Abstract
Objective
There is a need for clearly defined and widely applicable clinical criteria for the selection of patients who may benefit from hepatic resection for metastatic colorectal cancer. Such criteria would also be useful for stratification of patients in clinical trials for this disease.
Methods
Clinical, pathologic, and outcome data for 1001 consecutive patients undergoing liver resection for metastatic colorectal cancer between July 1985 and October 1998 were examined. These resections included 237 trisegmentectomies, 394 lobectomies, and 370 resections encompassing less than a lobe. The surgical mortality rate was 2.8%.
Results
The 5-year survival rate was 37%, and the 10-year survival rate was 22%. Seven factors were found to be significant and independent predictors of poor long-term outcome by multivariate analysis: positive margin (p = 0.004), extrahepatic disease (p = 0.003), node-positive primary (p = 0.02), disease-free interval from primary to metastases <12 months (p = 0.03), number of hepatic tumors >1 (p = 0.0004), largest hepatic tumor >5 cm (p = 0.01), and carcinoembryonic antigen level >200 ng/ml (p = 0.01). When the last five of these criteria were used in a preoperative scoring system, assigning one point for each criterion, the total score was highly predictive of outcome (p < 0.0001). No patient with a score of 5 was a long-term survivor.
Conclusion
Resection of hepatic colorectal metastases may produce long-term survival and cure. Long-term outcome can be predicted from five criteria that are readily available for all patients considered for resection. Patients with up to two criteria can have a favorable outcome. Patients with three, four, or five criteria should be considered for experimental adjuvant trials. Studies of preoperative staging techniques or of adjuvant therapies should consider using such a score for stratification of patients.
Surgical resection is the most effective therapy for metastatic colorectal cancer isolated to the liver. Several studies from major centers have demonstrated that resection of as much as 80% of the liver can be performed with an associated surgical mortality rate uniformly less than 5%. 1–6 Complete resection of detectable liver metastases results in 5-year survival for one third of patients. 1–6 The study by Scheele et al 1 reporting the experience of 434 liver resections for metastatic colorectal cancer from the University of Erlangen over a 32-year period between 1960 and 1992 documented a 10-year survival rate of 23% and a 20-year survival rate of 18%. Hepatic resection, therefore, is a safe and effective therapy for metastatic colorectal cancer and is the only therapy to date to be potentially curative. When compared with the natural history of this disease—untreated patients have a median survival of 6 to 12 months, 7,8 and the median survival with chemotherapy is 12 to 18 months 9,10 —it is clear why hepatic resection has become the standard therapy.
An aggressive approach to resection of hepatic metastases has been undertaken in many major centers. Indeed, as the safety of hepatic resection has improved, patients with multiple, bilobar, and large metastases routinely undergo resection. 1–3,6 As surgeons become more proficient in the technical aspects of resection, patient selection criteria based on biologic determinants of outcome are increasingly important. Criteria are needed to ensure that patients selected for surgery benefit from such invasive therapy. Because the increasing number of resections currently being performed allow for comparative studies of adjuvant therapies, of resection versus ablative therapies, and of preoperative imaging modalities, such criteria may also be of value in stratifying patients for clinical trials. Many prior studies have attempted to examine prognostic factors for tumor recurrence after resection. 2,5,6,11,12 Guided by these studies, we examined a large recent experience of liver resection for metastatic colorectal cancer at a tertiary referral center, with the aim of producing a clinically applicable scoring system for the selection of patients for surgery and for the stratification of patients for clinical studies.
MATERIALS AND METHODS
All patients admitted to the Memorial Sloan-Kettering Cancer Center for liver surgery during the 13-year period from October 1985 to October 1998 were identified in the Department of Surgery Liver Resection Database. One thousand one patients were identified who underwent hepatic resection for metastatic colorectal cancer. Selection criteria for liver resection were as follows:
• Medical fitness for major laparotomy
• No signs on preoperative imaging of disseminated disease
• Tumors anatomically confined within the liver such that adequate liver parenchyma could be preserved.
The routine imaging studies obtained before liver resection included abdominal and pelvic computed tomography scans and chest x-rays. The use of intraoperative ultrasound has been standard for the past 6 years.
Data for these patients were then extracted from the database, hospital and office charts, and interviews. Data examined included demographics (age, gender); site and pathology of primary colorectal lesion; presentation of liver metastases; extent and pathology of liver lesion; surgical details, including blood loss; hospital course, including complications; and outcome. Follow-up was by personal contact with the patient, the patient’s family, or the attending physician. One hundred twenty-three patients did not have determination of preoperative carcinoembryonic antigen (CEA).
Definitions
Nomenclature for the extent of hepatic resection is that defined by Goldsmith and Woodburne. 13 An extended right hepatectomy refers to resection of Couinaud’s 14 segments 4 through 8; an extended left hepatectomy refers to resection of segments 2, 3, 4, 5, and 8. Others have referred to these resections as right trisegmentectomy or left trisegmentectomy, respectively. 15 A right lobectomy is resection of segments 5 through 8; a left lobectomy is resection of segments 2 through 4. The presence of tumor to both the right and the left of the middle hepatic vein was considered bilobar tumor involvement.
Statistics
The chi square test or Fisher’s exact test, where appropriate, was used for univariate comparisons. 16 Proportional hazards regression was used to incorporate all the explanatory variables in the same model. 17 Statistical analysis was performed using the True Epistat Statistical package (Richardson, TX). Differences were considered significant at p = 0.05. All deaths within 30 days of surgery were considered surgical mortality.
RESULTS
Patient Demographics and Follow-Up
One thousand one patients underwent liver resection in the 13-year period of the study. There were 581 men and 420 women. The median age was 61 (range 27 to 87); 199 were age 70 or older. Three hundred ninety-three patients had died at the time of last follow-up, with the median time to death from liver resection 22 months (range 0 to 89 months). Median follow-up of survivors was 32 months.
Primary Lesions
Primary lesions were in the colon in 741 patients and in the rectum in 260. In 581 patients, the primary tumor was associated with regional lymph node metastases. Tumor in the liver was found synchronous with the primary in 287 patients and within 12 months of the primary colorectal cancer in 467.
Liver Tumors
The median number of liver tumors was 2 (range 1 to 20). Five hundred seventeen patients had a solitary liver tumor. Three hundred thirty had two or three tumors, whereas 154 had four or more liver tumors. The median size of liver tumors was 4.2 cm (range 1 to 26 cm). Ninety-four patients had tumors larger than 10 cm; 445 had tumors larger than 5 cm.
