Abstract
Objective
To assess the complications of level I and II axillary lymph node dissection in the treatment of stage I and II breast cancer, with breast-conservation surgery and mastectomy.
Summary Background Data
The role of axillary dissection for staging, and as an effective means of controlling regional nodal disease, has long been recognized. As small and low-grade lesions have been detected more frequently, and as its therapeutic impact has been questioned, axillary dissection has increasingly been perceived as associated with significant complications.
Methods
Two hundred patients, 112 of whom had breast-conservation surgery with axillary dissection and 88 of whom had total mastectomy with axillary dissection, were evaluated 1 year or more after surgery for arm swelling as well as nonedema complications. All patients had arm circumference measurements at the same four sites on both the operated and nonoperated sides.
Results
No patient had an axillary recurrence. The mean difference in circumference on the nonoperated versus operated side was 0.425 cm ± 1.39 at the midbiceps (p < 0.001), 0.315 cm ± 1.27 at the antecubital fossa (p < 0.001), 0.355 cm ± 1.53 at the midforearm (p < 0.005), and 0.055 cm ± 0.75 at the wrist (n.s.). Seven patients (3.5%) had mild swelling of the hand. Heavy and obese body habitus were the only significant predictors of edema on multivariate analysis. One hundred fifty-three (76.5%) patients had numbness or paresthesias of the medial arm and/or axilla after surgery; in 125 (82%) of these, the problem had lessened or had resolved on follow-up assessment.
Conclusions
The characterization of a level I and II axillary dissection as a procedure with significant complications does not appear justified based on this experience.
The role of axillary dissection in the treatment of invasive breast cancer has come under particular scrutiny in recent years as its impact on survival has been questioned. 1,2 Although the presence of nodal metastases remains the most significant predictor of prognosis, some contend that the widespread application of adjuvant systemic therapy lessens the impact of this information on therapeutic decision making. 3,4 Further, although axillary dissection is recognized as effective at controlling regional nodal disease, advocates of more limited sentinel lymphadenectomy contend that full axillary dissection for all patients with invasive breast cancer risks significant complications and might be more selectively applied only to patients harboring micrometastases to the sentinel nodes. 5–7
Since originally being described as a component of the radical mastectomy, axillary dissection for breast cancer persisted with modifications of the radical mastectomy, such as those of Patey and Dyson. 8 In that context, it connoted a complete resection of lymph nodes at all three levels, with efforts as well to ensure the inclusion of all external mammary nodes extending from the medial border of the axilla along the chest wall from the second to sixth ribs. 9 Dissection of the level III apical nodes was facilitated by transecting the attachment of the pectoralis minor muscle to the coracoid process, 10 and even the sternal portion of the pectoralis major might be detached laterally as well for further axillary exposure, after which the muscle would be reconstructed. 11
As breast-conserving procedures became accepted, and as additional data on the distribution of nodal micrometastases accumulated, the extent of elective axillary dissection was often modified. The incidence of level III micrometastases in the absence of level I or II metastases was found to be 1% or less. 12,13 Although the incidence of isolated metastases to level II was still <2% in some series, 14,15 the incidence of “skip” metastases did exceed 20% in other reports. 16,17 As a result, level I and II dissection, with preservation of the pectoralis minor, has come to represent the anatomic extent of axillary lymphadenectomy in most reports. 18
Curiously, there are limited data, either with breast-conservation surgery or with mastectomy, on the complications of level I and II lymphadenectomy. Discussions of the complications of axillary dissection that reflect the experiences from the era of the radical or Patey mastectomy, with or without postoperative radiation therapy, often present rates of arm edema that exceed 50%. 19,20 Clearly, that experience may exaggerate the potential complications of axillary lymph node dissection.
To assess the complications of a level I and II axillary lymph node dissection, we evaluated the surgical, cosmetic, and functional consequences in 200 patients consecutively seen in follow-up evaluation >1 year after surgery for stage I or II breast cancer. All axillary dissections followed a uniform surgical protocol, whether performed with breast-conservation surgery or with mastectomy.
MATERIALS AND METHODS
Two hundred patients were consecutively evaluated after surgical treatment for unilateral breast cancer that included a level I and II axillary dissection performed by one of three surgeons (DFR, MNH, RLS). All of these patients’ postoperative surveillance protocols included evaluation clinically by the surgeon every 3 months during the first 2 years after surgery, with more extended intervals beyond that but never exceeding 6 months. Only patients seen for the first annual examination or beyond were included in this analysis.
Patients having a level I and II axillary dissection in conjunction with a segmental excision had their procedure performed through an incision that followed a natural skin crease below the hair-bearing area of the axilla. The medial extent of the incision was kept lateral to the edge of the pectoralis major muscle; laterally it extended to the posterior axillary line. When a mastectomy was performed, the extent of skin incisions varied, but when lateral to the anterior axillary line they were also always placed below the hair-bearing area of the axilla.
