Abstract
Objective
To review the 10-year clinical experience of a single institution’s adult lung transplant program.
Methods
Since July 1988, 450 lung transplants have been performed in 443 patients. Recipient diagnoses included emphysema in 229 patients, cystic fibrosis in 70 patients, pulmonary fibrosis in 48 patients, pulmonary hypertension in 49 patients, and miscellaneous end-stage lung diseases in 47 patients. Single-lung transplant was performed in 157 cases, bilateral sequential lung transplant in 283 cases, en bloc double-lung transplant in 8 cases, and heart-lung transplant in 2 cases. Graft lungs were obtained from local donors in 24% of cases and from distant donors in 76% of cases. Ideal donors were used in 74% of cases; in 26%, the donor was classified as marginal based on objective criteria.
Results
Four hundred six (91.6%) lung transplant recipients survived to hospital discharge. There were 37 hospital deaths from cardiac events (n = 8), primary graft failure (n = 8), sepsis (n = 6), anastomotic dehiscence (n = 6), and other causes (n = 9). A diagnosis of chronic rejection (bronchiolitis obliterans syndrome [BOS]) was made in 191 patients (42.5%). BOS has not been improved by any specific therapy. Rates of freedom from BOS at 1, 3, and 5 years after the transplant are 82%, 42%, and 25%. One-, 3-, and 5-year actuarial survival rate for the entire group are 83%, 70%, and 54%. There is no statistical difference in survival according to diagnosis or type of lung transplant. Recipient waiting time was 116 days in the first 90 patients and 634 days in the most recent 90 patients.
Conclusions
Lung transplantation offers patients with end-stage lung disease acceptable prospects for 5-year survival. Chronic rejection and long waiting lists for donor lungs continue to be major problems facing lung transplant programs. The use of marginal and distant donors is a successful strategy in improving donor availability.
Clinical lung transplantation has undergone significant evolution during the past 15 years. After dozens of attempts by multiple surgeons resulting in only a single patient 1 surviving to hospital discharge, Reitz et al 2 reported the first successful pulmonary transplant in 1982 with a series of combined heart-lung transplantation procedures. In 1983, the University of Toronto group performed successful isolated single-lung transplantation and reported a small series of single-lung transplants conducted for pulmonary fibrosis. 3 Three years later, the same group described the first successful series of en bloc double-lung transplants, 4 and in 1989 the Washington University group described the technique of bilateral sequential single-lung transplant, a procedure that has required only minor modifications during the past decade. 5
During the era of these early reports, attention was focused on technical details of the transplant procedure to avoid early postoperative complications such as allograft dysfunction 6–8 and ischemic donor airway complications. 9–12 Progress was made quickly because the learning curve was steep for many of the problems faced. Griffith et al 13 reported a 10-year experience with 232 lung recipients spanning the years 1982 to 1992 and noted an improvement in the 1-year survival rate from 53% to 70% when the experienced was stratified into groups of early (n = 125) and recent patients (n = 107). Immunosuppression was imprecise; so too were the diagnosis and treatment of acute allograft rejection. This situation changed with evolution of technique, better graft preservation, and adoption of transbronchial biopsy as the gold standard for the diagnosis of acute allograft rejection. 14 In addition, opportunistic infection, especially cytomegalovirus, has been much better controlled with the routine use of acyclovir, ganciclovir, and newer antifungal agents.
As a result, in experienced lung transplant centers, early morbidity and mortality rates have been decreased. The current challenges facing clinical lung transplant programs are no longer technical; rather, they involve the critical shortages of suitable donor lungs and the relentless progression of chronic allograft rejection. 15–17
The adult lung transplant program at Washington University Barnes-Jewish Hospital began operation during the latter stages of the technical development phase of lung transplantation. The purpose of this report is to review the experience of an adult lung transplant program in a single institution during this critical period of lung transplant evolution.
MATERIALS AND METHODS
All adult lung transplants performed in our center between July 1998 and November 1998 were included in this review. A minimum of 3 months of follow-up is available in every patient. Retrospective analysis of transplant records and a computerized database was conducted.
Recipient Selection
Our program has used standard criteria for patient selection and listing for transplant as outlined by the American Thoracic Society and the International Society for Heart and Lung Transplantation. 18 The details of our waiting list regimen have been published elsewhere. 19,20
Donor Selection
The general selection criteria have been previously published. The majority of donors were characterized as ideal—in other words, clear chest x-ray, good gas exchange, minimal smoking history, and normal bronchoscopic examination. Increasingly, we have relied on marginal donors, those whose evaluation reveals surmountable defects in one or more of the standard criteria listed above. The exact definition of marginal donors, and our positive experience with the use of such donor lungs, have been outlined in a report from our group. 21 Donors located within our own region were considered local; donors from locations outside the catchment area of our organ-procurement organization were deemed distant.
Donor Harvest Technique
The technique of lung harvest used by our team has been previously reported 22 and has not changed in recent years. Donors are pretreated with systemic heparin (4 mg/kg) and a bolus dose of PGE1 (500 micrograms) administered directly into the pulmonary artery immediately before donor inflow occlusion. Antegrade pulmonary artery flush of 4 to 6 L modified Euro-Collins (5.6% glucose) (4.7 mmol/L MgSO4). In the past 2 years, we have added nitroprusside 10 mg/L to the flush solution. After cardiac extraction, the trachea is stapled with the lungs at functional residual capacity. We had previously demonstrated that hyperinflation of donor lungs during storage increased pulmonary capillary endothelial injury. 23 Lungs are harvested en bloc and separated into separate lung allografts after extraction.