Surgical Resections
The distribution of hepatic resections is shown in Table 1. A total of 394 lobectomies and 237 extended hepatectomies were performed. Many patients underwent more than one liver procedure. One hundred ninety-one patients had a simultaneous liver resection contralateral to the primary procedure. Ninety-seven patients underwent insertion of an arterial infusion pump. No patient in this series had ablative therapy performed as a primary liver procedure. The only use of cryotherapy was as technical assistance for resection as part of a cryoassisted resection. 18
Table 1. PROCEDURES PERFORMED
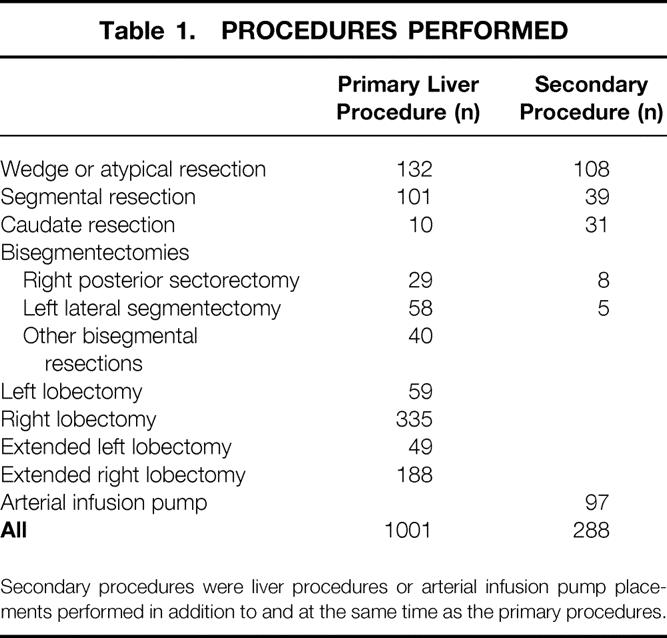
Secondary procedures were liver procedures or arterial infusion pump placements performed in addition to and at the same time as the primary procedures.
This series of liver resections is weighted toward extensive resections: 63% of the resections involved removal of a lobe of liver or more. There has also been a trend toward more resections and more extensive resections in recent years (Fig. 1). During the first 5 years of this study period, the average number of liver resections was 68 per year, with 13% consisting of trisegmentectomies. During the final 5 years of the study period, the average number of liver resections was 94 per year, with 31% consisting of trisegmentectomies.
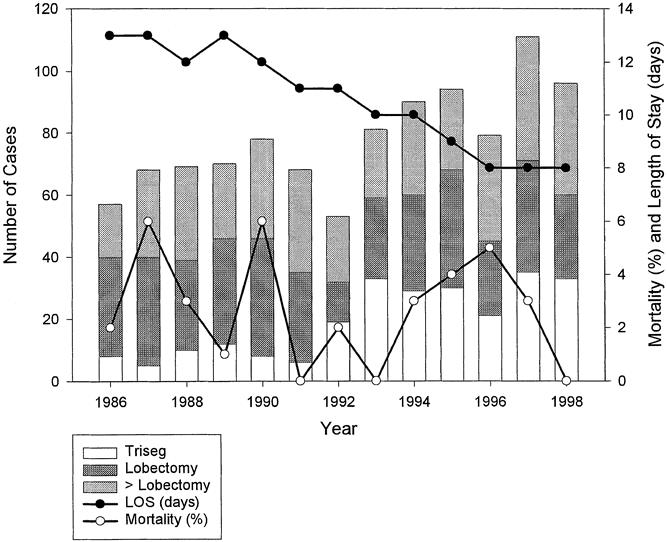
Figure 1. Changes in pattern of practice of liver resection for metastatic colorectal cancer. Bar graph represents the number of resections per year.
Perioperative Results
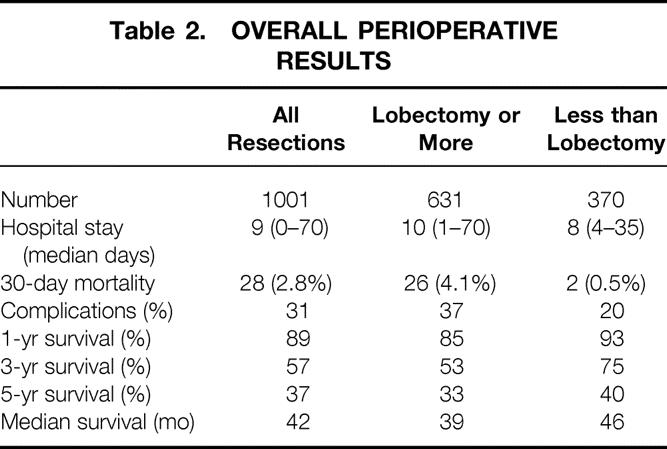
Median hospital stay was 11 days (range 1 to 70), and 28 patients (2.8%) died within 30 days of the liver resection (Table 2). Patients undergoing a lobectomy or more (n = 631) had a significantly longer hospital stay (p = 0.01) and a greater 30-day mortality rate (p = 0.02) than those undergoing resection of less than a lobe. Although the extent of liver resections have increased over the years, the surgical mortality rate has remained stable (see Fig. 1).
Table 2. OVERALL PERIOPERATIVE RESULTS
The length of hospital stay has significantly decreased from a median of 13 days in 1986 to a median of 8 days in 1998 (see Fig. 1). Some of the decrease in hospital stay is the result of an administrative change in clinical practice: since 1994, patients are no longer routinely admitted to the hospital before liver resection, thereby shortening the hospital stay by 2 days. Nevertheless, despite the increasing complexity of resections, the postoperative hospital stay has decreased from a median of 11 days in 1986 to 8 days in 1998.
Long-Term Survival
Kaplan-Meier survival curves (Fig. 2 ) illustrate the long-term survival after liver resection. Median survival calculated from the time of resection of the primary colorectal cancer was 69 months; calculated from the time of the liver resection, it was 42 months. Actuarial survival was 89% at 1 year after liver resection, 57% at 3 years, 37% at 5 years, and 22% at 10 years. Of the 551 patients who underwent resection before Jan. 1, 1994, 136 have been documented to be actual 5-year survivors. There was a difference (p = 0.003) in long-term outcome when comparing resections of a lobe or more (5-year survival of 33%) with resections of less than a lobe (5-year survival of 40%) (Table 3). This difference could be explained partly by the higher perioperative mortality rate of more extensive resections (4.1% vs. 0.5%) (see Table 2). However, even when perioperative deaths were excluded from analysis, there was still a small (p = 0.03) but significant difference in long-term outcome as related to the extent of resection.
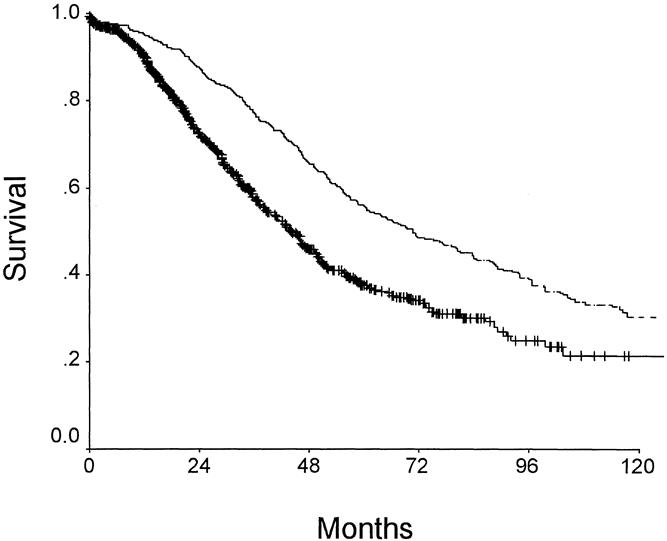
Figure 2. Survival after treatment for metastatic colorectal cancer to the liver. Bottom curve depicts survival as calculated from the time of liver resection. Top curve represents survival calculated from the time of resection of the primary colorectal cancer.
