Abstract
Objective
To examine the possibility of reducing ischemia-reperfusion injury (I/R injury) to the mouse liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic human Bcl-2 gene.
Summary Background Data
Ischemia-reperfusion injury has been demonstrated in a number of clinically relevant diseases such as myocardial infarction, cerebrovascular disease, sepsis, peripheral vascular disease, and organ transplantation. In this regard, apoptosis plays a central role.
Methods
Normal C57BL/6 mice were used. An adenovirus (ΔE1) vector containing the human Bcl-2 gene was developed in the authors’ laboratory. An adenovirus vector encoding an irrelevant gene (β-galactosidase, AdCMVLacZ) was used as a control. Taking advantage of the hepatotropic properties of adenovirus vectors, gene transfer was performed with 1 × 109 plaque-forming units by intravenous tail injection, 48 hours before the ischemic injury. Ischemic-reperfusion injury was induced by temporal and segmental occlusion of hepatic blood flow. Aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase activity was measured using standard assays. Liver biopsies were obtained before and 6 hours after I/R injury for morphologic assessment, and apoptosis was determined in situ with a histochemical assay.
Results
The expression of AdCMVhBcl-2 vector was confirmed by reverse transcription–polymerase chain reaction and functionally validated in apoptotic studies in endothelial cells. Expression of the Bcl-2 gene protects against I/R injury, as shown by a significant decrease in transaminases (p < 0.05) and necrosis and apoptosis (p < 0.001), and permanent survival (p < 0.0001), compared with sham-operated animals and animals treated with AdCMVLacZ.
Conclusions
Genetic modification of the liver to induce cytoprotection has potential applications to prevent I/R injury to the liver in surgical interventions, including liver transplantation.
Ischemia-reperfusion injury (I/R injury) has been demonstrated in a number of clinically relevant diseases, such as myocardial infarction, cerebrovascular diseases, sepsis, peripheral vascular disease, and organ transplantation. The exact mechanisms and mediators involved in I/R injury remain unknown, although various etiologic factors have been identified. These factors include activation of proteases and phospholipases, 1,2 alteration in calcium concentrations, 3–5 ATP depletion, 6 cell damage by free radicals, 7–9 inhibition of nitric oxide synthesis, 10 cytokines, 11 chemokines, 12 and endothelins. In addition, an active role of cells of the immune system, such as neutrophils, has been defined. 13–15
In this regard, apoptosis, or programmed cell death, plays a central role in many pathologies, including toxic, metabolic, viral, and immune-mediated diseases. 16–19 Although necrosis is the classical manifestation of hypoxia-induced cell damage, apoptosis is a common event in postreperfusion biopsy specimens of human liver grafts. In this context, the main cellular targets of apoptosis are hepatocytes and sinusoidal lining cells, and the intensity of apoptosis correlates with signs of hepatocyte injury. 16,17,20,21 In this regard, apoptosis is controlled through the expression of specific genes that are conserved in nematodes through mammals. Among these, the Bcl-2 gene family is the most important. 22,23 The protein encoded by the Bcl-2 gene has been implicated in the prolongation of cell survival by blocking the apoptosis and necrosis process. 22,24–27 Given the functional importance of the Bcl-2 gene in death cell control, it constitutes a prime target for cytoprotective therapeutic interventions in numerous disease states. Gene transfer studies in several types of mammalian cells have shown that elevated Bcl-2 protein levels inhibit the early mitochondrial changes associated with apoptosis and can protect cells from death induced by a wide variety of insults and stimuli. 22,28 Overexpression of Bcl-2 can protect neurons against focal ischemia, hypoglycemia, oxygen radical generators such as Adriamycin, can reduce infarction after occlusion of the midbrain artery, and can prevent apoptosis in insulinoma cells exposed to cytotoxic cytokines. 22,24,25,28–32 Whereas Bcl-2 is normally not expressed in the hepatocytes, de novo Bcl-2 expression has been observed after bile duct ligation; this suggests an adaptive phenomenon to resist apoptosis by toxic bile salts. 34 Moreover, the apoptosis protector, Bcl-2, is downregulated in the bile duct epithelial cells of human liver allografts in acute rejection, 35 and might therefore play a role in the increased apoptosis of these cells during acute rejection. 36,37
These observations raised the possibility that the liver injury during I/R can be prevented by temporal overexpression of Bcl-2. To this end, a successful gene transfer into the liver can be achieved in vivo using recombinant adenovirus vectors. The aim of this study was to evaluate whether in vivo adenovirus-mediated transfer of the antiapoptotic Bcl-2 gene into the liver could confer a cytoprotective effect against I/R injury.
METHODS
Generation of Recombinant Adenovirus Vector
A recombinant, E1-deleted adenovirus carrying the human Bcl-2 gene (AdCMVhBcl-2) without the transmembrane domain was constructed using the two plasmids homologous recombination method developed by Graham et al. 38 The plasmid encoding the hBc12 open reading frame without the transmembrane domain (generously provided by Dr. J. Reed, La Jolla Cancer Research Foundation, La Jolla, CA), was used as a source of the human Bcl-2 gene. The hBcl-2 without the transmembrane domain gene was cloned into the plasmid pcDNA3-Bcl-2 and excised with EcoRI and XhoI to release 0.7 kb fragment containing hBc12 open reading frame, and then subcloned into the EcoRI and XhoI site of the shuttle plasmid pCA13 (Microbix, Inc., Ontario, Canada). The resultant plasmid, pCAhBcl-2, contained 0.5 map units of sequence from the left end of the human adenovirus serotype 5 genome, the cytomegalovirus promoter, the human Bcl-2 open reading frame, followed by the SV40 poly(A) sequence (Fig. 1 ). The number of plaque forming units (pfu) per preparation was determined by plaque assay on 293 cells. Plaque assay on HeLa cells confirmed the absence of contaminating replication-competent adenovirus. As a control, we used an E1A/B-deleted, replication-incompetent, recombinant adenovirus vector that encodes the reporter gene E. coli β-galactosidase under the control of the cytomegalovirus promoter (AdCMVLacZ).