Recipient Procedure
Monitoring Lines
All patients receive a pulmonary artery catheter and quadruple-lumen venous line in the right internal jugular vein. Radial artery and right femoral artery catheters are placed in each patient. A Foley catheter and transesophageal echocardiography probe are also placed.
Incision
The majority of single-lung transplant procedures were conducted through a posterolateral thoracotomy. However, recently, an anterolateral fourth intercostal incision or a median sternotomy have been used in selected cases. For bilateral procedures, the standard exposure is through a bilateral anterolateral fourth interspace thoracotomy without sternal division. For single-lung transplant and the first lung extraction of a bilateral sequential procedure, the lung with least function, as judged by quantitative ventilation-perfusion scintigraphy, is removed first.
Technical Details
The explantation and implantation are performed as previously described. 5,22,24 During implantation, the lung is kept cold by topical application of saline with slush (saline ice slush). The bronchial closure technique has remained standard for the past 5 years. 25 Specifically, a running suture technique is used to close the posterior membranous wall, and the anterior cartilaginous bronchus is opposed with widely spaced interrupted figure-of-eight sutures. Typically, four or five figure-of-eight sutures are required to oppose the anterior airway. Donor and recipient peribronchial soft tissue is used to cover the anastomosis. The entire bronchial anastomosis is performed using 4-0 PDS material. The pulmonary artery anastomosis is constructed with 5-0 polypropylene. The donor and recipient left atrial anastomoses are constructed with 4-0 polypropylene, using an everting technique as much as possible.
When required, cardiopulmonary bypass is accomplished using intrathoracic cannulation: the right atrium and ascending aorta for right single and bilateral procedures, and the pulmonary artery and distal aorta for left-sided transplants.
General Postoperative Management
Ventilation
In general, patients are ventilated with standard ventilatory techniques. Extubation is performed in accordance with standard requirements of satisfactory gas exchange and mechanics. Most patients are extubated within 24 to 48 hours of transplantation after standard intermittent mandatory ventilation or pressure support weaning.
In patients with pulmonary vascular disease, we use a prolonged period—48 to 72 hours—of elective ventilation. Patients are kept heavily sedated and often paralyzed for that period of time. Tidal volumes are standard, but positive end-expiratory pressure of 7.5 to 10 cm H2O is applied.
In some patients, early graft dysfunction, rejection, or infection necessitates a prolonged period of postoperative mechanical ventilation. We have no hesitation about performing tracheostomy in these patients to facilitate mobilization of the patient, improve oral nutrition, and generally effect a more positive attitude in the ventilator-weaning patient.
Infection Prophylaxis
All patients are given routine antibacterial prophylaxis, most recently with cefepime and vancomycin. Administration of these agents is continued for several days, with adjustments to these empiric antibiotics based on the results of donor and recipient bronchial cultures. In the cystic fibrosis population, aerosolized colistin or tobramycin is also used. In addition to the broad-spectrum antibiotics, patients with cystic fibrosis also require specific antipseudomonal coverage, as dictated by the sensitivities of their preoperative sputum cultures.
Herpes simplex was formerly a frequent cause of postoperative complications because of oral ulcerations as well as occasional pneumonitis. Routine use of acyclovir prophylaxis, however, has eliminated herpes infection as a frequent postoperative complication. Cytomegalovirus remains a significant problem in pulmonary transplant recipients. 26 Most programs have adopted the strategy of matching seronegative donors to seronegative recipients. The highest incidence of severe cytomegaloviral infection occurs in seronegative recipients receiving lungs from seropositive donors. In these patients, prophylaxis with intravenous ganciclovir is used routinely according to the following protocol: weeks 2 to 8, 5 mg/kg intravenously twice daily; weeks 8 to 12, 5 mg/kg intravenously once daily; weeks 12 to 16, 5 mg/kg intravenously three times weekly. It is reasonable to apply this prophylaxis regimen in any circumstance in which either the donor or the recipient is seropositive.
It has not been our practice to use routine fungal prophylaxis. If a heavy growth of yeast is identified after the transplant in the donor bronchial culture, however, the use of prophylactic low-dose amphotericin is justified. Pneumocystis carinii was an occasional cause of postoperative pulmonary infection until the routine use of trimethoprim-sulfamethoxazole prophylaxis eliminated this as a significant pathogen. Alternative agents, such as inhaled pentamidine, are administered when an allergy to sulfa medications is present.
Immunosuppression
Most clinical lung transplant programs rely on triple-agent immunosuppression consisting of cyclosporine, azathioprine, and corticosteroids. In our program, cyclosporine administration is delayed, typically until the second postoperative day. By that time, the patient is generally extubated and taking oral medications, and we have found this avoidance of intravenous cyclosporine useful in minimizing or avoiding perioperative renal dysfunction.
Azathioprine therapy is also commenced during the immediate postoperative period. Patients receive 1 to 2 mg/kg intravenously daily; when an oral diet is initiated, the same dose level can be administered orally. The dose must be adjusted to maintain a white blood cell count >3500.
