzymes and high CEA levels. 6,19 Cytologic analysis can reveal the presence of mucin-containing cells 29 but is frequently uninformative. In practice, the main risk is misdiagnosis of pseudocyst; this rate has reached 37% to 57% in some series 21,22 but seems at present to be approximately 9%, 1 as in our series. The risk of confusion increases in case of a pancreatic duct connection, which can result in recurrent pancreatitis and high levels of pancreatic enzymes in cyst fluid. Endosonography may detect mucinous content, especially in small MCAs. 30 Endoscopic pancreatography or magnetic resonance pancreatography can show the communication between a normal pancreatic duct in the absence of changes suggestive of chronic pancreatitis. 31 Discovery of a pancreatic duct communication is infrequent (6% in our series). A review of the literature showed only 27 cases of such communicating MCAs, 28 which raises the question of a possible intraductal papillary mucinous tumor. 32
At laparotomy, when the distinction between pseudocyst and MCA is unclear, intraoperative biopsy of the cystic wall can be useful. The presence of epithelium indicates that the cyst is neoplastic and should be resected. However, epithelium is incomplete in 72% of cases, and the absence of epithelium does not rule out a mucinous neoplasm. 1 Intraoperative biopsy in our experience allowed correct histologic diagnosis in only half of cases.
Management of MCA is less controversial than that for SCA; it involves complete resection, which is often relatively straightforward, given the preponderance of distal lesions. 15 A recent study has suggested that enucleation of MCA can be performed, especially for lesions located in the head or uncinate process. These cases showed a low rate of recurrence but a high rate of pancreatic fistulas. 3 Enucleation appears to be a debatable procedure because of the risk of the malignancy of these tumors and the high rate of postoperative complications. The prognosis after pancreatic resection is excellent, even for borderline mucinous cystic tumors, 1 as in our series. Follow-up is recommended by morphologic explorations because of the malignancy potential and the difficulty in performing a complete histologic examination. Recurrence is rare but possible. 8 During follow-up, the discovery of a pancreatic cyst lesion can be related to postoperative pseudocyst, a recurrence of mucinous tumor linked to incomplete resection, a new mucinous neoplasm, or, as in one case in our series, a cystadenocarcinoma after inadequate histopathologic examination. 8
Mucinous Cystadenocarcinoma
Mucinous cystadenocarcinoma, the malignant form of a mucinous cystic neoplasm, is a cystic carcinoma composed at least of severely dysplastic mucin-producing columnar epithelium. 7 Objective findings of invasive malignancy should be required before lesions are designated as MCAC. 8 Malignant transformation of benign MCA is recognized but difficult to prove. There are at least three factors in favor of malignant transformation of a benign mucinous neoplasm. First, there are documented observations of mucinous cystic neoplasms that were quiescent for many years before acquiring malignant features. 1,27,33,34 In this survey, a cystic tumor was known to exist in three patients for 8, 11, and 17 years before it was diagnosed as MCAC. Second, the median age at diagnosis is higher for MCAC than MCA, 8,17,35 with a difference of 14.5 years in our survey. Third, the coexistence of benign-appearing and malignant epithelia on the histologic examination of resected tumors, 1,27 as in 55% of our cases, is an important factor. However, paradoxically, sex distribution and the anatomic location of lesions differ between malignant lesions and their benign counterparts. Female predominance is less noticeable for MCAC, 8 and some recent series have concerned predominantly male cases. 35,36 According to an analysis of 13 series published since 1978, which collected 156 cases of MCAC, 28 the tumor site was in the head of the pancreas in 46% of cases. This preferential location for MCAC in the head is contrary to the usual location of MCA in the body or tail of the pancreas. It is difficult to explain these differences concerning sex distribution and location, which were also observed in our series.
Almost all patients are symptomatic, 37,38 but the absence of symptoms does not rule out a diagnosis of MCAC, as for 14% of cases in this survey. Obstructive jaundice in 25% to 54% of cases, 8,17,34 as well as bleeding related to gastric involvement, portal hypertension, hemobilia, or hemosuccus, 39–41 can be indicative of an aggressive tumor. A palpable abdominal mass is present in 25% of cases. 8,17 The presence of diabetes mellitus, noted in 16% of our cases, seems strongly suggestive of a malignant mucinous neoplasm. 42 A particular situation is the case of patients who had undergone a previous surgical procedure, excluding pancreatic resection, because of inaccurate diagnosis (15% in this series). A review of the literature focused on this problem collected 51 cases of mucinous cystic neoplasm mistreated by a drainage procedure or prolonged observation. In 53% of cases, the final diagnosis was malignant mucinous neoplasm. 28 In exceptional cases, MCAC is discovered during pregnancy 43 or within the context of a polycystic disease of the kidney 44 (one case of each in our survey).
