Abstract
Objective
To investigate the relation of the biallelic Nco1 restriction fragment length polymorphism in the first intron of the tumor necrosis factor (TNF) β gene with the development of severe sepsis in multiply injured patients.
Summary Background Data
The biallelic Nco1 polymorphism of the TNFβ gene has been described to be associated with autoimmune diseases and with the mortality rate in severe sepsis. Therefore, the Nco1 polymorphism may be associated with the clinical finding that despite comparable risk factors, posttraumatic sepsis develops in some patients but not others.
Methods
The study group consisted of 110 patients with severe blunt trauma (Injury Severity Score ≥ 17). Typing of each patient for the biallelic Nco1 polymorphism was performed by analyzing restriction fragments of an Nco1-digested DNA fragment obtained using polymerase chain reaction. Genotypes were then related to the occurrence of severe posttraumatic sepsis and TNFα serum concentrations.
Results
Fifty-seven patients showed an uncomplicated posttraumatic recovery, and severe sepsis developed in 53 patients. The overall allele frequency (TNFB1 0.29, TNFB2 0.71) and genotype distribution (TNFB1 homozygous 7.3%, TNFB1/TNFB2 42.7%, TNFB2 homozygous 50%) were in agreement with the distribution in healthy volunteers. Genotype distribution in patients with an uncomplicated clinical course was significantly different from that in patients with severe posttraumatic sepsis. Development of severe posttraumatic sepsis was significantly increased in patients homozygous for the allele TNFB2. In patients with severe posttraumatic sepsis, TNFα serum concentrations were significantly higher in TNFB2-homozygous individuals compared with heterozygous and TNFB1-homozygous individuals. The age- and injury-matched odds ratio for the homozygous TNFB2 genotype compared with the heterozygous genotype was 5.22 (p = 0.007, 95% confidence interval 1.6 to 17.9).
Conclusions
In multiply injured patients, the Nco1 polymorphism within the TNFβ gene is associated with the development of severe posttraumatic sepsis and with increased TNFα serum levels when severe sepsis has occurred. This suggests a genetic determination of the individual inflammatory response after infection or tissue damage, which significantly influences susceptibility to severe nosocomial infections.
Development of sepsis and multiple organ failure (MOF) significantly impairs the prognosis of critical ill patients. Sepsis is currently regarded as the most common cause of death in the noncoronary intensive care unit 1–5 and has been reported to be the 13th leading cause of death in the United States. 6 Although our knowledge of the pathophysiologic events related to sepsis and MOF has grown enormously, new therapeutic approaches develop slowly, and the clinical outcome in severe sepsis remains poor. 5,7
Cytokine networks are thought to be of major importance in the pathogenesis of infectious diseases; in particular, tumor necrosis factor (TNF) appears to play a crucial role. 8,9 Because multiply injured patients are at high risk for the development of sepsis and MOF, circulating pro- and antiinflammatory cytokines have been studied extensively after severe blunt trauma and were found to correlate with severity of injury and clinical outcome. 10–13
Recently, it has been shown that a genomic restriction fragment length polymorphism (RFLP) within the TNF locus is correlated with increased TNFα plasma concentrations and a high mortality rate in patients with severe sepsis. 14 This biallelic RFLP in the first intron of the TNFβ gene at position 1064–1069, which is identified by the restriction fragments TNFB1 (5.5 kb) and TNFB2 (10.5 kb) by Nco1 digestion, has previously been described in connection with several autoimmune diseases. 15–17 The Nco1 polymorphism was found to be correlated with an amino acid variation of the TNFβ sequence at position 26, which is asparagine for the TNFB1 sequence and threonine for the TNFB2 sequence. 18 With regard to the functional consequences of TNF synthesis, it was found that phytohemagglutinin-stimulated peripheral blood mononuclear cells from TNFB1-homozygous individuals showed a significantly increased TNFβ production, whereas phytohemagglutinin- and endotoxin-stimulated monocytes from TNFB2-homozygous individuals showed a significantly higher production of interleukin-1b and TNFα. 15,16,18,19
Therefore, it seems reasonable to assume that a genetic determination of the individual inflammatory response to a variety of insults may contribute to the clinical finding that despite comparable risk factors, posttraumatic organ failure and sepsis develop in some patients but not others. Because it would be important for the treatment of patients with multiple trauma to identify those with increased susceptibility to sepsis, the purpose of our study was to determine whether a relation of the biallelic Nco1 polymorphism to the development of severe sepsis exists in patients with severe blunt trauma.