The axillary dissection was begun by defining the lateral edge of the pectoralis minor and the medial pectoral nerve branches and vessels, and incising the clavipectoral fascia parallel to the lateral edge of the pectoralis minor muscle. The clavipectoral fascia was further incised laterally, thereby exposing the lymphoadipose contents of the axilla. A concentrated effort to dissect all the soft tissue off the brachial plexus and the adventitia of the axillary vein was avoided. The medial pectoral nerve and vascular bundle lateral to the pectoralis minor muscle were protected.
In dissecting subjacent to the pectoralis minor muscle, the forearm was supported in a position directed toward the contralateral side and parallel to the patient, thereby facilitating medial retraction of the pectoral muscles. All branches of the axillary vein were divided and ligated from the level of the thoracoacromial vessels. Posteriorly directed subscapular vessels were not ligated. The inferior axillary contents were dissected off the serratus anterior fascia. The intercostobrachial nerve was ligated as it exited from the second intercostal space, although in the more recently treated patients it was often preserved. The long thoracic nerve and the thoracodorsal nerve and vessels were preserved and the axillary contents further dissected to the edge of the latissimus dorsi and the lateral extent of the axillary vein, where the specimen was transected.
All axillary dissections were drained using a closed suction system. Drains were kept in place for 4 to 5 days, at which time serous drainage was invariably <50 cc per 24 hours. Antibiotics were begun before the incision and maintained until the drain was discontinued.
Radiation to the conserved breast followed a uniform policy. Axillary radiation and chest wall radiation after mastectomy was not applied unless there were extensive nodal metastases. Reconstruction after mastectomy was performed by placement of a tissue expander or by autologous tissue reconstruction using a pedicle transverse rectus abdominis myocutaneous or latissimus dorsi flap. All patients were instructed in arm care and lymphedema precautions. All were given range-of-motion exercises after drain removal and an absence of seroma formation.
Patient variables included age at time of surgery, body habitus (defined as normal if the patient was within the range of 10% below to 20% over ideal body weight [IBW]; thin if >10% below IBW; heavy if 20% to 40% over IBW; and obese if >40% over IBW), intercurrent cardiac disease or diabetes, smoking history, postoperative seroma formation and the frequency of seroma aspiration after drain removal, wound infection, arm infections, paresthesias as outlined by the patient after surgery and in follow-up, pain requiring analgesia beyond the immediate postoperative period and in follow-up, range of motion, and lymphedema on follow-up assessment.
Treatment and pathologic variables included type of surgery, reconstruction, sacrifice of the intercostobrachial nerve, clinical and pathologic nodal status, total nodes dissected, tumor size, and the use of postoperative radiation therapy.
Lymphedema was assessed by measuring both arms at the same site in the midbiceps region, the antecubital fossa, the midforearm, and the wrist. The measurements were made with the arm straightened in a recumbent position. Care was taken not to pinch the skin when measuring the circumference at the different sites. Hand swelling was rated as nonexistent, minimal, or significant. All patients were carefully assessed for axillary recurrence throughout the follow-up examinations.
Nonlymphedema complications were monitored by use of a standardized interview protocol to elicit specific complaints and by evaluation of the patient’s medical record. Range of motion was assessed by the surgeon as active ranging at the shoulder joint, which was scored as equal to or decreased relative to the nonoperated side. Numbness and paresthesias were evaluated by standard questions asked of each patient regarding the location and severity of the symptoms at the time of evaluation and compared with those in the postoperative period (unchanged, improved, significantly improved, or completely resolved). Zones of persistent numbness were outlined by the examining physician and confirmed by the patient.
All values were reported as mean ± standard deviation or median with range. A paired t test was used to compare differences between operated and nonoperated arm circumferences. Statistical analysis of univariate associations was performed using chi square for categorical variables and Pearson’s linear correlation for continuous variables. A forward conditional logistic regression model was used for the multivariate analysis. The multivariate analysis incorporated the following variables: age; surgical procedure; tumor size; number of nodes; incidence of positive nodes; use of regional adjuvant radiation therapy; body habitus; presence of diabetes, hypertension, cardiac disease, or smoking history; and presence of seroma, wound infection or cellulitis. A probability value of <0.05 was accepted for statistical significance. Relative risk was reported with a 90% confidence interval.