Typical MCAC is a thick-walled macrocyst with a solid component and a peripheral rim of calcifications. 22 Dilatation of the main duct, noted on CT scan in 87% of cases in a recent series, 37 can reveal a pancreatic duct communication. Endosonography is useful in detecting intracystic mural nodules or extracystic solid components in small tumors. 36 In practice, correct prediction of tumor type is achieved in 32% to 43% of cases. 17,36 The main risk is misdiagnosis of pseudocyst subsequent to inappropriate treatment and prolonged observation, which jeopardizes the chances for cure. 1 Some biologic and morphologic investigations can help prevent this mistake. Elevated serum CA19-9 levels have been reported in 75% of cases. 20,45 Serum amylase levels are normal except in cases of communication with the pancreatic duct (10% of cases in our survey). Endoscopic retrograde cholangiopancreatography can suggest a malignant lesion by demonstrating duct obstruction or occlusion or showing a communication between the pancreatic or biliary duct and the cyst cavity. 1,46 Endoscopic retrograde cholangiopancreatography should probably be replaced by noninvasive magnetic resonance pancreatography, which allows complete opacification of the pancreatic duct system. 47
Preoperative cyst fluid analysis is debatable because of the theoretical risk of tumor cell seeding. 6,19 In our experience, no cases of malignant cell seeding along the needle tract were observed in 21 fine-needle aspirations for MCAC. Cytologic examination is helpful only when it reveals obviously malignant cells. 29 Cytology results were positive in only 29% of our cases. In a recent study, Bartsch et al 37 emphasized the possibility of determining the malignant potential of cystic tumors of the pancreas by detection of K-ras mutations in the aspirates of MCAC, although their experience was limited. High levels of CEA (>400 ng/ml) and CA19-9 (>50,000 U/ml) in cyst fluid have a good specificity for differentiating pseudocysts from mucinous tumors but do not provide reliable determination of malignant tumors. 6
Finally, intraoperative biopsy of the cyst wall usually allows diagnosis of the carcinoma, although an error could occur when the malignant component is focal. 19 In our series, a correct preoperative diagnosis was established in only 62% of cases.
An aggressive surgical approach to MCAC is warranted for at least three reasons. First, curative resection was possible in 65% of cases in a review of the literature collecting 173 cases of MCAC since 1978, 28 and 74% in our survey. Unlike ductal adenocarcinomas, MCAC tend to be “pushers” rather than “invaders,” 8 However, in certain conditions, a resection extended to surrounding viscera or the mesentericoportal vein is warranted, as it was in 26% of our cases. If metastases are present and resectable, they should be removed together with the tumor. 1 An apparently unresectable tumor with no metastases can become resectable after combined chemoradiation therapy, 48 as with two patients in our survey. Secondly, lymph node involvement, which decreases survival, is less common for MCAC than for ductal adenocarcinomas. In a recent impressive article, 49 node-negative resections were more common for MCAC (64%) than for ductal adenocarcinomas (30%). In our series, the incidence of positive lymph nodes was 25% for documented cases. Third, the prognosis after pancreatic resection is significantly better for patients with MCAC than for those with adenocarcinoma of the pancreas. 49,50 For resected MCAC, the 5-year actuarial survival rate has exceeded 50% 22,39,50 and has even reached 72% 34 (it was 63% in our survey). However, the prognosis for unresected MCAC is as poor as that for unresected pancreatic adenocarcinoma. 1 According to a preliminary report, survival seems to be correlated with DNA cytometry. 51 Patients with aneuploid aggressive tumors could benefit most from adjuvant chemoradiation therapy. 35
Acknowledgments