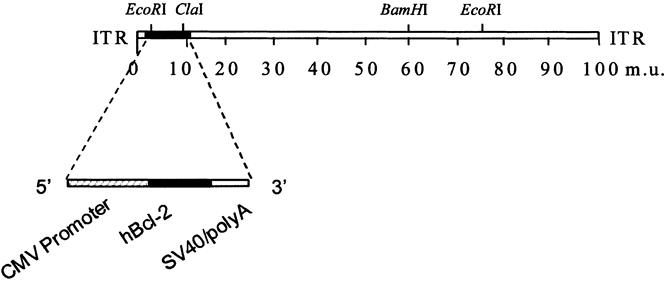
Figure 1. Generation of a recombinant adenovirus vector encoding the human Bcl-2 gene.
Animals
Experiments were performed in normal male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) 9 to 12 weeks old weighing 25 to 30 g, fed a laboratory diet of water and food ad libitum, in compliance with the University of Alabama at Birmingham Animal Care and Use Committee, which adheres to the NIH guidelines for the use of experimental animals. All surgical procedures were performed at the same hour to avoid circadian variations.
Surgical Procedures
A model of segmental hepatic ischemia was used in all experiments. Briefly, animals were anesthetized with ketamine (100 mg/kg) and xylazine (8 mg/kg). After midline laparotomy, the median lobe of the liver (approximately 45% of the liver mass) was clamped at its base using a microvascular clamp for 30 to 40 minutes. 15,39 For the survival analysis, the superior mesenteric artery was exposed and occluded with a microvascular clamp for 20 to 30 minutes. Estimates of blood flow using laser Doppler flowmetry indicated that occlusion of the superior mesenteric artery results in an approximately 70% reduction in blood flow in the mouse liver. 40 Reperfusion was initiated by gentle removal of the clamp. Sham-operated animals were treated in an identical fashion except for the omission of vascular occlusion.
In Vivo Gene Transfer
Adenovirus-mediated gene transfer to the liver was carried out after tail vein injection of 1 × 109 pfu of AdCMVLacZ or AdCMVhBcl-2 48 hours before the I/R injury. Three experimental groups were evaluated. Group 1 (n = 8) animals received intravenous injection of phosphate-buffered saline (PBS) alone. Group 2 (n = 8) animals received a recombinant adenovirus vector encoding a reporter gene (AdCMVLacZ). Group 3 (n = 8) animals received AdCMVhBcl-2.
Expression of Bcl-2 in Liver Grafts
The total RNA was extracted from snap-frozen liver biopsies using an RNA STAT-60 kit (Tel-Test B, Inc., Friendswood, TX) according to the manufacturer’s recommendations. Briefly, after homogenization in RNA STAT-60 (1 ml/50 mg tissue), the samples were incubated for 5 minutes at room temperature to allow dissociation of nucleoprotein complexes. Next, 0.2 ml of chloroform per milliliter of RNA STAT-60 was added. After 2 to 3 minutes at room temperature, the samples were centrifuged at 12,000g for 15 minutes at 4°C. RNA was precipitated with isopropanol at −70°C overnight, washed in 70% ethanol, and resuspended in RNase-free water. The first-strand cDNA synthesis was catalyzed by Moloney Murine Leukemia Virus reverse transcriptase with 15 mg of total RNA; random hexamer primers were used. The First-Strand cDNA Synthesis Kit (Pharmacia Biotech, Inc., Milwaukee, WI) was used according to the manufacturer’s recommendations. The cDNA was then employed as a template for polymerase chain reaction amplification to generate human Bcl-2-specific fragment (∼590 bp) using the primers AGT GGG ATG CGG GAG ATG TG and GGG GCC GTA CAG TTC CAC AA.
Liver Function Tests
Blood samples for aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) assessment were obtained after 6 hours of reperfusion and were analyzed using a serum analyzer (Amos Seralyzer, Miles Inc, Diagnostics Division, Elkhart, IN).
Morphologic Assessment
Liver biopsies for histologic assessment were obtained both before ischemia and 6 hours after reperfusion. Liver specimens were fixed in 10% formalin and embedded in paraffin. Six-micrometer sections stained with hematoxylin and eosin were evaluated at 200× magnification by a point-counting method for severity of hepatic injury using an ordinal scale, as previously described 39 :
• Grade 0: minimal or no evidence of injury
• Grade 1: mild injury consisting of cytoplasm vacuolation and focal nuclear pyknosis
• Grade 2: moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders
• Grade 3: severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration.
Detection of Apoptosis
A commercial in situ histochemical assay (Klenow-FragEL, Oncogene Research Products, Cambridge, MA) was used to detect the DNA fragmentation characteristic of apoptosis. In this assay, Klenow binds to exposed ends of DNA fragments generated in response to apoptotic signals and catalyzes the template-dependent addition of biotin-labeled and unlabeled deoxynucleotides. Biotinylated nucleotides are detected using a streptavidin–horseradish peroxidase conjugate. Diaminobenzidine reacts with the labeled sample to generate an insoluble colored substrate at the site of DNA fragmentation. Counterstaining with methyl green aids in the morphologic evaluation of normal and apoptotic cells. The results were scored semiquantitatively by averaging the number of apoptotic cells per microscopic field at 25× magnification. Six fields were evaluated per tissue sample.