MATERIALS AND METHODS
Subjects
The study was approved by the ethics committees of the Universities of Essen, Kiel, and Lübeck. We studied 110 patients (33 [30%] women and 77 [70%] men; age 38 ± 15 years [mean ± SD]) sustaining blunt trauma with an Injury Severity Score (ISS) 20 of ≥17 (mean 32 ± 12). Subjects fulfilled the following criteria: age 18 to 65 years, no penetrating injuries, and no preexisting chronic illness.
Injuries of the various body regions (head and neck, face, thorax, abdomen, extremities, skin) were classified using the Abbreviated Injury Scale (AIS). 20 The clinical course was followed prospectively in all patients. The MOF score was calculated daily according to Goris et al. 21 Severe sepsis was defined according to the ACCP/SCCM consensus conference criteria 4 and was diagnosed not before day 5 after trauma. Antibiotic treatment was documented. Based on the high affinity for the penicillin-binding protein (PBP) 3 in the bacterial cell wall and on in vitrofindings of high endotoxin release and TNF production, the antibiotics aztreonam, ceftazidime, and cefotaxime were classified as PBP3/TNF type; all others were classified as non-PBP3/TNF antibiotics. 22
Eighty-one patients were admitted to the emergency room of the University Hospital Essen, and 29 patients were admitted to the intensive care units of the Universities of Lübeck and Kiel with severe posttraumatic sepsis. All patients requiring surgical intervention received standard surgical care and postoperative intensive care unit treatment.
In patients admitted to the intensive care unit with severe posttraumatic sepsis, serum was collected daily at 7 to 8 A.M. during a 7-day period. Sera were stored frozen at −80°C until further analysis. Because our previous studies showed that TNFα serum levels were not detectable in the patients without posttraumatic sepsis, 23,24 sera were assayed for TNFα concentrations only in patients with severe posttraumatic sepsis.
Genotypic Analysis of the Nco1 RFLP Within the TNFβ Gene Locus
The genotype of the Nco1 RFLP of the TNFβ gene was determined by polymerase chain reaction (PCR) amplification and enzymatic digestion of the products with Nco1. Blood samples were drawn, and each patient’s genomic DNA was extracted by standard phenol-chloroform extraction 25 from peripheral blood mononuclear cells obtained by Ficoll density gradient centrifugation or from whole blood using a commercially available DNA isolation kit (QIAmp blood kit, Quiagen, Krefeld, Germany), according to the manufacturer’s instructions.
Polymerase chain reaction was performed using 1 unit of Thermus aquaticus thermostable DNA polymerase (Boehringer, Mannheim, Germany), 0.2 μmol dNTP, 200 pmol of each primer, 1 or 0.1 μL of chromosomal DNA, 50 mM KCl, 10 mM Tris/HCl, 1.5 mM MgCl2(pH 8.3), in a total volume of 100 μl. A 782-bp fragment of the TNFβ gene, including the first intron, was amplified by 37 cycles of denaturation (30 sec at 95°C), primer annealing (30 sec at 50°C), and DNA extension (45 sec at 72°C) in an automated PCR cycler (Gene Amp PCR System 2400, Perkin Elmer, Darmstadt, Germany). The following nucleotide sequences were used for PCR amplification 14 : 5′CCGTGCTTCGTGCTTTGGACTA3′ and 5′AGAGGGGTGGATGCTTGGGTTC3′. Seventeen microliters of the PCR product was digested with 1 μl Nco1 endonuclease (10 units/μl; Gibco, Eggenstein, Germany) and 2 μl 50 mM Tris-HCl, 10 mM KCl, 1 mM dithiothreitol, 0.1 mg/ml bovine serum albumin for 2 hours at 37°C. This resulted in complete digestion of the PCR product, because extension of incubation time and addition of fresh enzyme did not change the result (Fig. 1 ). The TNFB1 allele includes a restriction site for Nco1, which results in a 196-bp and a 586-bp fragment after digestion. After Nco1 digestion, 10 μl of digested and undigested samples were electrophoresed in a nonreducing 1.5% agarose gel (160 V, 1 hour) and stained with ethidium bromide. The bands were visualized under ultraviolet light and digitized with a videocamera (VarioCam, Phase, Lübeck, Germany).
