Abstract
Objective
To review the authors’ clinical experience with transhiatal esophagectomy (THE) and the refinements in this procedure that have evolved.
Background
Increased use of THE during the past two decades has generated controversy about the merits and safety of this approach compared with transthoracic esophageal resection. The authors’ large THE experience provides a valuable basis for benchmarking data regarding the procedure.
Methods
The results of THE were analyzed retrospectively using the authors’ prospectively established esophageal resection database and follow-up information on these patients.
Results
From 1976 to 1998, THE was performed in 1085 patients, 26% with benign disease and 74% with cancer. The procedure was possible in 98.6% of cases. Stomach was the esophageal substitute in 96%. The hospital mortality rate was 4%. Blood loss averaged 689 cc. Major complications were anastomotic leak (13%), atelectasis/pneumonia (2%), intrathoracic hemorrhage, recurrent laryngeal nerve paralysis, chylothorax, and tracheal laceration (<1% each). Actuarial survival of patients with carcinoma equaled or exceeded that reported after transthoracic esophagectomy. Late functional results were good or excellent in 70%. With preoperative pulmonary and physical conditioning, a side-to-side stapled cervical esophagogastric anastomosis (<3% incidence of leak), and postoperative epidural anesthesia, the need for an intensive care unit stay has been eliminated and the length of stay reduced to 7 days.
Conclusion
THE is possible in most patients requiring esophageal resection and can be performed with greater safety and fewer complications than the traditional transthoracic approaches.
In our initial 1978 report on transhiatal esophagectomy (THE) without thoracotomy, we proposed that this technique offered important advantages over traditional transthoracic esophagectomy, primarily avoidance of combined thoracoabdominal incisions and mediastinitis associated with an intrathoracic esophageal anastomotic leak. 1 Since this report, numerous publications have discussed the risks and merits of THE. Katariya et al 2 reviewed 1353 patients undergoing THE who were reported in the surgical literature between 1981 and 1992. Sixteen of the papers referenced (69.5%) were series of ≤50 patients and as such were not a true reflection of the type of results that can be achieved by more experienced surgical teams. The collective review by Gandhi and Naunheim 3 of the complications of THE in 1192 patients cited eight papers published between 1992 and 1994. Four of these papers were series of ≥100 patients (118, 131, 141, and 583, the latter being our most recent review of the subject 4 ).
Since 1976, THE without thoracotomy has been performed by the General Thoracic Surgery Service of the University of Michigan in 1085 patients for pathology of the intrathoracic esophagus. The refinements in surgical technique and pre- and postoperative management that have evolved over the years have been substantial. This report reviews this clinical experience and provides the basis for the authors’ position that THE and a cervical esophagogastric anastomosis is currently the safest, most efficient means of removing and replacing the esophagus, both for benign and malignant disease.
METHODS
Between 1976 and June 30, 1998, THE, as described previously, 1,5–7 has been performed by the General Thoracic Surgery Service at the University of Michigan Medical Center in 1085 patients with pathology of the intrathoracic esophagus. The results have been analyzed retrospectively using our esophageal resection database and follow-up through personal interviews and examinations, written correspondence, and telephone contacts with patients and families. Of these patients, 285 (26%) had benign disease necessitating esophageal replacement and 800 (74%) had carcinoma (Table 1). The patients with benign disease included 143 male patients (13%) and 142 female patients (13%), ranging in age from 14 to 89 years (average 52 years). Six hundred fifty-one (60%) of the patients with carcinoma were men and 149 (14%) were women; their ages ranged from 29 to 92 years (average 64 years). Two hundred thirty-nine (22%) of this entire series of patients were 71 years of age or older. Among the patients with carcinoma, 555 (69%) had adenocarcinomas (5 upper third, 53 middle third, and 497 lower third) and 225 (28%) had squamous cell carcinomas (28 upper, 121 middle, and 76 lower third). Additional cell types included adenosquamous (12), signet ring cell (11), anaplastic (2), poorly differentiated (2), small cell (2), and undifferentiated (1) carcinoma.