RESULTS
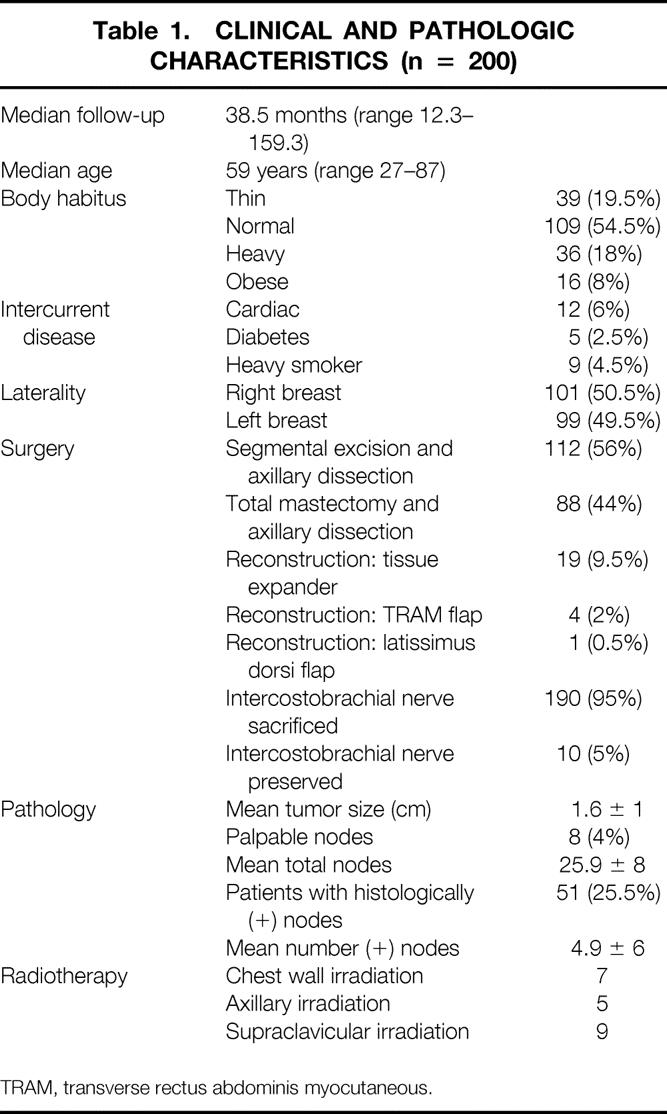
Two hundred patients were evaluated with a median follow-up time of 38.5 months (range 12.3 to 159.3 months). The clinical and pathologic characteristics of the patients studied are detailed in Table 1. One hundred twelve patients had breast-conservation surgery with axillary dissection, and 88 had total mastectomy and axillary dissection. One hundred seventy procedures were performed by one surgeon (DFR) and the remainder by the others (MNH or RLS).
Table 1. CLINICAL AND PATHOLOGIC CHARACTERISTICS (n = 200)
TRAM, transverse rectus abdominis myocutaneous.
There was no recurrent axillary disease in any patient within the follow-up period. All patients having breast-conservation surgery received breast irradiation. Five patients also received axillary irradiation after surgery. Seven patients who underwent mastectomy had chest wall irradiation. Nine patients had supraclavicular irradiation.
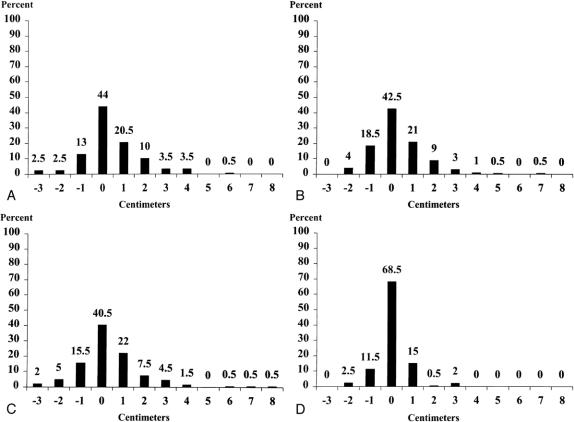
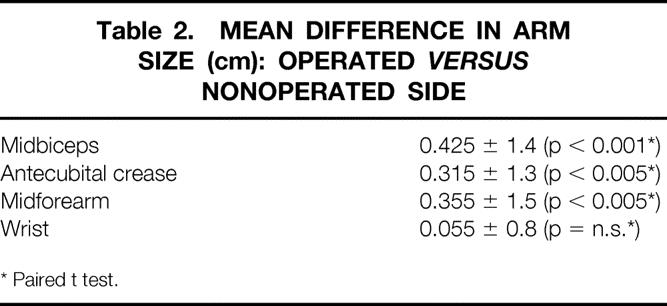
The difference in arm circumference at the midbiceps, antecubital crease, midforearm, and wrist are shown in Table 2. No statistically significant difference was demonstrated at the wrist; the most significant difference of 0.4 ± 1.4 cm (p < 0.001) was demonstrated at the midbiceps region. The frequency of differences in arm circumference is detailed in Figure 1. The frequency of swelling >2 cm in one or more regions, as well as the incidence of hand swelling, is noted in Table 3.
Table 2. MEAN DIFFERENCE IN ARM SIZE (cm): OPERATED VERSUS NONOPERATED SIDE
* Paired t test.
Figure 1. Percentage distribution of difference in circumference (cm) between operated and nonoperated arms at midbiceps (A), antecubital crease (B), midforearm (C), and wrist (D). A negative value indicates that the nonoperated circumference was larger.