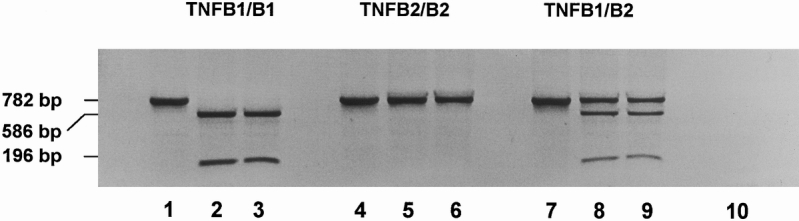
Figure 1. Genotypic analysis of the Nco1 RFLP. A 782-bp fragment of the TNFβ gene, including the first intron, was amplified by PCR. The PCR product was digested with Nco1 endonuclease. Lanes 1–3: TNFB1 homozygous genotype (TNFB1/B1). Lane 1: Undigested PCR product. Lane 2: Nco1-digested PCR product (2 hours, 37°C). Lane 3: Nco1-digested PCR product (4 hours, 37°C; addition of 10 U Nco1 endonuclease at t = 0 and t = 2 hours). Lanes 4–6: TNFB2 homozygous genotype (TNFB2/B2). Lane 4: Undigested PCR product. Lane 5: Nco1-digested PCR product (2 hours, 37°C). Lane 6: Nco1-digested PCR product (4 hours, 37°C; addition of 10 U Nco1 endonuclease at t = 0 and t = 2 hours). Lanes 7–9: Heterozygous genotype (TNFB1/B2). Lane 7: Undigested PCR product. Lane 8: Nco1-digested PCR product (2 hours, 37°C). Lane 9: Nco1-digested PCR product (4 hours, 37°C; addition of 10 U Nco1 endonuclease at t = 0 and t = 2 hours). Lane 10: Control (PCR without chromosomal DNA).
TNFα Serum Levels
TNFα was measured in serum using a commercially available enzyme-linked immunosorbent assay kit (Medgenix, Germany; lower detection limit 2.5 pg/ml).
Statistical Analysis
If not otherwise mentioned, data are expressed as mean ± standard deviation. Chi square test, Kruskal-Wallis H test, and Bonferroni corrected one-way analysis of variance were used to test for significant differences between the groups and were calculated with the SPSS for Windows Release 6.1.3 program. A two-tailed p < 0.05 was considered significant.
Statistical significance of the odds ratio (OR) associated with a particular genotype (OR = n1n4/n2n3, where n1 is the number of patients with sepsis carrying the genotype, n2 is the number of controls carrying the genotype, and n3 and n4 are the corresponding numbers of patients with sepsis and control patients not carrying the genotype) was calculated using a chi square distribution with one degree of freedom, 26 where:
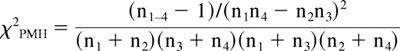 |
must exceed 3.841 to attain p < 0.05. A 95% confidence interval (OR(1±1.96/√χ2PMH)) excluding the value 1 shows statistical significance.
RESULTS
Characteristics of the Study Subjects
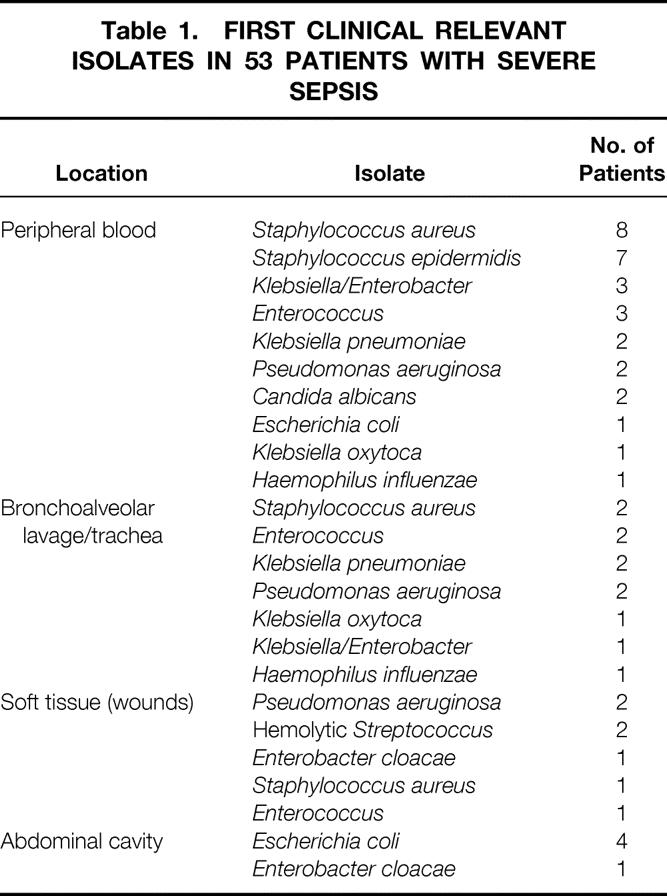
Severe posttraumatic sepsis was diagnosed in 53 patients (48.2%). In 26 (49%) of the 53 patients with severe sepsis, gram-positive microorganisms were isolated; in 25 (47%) of the 53, gram-negative microorganisms were isolated. In two cases, sepsis was caused by fungi. In most of the patients, several isolates were found subsequently during intensive care unit treatment (e.g., in peripheral blood cultures). The source of infection was predominantly pneumonia. Soft tissue infection (n = 7) and posttraumatic peritonitis (n = 5) as a source for severe sepsis were found less frequently. The first relevant microbiologic finding from each patient with severe posttraumatic sepsis is shown in Table 1. Four of the 53 patients with severe posttraumatic sepsis received PBP3/TNF antibiotics (3 patients ceftazidime, 1 patient both ceftazidime and cefotaxime). One of the 57 patients without posttraumatic sepsis received cefotaxime for perioperative prophylaxis. None of the patients received aztreonam.