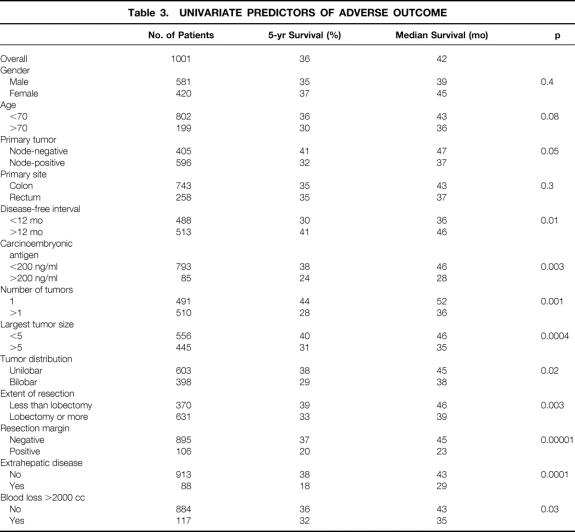
Table 3. UNIVARIATE PREDICTORS OF ADVERSE OUTCOME
Univariate Predictors of Long-Term Outcome
Patient Demographics
Neither age at the time of liver resection nor gender predicted long-term outcome.
Characteristics of the Primary Tumor
Nodal status of the primary cancer predicted outcome. Lymph node involvement by tumor was an adverse prognostic factor for outcome, although the 5-year survival rate for the node-positive group was still 32%. Tumor site or grade (data not shown) was not predictive of outcome.
Presentation and Treatment of Liver Metastases
Presentation of liver metastases with a disease-free interval of <12 months after resection of the colorectal primary or with a CEA level >200 ng/ml was predictive of adverse outcome. The extent of resection and surgical blood loss were predictive of long-term outcome (see Table 3). This difference in outcome related to blood loss could be explained completely by the higher perioperative mortality rate: when perioperative deaths were excluded from analysis, there was no longer a significant (p = 0.3) difference in long-term outcome.
Even when the presentation of liver tumor was synchronous or within 12 months of the resection of the colorectal primary, the 5-year survival rate was 30%. Further, even when the CEA level exceeded 200 ng/ml, the 5-year actuarial survival rate was 24% (see Table 3). Of the 85 patients with a preoperative CEA level >200 ng/ml, to date 12 are alive beyond 5 years.
Pathologic Features of Liver Tumor
Patients with a solitary metastasis from a colorectal primary tumor had a 5-year survival rate of nearly 44% after resection. Multiple liver metastases, size of tumor >5 cm, bilobar disease, and extrahepatic disease were all predictors of adverse outcome. The 5-year survival rates of patients with more than three tumors (23%), largest tumor >5 cm (40%), and bilobar tumor involvement (29%) were nevertheless sufficiently favorable to justify the risks of the procedure, if these were the only positive criteria.
In this study, 88 patients with extrahepatic disease underwent liver resection. The majority (n = 45) had involvement of other organs by direct extension: diaphragm (n = 22), perinephric fascia (n = 3), inferior vena cava (n = 9), ligamentum teres (n = 2), extrahepatic portal vein (n = 1), extrahepatic biliary tree (n = 2), and abdominal wall (n = 6). Liver resection was performed in the setting of discontiguous extrahepatic metastases in 43 cases: documented portal nodal disease (n = 10), lung metastases (n = 21), peritoneal disease (n = 7), and pelvic metastases (n = 5). Except for the cases of simultaneous lung metastases, all cases of extrahepatic disease were discovered during the laparotomy. These patients with extrahepatic disease had a 5-year actuarial survival rate of only 18%.
The major surgical factor that influenced long-term outcome was clearance of tumor at histologic examination. Patients with a positive margin had a 5-year survival of only 20%.
Multivariate Analysis of Outcome
Using surgical clearance margin, presence of extrahepatic disease, number of tumors, preoperative CEA level, size of the largest tumor, nodal status of primary, disease-free interval from the primary to discovery of the liver metastases, and bilateral tumors as variables, analysis by proportional hazards was performed. The first seven of these eight parameters were independent predictors of outcome (Table 4). Of these, positive margin and presence of extrahepatic disease were clearly the most influential, with an increase in the likelihood of death of 1.7 times if either was positive. These characteristics should be considered contraindications to liver resection.
Table 4. MULTIVARIATE PREDICTORS OF RECURRENCE
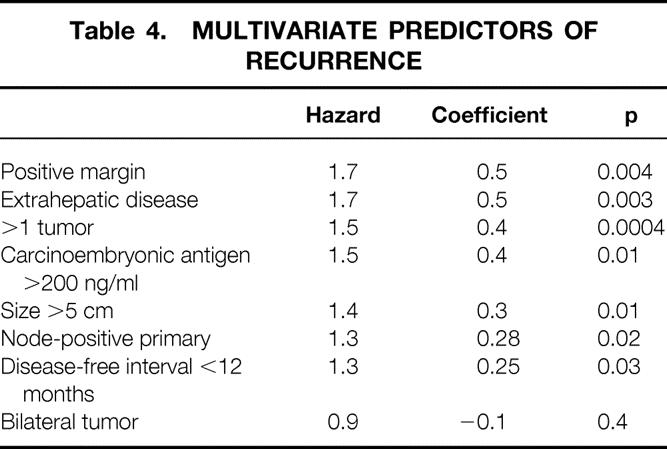
Although predictive of outcome, number of tumors, preoperative CEA level, size of the largest tumor, nodal status of primary, and disease-free interval from the primary to discovery of the liver metastases cannot be considered complete contraindications to resection, because each alone was still associated with a sufficiently favorable outcome to justify a major surgical procedure. These criteria were, therefore, used in the following clinical scoring scale to determine whether a combination of these criteria would dictate the choice of clinical options.
Clinical Risk Score
The five clinical criteria—nodal status of primary, disease-free interval from the primary to discovery of the liver metastases of <12 months, number of tumors >1, preoperative CEA level >200 ng/ml, and size of the largest tumor >5 cm—were chosen as criteria for a clinical risk score (CRS) (Table 5). Each criteria was assigned one point, and the total score was compared with the clinical outcome of each patient after liver resection. The total score was found to be highly predictive of long-term outcome (p < 0.0001) (Fig. 3 ). The 5-year actuarial survival rate for patients with 0 points was 60%, whereas that for patients with 5 points was 14% (see Table 5). In fact, no patient with 5 points has survived 5 years.
Table 5. CLINICAL RISK SCORE FOR TUMOR RECURRENCE
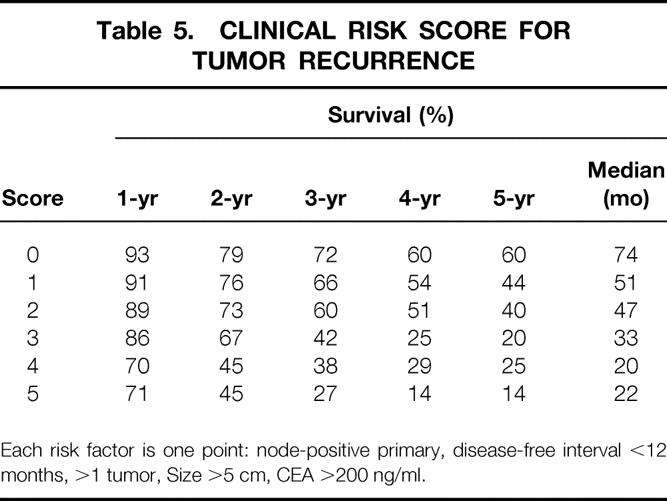
Each risk factor is one point: node-positive primary, disease-free interval <12 months, >1 tumor, Size >5 cm, CEA >200 ng/ml.