Table 3. EDEMA AND CELLULITIS
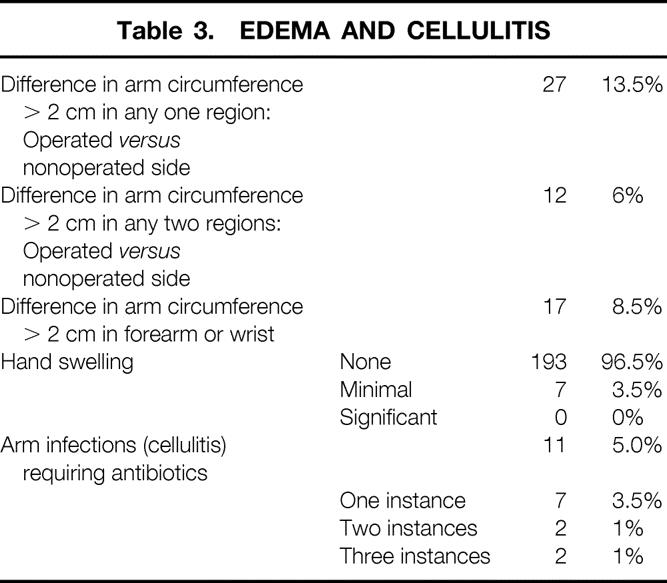
Seven patients (3.5%) had minimal hand swelling. No patient had significant swelling. Although 27 patients (13.5%) had swelling of >2 cm in any one area, an increase of >2 cm at the forearm or wrist level was experienced by 17 patients (8.5%); 4 of these (23.5%) also had swelling of the hand. Twelve patients (6%) had swelling of >2 cm in two regions. Thirty-one (15.5%) patients had an increase in arm circumference >1 cm on the side contralateral to the surgery.
The variables predictive of edema >2 cm in any location with or without hand edema are listed in Table 4. Only heavy and obese body habitus emerged as significant predictors of an increase in arm circumference on the operated side. The number of episodes of arm cellulitis approached significance (p = 0.054) as a predictor of edema.
Table 4. VARIABLES PREDICTIVE OF LYMPHEDEMA* ON UNIVARIATE AND MULTIVARIATE ANALYSIS
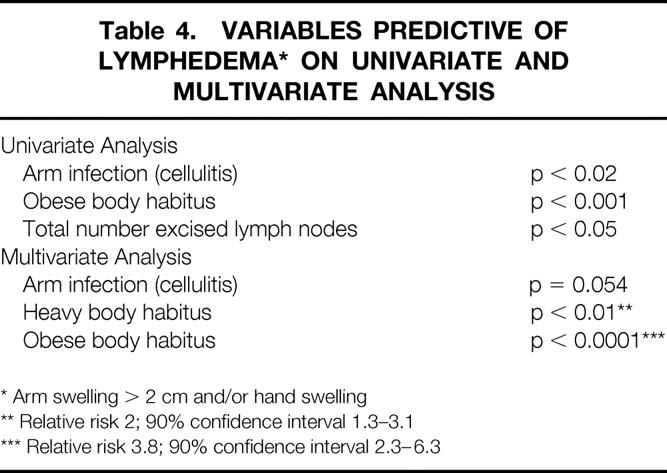
* Arm swelling > 2 cm and/or hand swelling
** Relative risk 2; 90% confidence interval 1.3–3.1
*** Relative risk 3.8; 90% confidence interval 2.3–6.3
The nonlymphedema variables that were evaluated are listed in Table 5. One hundred nineteen patients (59.5%) had seromas treated by aspiration after drain removal. Eleven patients (5.5%) had infections of the arm after surgery; four had more than one infection.
Table 5. NONLYMPHEDEMA COMPLICATIONS
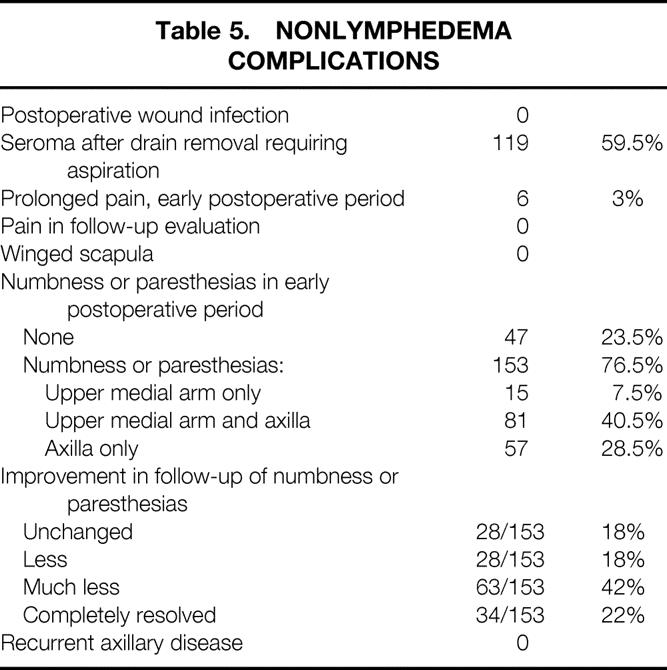
Five (2.5%) patients had prolonged pain requiring analgesic medications in the postoperative period, but no patient had pain at the time of follow-up. Numbness or paresthesias to the upper medial arm and/or axillary skin were reported by 153 (76.5%) patients in the early postoperative period. In follow-up evaluation, 34 (22%) reported complete resolution; an additional 91 (60%) reported improvement, 63 (42%) of whom reported that the improvement was significant. Preservation of the intercostobrachial nerve in 10 patients was associated with numbness or paresthesias in the postoperative period in 8 patients (80%). In follow-up, these were resolved in seven patients (70%) (see Table 5).