Table 1. FIRST CLINICAL RELEVANT ISOLATES IN 53 PATIENTS WITH SEVERE SEPSIS
Patients in whom severe posttraumatic sepsis developed were significantly older (43 ± 16 years vs. 33 ± 13 years; p = 0.0014), and the ISS was significantly greater than in patients with an uncomplicated clinical course (40 ± 12 vs. 25 ± 9; p < 0.001). The latter was caused by more severe injuries of the abdomen (AIS 2.6 ± 2 vs. 1.0 ± 1.6; p < 0.001), chest (3.0 ± 1.7 vs. 1.8 ± 1.7; p < 0.001), and extremities (AIS 3.3 ± 0.8 vs. 2.7 ± 1.1; p = 0.0041) in patients with severe posttraumatic sepsis. There were no differences in severity of injuries of the head and neck (AIS 2 ± 1.7 vs. 1.5 ± 1.6), face (AIS 1.1 ± 1.2 vs. 1 ± 1.5), and skin (AIS 0.5 ± 0.8 vs. 0.4 ± 0.9) between patients with and without severe posttraumatic sepsis. As expected, patients with severe posttraumatic sepsis showed a significantly increased length of mechanical ventilation (24 ± 33 days vs. 8 ± 8 days; p < 0.001), duration of intensive care unit treatment (29 ± 35 days vs. 11 ± 11 days; p < 0.001), and higher maximal MOF scores (8 ± 3 vs. 5 ± 3; p < 0.001). In patients with severe posttraumatic sepsis, the mortality rate was 32% (17/53) versus 7% (4/57) in patients without severe sepsis (p < 0.001). Death in patients without severe posttraumatic sepsis was caused by the severity of injury without clinical signs or laboratory data indicating sepsis.
Allele Frequency and Genotype Distribution
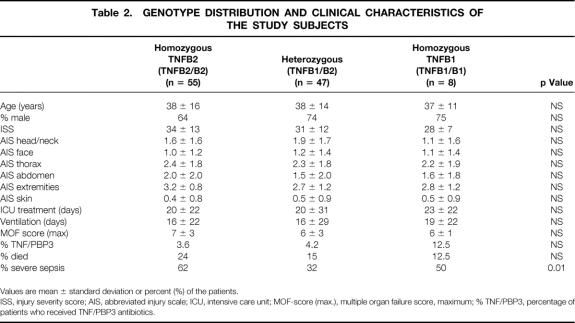
The overall allele frequency was TNFB1 0.29 and TNFB2 0.71. Eight (7.3%) patients were homozygous for the allele TNFB1, 55 (50%) were homozygous for the allele TNFB2, and 47 (42.7%) were heterozygous (TNFB1/TNFB2). The three genotypic groups were comparable in age, gender, ISS, injury pattern measured using AIS of the specific body regions, and survival. There were no differences in type of antibiotic treatment, length of mechanical ventilation, duration of intensive care treatment, and maximal MOF scores between the genotypic groups. In contrast, between the three genotypic groups, significant differences (p = 0.01) were detectable for the frequency of severe posttraumatic sepsis (Table 2).
Table 2. GENOTYPE DISTRIBUTION AND CLINICAL CHARACTERISTICS OF THE STUDY SUBJECTS
Values are mean ± standard deviation or percent (%) of the patients.
ISS, injury severity score; AIS, abbreviated injury scale; ICU, intensive care unit; MOF-score (max.), multiple organ failure score, maximum; % TNF/PBP3, percentage of patients who received TNF/PBP3 antibiotics.
When compared with patients carrying at least one TNFB1 allele (TNFB1 homozygous and heterozygous genotype), the TNFB2 homozygous genotype was associated with an OR of 3.07 (p = 0.004; χ2PMH = 8.12; 95% CI 1.42 to 6.63) for the development of severe sepsis. Compared with the heterozygous genotype, the OR for the homozygous TNFB2 genotype was 3.45 (p = 0.0026; χ2PMH = 8.99; 95% CI 1.54 to 7.77) (Table 3).