Table 1. INDICATIONS FOR TRANSHIATAL ESOPHAGECTOMY (1085 PATIENTS)
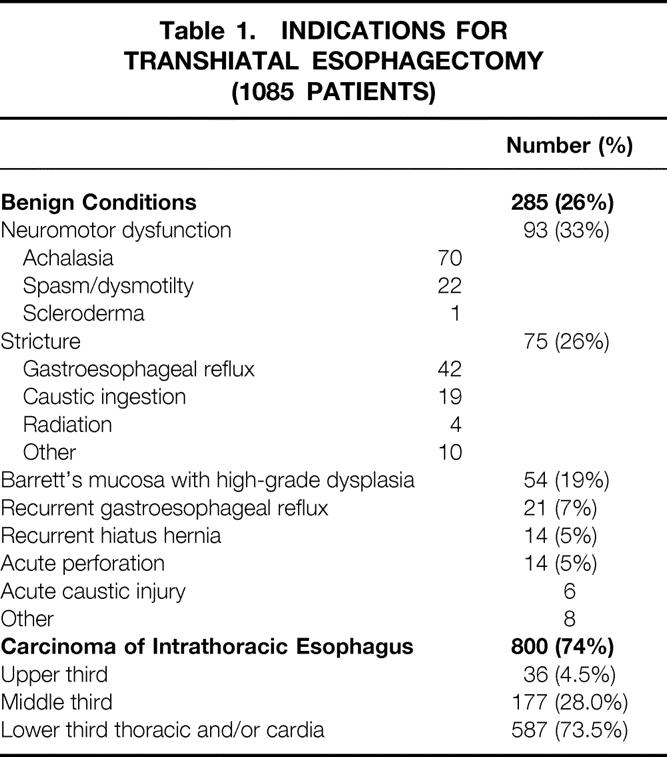
Among 1100 patients in whom THE was attempted, it was possible in 98.6%. Fifteen patients required conversion to a transthoracic esophagectomy because of intrathoracic esophageal fixation or bleeding. Transhiatal esophagectomy has been possible despite the presence of mediastinal inflammation from prior surgical procedures, perforations, or radiation therapy. Of the patients with cancer, 234 (29%) had undergone radiation therapy before THE. Of the patients with benign disease, 146 (52%) had undergone one or more prior esophageal or periesophageal surgical procedures, including antireflux repairs (85), esophagomyotomy (60), vagotomy (15), esophagotomy (4), colonic interposition (4), repair of perforation (3), esophageal exclusion (3), esophagoplasty (2), laryngopharyngectomy (2), proximal gastrectomy (3), and sclerotherapy and resection of leiomyoma (1 patient each). Four patients with acute caustic injuries underwent an emergent THE, cervical esophagostomy, and feeding jejunostomy followed by delayed esophageal reconstruction 2 to 8 weeks later. One patient with a malfunctioning antiperistaltic retrosternal colonic bypass of a caustic esophageal stenosis underwent removal of the retrosternal colon, THE, and a cervical esophagogastric anastomosis.
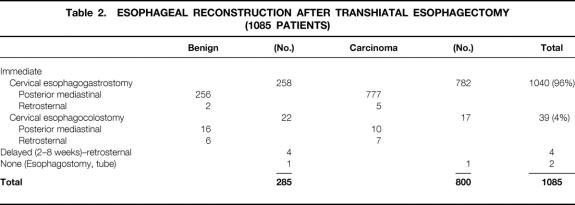
Esophageal resection and reconstruction were performed at the same sitting in all but six patients, five with benign disease and one with carcinoma (Table 2). The stomach was used as the esophageal substitute in 1040 (96%) of the patients who underwent their esophagectomy and reconstruction at one sitting. Six of the 25 patients with acute or chronic esophageal caustic injuries required either partial or total gastric resection for the caustic gastric burn. Colon was used to replace the esophagus in these latter 6 patients and in 33 others who had undergone prior gastric resections for peptic ulcer disease that precluded cephalad reach of the stomach for a cervical esophagogastric anastomosis. The original esophageal bed in the posterior mediastinum was used for esophageal substitution in all but 20 patients, in whom either residual posterior mediastinal tumor or fibrosis and narrowing prevented comfortable, tension-free positioning of the stomach for a cervical anastomosis. In these latter 20 patients, the retrosternal route was used. In 100 patients (9%), 37 with benign disease and 63 with carcinoma, a partial upper sternal split, as described previously, 8 was added to the standard left cervical incision to obtain exposure of the cervicothoracic esophagus because of inability to extend the neck as a result of cervical osteoarthritis or a “bull neck” habitus and essentially no available supraclavicular length of cervical esophagus. The patients with carcinoma routinely underwent sampling of accessible subcarinal, paraesophageal, and celiac axis lymph nodes, but no attempt was made to perform an en bloc wide resection of the esophagus with its contiguous lymph node-bearing tissues.