The early use of perioperative corticosteroids is controversial. Most physicians have adopted the use of a moderate dose of corticosteroid—methylprednisolone 0.5 to 1 mg/kg/day intravenously—for several days before initiating an oral dose of prednisone, 0.5 mg/kg/day. We have demonstrated, however, as reported by Miller et al, 27 that withholding the first few days of prednisone has neither an adverse nor a beneficial effect on bronchial healing and may, in fact, lessen the risk of perioperative sepsis. We continue to use the moderate doses described above, and we begin using them on the day of the transplant.
For most patients, chronic immunosuppression consists of cyclosporine, prednisone, and azathioprine. Doses of cyclosporine are chosen to maintain blood levels in the range of 250 to 300 ng. Prednisone administration is also gradually reduced to minimize the complications of long-term steroid use.
A matter of considerable controversy is the use of postoperative cytolytic therapy with antithymocyte globulin or OKT3. We use Atgam (Upjohn, Kalamazoo, MI) in our clinical program. It has been argued that these agents, particularly OKT3, predispose patients to a higher incidence of cytomegaloviral infection. This experience was accumulated, however, at a time when cytomegalovirus matching was not widely practiced and cytomegalovirus prophylaxis was not routine. It is probable that the early use of cytolytic therapy does reduce the frequency and severity of early postoperative rejection episodes. We also favor the early use of ATGAM because it allows us to withhold the initial intravenous doses of cyclosporine, thereby enhancing early renal function.
Infection-Rejection Surveillance
As discussed by Trulock, 28 the use of radiographic, clinical, and physiologic criteria has been insufficient to delineate infection from rejection in the early posttransplant lung recipient. For this reason, we perform routine fiberoptic bronchoscopy whenever there is a clinical indication in the absence of recently identified, untreated organisms identified by sputum culture. The advantage of routine flexible bronchoscopy is that it enables performance of bronchoalveolar lavage and transbronchial biopsy. Although bronchoalveolar lavage has not proven useful in the diagnosis of rejection, it is invaluable in the identification of opportunistic infection, which is commonly encountered in transplant recipients. Transbronchial biopsy has proven to be the major tool in the diagnosis of pulmonary rejection. We have used this procedure frequently: both for routine surveillance and in patients with unexplained pulmonary infiltrates. Routine bronchoalveolar lavage and transbronchial biopsies are performed at 3 weeks, 3, 6, 9, and 12 months, and on an annual basis thereafter.
Regardless of the method of diagnosis, if rejection is indeed the problem, a dramatic improvement in clinical findings, radiographic evidence, and PaO2 will be observed within 8 to 12 hours after administration of 15 mg/kg of methylprednisolone.
Complications
Rejection
In the majority of patients, acute rejection episodes were confirmed histologically by transbronchial biopsy. Rejection was graded according to the International Society for Heart-Lung Transplantation (ISHLT) grading system. 29,30 Chronic allograft rejection (bronchiolitis obliterans syndrome [BOS]) was diagnosed on the basis of a progressive fall in one second forced vital capacity from a prior posttransplant baseline, as defined and graded by the ISHLT grading system. 31
Airway Complications
Recipients were judged to have an airway complication when some surgical intervention (e.g., dilatation, stenting, laser fulguration) was used to manage the complication.
Death
Deaths from any cause are included in this analysis. Perioperative mortality included all deaths during the transplant admission or during the first 30 days after the transplant in patients discharged from the hospital.
RESULTS
Lung transplantation was performed in 443 patients, with 7 patients receiving transplants on two occasions, for a total of 450 total transplant procedures. The data for these patients are summarized in Table 1. The patients were evenly distributed by sex. The breakdown by diagnosis was dominated by emphysema (both chronic obstructive pulmonary disease and alpha-1 antitrypsin deficiency), with more than half of the patients falling into this group. The remainder of the patients were fairly evenly distributed among the remaining diagnostic classifications. Preoperative functional status has been well described in previous publications. 32 No single measured parameter captures the functional status of all recipients, but the 6-minute walk comes closest to approaching a diagnosis-independent descriptor of impairment.
Table 1. TRANSPLANT RECIPIENTS
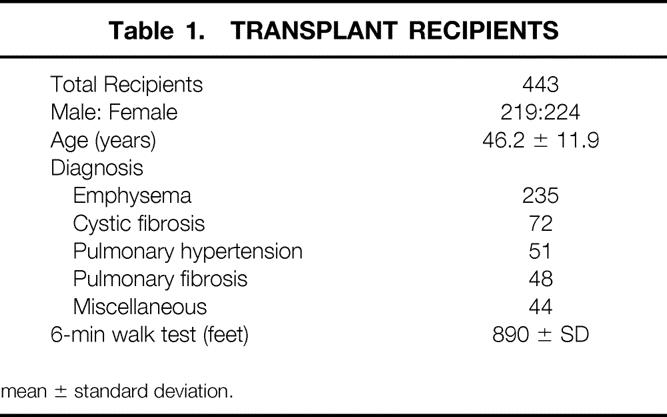
mean ± standard deviation.
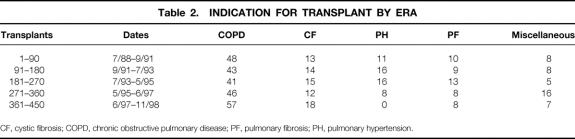
The frequency of each of the diagnoses leading to transplantation has evolved over the course of our experience. Table 2 lists the frequency of the major diagnoses for each block of 90 transplants. The rise in emphysema as a reason for transplant and the virtual disappearance of pulmonary hypertension as an indication are notable. Cystic fibrosis and pulmonary fibrosis have remained stable in their proportional representation throughout the decade.