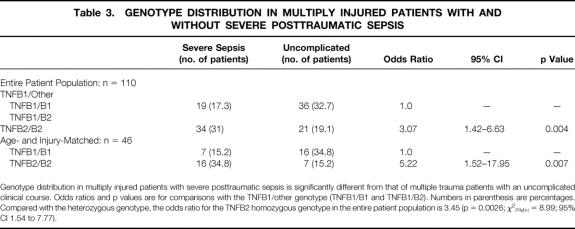
Table 3. GENOTYPE DISTRIBUTION IN MULTIPLY INJURED PATIENTS WITH AND WITHOUT SEVERE POSTTRAUMATIC SEPSIS
Genotype distribution in multiply injured patients with severe posttraumatic sepsis is significantly different from that of multiple trauma patients with an uncomplicated clinical course. Odds ratios and p values are for comparisons with the TNFB1/other genotype (TNFB1/B1 and TNFB1/B2). Numbers in parenthesis are percentages. Compared with the heterozygous genotype, the odds ratio for the TNFB2 homozygous genotype in the entire patient population is 3.45 (p = 0.0026; χ2PMH = 8.99; 95% CI 1.54 to 7.77).
To study the association of the TNFB2/B2 genotype with the development of severe posttraumatic sepsis independent from known risk factors for sepsis, such as age, ISS, or injury pattern, 1,27,28 a matched pairs analysis was performed. Twenty-three patients with severe posttraumatic sepsis were matched with patients without severe posttraumatic sepsis based on age, ISS, and pattern of injury. Between the individual pairs, the mean difference in age was 1.3 ± 2.9 years; the mean difference was 1.2 ± 4 in ISS and 0 ± 2 in AIS. The allele frequencies of the matched groups were 0.15 TNFB1 and 0.85 TNFB2 in patients with severe posttraumatic sepsis and 0.35 TNFB1 and 0.65 TNFB2 in patients without severe sepsis. Sixteen of the 23 patients with severe posttraumatic sepsis and 7 of the 23 patients without severe posttraumatic sepsis were homozygous for the allele TNFB2 (p = 0.007). Therefore, the age- and injury-adjusted OR for the homozygous TNFB2 genotype compared with the heterozygous genotype was 5.22 (χ2PMH = 6.89; 95% CI 1.52 to 17.95; see Table 3).
TNFα Serum Concentrations in Patients with Severe Sepsis
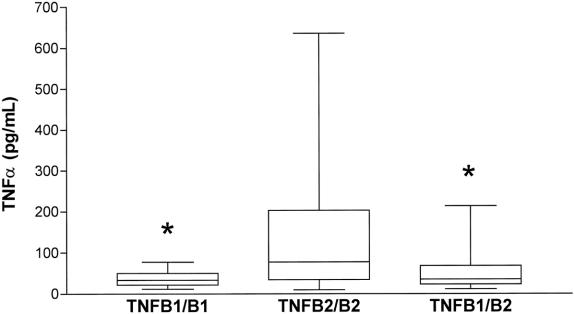
The TNFα levels found in serum from patients with severe posttraumatic sepsis are shown in Figure 2. Mean TNFα serum concentrations were significantly higher in patients homozygous for the TNFB2 allele (143 ± 149 pg/ml) than in heterozygous (54 ± 48 pg/ml) and TNFB1-homozygous patients (39 ± 21 pg/ml) during the 7-day observation period after admission to the intensive care unit.
Figure 2. TNFα serum concentrations in patients with severe posttraumatic sepsis during a 7-day observation period after admission to the intensive care unit. TNFB1/B1: n = 14 values; TNFB2/B2: n = 126 values, TNFB1/B2: n = 63 values. The box extends from the 25th percentile to the 75th percentile; the horizontal line shows the median (50th percentile). Error bars show the smallest and the largest values. *, p < 0.05 vs. TNFB2-homozygous subjects (TNFB2/B2) (one-way analysis of variance, Bonferroni correction).