The authors thank Dr. Pascal Glémain. The following surgeons collaborated in this report: B. Allagnat (Bourgouin-Jallieu), J.P. Arnaud, J. Ronceray (Angers), M. Allouis, F. Maurus (Lorient), X. Barth (Lyon), J. Baulieux, E. Delaroche (Lyon), W. Beany (La Rochelle), J. Belghiti, A. Sauvanet, J.H. Maillochaud (Clichy), P. Boissel, B. Bresler (Nancy), B. Bouchet (Chambéry), J. Boulez (Lyon), J.C. Brissiaud (Lyon), P. Cabanis (Créteil), P. Caillon (Villeurbanne), J. Carles, J. Saric, E. Rullier (Bordeaux), M. Carretier, J. Barbier (Poitiers), J.F. Charles (Brest), P.A. Coiffard (Nantes), P. Cougard (Dijon), J. Cuilleret, O. Tiffet (Saint-Etienne), P. Cubertafond, F. Mathonet (Limoges), A. Dabrowski (Seclin), T. De Cervens (Saint-Nazaire), T. Panou de Faymoreau (Quimperlé), J.R. Delpéro (Marseille), B. Descottes, D. Valleix (Limoges), J. Domergue (Monptelier), T. Dodart (LeHavre), A. Durand (Roanne), J.L. Faure (Bourgouin-Jallieu), J.B. Flament, B. Delattre, J.P. Cailliez-Thomas (Reims), B. Gayet, A. Errougani, P. Lévy (Paris), C. Gautier-Benoit (Lens), M. Gignoux, P. Ségol, N. Girard (Caen), G. Godlewski, M. Prudhomme (Nîmes), C. Gouillat, P. Bérard (Lyon), J.L. Gouzi, L.Demasles (Toulouse), M. Guivarc’h, H. Mosnier (Paris), P. Herbière (Albi), R. Houdard (Paris), C. Huguet, N. Ambrosiani (Monaco), M. Hugier (Paris), D. Jaeck, P. Bachelier (Strasbourg), M. Julien, P.L. Fagniez (Créteil), J. Kahn (Epinal), J.P. Lechaux (Paris), P. Ledouarec (Dieppe), M. Legoff (La Rochelle), J.P. Lenriot (Longjumeau), C .Letoublon, M. Pécher (Grenoble), Y.P. Letreut (Marseille), J.C. Levasseur (Reims), G. Lorimier (Angers), P. Marre (Marly-le-Roi), C. Meyer, S. Rohr (Strasbourg), B.Millat (Monptelier), A. Morin (Nantes), H. Ollivier (Saint-Brieuc), J. Paineau, J. Visset (Nantes), J.L. Peix (Lyon), L. Potiron (Nantes), C. Proye, J.P. Triboulet, J.L. Houpeau (Lille), P. Quandalle (Lille), H. Richelme (Nice), J. Rivière (Nantes), B. Signouret (Marseille), P. Tenière (Rouen), J. Testart, C. Peillon (Rouen), E. Thoulouzan (La Rochelle), P. Verhaeghe (Amiens), and J.A. Warlin (Enghien).
Footnotes
Correspondence: Joël Le Borgne, MD, Clinique Chirurgicale 2, Hôtel Dieu, 1 Place Alexis Ricordeau, 44093 Nantes Cedex 01, France.
Accepted for publication March 12, 1999.
References
- 1.Fernandez-Del Castillo C, Warshaw AL. Cystic tumors of the pancreas. Surg Clin North Am 1995; 75: 1001–1016. [DOI] [PubMed] [Google Scholar]
- 2.Funabiki T, Kamei K. Serous cystadenocarcinoma. In: Pour PM, ed. Atlas of exocrine pancreatic tumors. Tokyo: Springer; 1994: 101–116.
- 3.Talamani MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas. Is enucleation an adequate operation? Ann Surg 1998; 227: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyke CM, Van Heerden JA, Colby TV, et al. The spectrum of serous cystadenoma of the pancreas. Clinical, pathologic, and surgical aspects. Ann Surg 1992; 215: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loser C, Folsch UR, Creutfeldt W. Serous cystadenoma and mucinous cystadenoma/cystadenocarcinoma of the pancreas. Clinical manifestation, diagnostic procedure and therapeutic concept [in German]. Leber Magen Darm 1990; 20: 173–174. [PubMed] [Google Scholar]
- 6.Hammel P, Levy P, Voitot H, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology 1995; 108: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 7.Kloppel G, Solcia E, Longnecker DS, et al. Histological typing of tumors of the exocrine pancreas, 2d ed. Berlin: Springer; 1996.
- 8.Sarr MG, Prabhakar LP, Loftus EV Jr. The spectrum of cystic neoplasms including mucinous ductal ectasia. In: Advances in pancreatic disease. New York: Thieme; 1996: 352–370.
- 9.Furukawa H, Takayasu K, Murai K, et al. Serous cystadenoma of the pancreas communicating with a pancreatic duct. Int J Pancreatol 1996; 19: 141–144. [DOI] [PubMed] [Google Scholar]
- 10.Iacono C, Bortolasi L, Scarpa A, et al. Cystadenomas of the pancreas are frequently associated with malignant tumors [abstract]. In: Proceedings of the Digestive Week of the American Gastroenterological Association, San Francisco, 1996.