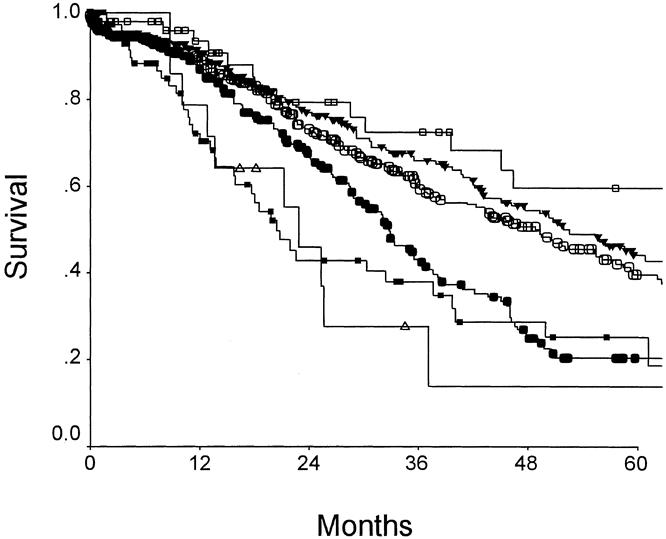
Figure 3. Survival after hepatic resection as related to clinical risk score. Open box: score = 0 (n = 52); filled triangle: score = 1 (n = 262); open circle: score = 2 (n = 350); filled circle: score = 3 (n = 243); filled box: score = 4 (n = 80); open triangle: score = 5 (n = 14). p < 00001.
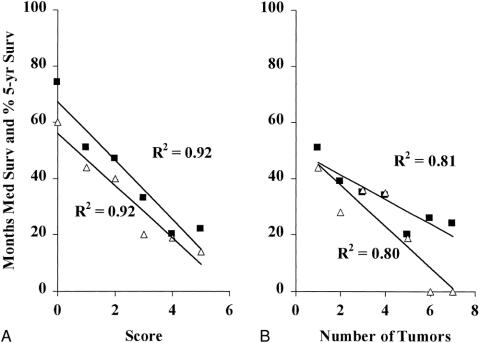
Others have proposed using the total number of tumors as a criterion for stratifying patients for clinical trials. 19,20 This makes some sense, because the number of tumors correlates with long-term outcome (Fig. 4). However, when the CRS is compared with the number of tumors for correlation to outcome, it is clear that the CRS is more closely predictive of prognosis. Figure 5 presents the clinical risk score as plotted against median survival or 5-year survival; this is compared with a plot of the number of tumors versus median survival and 5-year survival. The CRS correlated much more closely with outcome (r2 = 0.92 vs. 0.80 for 5-year survival; r2 = 0.92 vs. 0.81 for median survival). In addition, the slopes of the CRS correlations are much steeper (m = −10.3 and −9.1 vs. m = −7.3 and −4.4). Thus, not only does the CRS predict survival more accurately, it also distributes patients along a much wider range of survival.
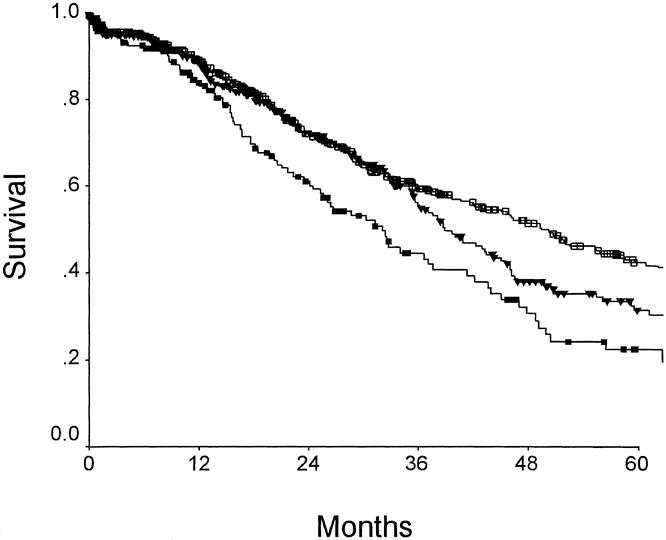
Figure 4. Survival after hepatic resection for colorectal metastases as related to number of liver tumors. p = 0.004. Open squares: number = 1 (n = 517); filled triangles: number = 2 or 3 (n = 330); filled squares: number = ≥4 (n = 154).
Figure 5. Predictors of long-term outcome. (A) Correlation of clinical risk score to median survival (square) (r2 = 0.92, m = −10.3) and to 5-year survival (triangle) (r2 = 0.92, m = −9.1). (B) Correlation of number of tumors to median survival (square) (r2 = 0.81, m = −7.3) and to 5-year survival (triangle) (r2 = 0.80, m = −4.4).
DISCUSSION
The liver is the first major organ reached by venous blood draining from the gastrointestinal tract. Cancer cells traveling by hematogeneous spread, therefore, have a high likelihood of arriving and lodging within the sinusoids of the liver. This would explain the observation that the liver is the most common organ of distant metastases from colorectal cancer. 21 Such liver metastases are classified by the AJCC staging criteria as stage IV disease, 22 and treatment was long accompanied by nihilism; surgical therapy in particular was regarded with great skepticism. 23 However, it was also noted from autopsy studies that the liver often is the sole site of metastases. 24 Such findings encouraged the initial attempts at resection of limited liver metastatic disease. 25–29 During the past two decades, a large body of data 1–6,30–35 has confirmed that long-term survival can result from resection of hepatic colorectal metastases. The current report is a large, single-institution experience in the surgical management of hepatic colorectal metastases and presents data to support the notion that hepatic resection is safe and effective therapy. Despite the increasing complexity of resections, the surgical mortality rate remains <3%. The long-term results mirror those of previous studies 1–6,30–35 and document that liver resection produces 5-year survival in more than one third of patients. The current study is also of sufficient size and duration to document a 10-year survival rate of 22%. This agrees well with the only other published study with sufficiently long follow-up to document 10-year survival 1 and demonstrates that long-term survival and potential cure are anticipated outcomes for a significant proportion of patients who undergo liver resection. Because no other therapy to date provides a cure, hepatic resection is the treatment of choice and serves as the standard of comparison for treatment of metastatic disease isolated to the liver.
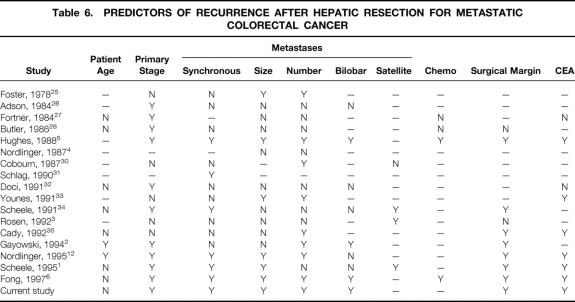
The evolution of liver surgery documented in our tertiary cancer referral center reflects well the growth of hepatic resections for metastatic colorectal cancer on a national scale. Increasing numbers of such procedures are being performed (see Fig. 1), 1,2,12 indicating increasing acceptance of hepatic resection for the treatment of metastatic colorectal cancer. There is also increasing safety associated with liver operations: most series from major centers are reporting a surgical mortality rate <5%, even for the most extensive hepatic resections. 1–6,30–35 These factors have combined to result in an ever-increasing complexity of the surgical approach. Resections involving the removal of greater than two thirds of the liver parenchyma are common, as are resections of more than four hepatic metastases. 1,2,12 The favorable perioperative outcomes for these extensive resections testify to the increasing technical prowess of hepatobiliary surgeons but cannot in and of themselves serve as a justification for these complex procedures. There is an ongoing need for scrutiny of long-term outcome data for the biologic justification of such invasive therapy. The current data confirm a multifactorial determinant of long-term outcome (Table 6 ). 1–6,30–35 Patient characteristics, primary tumor factors, presentation of metastatic disease, and surgical findings combine to influence disease outcome, and distilling these data to formulate a prognostic scoring system to guide daily practice is the goal of the current study.