DISCUSSION
TNFα is believed to be a pivotal proinflammatory mediator in host defense to infection. 29,30 Moreover, an innate profile of cytokine production as well as a genetic influence for fatal infectious diseases has been proposed. 31,32 Several polymorphic elements in the TNF gene region as well as the human leukocyte antigen type have been related to TNF production, but the precise location of the genetic elements controlling TNF response remain unclear, and TNF gene expression has been shown to be regulated only in part by transcriptional events. 33–35 Two polymorphisms of the human TNF gene that might influence TNFα secretion have been described to be associated with severe clinical forms of infectious diseases 14,35–37 : the Nco1 polymorphism of the TNFβ gene and a G/A polymorphism at position −308 in the TNFα promoter region. In patients with cerebral malaria, the −308G/A polymorphism has been shown to be associated with outcome and clinical course of the disease. The OR for death or severe neurologic sequelae from cerebral malaria was 7 in patients carrying the homozygous form of the −308A allele. 35 Similar to cerebral malaria, the −308A allele and the Nco1 polymorphism were found to be significantly associated with the development of mucocutaneous leishmaniasis. 36 In contrast, in patients with meningococcal disease 31 and in patients with severe sepsis of various origins, 38 the −308G/A polymorphism was not associated with outcome, indicating that the significance of the −308G/A polymorphism does not in general extend to infectious diseases. Previously, the mortality rate in severe sepsis was found to be significantly increased in patients homozygous for the allele TNFB2 of the Nco1 polymorphism compared with heterozygous patients. 14
The genotype distribution determined in multiply injured patients presented here was found to be in agreement with the previously described distribution in unrelated and unselected healthy volunteers. 15,16,19,36,39–43 Because most of our patients were victims of traffic accidents, there is no reason to assume that they came from a distinct population. Several risk factors for severe sepsis such as age, gender, ISS, and injury pattern were studied within the three genotypic groups, but significant differences were not detectable.
Recently, the association of the type of antibiotic treatment and the mortality rate in severely injured trauma patients at high risk for sepsis was described. In trauma patients treated with aztreonam, ceftazidime, or cefotaxime (PBP3/TNF antibiotics), the mortality rate was found to be significantly increased when compared with patients who had received non-PBP3/TNF antibiotics. 22,44 In our study, only 4.5% of patients received PBP3/TNF antibiotics, and there were no differences between the three genotypic groups.
The major finding of the present study is that a statistically highly significant association exists between the genotype of the biallelic Nco1 polymorphism of the TNFβ gene and the development of severe sepsis after severe blunt trauma. Subjects homozygous for the allele TNFB2 have a significantly increased risk for the development of severe posttraumatic sepsis, with an age- and injury-matched OR of 5.22 compared with patients with the heterozygous genotype. Despite the possibility that the high frequency of TNFB1-homozygous patients with severe posttraumatic sepsis is a chance observation from the small sample size, it cannot be ruled out that both homozygous genotypes are at high risk for posttraumatic sepsis when compared with heterozygotes. Therefore, a larger cohort of patients is needed to study the relation of the TNFB1 homozygous genotype to the development of severe posttraumatic sepsis definitively.
In contrast to multiply injured patients without posttraumatic complications, relevant systemic TNFα concentrations can be detected only in patients with posttraumatic complications, such as sepsis, adult respiratory distress syndrome, or MOF. 45–47 In agreement with our previous findings on patients with severe sepsis of various origin, 14 TNFα serum concentrations in patients with severe posttraumatic sepsis were significantly higher in TNFB2-homozygous subjects than in heterozygous and TNFB1-homozygous subjects. Although the source of TNFα measured in plasma or serum from patients with severe sepsis is unknown, the differences in TNFα serum concentrations between the genotypic groups detected here may correspond to the distinct capacity of peripheral blood mononuclear cells or monocytes to produce TNFα in response to stimulating agents; this has been shown to be associated with the Nco1 polymorphism. In terms of TNFα as a pivotal mediator for the development of sepsis, the low TNFα levels in TNFB1-homozygous patients with severe sepsis may argue against the theory of increased susceptibility for severe sepsis of both homozygous genotypes.
In conclusion, our data provide evidence of a genetic determination for susceptibility to severe nosocomial infections in severely injured, critically ill patients. Detection of the Nco1 polymorphism appears to be a useful tool for evaluating patients at high risk for sepsis immediately after severe blunt trauma and for identifying those who could profit from anti-TNF strategies. It cannot be ruled out that the Nco1 polymorphism is not directly related to sepsis susceptibility but serves as a linked major histocompatibility complex (MHC) marker because of its location in the class III region of the MHC. 33,34 Therefore, additional studies to investigate the mechanism of increased susceptibility to severe sepsis influenced by the Nco1 polymorphism are needed to obtain a rational basis for therapy, which should be adapted to the genetic background of the patient with severe blunt trauma.
Acknowledgment
The authors thank Mrs. Heike Löffler for her excellent technical help.
Footnotes
Correspondence: F. Ulrich Schade, PhD, Klinische Forschergruppe “Schock und Multiorganversagen,” Zentrum für Chirurgie, Universitätsklinikum Essen, Hufelandstr. 55, 45122 Essen, Germany.