Table 2. ESOPHAGEAL RECONSTRUCTION AFTER TRANSHIATAL ESOPHAGECTOMY (1085 PATIENTS)
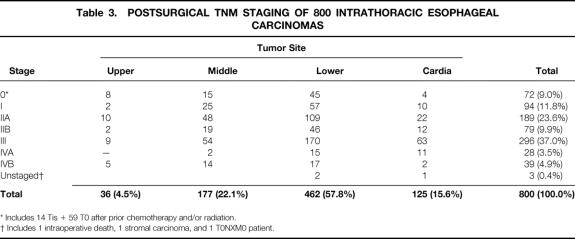
Postsurgical TNM staging 9 of the carcinomas based on histologic examination of the resected specimen is shown in Table 3. Among the 72 patients with stage 0 tumors, 15 had carcinoma in situ and 57 had no residual tumor after preoperative radiation therapy or chemotherapy or both. Forty-six percent of tumors were transmurally invasive into the periesophageal adventitia or were metastatic beyond regional lymph nodes (stage III or IV tumors). A pyloromyotomy in all patients undergoing esophageal replacement with stomach and a feeding jejunostomy in all patients undergoing THE were performed routinely.
Table 3. POSTSURGICAL TNM STAGING OF 800 INTRATHORACIC ESOPHAGEAL CARCINOMAS
* Includes 14 Tis + 59 T0 after prior chemotherapy and/or radiation.
† Includes 1 intraoperative death, 1 stromal carcinoma, and 1 T0NXM0 patient.
RESULTS
There were three intraoperative deaths from uncontrollable mediastinal bleeding that occurred during mobilization of the esophagus (one upper and two distal-third carcinomas). Six additional patients had inordinate intraoperative bleeding—two intraabdominal associated with portal hypertension from cirrhosis, three from a torn azygos vein during mobilization of a mid-third carcinoma, and one from splenic injury. Excluding these latter six patients, intraoperative blood loss averaged 689 cc (Table 4).
Table 4. MEASURED INTRAOPERATIVE BLOOD LOSS WITH TRANSHIATAL ESOPHAGECTOMY
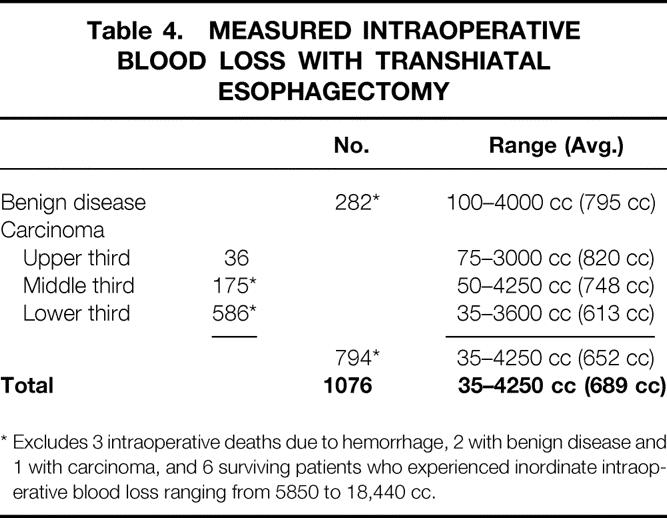
* Excludes 3 intraoperative deaths due to hemorrhage, 2 with benign disease and 1 with carcinoma, and 6 surviving patients who experienced inordinate intraoperative blood loss ranging from 5850 to 18,440 cc.
With increasing experience with THE, much of the esophageal mobilization is performed not bluntly but under direct vision through the retracted diaphragmatic hiatus using long right-angle clamps applied to the periesophageal tissues. This is reflected by the decrease in average blood loss during various phases of our experience with this procedure: 1166 cc for the first 50 procedures (1978 to 1980), 505 cc for 50 consecutive procedure in 1991 and 1992, and 360 cc for 114 consecutive procedures in 1996 and 1997.
Intraoperative Complications
Entry into one or both pleural cavities was identified during surgery at the time of routine inspection through the diaphragmatic hiatus after the esophagectomy and treated with a chest tube(s) in 831 (77%) of the patients. A splenectomy was required because of intraoperative injury in 34 patients (3%). There were four (<1%) intraoperative membranous tracheal lacerations, three involving the high trachea (repaired through a partial upper sternal split), and one involving the carina (requiring a right thoracotomy for repair). Entry into the gastric or duodenal mucosa during performance of the pyloromyotomy occurred in <2% and was managed by repairing the hole with interrupted 5-0 polypropylene and buttressing the repair with adjacent omentum.
Postoperative Complications
Postoperative mediastinal bleeding requiring a thoracotomy for control within 24 hours of THE occurred in five (<1%) patients, three with carcinoma and two with a megaesophagus of achalasia; each had arterial bleeding in the esophageal bed.
Recurrent laryngeal nerve injury, as manifested by hoarseness, occurred in 74 patients (7%). This hoarseness was transient in 50 of these patients, resolving spontaneously in 2 to 12 weeks. In 24 patients (<1%), there was persistent hoarseness, and 7 of these required vocal cord medialization procedures. The incidence of recurrent laryngeal nerve injury has progressively declined with greater experience with cervical esophageal mobilization and strict avoidance of placement of metal retractors against the tracheoesophageal groove. The average incidence of recurrent nerve injury was 32% from 1978 to 1982 (average of 23 THE procedures performed annually); 5% from 1983 to 1987 (average of 45 THE procedures performed annually); 3% from 1988 to 1992 (average of 55 THE procedures performed annually); and 2% from 1993 to 1997 (average of 82 THE procedures performed annually).