Table 6. PREDICTORS OF RECURRENCE AFTER HEPATIC RESECTION FOR METASTATIC COLORECTAL CANCER
Other investigators have proposed prognostic scoring systems of varying complexity to improve patient selection for surgical therapy and as criteria for stratification of patients in clinical trials. At the one extreme, clinical selection may be based on a single clinical criterion. In the two major clinical trials of adjuvant chemotherapy for patients undergoing liver resection, the number of tumors was the main clinical criterion used for stratification of patients. 19,20 Gayowski et al 2 included size and distribution of tumors and proposed a staging system for hepatic colorectal metastases based on size of liver metastases >2 cm, number of lesions >1, and bilobar distribution of tumors. Cady and Stone 36 proposed a scoring index based on four risk factors: disease-free interval after treatment of the primary, number of liver tumors, CEA level, and margin of resection. Nordlinger et al 12 attempted to incorporate additional prognostic criteria for outcome and proposed a system based on seven criteria: age older than 60 years, stage of primary, >4 liver metastases, synchronous disease, size of largest lesion >5 cm, CEA level >30 ng/ml, and positive margins. We agree with this attempt to incorporate as many prognostic factors as reasonable to allow a wide stratification of outcome. In our current analysis, seven criteria were found to be independent prognostic factors for outcome. However, the presence of extrahepatic disease by itself must be regarded as a relative contraindication to liver resection, and positive surgical margin is not a useful preoperative patient selection criteria. The remaining five criteria—nodal status of primary, disease-free interval from the primary to discovery of the liver metastases <12 months, number of tumors >1, preoperative CEA level >200 ng/ml, and size of the largest tumor >5 cm—were incorporated into the proposed clinical risk scoring system. These not only represent the factors most commonly found by others to be prognostic for clinical outcome (see Table 6), but are also universally available in the preoperative evaluation of patients. No doubt other, more sophisticated markers, such as tumor ploidy, 35 oncogene expression, or tumor suppressor gene expression, 37 may further stratify patients with regard to outcome. However, such cellular or genetic markers are not likely to be readily available except at a few academic centers; this limits their usefulness and also dooms any system incorporating their use in terms of wide applicability. The choice of criteria for the current CRS is therefore based on the importance of these five factors as prognostic indicators for long-term outcome, and on the wide preoperative availability of the relevant information. The proposed CRS, therefore, has potentially wide applicability in multiinstitutional trials or in the multiinstitutional evaluation of data.
Although the relative risk for cancer-related death varied somewhat for these five criteria, we decided to assign each criterion one point for simplicity and thus enhanced utility. This design is sustained by the current data. The long-term outcome correlates closely with the CRS (see Fig. 3). Further, when compared directly with the most commonly used single factor for patient selection—number of tumors—the utility and advantage of the CRS are particularly evident (see Fig. 4). Not only is there a closer correlation to median and long-term survival, but the wider range of survivals related to CRS and the steeper slope of the correlation between score and outcome allows for more discrete segregation and stratification of patients for therapy. Patients with a CRS of 0, 1, or 2 have a highly favorable outcome, and surgical resection is undoubtedly rational therapy for such patients (see Table 5). Patients with scores of 3 or 4 have a much more guarded prognosis, and resection should be planned in the context of adjuvant therapies. Patients with a score of 5 have very poor outcomes, and resection without additional effective adjuvant therapy or outside of adjuvant trials is highly questionable. Patients with a CRS of 3, 4, or 5 are also ideal candidates for study of any novel imaging modality designed to improve the detection of occult disease. These would be patients most likely to benefit from 18F-FDG whole-body positron emission tomography scanning 38 or from radioimmunoimaging. 39
As a practical illustration of the utility of the proposed CRS, we will examine the clinical setting of the patient who has small metastatic disease within the liver. The therapeutic dilemma faced by the hepatobiliary surgeon in this setting is the timing of hepatic resection. When an early operation is undertaken, there is the risk of missing tumors that are even smaller than those triggering intervention, and there is the worry that additional disease will manifest soon after hepatic resection and prove the operation to have been unjustified or inadequate. The alternative approach of delaying surgery to allow any hidden disease to manifest and to direct surgical planning runs the risk of allowing new metastases from established metastatic sites. 40 The application of the CRS to patients in the current study with largest tumors <3 cm is presented in Figure 6. Patients with scores of 0, 1, or 2 have a very favorable outcome (median survival 55 months, 5-year survival rate 47%). These patients are clearly good candidates for hepatic resection, and immediate surgery should be considered. In patients with 3 or 4 points, however, the outlook is more guarded. In these patients, delayed surgery to allow better assessment for additional disease, surgery in the context of aggressive adjuvant therapy, or neoadjuvant therapy to document effective adjuvant therapy before resection are all rational courses of action. This scoring system may allow selection of patients for trials comparing these approaches.
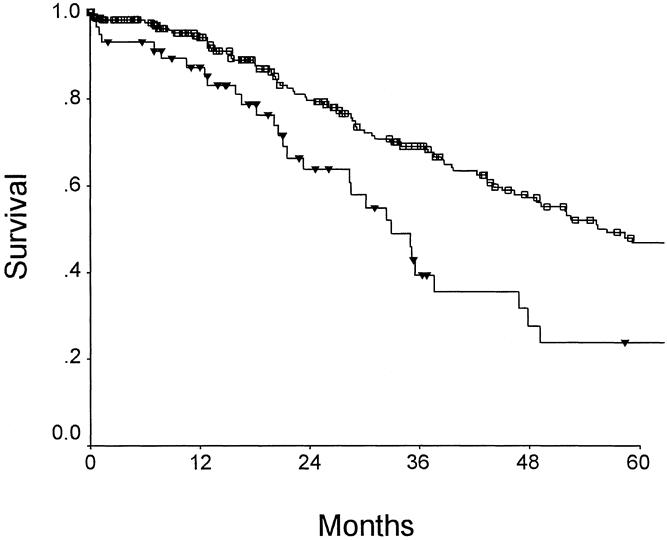
Figure 6. Prediction of long-term outcome for small (<3 cm) metastatic deposits (n = 293). Correlation of long-term outcome with clinical risk score. For a score of 0 to 2 (n = 236) (open box), the median survival was 56 months and the 5-year survival rate was 47%. For a score of 3 or 4 (n = 57) (filled triangles), the median survival was 32 months and the 5-year survival rate was 24%.
Many ablative methods have been proposed for the treatment of liver tumors. Of these, cryoablation 41 and radiofrequency ablation 42 show the greatest promise as treatment for metastatic colorectal cancer. Although unlikely to be curative for large lesions, it is possible that these ablative techniques may produce complete destruction of smaller (<3 cm) tumors. What role these ablative techniques will play in the clinical care of the patient with metastatic colorectal cancer, and more specifically how these modalities will compare with surgical resection in the treatment of patients with small tumors are questions of considerable importance. Data from the current study demonstrate that patients with tumors ≤3 cm have a very favorable outcome after resection. The median survival exceeds 50 months, and 5-year survival can be expected in 44% of patients. Results of any study of ablative treatments of small liver metastases must be compared against such favorable results, which at present remain the gold standard. The wide range of outcomes in patients with small tumors as related to the CRS proposed here demonstrate that a stratification score such as this must be used in comparative studies of ablative therapies.