Statistical Analysis
All data are expressed as mean ± SEM. Treatment groups were compared statistically using the unpaired two-tailed Student’s test. The Kaplan-Meier method was used to plot the survival distributions of each group. The log-rank test was used to compare the survival distributions of the groups.
RESULTS
Generation of Recombinant Adenovirus Vector and Expression of Human Bcl-2 in Liver
A recombinant adenovirus vector encoding the human Bcl-2 gene driven by the CMV promoter was generated (see Fig. 1). AdCMVhBcl-2 can cytoprotect human endothelial cells and liver grafts in hypothermia during standard preservation time for organ transplantation. 41 We first wished to demonstrate that AdCMVhBcl-2 could mediate transfer of the Bcl-2 gene to the liver. To this end, mice were injected intravenously with AdCMVhBcl-2, with a control vector expressing the E. coli β-galactosidase gene AdCMVLacZ, or with PBS.
At 48 hours after infection, total RNA was extracted from the livers and subjected to reverse transcription–polymerase chain reaction. As shown in Figure 2, expression of the Bcl-2 gene could be detected in the liver of mice injected with AdCMVhBcl-2 but not in the liver of mice injected with the control vector or PBS. High levels of Bcl-2 were obtained 8 hours after intravenous AdCMVhBcl-2 administration (data not shown). As previously reported, the intravenous administration of adenoviral vectors, 1 × 109 pfu, transduces >95% of hepatocytes with 20 to 40 viral genome copies per cell. 42,43 Thus, Bcl-2 expression in the liver can be accomplished in vivo after systemic administration of a recombinant adenovirus encoding Bcl-2.
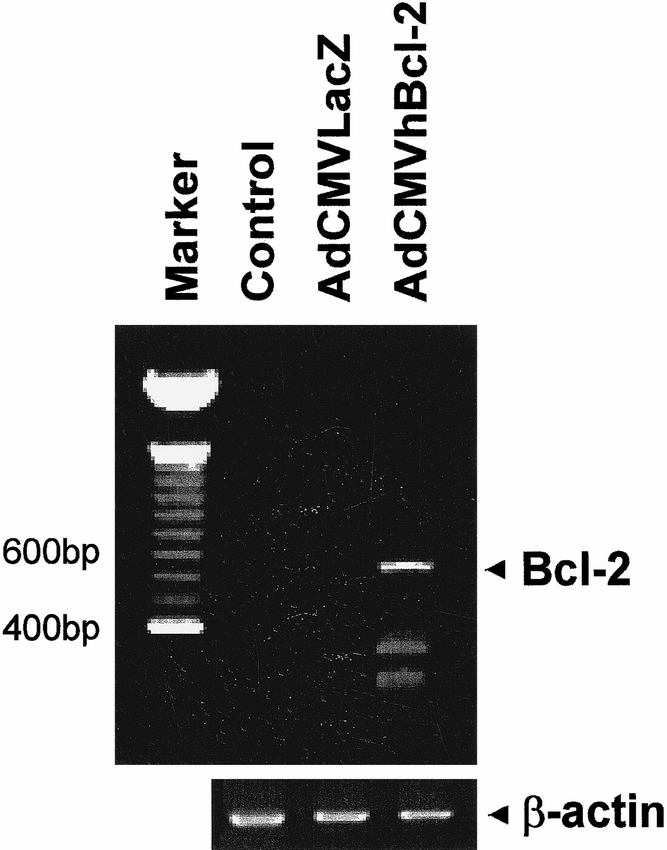
Figure 2. Expression of human Bcl-2 protein in liver 48 hours after in vivo gene transfer with AdCMVhBcl-2.
Reduced I/R Injury with the Antiapoptotic hBcl-2 Gene
Having confirmed that injection of AdCMVhBcl-2 led to expression of the antiapoptotic Bcl-2 gene in the liver, we sought to investigate whether this could induce cytoprotection after I/R injury of the liver. Mice were injected with AdCMVhBcl-2, the control vector AdCMVLacZ, or PBS. For these studies, we used a previously described model of segmental hepatic warm ischemia. Ischemia-reperfusion injury was induced by occlusion of the median lobe of the liver (approximately 45% of the liver mass) for 30 to 40 minutes.
Serum AST and ALT levels were used to assess liver injury in the three experimental groups. AST is an established marker of hepatic damage after warm ischemia and reperfusion injury. 39,44 In our preliminary studies, we showed that the peak of AST concentration in animals subjected to 30 to 40 minutes of ischemia of 45% of the liver mass occurs 5 to 7 hours after injury (data not shown). Therefore, in our experiments, we measured serum AST levels 6 hours postinjury. Animals from the control group injected with PBS showed a significant increase in serum AST levels 6 hours after the I/R injury (Fig. 3 ). Animals injected with an adenovirus vector encoding an irrelevant gene (AdCMVLacZ) shown a similar increase in AST levels. In contrast, animals injected with the AdCMVhBcl-2 vector showed a significant reduction in AST. An increase in AST was observed in animals given AdCMVLacZ followed by sham operation (1.3 ± 0.2 IU/L) compared with PBS (0.6 ± 0.2 IU/L) or AdCMVhBcl-2 (0.8 ± 0.22 IU/L). This reflects the hepatotoxic effect of adenovirus vectors, as previously reported. 45,46 Serum ALT levels followed a similar pattern (data not shown). A significant reduction of the LDH peak 6 hours after the I/R injury was observed in animals given AdCMVhBcl-2 (Fig. 4). These results demonstrate that adenovirus-mediated gene transfer of the antiapoptotic hBcl-2 gene induces hepatoprotection against I/R injury.