Chylothorax occurred in 18 patients (<1%), 12 with carcinoma and 6 with benign disease. Transthoracic ligation of the injured thoracic duct within 7 to 10 days of surgery, as described previously, 10 was used to manage the problem successfully in each case. Twenty-nine patients (3%) had abdominal wound infections or dehiscences. Clinically significant atelectasis or pneumonia prolonging the hospital stay beyond 10 days occurred in 17 patients (2%).
The overall anastomotic leak rate after a cervical esophagogastric anastomosis was 13% (146 patients). Twenty-five percent of these leaks (36) occurred in patients with benign disease, whereas 75% (110) occurred in patients undergoing THE for carcinoma. Eleven (30%) of the patients with benign disease who had anastomotic leaks had undergone prior hiatal hernia surgical procedures, which may have jeopardized the healing of the anastomosis after remobilization of the gastric fundus for esophageal replacement. Among the 1030 surviving patients in whom the stomach was positioned in the posterior mediastinum in the original esophageal bed, there were 137 leaks (13%) compared with 6 leaks (86%) in the seven patients with retrosternal placement of the stomach. All but 9 of the 146 cervical esophagogastric anastomotic leaks in this series were successfully managed by opening the cervical wound at the bedside and local packing until healing occurred. Early initiation of esophageal bougienage within 7 to 10 days to prevent late stenosis, as described previously, 11 was routine. Of the 110 patients with carcinoma who had anastomotic leaks after THE and cervical esophagogastric anastomosis, 38 (35%) had undergone preoperative radiation or chemoradiation therapy, which may have jeopardized the healing of the radiated gastric fundus. Necrosis of the upper stomach necessitating takedown of the stomach from the chest, resection of the devitalized stomach, and a cervical esophagostomy occurred in nine patients, eight with carcinoma and one with benign disease.
Mortality Rate
Among these 1085 patients undergoing THE, the overall hospital mortality rate was 4% (44 deaths)—36 (4.5%) among the 800 patients with carcinoma and 8 (2.8%) among the 285 patients with benign disease. The causes of death among the patients with cancer included hepatic failure (6), respiratory insufficiency (5), myocardial infarction (4), intraoperative hemorrhage (3), pneumonia (3), sepsis (3), intestinal ischemia (3), sudden death/cardiac arrest (3), pulmonary embolus (2), and posterior mediastinal abscess, retroperitoneal abscess, unrecognized brain metastasis, and delayed pyloromyotomy leak (1 each). The causes of death among the patients with benign disease included sepsis (5), myocardial infarction (1), respiratory insufficiency (1), and portal vein thrombosis (1).
The hospital mortality rate after THE averaged 9% from 1978 to 1982 (average number of THE procedures, 23 per year); 4% from 1983 to 1987 (average number of THE procedures, 45 per year); 2% from 1988 to 1992 (average number of THE procedures, 55 per year); and 4% from 1993 to 1997 (average number of THE procedures, 82 per year).
Length of Stay
Of the 748 patients discharged alive from the hospital after a THE and cervical esophagogastric anastomosis for carcinoma, 400 (53%) were discharged by the 10th postoperative day, 201 (27%) from 11 to 14 days, 81 (11%) from 15 to 21 days, and 66 (9%) after 21 days. Of the 251 patients discharged after THE for benign disease, 116 (46%) were discharged by the 10th postoperative day, 76 (30%) from 11 to 14 days, 30 (12%) from 15 to 21 days, and 29 (12%) after 21 days. Overall, 52% of these patients were discharged within 10 days of THE, 28% within 2 weeks, and 11% within 3 weeks. In the past 2 years, the average length of stay after an uncomplicated THE has decreased to 7 days.
Functional Results
Of the 39 patients undergoing esophageal replacement with colon after THE, 11 of the 17 with cancer died within an average period of 18.5 months. Of the 22 patients with benign disease, there are only 10 surviving patients. As a result of the small number of patients with colon interpositions after THE available for follow-up, they are not considered in this assessment of functional results of esophageal substitution.
Esophageal Substitution With Stomach for Benign Disease
Patients with benign disease requiring esophageal resection and reconstruction have a considerably longer life expectancy than those with carcinoma and as such serve as a better indicator of the functional results of visceral esophageal substitution. Among the 251 hospital survivors of THE and esophageal replacement with stomach, for benign disease, follow-up information regarding functional results was available for up to 213 months (average 47 months) after operation for 242. In assessing the functional results of esophageal substitution, the following factors were analyzed: presence and degree of dysphagia, regurgitation, and postvagotomy diarrhea and cramping (“dumping”).