The data presented are further documentation that resection of hepatic colorectal metastases represents safe and effective therapy. An ever-more-aggressive approach is being undertaken based on the improving safety of hepatic resection and as a result of the inability of other therapies to produce long-term survival. It is also clear that systemic chemotherapy may have the greatest impact in the adjuvant setting where residual disease is minimal and microscopic. In a recent study of combined regional and systemic adjuvant chemotherapy, patients with an unfavorable CRS benefited most from such aggressive adjuvant chemotherapy. 20 A CRS such as that proposed here may well find utility not so much in eliminating candidates for resection, but in selecting adjuvant therapy.
Many promising, novel cancer therapies in the preclinical stages of development are likely to reach fruition and clinical trial in the near future. These include oncolytic viral therapy, 43 radionucleid therapeutic modalities, 44 and immune strategies. 45 As these and other therapies are evaluated clinically, rational comparisons among the large number of treatment approaches will be possible only if well-defined and proven criteria for patient stratification are available. The current study presents a CRS that may fill this role. It incorporates in a straightforward and easily applicable scale widely accepted prognostic factors that are available for all patients at all centers. The current study attempts to validate this system using the largest single-institution experience in this disease yet reported. We hope that this CRS will not only contribute to the routine care of patients with metastatic colorectal cancer, but also will assist in the planning and execution of clinical trials, particularly in the multicenter setting.
Discussion
Dr. Henry A. Pitt (Milwaukee, Wisconsin): I would like to thank Drs. Blumgart and Fong and their colleagues for the opportunity to review their manuscript and to discuss this excellent paper.
As you just heard, their experience is extensive. Their short- and long-term results are excellent, and their analysis is very complete. Their data are very important because their analysis includes a large number of patients from a single institution managed by a small number of surgeons. With this power, they confirmed that all of the factors suggested in other analyses are predictors of outcome in their patients. With this information, they have proposed a clinical risk score that should be useful in the design of future studies. Analysis of this and other studies suggests that extrahepatic disease, positive margins, and the number of liver lesions are the best predictors of outcome.
Among these three factors, the one that can be assessed most accurately preoperatively is the number of liver lesions. Thus, in this and most other similar analyses in the literature, the vast majority of patients who have been explored for surgical therapy have had four or fewer lesions on preoperative studies. However, with the introduction of new techniques such as cryotherapy and radiofrequency ablation, the value of the number of lesions as a predictor of outcome must be questioned.
To address this issue, a more aggressive surgical approach to colorectal liver metastases was undertaken by Dr. Edward Quebbeman at the Medical College of Wisconsin in 1993. With the addition of cryoablation to the surgical armamentarium, 28% of 106 patients undergoing a potentially curative procedure had more than four metastases.
This slide demonstrates the survival by the number of tumors treated. As you can see, the median and 3-year survival was actually slightly greater in the patients with five to eight lesions compared to those with one to four, although this difference was not statistically significant. With this approach, the presence of extrahepatic disease and the ability to perform a curative procedure were predictors of outcome in a multivariate analysis, but the number of lesions was not predictive of outcome.
On the basis of these data, which were presented at the Central Surgical Association last month, we recommended that patients with up to eight liver lesions should be explored, with the goal to resect and/or ablate all of the liver lesions. Therefore, my two questions are whether you have used ablative procedures in any of your patients and whether you believe that the number of metastases should be used as a criterion for exploration now that we have the availability of ablative procedures.
Presenter Dr. Leslie H. Blumgart (New York, New York): Thank you, Dr. Pitt. Of course we have used ablative procedures. But the patients are not included in this series of resection. The purpose of the study was to examine exactly what you are suggesting, that is, to assess these factors in such a manner that we could define a reasonable scoring system to allow stratification of patients for study with such modalities as cryosurgery and other ablative procedures.
Dr. Robert M. Beazley (Boston, Massachusetts): To my knowledge, the first reported resection for colorectal metastasis to the liver occurred in 1941 by Richard Catell at the Lahey Clinic, and since that time, a considerable body of literature has developed in this area. Dr. Fong’s paper is a notable addition in that it is the largest single-institution experience, one accumulated during a relatively short period of time by a small number of surgeons. Their conclusions are straightforward and clinically quite useful. It is especially helpful from a clinical standpoint that their proposed clinical risk score can be applied in making preoperative decisions and thus permit appropriate focusing of our resources.
For example, patients presenting with liver metastasis 4 to 6 months after a primary resection currently present a bit of a dilemma. I have always suggested some chemotherapy and a watch-and-wait attitude before surgery. The clinical risk score provides us with selection guidance, suggesting that a low-score patient is an immediate resection candidate, while those patients who might be three, four, or five are not, and perhaps watch-and-wait is the best course for them.
I have a few questions for the authors. First, in that you have done a large number of extended liver resections with an extremely acceptable mortality, is there any special testing that you do preoperatively to predict liver reserve in those patients you anticipate extended resection? Should age be a consideration in major extended resection, particularly in the elderly where you may be anticipating a trisegmentectomy? Would you suggest that we not resect or delay resection in those who have scores in the 4 and 5 range? In your study, this constituted a small group of patients, and I would like to know how you feel about that. Lastly, comment on your criteria for reresection of patients, the patient who is successfully resected and comes back a year or two later with another metastasis. How do you decide about that?
I think the authors are to be congratulated on their skill and perseverance in performing these demanding procedures. We are in debt to you for providing us with your data, which I predict will prove to be clinically quite useful.
Dr. Blumgart: Thank you very much, Dr. Beazley. I will take the points you mentioned one at a time and then I would like to take up one other comment you made.
First, in relation to liver reserve: We have not taken any particular refined measurements of liver functional capacity (these patients were not cirrhotic) but relied on the fact that we can see a good functional piece of liver on, for instance, a CT scan with good vascularization and good outflow. If we can resect these patients and maintain a good portal inflow and a good hepatic venous outflow, we have had no particular concerns even with very extensive resection.
In relation to age, we presented at this Society some years ago a paper relating age to mortality and outcome in pancreaticoduodenectomy and in liver resection, and showed no difference in outcome between patients older than or younger than 70 years.
The question of a delay in the timing of resection for patients with score of 4 or 5: I wonder if I could answer that by just relating it to the other comment you made in relation to patients who have a recurrence within the first year or have a synchronous metastasis. These are small metastases less than 3 cm in size, maybe one or two metastases present. You are quite right, these patients present a dilemma for us as well as for you.
It is of some interest that we took the proposed score and we looked at patients with tumors 3 cm or less in size and applied the score to the outcome. Those patients with a score of 0 to 2 had a 5-year survival of 47%, whereas patients with a score of 3 to 5 had only a survival of 24%.
This is a very significant difference and does suggest that the proposed scoring system may be of some value in selecting patients with such small lesions either for immediate resection or for study, for instance, in neoadjuvant regimes. I think that your question is relevant and important and it is one which the use of this scoring system may help answer.
You asked about the reresection. Yes, we have published in that area as well. But so have many others. Reresection has been shown to be safe, with no greater operative mortality than primary resection, and also to yield good results. I think you were asking how did we select such patients. I think the answer is, with some difficulty, but always looking to the feasibility of resection based on the anatomical distribution of the recurrence.