- 11.Tarpila E, Borch K, Franzen L, et al. Cystic neoplasms of the pancreas: A clinicopathological study of 38 cases. Dig Surg 1989; 6: 138–141. [Google Scholar]
- 12.Hammel P, Begeilman C, Chauveau D, et al. Variété des lesions pancréatiques observées au cours de la maladie de von Hippel Lindau. Présentation de 8 cas. Gastroenterol Clin Biol 1995; 19: 1011–1017. [PubMed] [Google Scholar]
- 13.Jonhson CD, Stephens DH, Charboneau JW, et al. Cystic pancreatic tumors: CT and sonographic assessment. AJR 1988; 151: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 14.MacCarty RL. Cyst fluid analysis and imaging of pancreatic cystic lesions. AJR 1995; 164: 820–821. [DOI] [PubMed] [Google Scholar]
- 15.Le Borgne J. Cystic tumors of the pancreas. Br J Surg 1998; 85: 577–579. [DOI] [PubMed] [Google Scholar]
- 16.Vilgrain V, Kazeroun F, Anglade MC, et al. Interobserver agreement and diagnostic accuracy of cystic pancreatic lesions at CT. Radiology 1995; 197(Suppl): 37. [Google Scholar]
- 17.De Calan L, Levrard H, Hennet H, et al. Pancreatic cystadenoma and cystadenocarcinoma: Diagnostic value of preoperative morphological investigations. Eur J Surg 1995; 161: 35–40. [PubMed] [Google Scholar]
- 18.Lewandrowski K, Warshaw AL, Compton CC. Macrocystic serous cystadenoma of the pancreas: A morphologic variant differing from microcystic adenoma. Hum Pathol 1992; 23: 871–875. [DOI] [PubMed] [Google Scholar]
- 19.Lewandrowski K, Lee J, Southern J, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: A new approach to the preoperative assessment of pancreatic cystic lesions. AJR 1995; 164: 815–819. [DOI] [PubMed] [Google Scholar]
- 20.Sperti C, Pasquali C, Guolo P, et al. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer 1996; 78: 237–243. [DOI] [PubMed] [Google Scholar]
- 21.Delcore R, Thomas JH, Forster J, et al. Characteristics of cystic neoplasms and results of aggressive surgical treatment. Am J Surg 1992; 164: 437–442. [DOI] [PubMed] [Google Scholar]
- 22.Warshaw AL, Compton CC, Lewandrowski K, et al. Cystic tumors of the pancreas: New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg 1990; 212: 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zirinsky K, Abiri M, Baer JW. Computed tomography demonstration of pancreatic microcystic adenoma. Am J Gastroenterol 1984; 79: 139–142. [PubMed] [Google Scholar]
- 24.George DH, Murphy F, Michalski R, et al. Serous cystadenocarcinoma of the pancreas: A new entity? Am J Surg Pathol 1989; 13: 61–66. [DOI] [PubMed] [Google Scholar]
- 25.Kamei K, Funabiki T, Ochiai M, et al. Multifocal pancreatic serous cystadenoma with atypical cells and focal perineural invasion. Int J Pancreatol 1991; 10: 161–172. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimi N, Sugie S, Tanaka T, et al. A rare case of serous cystadenocarcinoma of the pancreas. Cancer 1992; 69: 2449–2453. [DOI] [PubMed] [Google Scholar]
- 27.Compagno J, Oertel J. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma): A clinicopathologic study of 41 cases. Am J Clin Pathol 1978; 69: 573–580. [DOI] [PubMed] [Google Scholar]
- 28.Le Borgne J, Bogomoletz WV, Vilgrain V. Les cystadénomes mucineux. Les cystadénocarcinomes. In: Le Borgne J, et al. Les tumeurs kystiques du pancréas. Paris: Arnette; 1997: 47–89.