Every patient who has had a cervical esophagogastric anastomosis is instructed to return for an outpatient anastomotic dilatation (with a 46F or larger bougie) if there is any degree of cervical dysphagia after discharge from the hospital. With this liberal use of dilatation therapy, only 56 of the patients with benign disease (23%) have not had a postoperative esophageal dilatation. However, at the time of their latest follow-up evaluation, 157 of the 242 followed patients (65%) ate a regular unrestricted diet and had no dysphagia, 38 (16%) had occasional mild dysphagia requiring no treatment, 36 (15%) underwent an occasional esophageal dilatation but swallowed well between treatments and were satisfied with their ability to eat, and 11 (4%) had “severe” dysphagia requiring regular dilatations (daily or weekly). The majority of these patients with dysphagia were able to swallow 46F or larger esophageal bougies.
One hundred forty-six patients (60%) said they had no regurgitation of gastric contents whatsoever. Seventy-seven (32%) had occasional, mild regurgitation only if they reclined or lay prone after a large meal; their reflux was not a major problem for them. Eighteen patients (7%) slept with their head elevated at night (with their bed propped up on blocks, on a wedge, or in a recliner chair) because of more frequent, troublesome nocturnal regurgitation. One patient (<1%) had had pulmonary complications resulting from aspiration.
At latest follow-up, 147 patients (61%) said they had no postprandial cramping or diarrhea. Ninety-five patients (49%) had had varying degrees of dumping syndrome (postprandial nausea, cramping, diaphoresis, or diarrhea); the symptoms generally subsided over time and were usually well controlled with diphenoxylate or tincture of opium. Forty-nine (20%) had “mild” diarrhea (infrequent, requiring no treatment), 16 (7%) had “moderate” diarrhea (occasionally requiring medication), and 10 (4%) had “severe” postprandial diarrhea requiring regular mediation (e.g., diphenoxylate, loperamide, tincture of opium). Mild postprandial cramping requiring no treatment was reported by 38 patients (16%) and moderate cramping requiring regular use of antispasmodics by 9 (4%).
On the basis of their most recent follow-up evaluation, overall functional results were scored as excellent (completely asymptomatic) in 71 (29%), good (mild symptoms requiring no treatment) in 93 (39%), fair (symptoms requiring occasional treatment, such as a dilatation or antidiarrheal medication) in 68 (28%), and poor (symptoms requiring regular treatment) in 10 (4%).
Esophageal Substitution With Stomach for Carcinoma
Among the 748 hospital survivors of THE and a cervical esophagogastric anastomosis, follow-up information regarding functional results for up to 194 months (average 29 months) after surgery was available for 721. Using the same liberal policy of postoperative anastomotic dilatation described above, 343 (48%) had never undergone an esophageal dilatation. Three patients required resection of an anastomotic stricture and construction of a new cervical esophagogastric anastomosis. At latest follow-up, 575 (80%) had no dysphagia whatsoever, 71 (10%) had occasional mild dysphagia requiring no treatment, 55 (8%) had moderate dysphagia requiring an occasional dilation, and 20 (2%) had severe dysphagia requiring regular dilatations. Five hundred seventy-one (79%) said they had no regurgitation; 124 (17%) had occasional mild regurgitation when recumbent after a large meal but generally sleep horizontally with their heads elevated on one or two pillows; and 25 (3.5%) had moderate troublesome regurgitation requiring that they sleep upright at night to avoid nocturnal regurgitation. One (<1%) had pulmonary complications of aspiration. At latest follow-up, 530 (74%) said they had no postprandial cramping or diarrhea. One hundred ninety-one (26%) had varying degrees of dumping syndrome. Five hundred seventy-one (79%) had no diarrhea, 117 (16%) had mild diarrhea requiring no treatment, 27 (14%) had moderate diarrhea requiring occasional medication, and 6 (<1%) had severe diarrhea requiring regular medication. Cramping postprandial abdominal discomfort was mild and necessitated no treatment in 83 patients (11.5%), or moderate, requiring periodic antispasmodic medication, in 13 (2%). Overall, at the latest follow-up, 389 with carcinoma (54%) had an excellent functional result (asymptomatic), 204 (28%) a good result (mild symptoms requiring no treatment), 108 (15%) a fair result (symptoms requiring occasional treatment), and 20 (3%) a poor result (severe dysphagia requiring regular dilatation).