DISCUSSION
The most commonly cited source of complications after axillary dissection is lymphedema. Our results demonstrate an objective difference in arm circumference at a single site of >2 cm in 13.5% of patients, not dissimilar to the incidence of 16% noted by Lin et al 21 in a more heterogeneous group of patients. It also conforms to the incidence noted by Kissin et al 22 in 200 patients; in that study, subjective lymphedema was noted in 14% of patients, but the incidence was 25.5% when objectively measured by a limb volume difference exceeding 200 ml. Predictably, heavy and obese patients in our experience were more likely to develop swelling; this was the only predictor of edema when the variables were evaluated in a multivariate analysis. Because of the infrequency of significant edema in our clinical experience, we relied exclusively on circumferential measurements at multiple sites, which correlated in the Kissin study with volumetric analysis. Subjective hand swelling was noted in only 3.5% of patients and was always minimal. No patient had significant hand swelling that was functionally disabling. Clearly, objective assessment reflects a higher incidence of arm swelling than does subjective assessment.
We did not measure the arm circumference before surgery, because changes in the extended follow-up period are also dependent on weight loss and gain, variables that are treatment-dependent as well. Comparison with the contralateral arm represents the reference point for patients in follow-up and is therefore more clinically relevant. Notably, 15.5% of patients in our series had an increase in arm circumference of >1 cm on the nonoperated side. Clearly, anatomic variability independent of surgical sequelae does exist. 23 Nevertheless, even a minimal degree of swelling or a sense of arm heaviness on the side of the surgery may become for the patient a constant physical reminder of the original cancer. The often-insidious and subtle initial presentation of lymphedema can develop into more obvious enlargement as increased lymphatic pressure prevents diffusion of lipids and protein; this promotes fibrosis and susceptibility to infection. In this regard, 11 (5.5%) patients had one or more episodes of arm cellulitis requiring treatment in follow-up, all of which resolved rapidly. Five (45.5%) of these patients had an increase in arm circumference exceeding 2 cm.
Recently reported incidences of lymphedema after axillary dissection demonstrate a wide range, from 5% to 25.5%. 21,22,24–29 This is due no doubt to the great variability of procedures, radiation treatments, objective assessment criteria, and duration of follow-up in these series. However, the retreat from radical mastectomy, as well as postoperative radiation therapy after mastectomy, has significantly decreased the incidence of the severe lymphedema that was a feared long-term sequelae of radical surgery.
Seroma formation was common, in our experience. A collection of serum and lymph is to be expected after axillary dissection. 30,31 Despite postoperative closed suction drainage to minimize prolonged seroma formation, 59.5% of patients required seroma aspirations after the discontinuance of a drain. However, no patient had a postoperative infection or prolonged and excessive seroma formation requiring reinsertion of a closed suction drain.
No patient in our series had any range-of-motion limitation. Further, because no patients reported a change in arm or hand strength, no objective measurement was made in this regard. Reports in the literature have assessed the influence of shoulder exercises on seroma formation after axillary dissection performed with both breast-conservation surgery or total mastectomy. 32–35 No apparent adverse effect has been observed from early arm mobilization. With suction drainage in place, we have advised only modest mobility of the arm to minimize stiffness and the risk of a frozen shoulder if immobility is prolonged. When seroma formation is controlled, we have then encouraged an active increase in the use of the arm, with full range-of-motion activity. This delay in full shoulder exercise has not been associated with arm dysfunction in our experience. Although some patients may participate in exercise programs in the follow-up period, this information was not included in our analysis.
Pain after axillary dissection is difficult to assess in the immediate postoperative period. Although 2.5% of patients reported pain requiring analgesia beyond the immediate postoperative period, no patient seen in follow-up noted pain requiring analgesia attributable to the axillary dissection. No patient had a winged scapula.
Numbness or paresthesias to the skin of the upper medial arm and/or axilla from intercostobrachial nerve sacrifice or injury have long been recognized. 36,37 In our experience, 76.5% of patients had such changes in the initial postoperative period, but with prolonged follow-up complete resolution was achieved in 22%, and the problems were improved in an additional 59%. This experience is similar to that reported by Salmon. 38 In the study of Paredes et al, 39 truncal section of the intercostobrachial nerve affected axillary and arm sensitivity in almost all patients when assessed after surgery; axillary anesthesia or analgesia persisted in >50% of patients. The great majority of patients had a return of arm sensation after 12 months, 30% having complete return of sensation to both the axilla and arm. With nerve preservation, >50% of patients had early anesthesia or analgesia to the axilla and arm, almost all resolving after 12 months. Although we now preserve the intercostobrachial nerve whenever possible, in the 10 recently treated patients numbness was experienced by 7.