Dr. John Terblanche (Cape Town, South Africa): It is a great privilege to be able to congratulate the authors on their seminal paper, which I think will alter our practice. Two questions for Dr. Blumgart.
The first is: In the abstract you have proposed six criteria, while in the presentation and the paper you use five criteria. I understand the reason for the change. Is there a subset of the patients who have either a score of 6 or of 5 where it is not worthwhile treating them? It would be important to identify those patients who we are currently treating, but who should be considered to be inoperable. They could then be considered for alcohol injection or other palliative treatment.
The second question is: In several published papers, other criteria have been presented as important. Why the difference? Is it because you have been able to assess a very large number of patients?
Dr. Blumgart: Thank you, Dr. Terblanche. Yes, the abstract is different from the presentation. This is because when we looked at the bilaterality of tumors, it was not, in fact, of significance. It is impossible to separate, as Dr. Fong said in the presentation, number and size of tumors from bilaterality, so we dropped bilaterality and concentrated on the five score points mentioned.
If I have the second question right, I think you are asking how this score differs from that of others. It doesn’t really. It simply takes a limited number of factors which are easily available preoperatively. It omits things which we cannot know preoperatively and allows anybody in any institution to use the system and to participate in the staging of patients in, for example, multicenter studies.
It is important that I emphasize that a score of 5 would not cause us to refuse resection to any one patient. The results are often good in spite of adverse factors. However, the score does give a good prognostic index. Its major value, however, will be in allowing stratification of patients in prospective studies of adjuvant or alternative treatment modalities.
Dr. Shunzaburo Iwatsuki (Pittsburgh, Pennsylvania): We also have a fair-sized database of hepatic metastasis in Pittsburgh and recently completed our study. Our prognostic scoring system will be published in the August issue of the Journal of the American College of Surgeons.
Our study revealed 12 risk factors to be significant by univariate analysis, and only four factors to be independently significant by a stepwise Cox regression model with likelihood ratio test, as shown in the slide. The two factors, CEA level and positive node at colon surgery, were not independent factors, and therefore both were eliminated from the scoring system.
High CEA level is known to have a close relationship with tumor load, such as size and number of tumor. Positive node at colon surgery, or stage of primary tumor has a close relationship to tumor-free interval (time to hepatic recurrence).
The inclusion of those two confounding factors in scoring system surely makes the grading less accurate by counting the same kind of risk factors repeatedly.
My questions are: One, did you use a stepwise Cox regression model or nonstepwise Cox regression model? Two, did you statistically confirm that all of your six risk factors in the grading system were independent to each other? Finally, I have a great concern in your statistical methods.
Dr. Blumgart: Thank you very much, Dr. Iwatsuki, for telling me what we should have done! At least I think I understand what you are telling me I should have done.
In point of fact, we did do a multivariate analysis and the data have been presented. I am not sure why it differs from the data that you say you are about to publish. I know that your publication—I think the author was Dr. Gayowski—in 1994 in fact mentioned many of the same factors we have discussed. Maybe the difference in opinion relates to the number of patients in the studies. It may be that with the large number of patients we have, we are seeing a different result to those which you would wish us to report.
Dr. Harold J. Wanebo (Providence, Rhode Island): Dr. Blumgart, I would like to congratulate you, Dr. Fong, and your coauthors for an outstanding series and for coming up with a clinical scoring system that has some statistical validity.
I have two questions. The first is regarding intraoperative selection factors that would preclude resection. What factors are you currently utilizing for patients on the operating table to exclude a resection and what do you subsequently do with that patient?
The second question refers back again to the problem of liver reserve. On a practical basis, what is the bottom line regarding the amount of liver that can be left behind in a patient without cirrhosis within the middle-aged group? Is 20% acceptable? Give us a bottom line.
Dr. Blumgart: Thank you, Dr. Wanebo. I will start with the last question first. As you know, I visited with you not long ago and we debated this point. I think it is all right if you want to do refined tests of hepatic functional reserve. We just don’t find them useful. If you look at our results, even with very extensive resection, I think our attitude is substantiated. It is quite different in the cirrhotic liver and you can make an argument for your approach there. However, I don’t really feel that the time, effort, and money spent doing these tests in the patient with a noncirrhotic liver justifies the effort. The results speak for themselves. We have a 2.8% mortality in 1000 cases. I don’t think more than three or four of these people died of liver failure. So I don’t think that refined liver functional assessment is very important.
I think the other question you raise is more interesting: What do we do at operation? Well, we resected 79% of all tumors in patients that we took to the operating room. In fact, in the recent year or so, we have been doing laparoscopy prior to laparotomy in these patients. Doing that, you pick up some patients who would not go on to open operation. Using this approach, the resectability rate is now in excess of 90%. This means that we have relatively few patients in which we have the problem you raise.
Dr. Wanebo: Finally, I guess the real question is a philosophical one regarding the management of nonresectable patients. Many of us believe in the use of hepatic artery infusion in patients who are not candidates for resection. This, of course, means that one has to plan for placing a hepatic artery catheter and pump up front. The question is whether the patient would benefit from placement of a hepatic artery catheter so the operation is not wasted. We are aware of the controversy regarding its contribution to improved survival, but believe there is confirmed benefit of controlling disease in the liver.
Dr. Blumgart: It really is a question of how you design clinical trials in relation to the use of infusaid therapy or other approaches. You say you “believe in it.” Yes, we probably are beginning to believe in it, too.
As you know, there has been a prospective randomized trial of infusaid pump chemotherapy running at Memorial Sloan-Kettering Cancer Center. That trial has recently closed. Initial results suggest that infusaid pump chemotherapy may improve survival, particularly in patients with more than two tumors. I think for those few patients that you cannot resect, it is appropriate to have some form of alternative therapy available. This is, in fact, what you are suggesting. However, I would again emphasize that a scoring system such as we propose is important in stratifying patients for prospective studies of adjuvant or alternative therapy.
Dr. Henri Bismuth (Villejuif, France): This is an important paper, and I congratulate Dr. Fong and Leslie Blumgart for it. My question concerns the group of patients who have more than three predictors of mortality. I am upset by the possibility that some physicians, or even surgeons, might take this score as an argument against liver resection. Indeed, what this paper shows is that, in this group of patients, the 5-year survival is around 20%, less than the 54% in Group 1, but certainly better than the 0% which would be expected in similar patients without resection. This is a very important point. My policy is to try to resect when it is technically possible in terms of anatomy and function of the remaining liver, and to use some kind of neoadjuvant treatment, especially chemotherapy. We must try to give the patient the chance, even small, of a cure. I am sure, Leslie, that you will agree with this.
Dr. Blumgart: Thank you very much, Professor Bismuth. As always, you make a very important point, and it is a pleasure to be able to agree with you. I don’t know what to do with these patients either. But you are absolutely right; the current results of chemotherapy leave much to be desired.
I think your approach, which you have published and championed recently, of trying to convert some patients who appear to be irresectable to resectability is an aggressive chemotherapeutic regime which you have shown can work. I think it is an appropriate approach which others should attempt to duplicate. We have recently operated on a few patients in whom we have had multiple small tumors in the right lobe and a very small hemiliver on the other side (i.e., a small left lobe). This has been of some concern coming back to the point that Dr. Wanebo raised in relation to liver function. We have used unilateral portal vein embolization to induce atrophy on the affected side and hypertrophy in the proposed remnant. This approach, first championed by Makuuchi in Japan, can be of value in this situation.