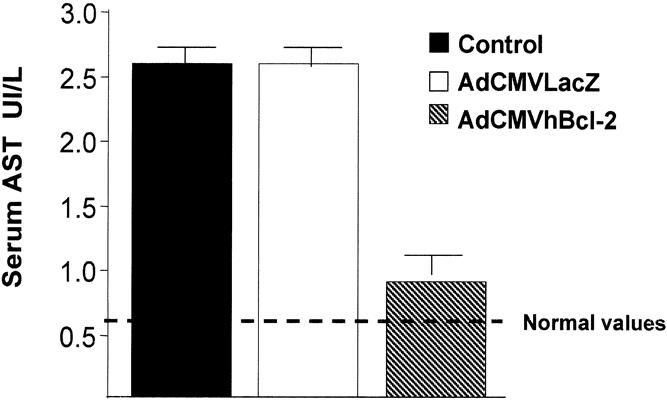
Figure 3. Reduced AST levels after I/R injury to the liver in animals injected with AdCMVhBcl-2.
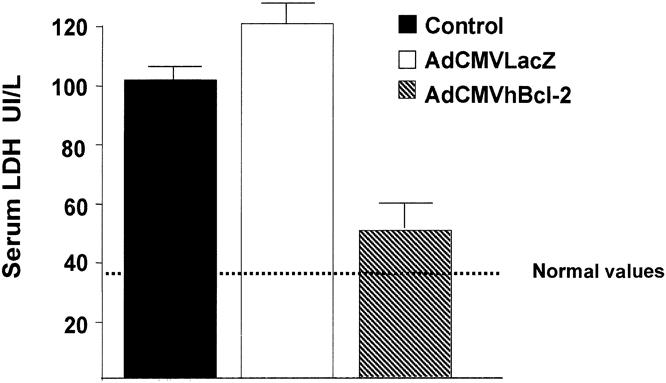
Figure 4. Reduced LDH levels in animals cytoprotected with AdCMVhBcl-2 after I/R injury to the liver.
Histologic Analysis After I/R Injury
Next we investigated the degree of hepatocyte injury in this experimental model. To this end, stained biopsy specimens were subjected to a point-counting method using an ordinal scale, as described. 39 Liver specimens obtained 6 hours after the I/R injury (median lobe of the liver) from control animals injected with PBS or the irrelevant vector AdCMVLacZ showed moderate to severe injury (grade 2 or 3) with nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders. Areas of necrosis with disintegration of hepatic cords and neutrophil infiltration were also evident (Fig. 5 ). Consistent with the levels of transaminases, animals injected with AdCMVhBcl-2 showed minimal evidence of injury, including a significant reduction of liver necrosis (grade 0 or 1). Mild neutrophilic infiltrate and apoptotic degeneration of hepatocytes were evident before the ischemic injury in samples obtained 48 hours after AdCMVLacZ administration. These morphologic alterations have been previously reported and represent an inflammatory response to the adenovirus vectors after intravascular administration. 45,46 Interestingly, livers infected with AdCMVhBcl-2 showed no significant difference before the ischemic injury compared with control animals treated with PBS. These results suggest that hBcl-2 might protect the host cell against the cytotoxic effects of the adenovirus vector or decrease the inflammatory response secondary to the viral infection.
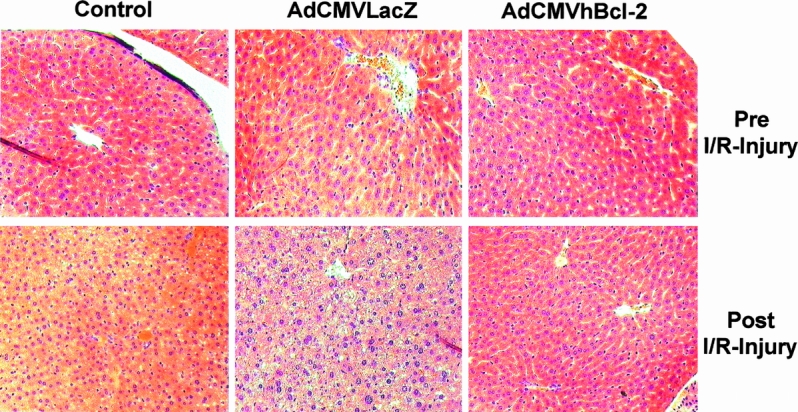
Figure 5. Histologic analysis after I/R injury to liver.
Reduced Apoptosis After I/R Injury
Having demonstrated that Bcl-2 protects the liver against I/R injury, we wished to examine whether this was the result of reduced apoptosis in the liver parenchyma cells. To investigate this, we employed an in situ histochemical assay to detect the DNA fragmentation characteristic of apoptosis. Apoptotic cells were more evident in postreperfusion samples from animals injected with PBS or with adenovirus carrying an irrelevant gene (Table 1 , Fig. 6 ). In contrast, significantly fewer apoptotic cells were present in the animals that received AdCMVhBcl-2 (p < 0.01). Animals treated with AdCMVLacZ showed significantly more apoptotic cells before the ischemic injury compared with animals that received AdCMVhBcl-2. Thus, these results demonstrated the ability of hBcl-2 gene to protect the host cell against the cytotoxic effects of the adenovirus vectors.