The explanation for the return of or improvement in sensation for the majority of patients no doubt reflects the richness of cutaneous sensory innervation to the axilla and upper medial arm. The intercostobrachial nerve, which supplies sensory fibers to the medial aspect of the upper arm, axillary skin, and upper lateral breast, arises as the lateral cutaneous branch of the ventral primary ramus of T2. In its course through the posterior axilla, it joins a filament of the medial brachial cutaneous nerve, the smallest branch of the medial cord of the brachial plexus. An additional contact has been described with the posterior brachial cutaneous branch of the radial nerve. 40 The size of the intercostobrachial nerve and the extent of its distribution appear to vary inversely with the size and distribution of the medial brachial cutaneous nerve. Anastomoses between the intercostobrachial and the lateral cutaneous branches of T1 and T3 may exist. The anastomosis between the intercostobrachial and medial brachial cutaneous nerves is rather constant and may be represented by two or more filaments connecting them. Anastomoses with branches of T1 and T3 and the posterior brachial cutaneous branch of the radial nerve appear to represent occasional or inconstant findings. The intercostobrachial nerve may give off two or more branches as it traverses the axillary fat. Another sensory nerve, the medial brachial cutaneous nerve, the smallest branch of the medial cord of the brachial plexus, is formed from fibers arising from cord segments C8 and T1 or from T1 alone. Descending through the axilla, it pierces the brachial fascia at about the middle of the arm. It innervates the skin and subcutaneous tissues of the posterior aspect of the lower third of the arm as far as the olecranon. The nerve occasionally arises as a branch of the medial antebrachial cutaneous nerve, which also arises from C8, T1. It may receive fibers from T2 and/or T3, or it may be absent. Because the medial brachial cutaneous nerve frequently has direct contacts and varies inversely in size with the intercostobrachial nerve, it should be considered as often sensory to the axilla as well. In addition, a second intercostobrachial nerve often arises from the lateral cutaneous branch of T3 and supplies the axilla and medial side of the arm. 41 The first intercostal nerve also provides a lateral cutaneous branch that supplies the skin of the axilla and may communicate with the intercostobrachial nerve and the medial brachial cutaneous as well. Although denervation or division of the intercostobrachial nerve will lead to numbness or paresthesias of the skin in the upper medial arm and axilla, this may be of varying degrees, distribution, and duration as a result of the richness of the sensory nerve supply to the axilla and upper arm. Preservation of the intercostobrachial nerve, particularly in the absence of lateral axillary lymphadenopathy, is recommended, but if the intercostobrachial nerve is transected, resulting numbness or paresthesias may well abate, as was true for most patients in our experience.
In no patient in this series did recurrent disease develop in the axilla. The rarity of axillary recurrence after a carefully performed axillary dissection is well established. In the NSABP B-04 study, axillary recurrence was reported in only 1.4% of node-negative patients and 1% of node-positive patients after axillary dissection, whereas clinically palpable nodal metastases developed in 17.8% of patients with clinically negative axillae randomized not to have an axillary dissection. Series of patients whose axillae were not treated have been reported by Cady et al 42 with an axillary failure rate of 16% and by Baxter et al 43 with a 10-year actuarial axillary failure rate of 28%. Radiation may be an effective alternative, as reported by Recht et al, 44 who noted a 0.8% incidence of axillary recurrence after axillary radiation in 355 clinically node-negative patients. With clinically positive axillae, as reported in the NSABP B-04 study, the axillary recurrence rate in patients with palpable nodal disease receiving axillary radiation rather than axillary dissection was 12%. An even higher rate of 19% was reported in a series by Osborne et al from the Royal Marsden Hospital. 45
Elective axillary dissection clearly provides significant prognostic information, but greater selectivity in the performance of the procedure is coming under closer scrutiny as the frequency of smaller, often nonpalpable and low-grade cancers rises as a result of more widespread mammographic screening. 45–48 Sentinel lymphadenectomy has been proposed as an alternative to elective axillary dissection. In reported series to date, sentinel node biopsies have most often been followed by completion axillary dissection. 5,6,7,49 Sentinel node biopsy allows a more exhaustive assessment of the sentinel node for micrometastasis. Whether sentinel lymphadenectomy will eliminate elective axillary dissection from the surgical treatment of breast cancer remains to be demonstrated. However, the characterization of level I and II axillary dissection as a procedure that should be abandoned because of a significant morbidity rate does not appear justified by our experience.
Footnotes
Correspondence: Daniel F. Roses, MD, New York University Medical Center, 530 First Ave., New York, NY 10016.
Accepted for publication March 19, 1999.