So I am sure you are right. As we get better at hepatic resection, we could use a scoring system like this to pick the cases more likely to have a poor outcome.
Footnotes
Correspondence: Yuman Fong, MD, Hepatobiliary Surgery Service, Dept. of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Supported in part by NIH grants RO1CA76416, RO1CA72632, and RO1CA61524 (YF).
Accepted for publication April 1999.
References
- 1.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 2.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathological risk factors. Surgery 1994; 116: 703–711. [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992; 216: 492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlinger B, Parc R, Delva E, Quilichini M, Hannoun L, Huguet C. Hepatic resection for colorectal liver metastases. Ann Surg 1987; 205: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes KS, Simons R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery 1988; 103: 278–288. [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997; 15: 938–946. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe BM, Donegan WL, Watson F, et al. Factors influencing survival in patients with untreated hepatic metastases. Surg Gynecol Obstet 1968; 127: 1–11. [PubMed] [Google Scholar]
- 8.Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver: I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 1969; 23: 198–202. [DOI] [PubMed] [Google Scholar]
- 9.Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional vs. systemic continuous 5-FU chemotherapy in the treatment of colorectal metastases. Ann Surg 1987; 206: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham D, Pyrhonen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer [see comments]. Lancet 1998; 352: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 11.Geller AI. A system, using neural cell lines, to characterize HSV-1 vectors containing genes which affect neuronal physiology, or neuronal promoters. J Neurosci Meth 1991; 36: 91–103. [DOI] [PubMed] [Google Scholar]
- 12.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie Cancer 1996; 77: 1254–1262. [PubMed] [Google Scholar]
- 13.Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet 1957; 105: 310–318. [PubMed] [Google Scholar]
- 14.Couinaud C. Bases anatomiques des hepatectomies gauche et droite reglées. J Chirurgie 1954; 70: 933–966. [PubMed] [Google Scholar]
- 15.Starzl TE, Bell RH, Beart RW, Putnam CW. Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet 1975; 141: 429–438. [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews DE, Farewell VT. Understanding medical statistics. New York: Karger; 1990.
- 17.Cox DR. Regression models and life tables (with discussion). J R Stat Soc B 1972; 187–220.
- 18.Polk W, Fong Y, Karpeh M, Blumgart LH. A technique for the use of cryosurgery to assist hepatic resection. J Am Coll Surg 1995; 180: 171–176. [PubMed] [Google Scholar]
- 19.Lorenz M, Muller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. Ann Surg 1998; 228: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemeny N, Huang Y, Cohen AM, et al. Randomized study of hepatic arterial infusion and systemic chemotherapy versus systemic chemotherapy alone as adjuvant treatment following resection of hepatic metastases from colorectal cancer [abstract]. Proceedings of the American Society of Clinical Oncology, Atlanta, GA, May 1999.
- 21.Kemeny N, Fong Y. Treatment of liver metastases. In: Holland JF, Frei E, Bast RC, Kufe DW, Morton DL, Weichselbaum RR, eds. Cancer medicine. Baltimore: Williams & Wilkins; 1997: 1939–1954.
- 22.Fleming ID, Cooper JS, Henson DE, et al. Manual for staging of cancer. 5th ed. Philadelphia: Lippincott-Raven; 1997.
- 23.Silen W. Hepatic resection for metastases from colorectal carcinoma is of dubious value. Arch Surg 1989; 124: 1021–1024. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert HA, Kagan AR. Metastases: incidence, detection, and evaluation without histologic confirmation. In: Weiss L, ed. Fundamental aspects of metastasis. Amsterdam: North Holland Publishing Co.; 1976: 385–405.
- 25.Foster JH. Survival after liver resection for secondary tumors. Am J Surg 1978; 135: 389–394. [DOI] [PubMed] [Google Scholar]
- 26.Adson MA, Van Heerden JA, Adson MH, Wagner JS, Ilstrup DM. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119: 647–651. [DOI] [PubMed] [Google Scholar]
- 27.Fortner JG, Silva JS, Golbey RB, Cox EB, Maclean BJ. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg 1984; 199: 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler J, Attiyeh FF, Daly JM. Hepatic resection for metastases of the colon and rectum. Surg Gynecol Obstet 1986; 162: 109–113. [PubMed] [Google Scholar]
- 29.Pagana TJ. A new technique for hepatic infusional chemotherapy. Sem Surg Oncol 1986; 2: 99–102. [DOI] [PubMed] [Google Scholar]
- 30.Cobourn CS, Makowka L, Langer B, Taylor B, Falk R. Examination of patient selection and outcome for hepatic resection for metastatic disease. Surg Gynecol Obstet 1987; 165: 239–246. [PubMed] [Google Scholar]
- 31.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer: competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol 1990; 16: 360–365. [PubMed] [Google Scholar]
- 32.Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozetti F. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg 1991; 78: 797–801. [DOI] [PubMed] [Google Scholar]
- 33.Younes RN, Rogatko A, Brennan MF. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg 1991; 214: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 1991; 110: 13–29. [PubMed] [Google Scholar]
- 35.Cady B, Stone MD, McDermott WV Jr, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg 1992; 127: 561–569. [DOI] [PubMed] [Google Scholar]
- 36.Cady B, Stone MD. The role of surgical resection of liver metastases in colorectal carcinoma. Semin Oncol 1991; 18: 399–406. [PubMed] [Google Scholar]
- 37.Belluco C, Guillem JG, Kemeny N, et al. p53 nuclear protein overexpression in colorectal cancer: a dominant predictor of survival in patients with advanced hepatic metastases. J Clin Oncol 1996; 14: 2696–2701. [DOI] [PubMed] [Google Scholar]
- 38.Lai DT, Fulham M, Stephen MS, et al. The role of whole-body positron emission tomography with [18F]fluorodeoxyglucose in identifying operable colorectal cancer metastases to the liver. Arch Surg 1996; 131: 703–707. [DOI] [PubMed] [Google Scholar]
- 39.Rivoire M, Yoshida K, Divgi C, Welt S, Cohen A, Sigurdson ER. Radioimmunodetection of hepatic metastases with gamma-detecting probe. Cancer Res 1990; 50: 877s–879s. [PubMed] [Google Scholar]
- 40.August DA, Sugarbaker PH, Schneider PD. Lymphatic dissemination of hepatic metastases: implications for the follow-up and treatment of patients with colorectal cancer. Cancer 1985; 55: 1490–1494. [DOI] [PubMed] [Google Scholar]
- 41.Morris DL, Ross WB. Australian experience of cryoablation of liver tumors. Surg Oncol Clin North Am 1996; 5: 391–397. [PubMed] [Google Scholar]
- 42.McGahan JP, Browning JP, Brock JM, et al. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990; 25: 267–270. [DOI] [PubMed] [Google Scholar]
- 43.Kooby DA, Carew JF, Halterman MW, et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multimutated herpes simplex virus type-1 (G207). FASEB J 1999 (in press). [DOI] [PubMed]
- 44.Macapinlac HA, Kemeny N, Daghighian F, et al. Pilot clinical trial of 5-[125I]-Iodo-2′-deoxyuridine in the treatment of colorectal cancer metastatic to the liver. J Nucl Med 1996; 37: 25S–32S. [PubMed] [Google Scholar]
- 45.Karpoff HM, D’Angelica M, Blair S, Brownlee MD, Federoff H, Fong Y. Prevention of hepatic tumor metastases in rats with herpes viral vaccines and gamma-interferon. J Clin Invest 1997; 99: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]