Table 1. REDUCED INCIDENCE OF APOPTOTIC CELLS AFTER REPERFUSION IN ANIMALS INJECTED WITH AdCMVhBcl-2
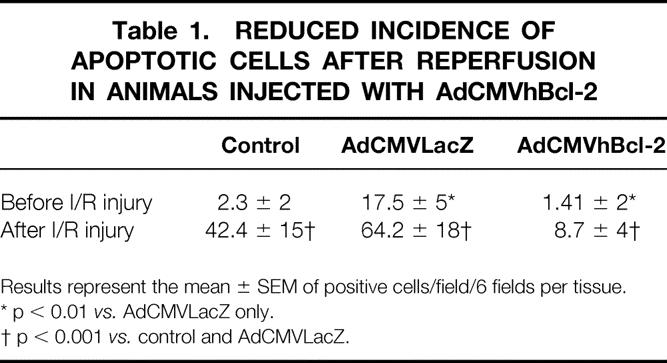
Results represent the mean ± SEM of positive cells/field/6 fields per tissue.
* p < 0.01 vs. AdCMVLacZ only.
† p < 0.001 vs. control and AdCMVLacZ.
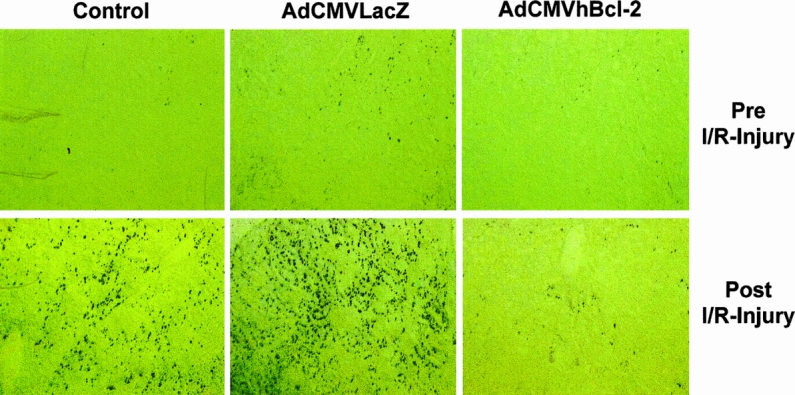
Figure 6. Apoptosis of the liver parenchyma cells after I/R injury.
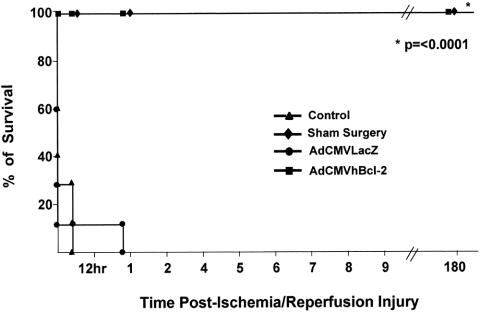
Prolonged Survival After I/R Injury
We next wished to determine whether the cytoprotective effect of the antiapoptotic Bcl-2 gene would translate into a survival advantage in animals with I/R injury. Thus, animals were injected with AdCMVhBcl-2, AdCMVLacZ, or PBS and then subjected to I/R injury. For these studies, the superior mesenteric artery was occluded for 20 to 30 minutes. Preliminary studies in our laboratory showed that the mortality rate of normal mice with 20 to 30 minutes of such occlusion is 97% at 24 hours. Moreover, serum AST and ALT levels increased significantly, and histologic examination demonstrated extensive hepatocellular damage (data not shown). The survival after 24 hours in control animals injected with PBS was zero, and only 20% of animals treated with AdCMVLacZ survived this length of time. However, all the animals treated with AdCMVhBcl-2 survived indefinitely (p < 0.0001) (Fig. 7). All the mice in the sham-operated group survived, which excludes the anesthetic and surgical procedure as the direct cause of death in this experimental model. Six months later, four survivors were killed for histologic analysis and Western blot studies for Bcl-2 protein expression; no expression of Bcl-2 was observed. In this regard, no evidence of malignant transformation was observed in the pathologic analysis of different organs, including the liver. The other four survivors are currently alive and in perfect condition after 8 months, with normal AST and ALT plasma levels (data not shown). These results show that the hBcl-2 gene confers a significant survival advantage after I/R injury to the liver.
Figure 7. Prolonged survival after I/R injury to the liver in animals genetically modified in vivo with AdCMVhBcl-2.
DISCUSSION
In this study, we demonstrated the possibility of in vivo genetic modification of the liver with a recombinant adenovirus vector expressing the human antiapoptotic gene Bcl-2. Expression of this gene provided genetic cytoprotection against I/R injury to the liver, as demonstrated by reduction of transaminases, minimal evidence of histologic injury, and a significant reduction in the apoptotic process after I/R injury. Multiple mechanisms have been implicated during I/R injury, but the final consequence is damage to the parenchymal liver cells. Previous studies have shown that the Bcl-2 gene protects a wide variety of cell types from undergoing apoptosis in response to such diverse stimuli as ionizing radiation, viral infection, growth factor deprivation, cytokine cytotoxicity, and ischemia. 22 Yamabe et al 26 recently demonstrated that transfection of the Bcl-2 gene through the HVJ-liposome method can be used to prevent liver cell necrosis induced by hypoxia in vitro and in vivo. Similar results have been reported in neuron cells, where Bcl-2 decreases the extent of necrosis after multiple neurologic insults. 27,28 In the present study, we demonstrated that hBcl-2 gene mediated a significant reduction in apoptosis and necrosis after I/R injury to the liver. In ex vivo and in vivo models of hypoxic liver damage, both oxidative phosphorylation and glycolysis are blocked by the lack of oxygen and substrates, resulting in a decrease in ATP levels and loss of mitochondrial function. 47,48 Subsequently, disturbance of calcium homeostasis and the cytoskeletal system leads to hepatocyte death. 49 Pastorino et al 50 and Watanabe et al 51 have demonstrated loss of mitochondrial function before hypoxic cell death, and protection of the mitochondrial function prevents it. Therefore, the mitochondria play an essential role in the hypoxic apoptosis pathway. Transfection of cells with Bcl-2 gene inhibits the early mitochondrial changes associated with apoptosis 22,52,53 and necrosis. 26 Therefore, the cytoprotection conferred by the expression of the hBcl-2 gene might be related to prevention of mitochondrial dysfunction.