Supported by Grants Schm 74/13-2 and Stu 185/2-2 by Deutsche Forschungsgemeinschaft, Bad Godesberg, Germany and Fonds der Chemischen Industrie, Frankfurt/Main, Germany (FUS).
Accepted for publication March 11, 1999.
References
- 1.Bone RC, Fisher CJ Jr, Clemmer TP, et al. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 1987; 317: 653–658. [DOI] [PubMed] [Google Scholar]
- 2.Parillo JE, Parker MM, Natanson C, et al. Septic shock: Advances in the understanding of the pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 1990; 113: 227–242. [DOI] [PubMed] [Google Scholar]
- 3.Niedermann MS, Fein AM. Sepsis syndrome, the adult respiratory distress syndrome, and nosocomial pneumonia: A common clinical sequence. Clin Chest Med 1990; 11: 633–650. [PubMed] [Google Scholar]
- 4.American College of Chest Physicians—Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20: 864–875. [PubMed] [Google Scholar]
- 5.Bone CR. Immunologic dissonance: A continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med 1996; 125: 680–687. [DOI] [PubMed] [Google Scholar]
- 6.Centers of Disease Control and Prevention, National Center for Health and Statistics. Mortality patterns—United States, 1990. Monthly Vital Stat Rep 1993; 41: 5. [Google Scholar]
- 7.Freeman BD, Natanson C. Clinical trials in sepsis and septic shock in 1994 and 1995. Current Opinion in Critical Care 1995; 1: 349–357. [Google Scholar]
- 8.Beutler B, Grau GE. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med 1993; 21: S423–435. [PubMed] [Google Scholar]
- 9.Strieter RN, Kunkel SL, Bone RC. Role of tumor necrosis factor alpha in disease states and inflammation. Crit Care Med 1993; 21: S447–463. [DOI] [PubMed] [Google Scholar]
- 10.DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg 1995; 130: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 11.Roumen RMH, Hendriks T, van der Ven-Jongenkrijk J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Ann Surg 1993; 218: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ertel W, Keel M, Bonaccio M, et al. Release of anti-inflammatory mediators after mechanical trauma correlates with severity of injury and clinical outcome. J Trauma 1995; 39: 879–887. [DOI] [PubMed] [Google Scholar]
- 13.Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996; 24: 392–397. [DOI] [PubMed] [Google Scholar]
- 14.Stüber F, Petersen M, Bokelmann F, Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-α concentrations and outcome of patients with severe sepsis. Crit Care Med 1996; 24: 381–384. [DOI] [PubMed] [Google Scholar]
- 15.Pociot F, Molvig J, Wogensen L, et al. A tumor necrosis factor beta gene polymorphism in relation to monokine secretion and insulin-dependent diabetes mellitus. Scand J Immunol 1991; 33: 37–49. [DOI] [PubMed] [Google Scholar]
- 16.Pociot F, Briant L, Jongeneel CV, et al. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex with the secretion of TNF-alpha and TNF-beta by human mononuclear cells: A possible link to insulin-dependent diabetes mellitus. Eur J Immunol 1993; 23: 224–231. [DOI] [PubMed] [Google Scholar]
- 17.Fugger L, Morling N, Ryder P, et al. Nco1 restriction fragment length polymorphism (RFLP) of the tumor necrosis factor (TNFα) region in four autoimmune diseases. Tissue Antigens 1989; 34: 17–22. [DOI] [PubMed] [Google Scholar]
- 18.Messer G, Spengler U, Jung MC, et al. Polymorphic structure of the tumor necrosis factor (TNF) locus: An Nco1 polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J Exp Med 1991; 173: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molvig J, Pociot F, Baek L, et al. Monocyte function in IDDM patients and healthy individuals. Scand J Immunol 1990; 31: 297–306. [DOI] [PubMed] [Google Scholar]
- 20.Greenspan L, McLellan BA, Greig H. Abbreviated injury scale and injury severity score: A scoring chart. J Trauma 1985; 25: 60–64. [DOI] [PubMed] [Google Scholar]
- 21.Goris RJ, te-Boekhorst TP, Nuytinck JK, et al. Multiple-organ failure: Generalized autodestructive inflammation? Arch Surg 1985; 120: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 22.Mock CN, Jurkovich GF, Dries D, Maier RV. The clinical significance of endotoxin released by antibiotics: What is the evidence? J Endotox Res 1996; 3: 253–259. [Google Scholar]
- 23.Majetschak M, Flach R, Heukamp T, et al. Regulation of whole blood tumor necrosis factor production upon endotoxin stimulation after severe blunt trauma. J Trauma 1997; 43: 880–887. [DOI] [PubMed] [Google Scholar]
- 24.Majetschak M, Flach R, Jennissen V, et al. The extent of traumatic damage determines a graded depression of the endotoxin responsiveness of peripheral blood mononuclear cells from patients with blunt injuries. Crit Care Med 1999; 27: 313–318. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual, 2d ed. New York: Cold Spring Harbor Laboratory Press; 1989: 14–21.