Table 2. INDICATION FOR TRANSPLANT BY ERA
CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; PF, pulmonary fibrosis; PH, pulmonary hypertension.
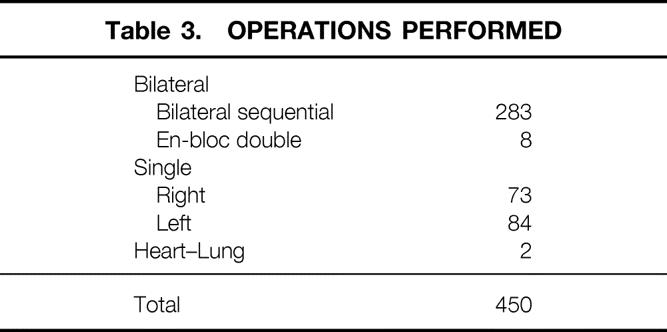
The 450 separate operations included 157 single-lung transplants, 291 double-lung transplants, and 2 heart-lung transplants. These procedures are summarized in Table 3. The bilateral experience includes eight en bloc double-lung transplants in which the airway anastomosis was made in the distal trachea, an approach that we discarded early in this experience.
Table 3. OPERATIONS PERFORMED
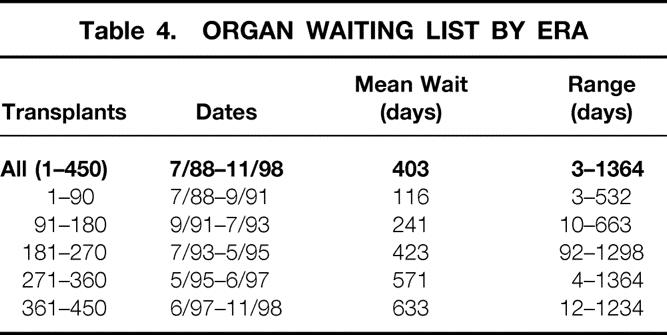
Although the mean time on the waiting list was 403 days for the entire experience, that statistic is deceptive in that it has been steadily lengthening throughout the period of these observations. Table 4 lists the mean waiting times for each block of 90 patients, showing that the waiting time for donor lungs has increased substantially in each successive block.
Table 4. ORGAN WAITING LIST BY ERA
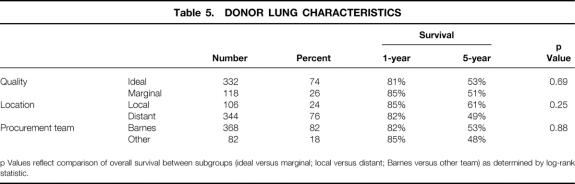
The impact of different strategies in lung allograft procurement is summarized in Table 5. Recipient survival was assessed according to stratification based on donor quality (ideal vs. marginal), donor location (local vs. distant), and procurement teams (Barnes vs. others). As depicted, there was no statistical difference in recipient survival with any of these stratifications.
Table 5. DONOR LUNG CHARACTERISTICS
p Values reflect comparison of overall survival between subgroups (ideal versus marginal; local versus distant; Barnes versus other team) as determined by log-rank statistic.
Table 6 reports the prevalence of airway complications that required intervention in each of the blocks of 90 transplants. As shown, the initial half of our experience was marked by a relatively high rate of airway complications, as the fine points of the surgical technique were evolving. Both our techniques and the surgical results have been stable over the past 6 years and nearly 300 transplants.
Table 6. AIRWAY ANASTOMOTIC COMPLICATIONS
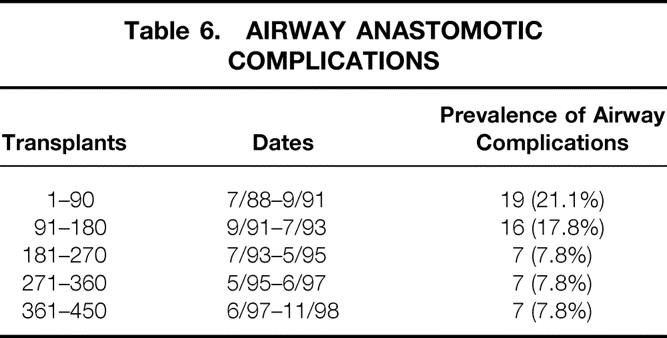
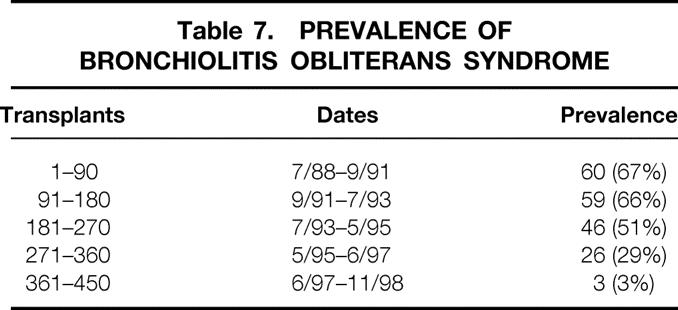
The prevalence of BOS for each block of 90 transplants is listed in Table 7. The progressive increase in the prevalence continues without evidence of plateau throughout the entire experience.