In vivo gene transfer of Bcl-2 48 hours before a lethal warm I/R injury to the liver conferred permanent survival, compared with 100% deaths in animals injected with PBS alone or with an adenoviral vector encoding an irrelevant gene. Normal liver function test results and normal liver histology were demonstrated 6 months after the liver injury in animals cytoprotected with Bcl-2.
Gene transfer of antiapoptotic genes with adenoviral vectors is an attractive alternative to ameliorate I/R injury to the liver because high efficient gene transfer can be accomplished after systemic administration and temporary transgene expression. The main limitation of this technology for I/R injury secondary to trauma, cerebral and myocardial infarction, and so forth, is the necessity of gene transfer before the injury. However, we are exploring the efficacy of gene transfer of Bcl-2 after the ischemic injury.
High levels of hBcl-2 gene expression can be demonstrated 8 hours after in vivo intravenous administration (data not shown). Therefore, gene transfer and expression of Bcl-2 in liver allografts could potentially be achieved hours before organ procurement. Liver damage has been demonstrated at various times of the transplantation procedure, including harvest of the organ, cold preservation time, warm ischemia, and organ reperfusion. Therefore, Bcl-2 overexpression might improve graft function after transplantation. Recently, Albert et al 54 and Rovere et al 55 demonstrated that apoptotic cells induce direct activation of dendritic cells, a critical step in the immunologic response. Therefore, reduced cell damage and apoptosis during the early steps of liver transplantation would potentially decrease the immunogenicity of the organ and therefore the incidence of rejection. Cells expressing Bcl-2 have been shown to block cytotoxicity mediated by allospecific cytotoxic T lymphocytes and to resist the cytotoxic effect mediated by perforin and granzymes. 56 Therefore, expression of Bcl-2 after liver transplantation would potentially confer protection against the immune system.
The duration of ischemia has been demonstrated to be one of the most important risk factors for postoperative complications in patients undergoing liver resection. 57,58 Gene transfer of antiapoptotic genes can be achieved before the surgical procedure to induce cytoprotection and potentially to decrease postoperative complications.
Gene-transduced hepatocytes with adenoviral vectors are rapidly eliminated; therefore, the expression of the transgene is limited. Fas-mediated apoptosis induced by adenoviral vectors has been shown to play a critical role. 59 Recent experiments from our laboratory have shown that coexpression of Bcl-2 and a reporter gene is an effective strategy in preventing the rapid elimination of adenoviral-transduced hepatocytes (Bilbao G, manuscript in preparation). Lacronique et al, 60 using hepatocytes from transgenic animals expressing human Bcl-2, showed that these hepatocytes can resist the administration of agonistic Fas antibody, whereas normal hepatocytes were killed. All these observations suggest that expression of Bcl-2 can block the Fas-mediated apoptosis induced by adenoviral vectors.
Bcl-2 was discovered by its association with the t(14;18) chromosomal translocations found in non-Hodgkin B-cell lymphomas. 61 No evidence of neoplasia has been demonstrated in transgenic mice with overexpression of Bcl-2. 62 We have demonstrated transient expression of hBcl-2 in liver after genetic modification in vivo with a recombinant adenovirus (data not shown). Further, in this study no evidence of liver dysfunction or malignant transformation in long-term survivors was observed.
In summary, we have demonstrated that an adenovirus vector carrying the antiapoptotic human Bcl-2 gene could mediate cytoprotection of the liver against I/R injury in vivo. Further, permanent survival was achieved after a lethal warm I/R injury. The beneficial effect of Bcl-2 on reperfusion injury seems to be related to its antiapoptotic and antinecrotic properties. Prevention or reduction of I/R injury with gene therapy could be an attractive therapeutic alternative in multiple clinical conditions, such as liver surgery and organ transplantation and other conditions associated with I/R injury.
Acknowledgment
The authors thank M. Joyce Pike for skilled animal care assistance.
Footnotes
Correspondence: David T. Curiel, MD, Gene Therapy Program, 1824 6th Ave. South, Room WTI 620, Birmingham, AL 35294.
Supported by National Institute of Health grants RO1-CA 72532-01 and the Med BioChem RO1-CA74242-01.
Guadalupe Bilbao, MD, is a USAMRC postdoctoral fellow.
Accepted for publication February 16, 1999.