Survival Rate of Patients With Carcinoma
Of the 800 patients undergoing THE for carcinoma, 764 left the hospital alive. No follow-up was available on 31 patients (4%). Patients were followed for up to 195 months after THE (mean follow-up 27 months). The Kaplan-Meier actuarial survival for the first 5 years after THE for carcinoma of the intrathoracic esophagus and cardia in these patients is shown in Figure 1. The overall 2-year survival rate was 47%; the 5-year survival was 23%. Patients with lower-third esophageal or cardia cancers had a 5-year survival rate of 26% compared with 13% for those with middle-third carcinomas and 24% for those with upper-third tumors (Fig. 2). Of 217 patients who received chemotherapy and radiation therapy before THE, 49 (23%) had T0N0 tumors (complete responders) on final pathology. The 2-year actual survival rate for these 49 patients was 86%; the 5-year survival rate was 48% (Fig. 3) . Not unexpectedly, the stage of the resected tumor was an important determinant of survival after THE: those with stage 0 or I tumors lived considerably longer than those with more advanced disease (Fig. 4, Table 5 ). The survival rate after THE for adenocarcinoma was better than after THE for squamous carcinoma. There was an overall statistically significant (p < 0.01) survival advantage for adenocarcinoma, and this advantage approached statistical significance (p = 0.06) at 5 years (Fig. 5 ).
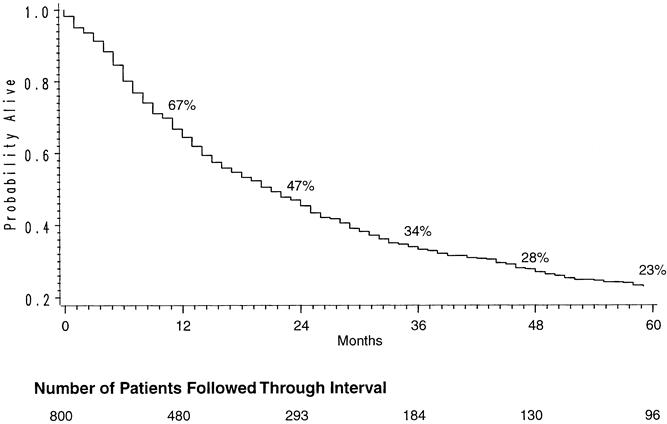
Figure 1. Kaplan-Meier actuarial survival curve of 800 patients undergoing transhiatal esophagectomy for carcinoma of the intrathoracic esophagus and cardia.
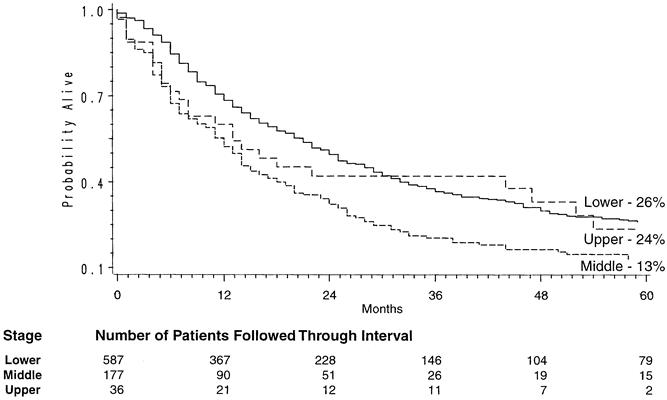
Figure 2. Site-dependent Kaplan-Meier survival curves in patients undergoing transhiatal esophagectomy for carcinoma of the intrathoracic esophagus and cardia.
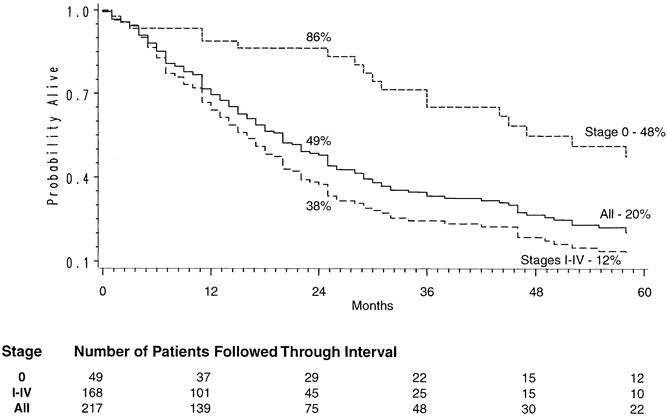
Figure 3. Kaplan-Meier survival curve in patients receiving chemotherapy and radiation therapy before transhiatal esophagectomy.