Table 7. PREVALENCE OF BRONCHIOLITIS OBLITERANS SYNDROME
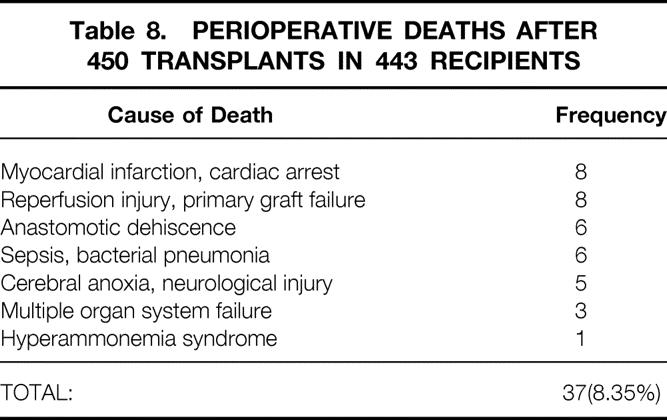
The perioperative deaths are described in Table 8. The 37 deaths occurred in the initial hospitalization or, if the patient was discharged, within the first 60 days after surgery. The deaths occurred on the day of surgery in two cases and as late as postoperative day 103 in one patient. Cardiac-related deaths and primary graft failure were the leading causes of death, with eight deaths attributed to each of these problems. Anastomotic dehiscence, sepsis, and neurologic injury were each noted as the cause of death in several patients. These 37 patients represented 8.35% of the entire cohort of 443 transplant recipients.
Table 8. PERIOPERATIVE DEATHS AFTER 450 TRANSPLANTS IN 443 RECIPIENTS
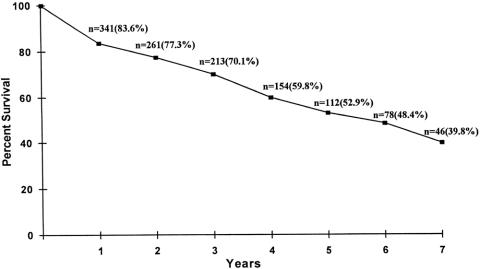
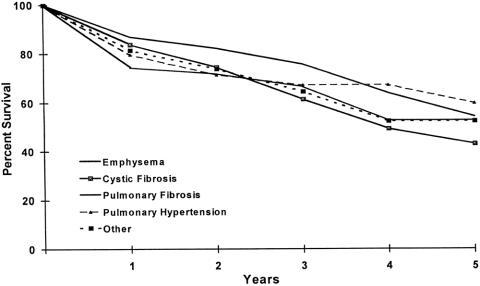
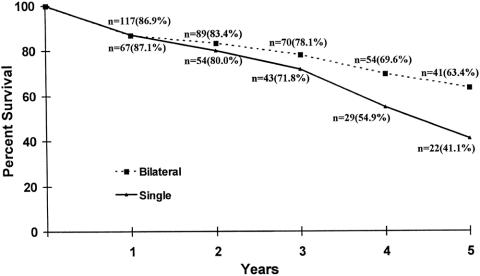
The long-term survival of lung transplant recipients is described in Figures 1, 2, and 3. Figure 1 is the Kaplan-Meier actuarial survival curve for the entire experience (450 transplants in 443 patients). Figure 2 shows survival broken down according to primary diagnosis (emphysema, cystic fibrosis, pulmonary fibrosis, pulmonary hypertension, and other diagnoses). Figure 3 depicts the survival of emphysema patients stratified according to procedure type (single-lung transplant vs. bilateral sequential transplant).
Figure 1. Lung transplantation performed on 443 recipients at the Washington University School of Medicine (July 1988–November 1998). Actuarial survival by time.
Figure 2. Survival of lung transplantation performed on 443 recipients at the Washington University School of Medicine (July 1988–November 1998), stratified according to underlying diagnosis leading to transplant. Actuarial survival by time.
Figure 3. Single (n = 78) versus bilateral (n = 152) lung transplantation performed on 235 recipients with emphysema at the Washington University School of Medicine (July 1988–November 1998). Actuarial survival by time.
DISCUSSION
The adult lung transplant program at Washington University Barnes-Jewish Hospital was conceived and implemented as a multidisciplinary program. Rigorous recipient selection criteria, preoperative preparation, intraoperative detail, and standard postoperative protocols have contributed to the low 8.4% perioperative mortality rate. In our experience, there has been no significant difference in the perioperative mortality rate by transplant indication.
In the international experience, 33 chronic obstructive lung disease is the most frequent indication for adult lung transplantation. This is also the case in our program. One reason for this predominance of emphysema may be that our program evaluates a larger number of patients with chronic obstructive lung disease as a result of our close association with a well-established lung-volume reduction surgery program. 34 In addition, the current algorithm for recipient priority on the waiting list is based largely on waiting time. It has been well documented that patients with chronic obstructive lung disease have a higher survival rate on transplant waiting lists than do patients with pulmonary fibrosis, cystic fibrosis, or pulmonary hypertension. 35 Because of this inequitable attrition on the waiting list, allocation of organs by waiting time favors the patient with chronic obstructive lung disease.
Our use of bilateral transplants in patients with chronic obstructive lung disease is controversial. Many other programs favor the single-lung option for these patients. However, our data suggest that bilateral recipients have a superior survival rate. Further, their perioperative management is simplified by the presence of bilateral grafts. Finally, the bilateral option enables the use of marginal donor lungs, a strategy that would not be acceptable in a single-lung procedure.