References
- 1.Kohli V, Gao W, Camargo CAJ, Clavien PA. Calpain is a mediator of preservation-reperfusion injury in rat liver transplantation. Proc Natl Acad Sci USA 1997; 94: 9354–9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lie TS, Seger R, Hong GS, Preissinger H, Ogawa K. Protective effect of aprotinin on ischemic hepatocellular damage. Transplantation 1989; 48: 396–399. [DOI] [PubMed] [Google Scholar]
- 3.Gasbarrini AP, Pasini P, Nardo B, et al. Chemiluminescent real time imaging of post-ischemic oxygen free radicals formation in livers isolated from young and old rats. Free Radic Biol Med 1998; 24: 211–216. [DOI] [PubMed] [Google Scholar]
- 4.Hochachka PW. Defense strategies against hypoxia and hypothermia. Science 1986; 231: 234–241. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CE, Reed DJ. Current status of calcium in hepatocellular injury. Hepatology 1989; 10: 375–384. [DOI] [PubMed] [Google Scholar]
- 6.Gores GJ, Flarsheim CE, Dawson TL, et al. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am J Physiol 1989; 257: C347–C354. [DOI] [PubMed] [Google Scholar]
- 7.Marubayashi S, Oshiro Y, Maeda T, et al. Protective effect of monoclonal antibodies to adhesion molecules on rat liver ischemia-reperfusion injury. Surgery 1997; 122: 45–52. [DOI] [PubMed] [Google Scholar]
- 8.Atalla SL, Toledo-Pereyra LH, MacKenzie GH, Cederna CP. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation 1985; 40: 584–590. [DOI] [PubMed] [Google Scholar]
- 9.Le MO, Louis H, Stordeur P, et al. Role of reactive oxygen intermediates in interleukin 10 release after cold liver ischemia and reperfusion in mice. Gastroenterology 1997; 113: 1701–1706. [DOI] [PubMed] [Google Scholar]
- 10.Negita M, Hayashi S, Koike S, et al. Protective effect of human superoxide dismutase cDNA transfection in the prevention of cold preservation injury. Transplant Proc 1997; 29: 1363. [DOI] [PubMed] [Google Scholar]
- 11.Simpson KJ, Lukacs NW, Colletti L, Strieter RM, Kunkel SL. Cytokines and the liver. J Hepatol 1997; 27: 1120–1132. [DOI] [PubMed] [Google Scholar]
- 12.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: Roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology 1998; 27: 507–512. [DOI] [PubMed] [Google Scholar]
- 13.Horie Y, Wolf R, Russell J, Shanley TP, Granger DN. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology 1997; 26: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Matsumura F, Takeya M, et al. Monocyte chemoattractant protein-1 enhances expression of intercellular adhesion molecule-1 following ischemia-reperfusion of the liver in rats. Hepatology 1998; 27: 727–734. [DOI] [PubMed] [Google Scholar]
- 15.Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 1997; 100: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghi-Scoazec G, Scoazec JY, Durand F, et al. Apoptosis after ischemia-reperfusion in human liver allografts. Liver Transpl Surg 1997; 3: 407–415. [DOI] [PubMed] [Google Scholar]
- 17.Galle PR. Apoptosis in liver disease. J Hepatol 1997; 27: 405–412. [DOI] [PubMed] [Google Scholar]
- 18.Patel T, Roberts LR, Jones BA, Gores GJ. Dysregulation of apoptosis as a mechanism of liver disease: An overview. Semin Liver Dis 1998; 18: 105–114. [DOI] [PubMed] [Google Scholar]
- 19.Patel T, Gores JG. Apoptosis in liver transplantation: A mechanism contributing to immune modulation, preservation injury, neoplasia, and viral disease. Liver Transpl Surg 1998; 4: 42–50. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Matsuno T, Tanaka N, Orita K. Activation of apoptosis during the reperfusion phase after rat liver ischemia. Transplant Proc 1996; 28: 1908–1909. [PubMed] [Google Scholar]
- 21.Sasaki H, Matsuno T, Ishikawa T, et al. Activation of apoptosis during early phase of reperfusion after liver transplantation. Transplant Proc 1997; 29: 406–407. [DOI] [PubMed] [Google Scholar]
- 22.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis [published erratum appears in Nat Med 1997 Aug;3(8):934]. Nat Med 1997; 3: 614–620. [DOI] [PubMed] [Google Scholar]
- 23.White E. Life, death, and the pursuit of apoptosis. Genes Dev 1996; 10: 1–15. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu S, Eguchi Y, Kosaka H, et al. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature 1995; 374: 811–813. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu S, Eguchi Y, Kamiike W, et al. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res 1996; 56: 2161–2166. [PubMed] [Google Scholar]
- 26.Yamabe K, Shimizu S, Kamiike W, et al. Prevention of hypoxic liver cell necrosis by in vivo human bcl-2 gene transfection. Biochem Biophys Res Commun 1998; 243: 217–223. [DOI] [PubMed] [Google Scholar]
- 27.Zhong LT, Sarafian T, Kane DJ, et al. Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A 1993; 90: 4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence MS, Ho DY, Sun GH, et al. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci 1996; 16: 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia I, Martinou I, Tsujimoto Y, Martinou JC. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science 1992; 258: 302–304. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Rabinovitch A, Suarez-Pinzon W, et al. Expression of the bcl-2 gene from a defective HSV-1 amplicon vector protects pancreatic b-cells from apoptosis. Hum Gene Ther 1996; 7: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 31.Martinou JC, Dubois-Dauphin M, Staple JK, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 1994; 13: 1017–1030. [DOI] [PubMed] [Google Scholar]
- 32.Shimazaki K, Ishida A, Kawai N. Increase in bcl-2 oncoprotein and the tolerance to ischemia-induced neuronal death in the gerbil hippocampus. Neurosci Res 1994; 20: 95–99. [DOI] [PubMed] [Google Scholar]
- 33.Tzung SP, Fausto N, Hockenbery DM. Expression of Bcl-2 family during liver regeneration and identification of Bcl-x as a delayed early response gene. Am J Pathol 1997; 150: 1985–1995. [PMC free article] [PubMed] [Google Scholar]
- 34.Kurosawa H, Que FG, Roberts LR, Fesmier PJ, Gores GJ. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol 1997; 272: G1587–G1593. [DOI] [PubMed] [Google Scholar]
- 35.Gapany C, Zhao M, Zimmermann A. The apoptosis protector, bcl-2 protein, is downregulated in bile duct epithelial cells of human liver allografts. J Hepatol 1997; 26: 535–542. [DOI] [PubMed] [Google Scholar]
- 36.Krams SM, Egawa H, Quinn MB, et al. Apoptosis as a mechanism of cell death in liver allograft rejection. Transplantation 1995; 59: 621–625. [PubMed] [Google Scholar]
- 37.Yamamoto H, Ohdan H, Shintaku S, et al. Analysis of apoptosis-associated genes: Fas ligand, bcl-2 and bax in the rat liver allograft. Transplant Proc 1998; 30: 22–24. [DOI] [PubMed] [Google Scholar]
- 38.Hitt MM, Addison CL, Graham FL. Human adenovirus vectors for gene transfer into mammalian cells. In: August JT, ed. Gene therapy. London: Academic Press; 1996: 138–206.