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–748. [PubMed] [Google Scholar]
- 27.Broos PL, D’Hoore A, Vanderschot P, et al. Multiple trauma in elderly patients. Factors influencing outcome: Importance of aggressive care. Injury 1993; 24: 365–368. [DOI] [PubMed] [Google Scholar]
- 28.Aufmkolk M, Majetschak M, Voggenreiter G, et al. Outcome and prognosis after multiple trauma in the aged. Unfallchirurg 1997; 100: 477–482. [DOI] [PubMed] [Google Scholar]
- 29.Bone RC. The pathogenesis of sepsis. Ann Intern Med 1991; 115: 457–469. [DOI] [PubMed] [Google Scholar]
- 30.Beutler B, Cerami A. Cachectin: More than a tumor necrosis factor. N Engl J Med 1987; 316: 379–385. [DOI] [PubMed] [Google Scholar]
- 31.Westendorp RGJ, Langermans JAM, Huizinga TWJ, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 1997; 349: 170–173. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen TIA, Nielsen GG, Andersen PK, Teasdale TW. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med 1988; 318: 727–732. [DOI] [PubMed] [Google Scholar]
- 33.Pauli U. Control of tumor necrosis factor gene expression. Crit Rev Eukar Gen Expr 1994; 4: 323–344. [DOI] [PubMed] [Google Scholar]
- 34.Trowsdale J. Genomic structure and function in the MHC. Trends Genet 1993; 9: 117–122. [DOI] [PubMed] [Google Scholar]
- 35.McGuire W, Hill AVS, Allsopp CEM, et al. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature 1994; 371: 508–511. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera M, Shaw MA, Sharples C, et al. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med 1995; 182: 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor α promoter polymorphism effects transcription. Mol Immun 1997; 34: 391–399. [DOI] [PubMed] [Google Scholar]
- 38.Stüber F, Udalova IA, Book M, et al. −308 tumor necrosis factor (TNF) polymorphism is not associated with survival in severe sepsis and is unrelated to lipopolysaccharide inducibility of the human TNF promoter. J Inflam 1996; 46: 42–50. [PubMed] [Google Scholar]
- 39.Fugger L, Morling N, Ryder LP, et al. Nco1 restriction fragment length polymorphism (RFLP) of the tumor necrosis factor (TNFα) region in primary biliary cirrhosis and in healthy Danes. Scand J Immunol 1989; 30: 185–189. [DOI] [PubMed] [Google Scholar]
- 40.Laitinen T, Lokki ML, Tulppala M, et al. Tumor necrosis β gene polymorphism in relation to complotype in couples with spontaneous abortions and in control families. Scand J Immunol 1992; 35: 131–135. [DOI] [PubMed] [Google Scholar]
- 41.Peruccio D, D’Alfonso S, Borelli I, et al. Distribution of tumor necrosis factor alleles (Nco1 RFLP) and their relationship to HLA haplotypes in an Italian population. Hum Hered 1993; 43: 103–110. [DOI] [PubMed] [Google Scholar]
- 42.Campbell DA, Nelson S, Madhok R, et al. TNF Nco-I RFLP is not an independent risk factor in rheumatoid arthritis. Eur J Immunogen 1994; 21: 461–467. [DOI] [PubMed] [Google Scholar]
- 43.Park KS, Mok JW, Kim MY. Analysis of the first intron of TNFβ gene by Nco1 RFLP in Koreans. Jpn J Hum Genet 1997; 42: 357–362. [DOI] [PubMed] [Google Scholar]
- 44.Mock CN, Jurkovich GJ, Dries DJ, Maier RV. Clinical significance of antibiotic endotoxin-releasing properties in trauma patients. Arch Surg 1995; 130: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 45.Cinnat M, Waxman K, Granger GA, et al. Trauma causes sustained elevation of soluble tumor necrosis factor receptors. J Am Coll Surg 1994; 179: 529–537. [PubMed] [Google Scholar]
- 46.Svodoba P, Kantorova I, Ochmann J. Dynamics of interleukin 1, 2, and 6 and tumor necrosis factor alpha in multiple trauma patients. J Trauma 1994; 36: 336–340. [DOI] [PubMed] [Google Scholar]
- 47.Donelly TJ, Meade P, Jagels M, et al. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit Care Med 1994; 22: 768–776. [DOI] [PubMed] [Google Scholar]