We have accumulated significant experience in the use of marginal lung grafts. 21 We have previously demonstrated that marginal donor lungs produce both early and late outcomes that are not different from those in ideal grafts. This observation has been confirmed in this current review of our entire experience and supports our continued use of lung allografts that do not meet the standard criteria for acceptability.
It is also evident from this review that our program is in large part dependent on donors from distant sites. Snell et al 36 reported that survival is adversely affected by long-distance harvest if the distance causes the ischemic time of the lungs to exceed 5 hours. Our data do not support this notion. Early and late results demonstrate that survival is unaffected by long-distance harvest. In addition, we previously demonstrated that the functional outcome of long-distance harvest by other teams is equivalent to that obtained by our team harvesting lungs at a distance. 37
Although lung allograft reperfusion injury represented a frequent cause of death and complications in the early days of transplant, this problem has been less frequently encountered in recent years. This is in all likelihood the result of improvements in preservation and implantation techniques. In addition, we have found inhaled nitric oxide to be of particular value in the treatment of early allograft dysfunction. 38 In our program, extracorporeal membrane oxygenation (ECMO) has not been frequently employed. However, our small experience suggests that the early application of ECMO in patients with reperfusion injury refractory to maximum medical therapy provides the best likelihood of success. Patients in whom ECMO was used immediately in the course of their reperfusion injury had a uniformly better outcome.
Chronic lung allograft rejection or BOS remains the major cause of late complications and death. Our group has previously reported that all patients, followed for a long enough period, will demonstrate some evidence of BOS. Management of this condition is complicated by our inability to predict which patients are destined to develop severe BOS, and the lack of effective treatment for the condition once it occurs. The Pittsburgh group has demonstrated that frequent and severe early lung allograft rejection episodes predict the subsequent development of BOS. 39 In addition, our group recently reported a higher frequency and greater severity of BOS in patients in whom donor-specific anti-HLA antibodies develop during their postoperative course. 40 It is possible that identification of such antibodies might enable an adjustment in immunosuppressive strategies that may affect the subsequent development of BOS. This question is under study by our group.
Discussion
Dr. Larry R. Kaiser (Philadelphia, Pennsylvania): It is a real honor for me to be asked to discuss this paper as it really brings me full circle. I was privileged to be part of the Washington University Lung Transplant Program at its inception in 1988 when Dr. Cooper and I first came to Barnes Hospital. I remained a part of that program until 1991, when I left to assume my current position. I note both in the manuscript and in the presentation that the first 90 or so transplants correspond to my tenure and then the results seem to get significantly better. I hope it is not causal, that relationship, but it gives me some cause for concern.
The series that you presented is a remarkable one, not only for its sheer size but for the superb results. It is a testimony to the vision and insight of Drs. Cooper and Patterson and the team they have assembled. Dr. Patterson, I have a couple of questions for you.
You have been a major proponent of bilateral lung transplants for patients with emphysema despite the scarcity of donor organs and the track record of single lung transplants for this disease. Your results justify this bias, but to what do you attribute the survival differential between those emphysema patients receiving one lung or two, since there really is no difference in early mortality?
How do you decide which patients with emphysema will get a single lung? Which patients with emphysema do you choose for lung volume reduction surgery, to bring up a controversial topic?
With the increasing time spent on the waiting list, when do you list a patient who has emphysema? It sounds like you ought to list them at the time of their first cigarette.
Can you comment on your philosophy regarding retransplantation?
Finally, do you think we can ever expect to see 5-year survival rise above 50% in light of the problem with obliterative bronchiolitis?
Needless to say, I enjoyed this paper very much and congratulate the authors on a superb job.
Presenter Dr. G. Alexander Patterson (St. Louis, Missouri): I should also mention that Dr. Kaiser and I were involved in the Toronto program when Dr. Kaiser did his training there and I was a junior faculty member. So Dr. Kaiser’s transplant experience goes back quite a long time.
The large number of bilateral transplants predominating in our program reflects my own personal bias that it is a better operation. It takes longer and is, I suppose, theoretically associated with a somewhat higher risk, having twice the number of anastomoses. But I believe that the perioperative care of these patients is made much simpler.
Furthermore, we use a lot of marginal donors. And virtually all of these marginal donors are utilized in patients with emphysema undergoing bilateral transplantation. It is an easy group to utilize these marginal donors in.
When we evaluate an emphysema patient, we view them as potential candidates for lung volume reduction surgery or transplantation. With respect to transplant, we favor the single-lung option for older patients of smaller stature and reserve the bilateral option for younger patients of larger stature for whom it is unlikely we would be able to get an oversized donor lung.
The patients who are ideally suited for volume reduction at any age receive that option, because I think it is a far preferable option, with much better long-term survival than we see in our transplant patients. For those patients who are entering into their sixth decade of life we favor transplant—if they are not an ideal candidate for volume reduction, we favor transplant because it will give them better physiologic results much sooner.
With respect to listing patients, we utilize standardized listing criteria. So that the majority of patients with, for example, emphysema who are listed have an FEV1 in the 25% range or less. I will say, however, that one of the possible explanations for the large number of emphysema patients in our program is the fact that, given the current algorithm for donor allocation and lung transplantation being based solely on waiting time, that favors the emphysema patient. If we look at the wait-list survival, patients with cystic fibrosis, pulmonary hypertension, and pulmonary fibrosis have a much higher waiting list mortality. So those emphysema patients, even though they are ill when they are first listed, they are still going to be there 2 years, 3 years later when a suitable donor becomes available.