- 39.Camargo CAJ, Madden JF, Gao W, et al. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 1997; 26: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 40.Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest 1997; 99: 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilbao G, Contreras JL, Gomez-Navarro J, et al. Genetic modification of liver grafts with an adenoviral vector encoding the bcl-2 gene improves organ preservation. Transplantation 1999; 67: 775–783. [DOI] [PubMed] [Google Scholar]
- 42.Vrancken Peeters MJ, Lieber A, Perkins J, Kay MA. Method of multiple portal vein infusions in mice: Quantification of adenovirus-mediated hepatic gene transfer. BioTechniques 1996; 20: 278–285. [DOI] [PubMed] [Google Scholar]
- 43.Vrancken Peeters MJ, Patjin GA, Lieber A, Meuse L, Kay MA. Adenovirus-mediated hepatic gene transfer in mice: Comparison of intravascular and biliary administration. Hum Gene Ther 1996; 7: 1693–1698. [DOI] [PubMed] [Google Scholar]
- 44.Iu S, Harvey PR, Makowka L, et al. Markers of allograft viability in the rat. Relationship between transplantation viability and liver function in the isolated perfused liver. [published erratum appears in Transplantation 1987 Dec;44(6):835]. Transplantation 1987; 44: 562–569. [DOI] [PubMed] [Google Scholar]
- 45.Gao GP, Yang Y, Wilson JM. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol 1996; 70: 8934–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jooss K, Yang Y, Wilson JM. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther 1996; 7: 1555–1566. [DOI] [PubMed] [Google Scholar]
- 47.Okabe H, Kurosawa K, Hatanaka N, et al. Protection of cellular and mitochondrial functions against anoxic damage by fructose in perfused liver. Biochim Biophys Acta 1991; 1098: 41–48. [PubMed] [Google Scholar]
- 48.Todo S, Zhu Y, Zhang S, et al. Attenuation of ischemic liver injury by augmentation of endogenous adenosine. Transplantation 1997; 63: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatanaka N, Kamike W, Shimizu S, et al. Ca2+ release from mitochondria induces cytosolic enzyme leakage in anoxic liver. J Surg Res 1995; 58: 485–490. [DOI] [PubMed] [Google Scholar]
- 50.Pastorino JG, Snyder JW, Serroni A, et al. Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J Biol Chem 1993; 268: 13791–13798. [PubMed] [Google Scholar]
- 51.Watanabe F, Kamiike W, Nishimura T, et al. Decrease in mitochondrial levels of adenine nucleotides and concomitant mitochondrial dysfunction in ischemic rat liver. J Biochem 1983; 94: 493–499. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu S, Eguchi Y, Kamiike W, et al. Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene 1996; 13: 21–29. [PubMed] [Google Scholar]
- 53.Zamzami N, Marchetti P, Castedo M, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 1995; 182: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392: 86–89. [DOI] [PubMed] [Google Scholar]
- 55.Rovere P, Vallinoto C, Bondanza A, et al. Cutting edge: Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol 1998; 161: 4467–4472. [PubMed] [Google Scholar]
- 56.Chiu VK, Walsh CM, Liu CC, et al. Bcl-2 blocks degranulation but not Fas-based cell-mediated cytotoxicity. J Immunol 1995; 209: 211–218. [PubMed] [Google Scholar]
- 57.Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections: Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg 1989; 209: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huguet C, Gavelli P, Chieco A, et al. Liver ischemia for hepatic resection: Where is the limit? Surgery 1992; 111: 251–259. [PubMed] [Google Scholar]
- 59.Okuyama T, Li X-K, Funeshima N, et al. Fas-mediated apoptosis is involved in the elimination of gene-transduced hepatocytes with E1/E3-deleted adenoviral vectors. J Gatroenterol Hepatol 1998; 13(Suppl): S113–118. [PubMed] [Google Scholar]
- 60.Lacronique V, Mignon A, Fabre M, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 1996; 2: 80–86. [DOI] [PubMed] [Google Scholar]
- 61.Tsujimoto Y, Gorham J, Cossman J, et al. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 1985; 229: 1390–1393. [DOI] [PubMed] [Google Scholar]
- 62.Katsumata M, Siegel RM, Louie DC, et al. Differential effects of Bcl-2 on T and B cells in transgenic mice. Proc Natl Acad Sci USA 1992; 89: 11376–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]