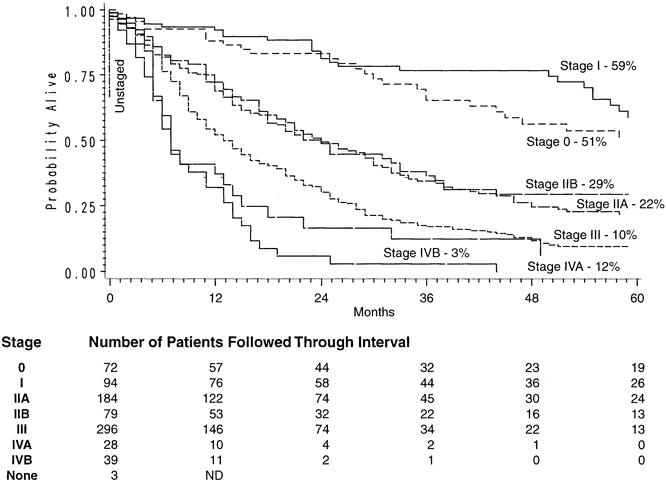
Figure 4. Stage-dependent Kaplan-Meier actuarial survival curves in patients undergoing transhiatal esophagectomy for carcinoma of the intrathoracic esophagus and cardia.
Table 5. KAPLAN-MEIER SURVIVAL AFTER TRANSHIATAL ESOPHAGECTOMY BY TUMOR STAGE
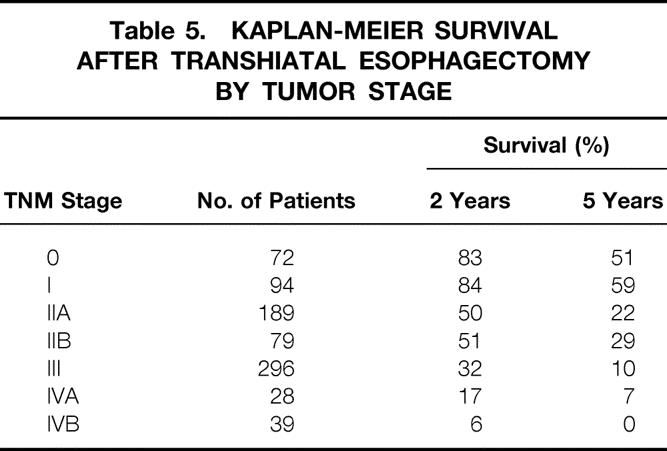
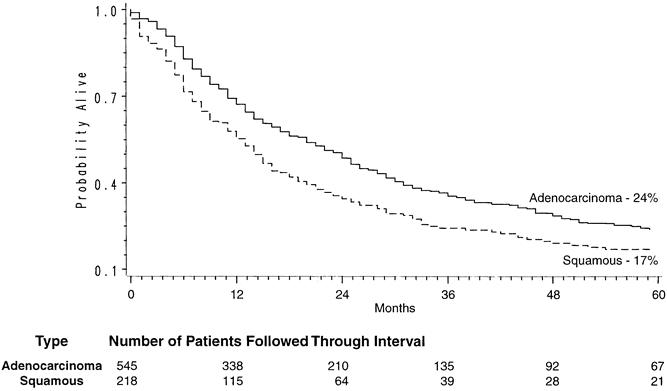
Figure 5. Histology-dependent Kaplan-Meier survival curves in patients undergoing transhiatal esophagectomy for carcinoma of the intrathoracic esophagus and cardia.
DISCUSSION
This experience with 1085 THE procedures underscores an increasingly recognized principle of surgery: outcomes after an operation inevitably improve with the number performed and the growing expertise of the surgeons involved. We have been able to perform a THE in 98.6% of all patients in whom it has been attempted over the past 20 years. Muller et al, 12 in an extensive collective review of the results of surgery in 76,911 patients with esophageal carcinoma, reported that the overall postoperative mortality rate after esophageal resection for carcinoma has been reduced by 50% in the last decade, and the lowest mortality (11% ± 8%) was in patients undergoing THE. In their collective review of the complications of THE in 1353 reported patients, Katariya et al 2 noted an overall 30-day mortality rate of 7.1%, a 1.3% incidence of massive intraoperative bleeding necessitating conversion to a transthoracic procedure, an 11.3% incidence of recurrent laryngeal nerve injury; a 50, incidence of “thoracic or pulmonary complications” (rather loosely defined as pneumothoraces, pleural effusions, pneumonias, empyemas, and respiratory failure), and a 15.1% anastomotic leak rate. Postoperative cardiac complications (arrhythmias, myocardial infarction, and pericardial tamponade) occurred in 11.9%. The more recent review by Gandhi and Naunheim 3 of the complications of THE included 1192 patients (583 from the University of Michigan series). The average reported mortality rate was 6.7%. The incidence of mediastinal hemorrhage was 3%; of recurrent laryngeal nerve injury, 9%; of respiratory complications, 12%; of anastomotic leaks, 12%; and of cardiac complications, 16%. Our data indicate that the hospital mortality rate for THE should be <5%; <1% of patients sustain major intrathoracic hemorrhage requiring conversion to a thoracotomy for control.