With respect to the long-term survival rate, I do think there is going to be progress made in the problems of bronchiolitis obliterans, and there are a couple of intriguing ideas. We have identified the presence of HLA antibodies in our group of patients suffering from OB and we think that predicts the subsequent development of bronchiolitis. There are a number of other centers around the world looking at bronchiolitis obliterans, so I do think that we will make progress in that area over the next few years.
Dr. Marlon Levy (Dallas, Texas): An excellent presentation and a wonderful series. I was intrigued by your report that marginal donors didn’t yield different results than ideal donors. I wonder why that is, since we don’t see that in other organs. I wonder if these results have led your group to be even less restrictive in the quality of the donors that they approve.
Dr. Patterson: Well, yes, we have liberalized our donor selection criteria enormously. The majority of donors that we utilize in our program have already been turned down by other sites. We are completely dependent on donors from distant regions. As I said, 75% of our donors come from outside our region. That is just a geographic fact. If you take the City of St. Louis out of our region, no one lives there. It is a fairly rural environment. So we do depend upon donors that are judged to be of inadequate quality. And these work just fine. As I said, we use these donors in the most easy cases, that is, the emphysema patients.
Dr. David M. Heimbach (Seattle, Washington): What sort of quality of life do your patients have, particularly after they start to get the bronchiolitis?
Dr. Patterson: All these patients preoperatively have a terrible quality of life. We certainly inform them that they are trading one disease for another disease, of their underlying lung disease for immunosuppression. Nonetheless, I think in the early postop follow-up all of these patients, the survivors, and the majority of patients have a marked improvement under quality of life.
Even patients who have relatively low rates or low grades of bronchiolitis obliterans still have a very good quality of life. Patients who develop severe bronchiolitis obliterans, particularly those with single-lung grafts, have a terrible quality of life. Fortunately, in most patients that doesn’t happen for several years after their transplant.
Dr. Ashokkumar B. Jain (Pittsburgh, Pennsylvania): I have a question. Some people believe obliterative bronchiolitis is a part of chronic rejection. Do you have any personal experience on this issue?
Dr. Patterson: I don’t have anything particularly to add to what you have suggested. It is generally accepted that bronchiolitis obliterans is a manifestation of chronic rejection. It is seen more frequently in patients who have early or persistent episodes of rejection during the early postoperative period. I don’t have any doubt that if we could somehow make more selective and more individualized our immunosuppression protocols, we would see less bronchiolitis obliterans. But unfortunately, we are not at that point yet.
Dr. David E. R. Sutherland (Minneapolis, Minnesota): Bronchiolitis obliterans, a manifestation of chronic rejection, is a major problem. You made no mention of the new drug, mycophenolate mofetil (CellCept), that might have an effect on chronic rejection. Are you using this drug?
You also mentioned that patients who have detectable HLA antibodies also have a higher incidence of bronchiolitis obliterans. Are the relevant antibodies detectable pretransplant or posttransplant? Do you require a negative crossmatch pretransplant on individuals who have reactivity to a panel of potential donors?
Dr. Patterson: Yes, we do a prospective crossmatch on all of our potential recipients, and those with a high PRA are separately crossmatched prior to transplant. Those are the negative PRA. We utilize just routine blood type and body size as the only matching requirement.
We have a small experience with mycophenolate. There is a trial ongoing. This series terminated in November of last year, and since that time we have had an increased experience with that agent. But it is not incorporated in this data.
Footnotes
Correspondence: G. Alexander Patterson, MD, Division of Cardiothoracic Surgery, One Barnes-Jewish Hospital Plaza, Suite 3108 Queeny Tower, St. Louis, MO 63110.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Derom F, Barbier F, Ringoir S. Ten-month survival after lung homotransplantation in man. J Thorac Cardiovasc Surg 1971; 61: 835–846. [PubMed] [Google Scholar]
- 2.Reitz B, Wallwork J, Hunt S, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982; 306: 557–564. [DOI] [PubMed] [Google Scholar]
- 3.Toronto Lung Transplantation Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986; 314: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 4.Patterson GA, Cooper JD, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg 1988; 45: 626–633. [DOI] [PubMed] [Google Scholar]
- 5.Pasque MK, Cooper JD, Kaiser LR, et al. An improved technique for bilateral lung transplantation: rationale and initial clinical experience. Ann Thorac Surg 1990; 49: 785–791. [DOI] [PubMed] [Google Scholar]
- 6.Novick RJ, Menkis AH, McKenzie FN. New trends in lung preservation: a collective review. J Heart Lung Transpl 1992; 11: 377–392. [PubMed] [Google Scholar]
- 7.Cooper JD, Vreim CE. Biology of lung preservation for transplantation. Am Rev Respir Dis 1992; 146: 803–807. [DOI] [PubMed] [Google Scholar]