References
- 1.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with and without radiation. N Engl J Med 1985; 312: 674–681. [DOI] [PubMed] [Google Scholar]
- 2.Cady D. The need to re-examine axillary lymph node dissection in invasive breast cancer. Cancer 1994; 73: 505–508. [DOI] [PubMed] [Google Scholar]
- 3. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. Early Breast Cancer Trialists Collaborative Group. Lancet 1992; 339: 1–15. [PubMed] [Google Scholar]
- 4.Haffty BG, Ward B, Pathare P. Reappraisal of the role of axillary lymph node dissection in conservative treatment of breast cancer. J Clin Oncol 1997; 15 (2): 691–700. [DOI] [PubMed] [Google Scholar]
- 5.Albertini JJ, Lyman GH, Cox CE, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 1996; 276: 1818–1822. [PubMed] [Google Scholar]
- 6.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 1993; 2: 335–339. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano AE, Jones RC, Brennan M, Statman RR. Sentinel lymphadenectomy and breast cancer. J Clin Oncol 1997; 15: 2345–2350. [DOI] [PubMed] [Google Scholar]
- 8.Patey DH, Dyson WH. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br J Cancer 1948; 2: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halsted WS. The results of operations for the cure of cancer of the breast performed at The Johns Hopkins Hospital from June 1889 to January 1894. Johns Hopkins Hosp Rep 1894–1895; 4: 297–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris MN, Gumport SL, Maiwandi H. Axillary lymph node dissection for melanoma. Surg Gynecol Obstet 1972; 135: 936–940. [PubMed] [Google Scholar]
- 11.Roses DF, Harris MN, Potter DA, Gumport SL. Total mastectomy with complete axillary dissection. Ann Surg 1981; 194: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veronesi U, Rilke F, Luini A, et al. Distribution of axillary node metastases by level of invasion. Cancer 1987; 59: 682–687. [DOI] [PubMed] [Google Scholar]
- 13.Boova R, Bonanne R, Rosato FE. Patterns of axillary nodal involvement in breast cancer. Ann Surg 1982; 196: 642–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veronesi U, Luini A, Galimberti V, et al. Extent of metastatic axillary involvement in 1,446 cases of breast cancer. Eur J Surg Oncol 1990; 16: 127. [PubMed] [Google Scholar]
- 15.Pigott J, Nichols R, Maddox WA, Balch CA. Metastases to the upper levels of the axillary nodes in carcinoma of the breast and its implications for nodal sampling procedures. Surg Gynecol Obstet 1984; 158: 255–259. [PubMed] [Google Scholar]
- 16.Danforth DN, Findlay PA, McDonald, et al. Complete axillary lymph node dissection for stage I-II carcinoma of the breast. J Clin Oncol 1986; 4: 655–662. [DOI] [PubMed] [Google Scholar]
- 17.Rosen PP, Lesser ML, Kinne DW, Beattie ET. Discontinuous or skip metastases in breast carcinoma. Analysis of 1228 axillary dissections. Ann Surg 1983; 197: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merson M, Pirovano C, Balzarini A, et al. The preservation of minor pectoralis muscle in axillary dissection for breast cancer: Functional and cosmetic evaluation. Eur J Surg Oncol 1992; 18: 215–218. [PubMed] [Google Scholar]
- 19.Britton RC, Nelson PA. Causes and treatment of postmastectomy lymphedema of the arm: Report of 114 cases. JAMA 1962; 180: 95–102. [DOI] [PubMed] [Google Scholar]
- 20.Hughes JH, Patel AR. Swelling of the arm following radical mastectomy. Br J Surg 1966; 53: 414. [DOI] [PubMed] [Google Scholar]
- 21.Lin PP, Allison DC, Wainstock J, et al. Impact of axillary lymph node dissection on the therapy of breast cancer patients. J Clin Oncol 1993; 11: 1536–1544. [DOI] [PubMed] [Google Scholar]
- 22.Kissin MW, della Rovere QG, Easton D, Westbury G. Risk of lymphedema following the treatment of breast cancer. Br J Surg 1986; 73: 580–584. [DOI] [PubMed] [Google Scholar]
- 23.Godal R, Swedborg I. A correction for the natural asymmetry of the arms in the determination of the volume of oedema. Scand J Rehab Med 1982; 193–195. [PubMed] [Google Scholar]
- 24.Ball AB, Thomas JM, Waters R. Radical axillary dissection in the staging and treatment of breast cancer. Ann R Coll Surg Engl 1992; 74: 126–129. [PMC free article] [PubMed] [Google Scholar]
- 25.Ivens D, Hoe AL, Podd CR, et al. Assessment of morbidity from complete axillary dissection. Br J Cancer 1992; 66: 136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hladiuk M, Huchcroft S, Temple W, Schmurr BE. Arm function after axillary dissection for breast cancer: A pilot study to provide parameter estimates. J Surg Onc 1992; 50: 47–52. [DOI] [PubMed] [Google Scholar]