- 29.Dodd LG, Farrell TA, Layfield LJ. Mucinous cystic tumor of the pancreas. An analysis of FNA characteristics with an emphasis on the spectrum of malignancy-associated features. Diagn Cytopathol 1995; 12: 113–119. [DOI] [PubMed] [Google Scholar]
- 30.Napoléon B, Souquet JC, Pujol B, Ponchon T. Pancreas and ampulla of Vater. In: Dubbins PA, Joseph AEA, eds. Ultrasound in gastroenterology. New York: Churchill Livingstone; 1994: 141–167. [PubMed]
- 31.Yeo CJ, Saar MG. Cystic and pseudocystic disease of the pancreas. Curr Prob Surg 1994; 31: 165–252. [DOI] [PubMed] [Google Scholar]
- 32.Warshaw AL. Mucinous cystic tumors and mucinous ductal ectasia of the pancreas. Gastrointest Endosc 1991; 37: 199–120. [DOI] [PubMed] [Google Scholar]
- 33.Remine SC, Frey D, Rossi RL, et al. Cystic neoplasms of the pancreas. Arch Surg 1987; 122: 443–446. [DOI] [PubMed] [Google Scholar]
- 34.Talamani MA, Fishman EK, Hruban RH, Pitt HA. Cystic neoplasms. In: Hepatobiliary and pancreatic disease. The team approach to management. Boston: Little, Brown and Company; 1995: 451–461.
- 35.Brenin DR, Talamonti MS, Yang EY, et al. Cystic neoplasms of the pancreas. A clinico-pathologic study including DNA flow cytometry. Arch Surg 1995; 130: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 36.Levy M, Levy P, Hammel P, et al. Diagnostic des cystadénomes et cystadénocarcinomes du pancreas: étude de 35 cas. Gastroenterol Clin Biol 1995; 19: 189–196. [PubMed] [Google Scholar]
- 37.Bartsch D, Bastian D, Barth P, et al. K-ras oncogene mutations indicate malignancy in cystic tumors of the pancreas. Ann Surg 1998; 228: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grieshop NA, Wiebke EA, Kratzer SS, et al. Cystic neoplasms of the pancreas. Am Surg 1994; 60: 509–515. [PubMed] [Google Scholar]
- 39.Hodgkinson DJ, Remine WH, Weiland LH. A clinical pathologic study of 21 cases of pancreatic cystadenocarcinomas. Ann Surg 1978; 188: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung KL, Lau WY, Cooper JE. Mucinous cystadenocarcinoma of the pancreas: An uncommon presentation with hemobilia. Gastrointest Endosc 1994; 632–634. [DOI] [PubMed] [Google Scholar]
- 41.Baruch M, Levy Y, Goldsher D, et al. Massive hematemesis presenting symptoms of cystadenocarcinoma of the pancreas. Postgrad Med J 1989; 65: 42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi K, Ogawa Y, Chijiiwa K, Tanaka M. Mucin hypersecreting tumors of the pancreas: Assessing the grade of malignancy preoperatively. Am J Surg 1996; 171: 427–431. [DOI] [PubMed] [Google Scholar]
- 43.Smithers BM, Welch C, Goodall P. Cystadenocarcinoma of the pancreas presenting in pregnancy. Br J Surg 1986; 73: 691. [DOI] [PubMed] [Google Scholar]
- 44.Niv Y, Turani C, Kahan E, Fraser GM. Association between pancreatic cystadenocarcinoma, malignant liver cysts, and polycystic disease of the kidney. Gastroenterology 1997; 112: 2104–2107. [DOI] [PubMed] [Google Scholar]
- 45.Shyr YM, Su CH, Tsay SH, et al. Mucin-producing neoplasms of the pancreas. Intraductal papillary and mucinous cystic neoplasms. Ann Surg 1996; 223: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayumi T, Hachizuka K, Yamagushi A, et al. A case of mucus-producing pancreatic cancer penetrating into the common bile duct and the duodenum previously diagnosed 10 years earlier. Pancreas 1993; 3: 390. [Google Scholar]
- 47.Koito K, Namieno T, Ichimura T, et al. Mucin-producing pancreatic tumors: Comparison of MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Radiology 1998; 208: 231–237. [DOI] [PubMed] [Google Scholar]
- 48.Wood D, Silberman AW, Heifetz L, et al. Cystadenocarcinoma of the pancreas: Neo-adjuvant therapy and CEA monitoring. J Surg Oncol 1990; 43: 56–60. [DOI] [PubMed] [Google Scholar]
- 49.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Pathology, complications, and outcomes. Ann Surg 1997; 226: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ridder GJ, Maschek H, Klempnauer J. Favourable prognosis of cystadenocarcinoma over adenocarcinoma of the pancreas after curative resection. Eur J Surg Oncol 1996; 22: 232–236. [DOI] [PubMed] [Google Scholar]
- 51.Southern JF, Warshaw AL, Lewandrowski KB. DNA ploidy analysis of mucinous cystic tumors of the pancreas. Correlation of aneuploidy with malignancy and poor prognosis. Cancer 1996; 77: 58–62. [DOI] [PubMed] [Google Scholar]