- 8.Kirk AJB, Colquhoun IW, Dark JH. Lung preservation: a review of current practice and future directions. Ann Thorac Surg 1993; 56: 990–1000. [DOI] [PubMed] [Google Scholar]
- 9.Schafers HJ, Haydock DA, Cooper JD. The prevalence and management of bronchial anastomotic complications in lung transplantation. J Thorac Cardiovasc Surg 1991; 101: 1044–1052. [PubMed] [Google Scholar]
- 10.Patterson GA, Todd TR, Cooper JD, et al. Airway complications after double lung transplantation. J Thorac Cardiovasc Surg 1990; 99 (1): 14–21. [PubMed] [Google Scholar]
- 11.Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994; 57: 506–511. [DOI] [PubMed] [Google Scholar]
- 12.Colquhoun IW, Gascoigne AD, Au J, et al. Airway complications after pulmonary transplantation. Ann Thorac Surg 1994; 57: 141–145. [DOI] [PubMed] [Google Scholar]
- 13.Griffith BP, Hardesty RL, Armitage JM, et al. A decade of lung transplantation. Ann Surg 1993; 218 (3): 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higenbottam T, Stewart S, Penketh A, Wallwork J. Transbronchial lung biopsy for the diagnosis of rejection in heart-lung transplant patients. Transplantation 1988; 46: 532–539. [DOI] [PubMed] [Google Scholar]
- 15.Reichenspurner H, Girgis RE, Robbins RC, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg 1995; 60: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan S, Trulock EP, Mohanakumar T, et al. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Ann Thorac Surg 1995; 60: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 17.Kroshus TJ, Kshettry VR, Savik K, et al. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg 1997; 114: 195–202. [DOI] [PubMed] [Google Scholar]
- 18.Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. J Heart Lung Transpl 1998; 17: 703–709. [PubMed] [Google Scholar]
- 19.Lynch JP, Trulock EP. Recipient selection. Sem Resp Crit Care Med 1996; 17: 109–117. [Google Scholar]
- 20.Trulock EP. Recipient selection. Chest Surg Clin North Am 1993; 3: 1–18. [Google Scholar]
- 21.Sundaresan S, Semenkovich J, Ochoa L, et al. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg 1995; 109: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 22.Sundaresan S, Trachiotis GD, Aoe M, et al. Donor lung procurement: assessment and operative technique. Ann Thorac Surg 1993; 56: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 23.Haniuda M, Hasegawa S, Shiraishi T, et al. Effects of inflation volume during lung preservation on pulmonary capillary permeability. J Thorac Cardiovasc Surg 1996; 112: 85–93. [DOI] [PubMed] [Google Scholar]
- 24.Meyers BF, Patterson GA. Technical aspects of adult lung transplantation. Semin Thorac Cardiovasc Surg 1998; 10: 213–220. [DOI] [PubMed] [Google Scholar]
- 25.Date H, Trulock EP, Arcidi JM, et al. Improved airway healing after lung transplantation: an analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg 1995; 110: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 26.Ettinger NA, Bailey TC, Trulock EP, et al. Cytomegalovirus infection and pneumonitis: impact after isolated lung transplantation. Am Rev Respir Dis 1993; 147: 1017–1023. [DOI] [PubMed] [Google Scholar]
- 27.Miller J, DeHoyos A, Group UoTLT, Group WULT. An evaluation of the role of omentopexy and of early perioperative corticosteroid administration in clinical lung transplantation. J Thorac Cardiovasc Surg 1993; 105: 247. [PubMed] [Google Scholar]
- 28.Trulock EP. Lung transplantation. Am J Respir Crit Care Med 1997; 155: 789–818. [DOI] [PubMed] [Google Scholar]
- 29.Yousem SA, Berry GJ, Brunt EM, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. J Heart Lung Transpl 1990; 9: 593–601. [PubMed] [Google Scholar]
- 30.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transpl 1996; 15 (1 Pt 1): 1–15. [PubMed] [Google Scholar]
- 31.Cooper JD, Billingham M, Egan T. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transpl 1993; 12: 713–716. [PubMed] [Google Scholar]
- 32.Cooper JD, Patterson GA, Trulock EP, Group WULT. Results of single and bilateral lung transplantation in 131 consecutive recipients. J Thorac Cardiovasc Surg 1994; 107: 460–471. [PubMed] [Google Scholar]
- 33.Hosenpud JD, Bennett LE, Keck BM, et al. The Registry of the International Society for Heart and Lung Transplantation: Fifteenth Official Report, 1998. J Heart Lung Transpl 1998; 17: 656–668. [PubMed] [Google Scholar]
- 34.Cooper JD, Patterson GA, Sundaresan RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996; 112: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 35.Hosenpud JD, Bennett LE, Keck BM, et al. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998; 351 (9095): 24–27. [DOI] [PubMed] [Google Scholar]
- 36.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: an important predictor of survival after lung transplantation. J Heart Lung Transpl 1996; 15: 160–168. [PubMed] [Google Scholar]
- 37.Shiraishi Y, Ochoa L, Richardson G, et al. Retrieval by other procurement teams provides favorable lung transplantation outcome. Ann Thorac Surg 1997; 64: 203–206. [DOI] [PubMed] [Google Scholar]
- 38.Date H, Triantafillou A, Trulock E, et al. Inhaled nitric oxide reduces human lung allograft dysfunction. J Thorac Cardiovasc Surg 1996; 111: 913–919. [DOI] [PubMed] [Google Scholar]
- 39.Bando K, Paradis IL, Similo S, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. J Thorac Cardiovasc Surg 1995; 110: 4–14. [DOI] [PubMed] [Google Scholar]
- 40.Smith M, Sundaresan S, Mohanakumar T, et al. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg 1998; 116: 812–820. [DOI] [PubMed] [Google Scholar]