- 27.Hoe AL, Iven D, Boyle GT, Taylor I. Incidence of arm swelling following axillary clearance for breast cancer. Br J Surg 1992; 79: 261–262. [DOI] [PubMed] [Google Scholar]
- 28.Keramopoulos A, Tsionou C, Minaretzis D, et al. Arm morbidity following treatment of breast cancer with total axillary dissection: A multivariate approach. Oncology 1993; 50: 445–449. [DOI] [PubMed] [Google Scholar]
- 29.Tadych K, Donegan WL. Postmastectomy seromas and wound drainage. Surg Gynecol Obstet 1987; 165: 483–487. [PubMed] [Google Scholar]
- 30.Jensen RFM, van Geel AN, de Goot HGW, et al. Immediate versus delayed shoulder exercises after axillary lymph node dissection. Am J Surg 1990; 160: 481–484. [DOI] [PubMed] [Google Scholar]
- 31.Knight CD, Griffen FD, Knight CD. Prevention of seromas in mastectomy wounds. The effect of shoulder immobilization. Arch Surg 1995; 130: 99–101. [DOI] [PubMed] [Google Scholar]
- 32.Dawson F, Stam L, Heslinga JM, Kalsbeek HL. Effect of shoulder immobilization on wound seroma and shoulder dysfunction following modified radical mastectomy: A randomized prospective trial. Br J Surg 1989; 76: 311–312. [DOI] [PubMed] [Google Scholar]
- 33.Petrek JA, Peters MM, Nori S, et al. Axillary lymphadenectomy: A prospective randomized trial of thirteen factors influencing drainage, including early or delayed arm mobilization. Arch Surg 1990; 125: 378–382. [DOI] [PubMed] [Google Scholar]
- 34.Shultz I, Barholm M, Grondal S. Delayed shoulder exercises in reducing seroma frequency after modified radical mastectomy: A prospective randomized study. Am Surg Oncol 1997; 4: 293–297. [DOI] [PubMed] [Google Scholar]
- 35.Temple WJ, Ketchum AS. Preservation of the intercostobrachial nerve during axillary dissection for breast cancer. Am J Surg 1985; 150: 585–588. [DOI] [PubMed] [Google Scholar]
- 36.Teicher I, Pouland B, Wise L. Preservation of the Intercostobrachial nerve during axillary dissection for carcinoma of the breast. Surg Gynecol Obstet 1982; 155: 891–892. [PubMed] [Google Scholar]
- 37.Salmon RJ, Insguer Y. Preservation vs. section of intercostobrachial nerve in axillary dissection. A prospective randomized trial [abstract]. Soc Surg Oncol 1996; 38. [Google Scholar]
- 38.Paredes JP, Puente JL, Potel J. Variations in sensitivity after sectioning the intercostobrachial nerve. Am J Surg 1990; 160: 525–528. [DOI] [PubMed] [Google Scholar]
- 39.Gardner E, Gray DJ, O’Rahilly R. Anatomy: A regional study of human structure, 5th edition. Philadelphia: Saunders; 1986: 106–107.
- 40.Dwight T, Hamann CA, McMurrich JP, Piersol GA, White JW. Human anatomy, 8th ed. Philadelphia: Lippincott; 1923: 1314.
- 41.Cady B, Stone M, Wayne J. New therapeutic possibilities in primary invasive breast cancer. Ann Surg 1993; 218: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baxter N, McCready D, Chapman J-A, et al. Clinical behavior of untreated axillary nodes after local treatment for primary breast cancer. Ann Surg Oncol 1996; 3: 235–240. [DOI] [PubMed] [Google Scholar]
- 43.Recht A, Pierce SM, Abner A, et al. Regional nodal failure after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol 1991; 9: 988–996. [DOI] [PubMed] [Google Scholar]
- 44.Osborne MP, Ormiston N, Harmer CL, et al. Breast conservation in the treatment of early breast cancer: A 20-year followup. Cancer 1984; 53: 349–355. [DOI] [PubMed] [Google Scholar]
- 45.Silverstein MJ, Gerson ED, Waisman JR, et al. Axillary lymph node dissection for T1a breast carcinoma: Is it indicated? Cancer 1994; 79: 664–667. [DOI] [PubMed] [Google Scholar]
- 46.Chontos AJ, Maher DP, Ratzer ER, Fenoglio ME. Axillary lymph dissection: Is it required in T1a breast cancer? J Am Coll Surg 1997; 184: 493–498. [PubMed] [Google Scholar]
- 47.White RE, Vezeridis MP, Konstadowlakis M, et al. Therapeutic options and results for the management of minimally invasive carcinoma of the breast: Influence of axillary dissection for treatment of T1a and T1b lesions. J Am Coll Surg 1996; 183: 575–582. [PubMed] [Google Scholar]
- 48.Pandelidis SM, Peters KL, Walusimbi MS, et al. The role of axillary dissection in mammographically detected carcinoma. J Am Coll Surg 1997; 184: 341–345. [PubMed] [Google Scholar]
- 49.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph nodes. Lancet 1997; 349: 1864–1867. [DOI] [PubMed] [Google Scholar]