In our initial 10 to 15 years of experience with THE, our focus was on the technique of the esophagectomy. 6,7 Greater use of long right-angle clamps applied to periesophageal soft tissues through the retracted hiatus has allowed more mobilization of the esophagus and hemostasis under direct vision, and our current intraoperative blood loss now averages 360 cc. Our blood bank no longer cross-matches blood for transfusion for THE patients on a routine basis. Avoidance of injury to the left recurrent laryngeal nerve during the cervical portions of the procedure by retracting the tracheoesophageal groove only with a finger instead of a metal retractor has reduced the incidence of postoperative hoarseness to <3%.
In more recent years, our focus has shifted from the esophagectomy to methods of avoiding an anastomotic leak and other complications associated with a cervical esophagogastric anastomosis. Although >98% of cervical esophagogastric anastomotic leaks are successfully managed by opening the neck wound at the bedside, irrigating the wound with swallowed water, and packing the wound until the fistula closes, some patients have disastrous complications associated with this anastomosis. 13 Among the worst of these latter problems are gastric tip necrosis as a result of ischemia of the upper portion of the mobilized stomach, epidural abscess due to bacterial seeding of the intervertebral disk from a suspension suture placed between the stomach and the cervical prevertebral fascia, and a cervical abscess causing a tracheogastric fistula. Further, although the acute postoperative complications of a cervical esophagogastric anastomotic leak are clearly less than those with an intrathoracic anastomotic leak, the long-term sequelae of a cervical esophageal leak are not so minor as was initially thought. In the review article by Katariya et al, 2 postoperative anastomotic strictures after THE occurred in 14.5% of patients, almost the same number as those with an anastomotic leak (15.1%). In our experience, nearly 50% of cervical esophagogastric anastomotic leaks eventually result in an anastomotic stricture as fibrosis associated with healing occurs. Although most cervical esophagogastric anastomotic strictures can be managed with periodic outpatient esophageal dilatations with 46F or larger bougies swallowed without the need for anesthesia or sedation, the need for chronic dilatations is clearly an unsatisfactory outcome of an operation intended to restore comfortable swallowing.
The intraoperative emphasis is now on performing mobilization of the stomach and manipulation of it through the chest as atraumatically as possible. The goal is to have as healthy and pink a gastric fundus as is in the abdomen available for the cervical anastomosis. As much as possible, we minimize the amount of stomach resected with the esophagus to preserve gastric submucosal collateral circulation; creation of a gastric “tube” is avoided. A traction drain sutured to the tip of the stomach to draw it through the posterior mediastinum as initially described is now avoided. We gently manipulate the mobilized stomach upward through the diaphragmatic hiatus and into the superior mediastinum with one hand until the tip can be grasped gently with a Babock clamp inserted by the other hand through the neck incision and then the fingertips. The clamp is not ratcheted closed to minimize trauma to the stomach, and 4 to 5 cm of stomach is delivered into the neck wound more by pushing from below in the chest rather than pulling from above in the neck. No suspension sutures are placed between the tip of the stomach and the cervical prevertebral fascia to avoid ecchymosis and local gastric trauma, which were seen in the past. Rather, the anterior gastric wall is gently tacked against the posterior esophageal wall on either side of the completed anastomosis.
Over the years, consistent with other reports in the literature, 2,3 our incidence of cervical esophagogastric anastomotic leak using a variety of manual suture techniques has varied from 10% to 15%. However, during the past year, the development of a side-to-side stapled cervical esophagogastric anastomosis by the authors (unpublished observation) has been a major technical advance. The Auto Suture Endo-GIA II 30-3.5 stapler (United States Surgical Corp., Norwalk, CT) applied directly through the cervical wound creates a 3-cm-long triple-stapled anastomosis. This anastomotic technique in 114 consecutive patients has been associated with a 2.6% incidence of anastomotic leak requiring drainage, and we are now comfortable discharging patients before their 10-day postoperative barium swallow, which was our standard for years.
With the current focus on efficient medical care and cost-containment, we require that THE patients completely abstain from cigarette smoking for 2 to 3 weeks before surgery, use an incentive inspirometer during this time to provide preoperative pulmonary physiotherapy that is continued after surgery, and when possible walk 1 to 2 miles a day to condition themselves for early postoperative ambulation. This policy, combined with the use of epidural anesthesia to facilitate deep breathing, has allowed our THE patients to be extubated in the operating room. In contrast to the reports of others regarding the adverse effects on pulmonary function of THE and methods of reducing these pulmonary complications, 14,15 postoperative mechanical ventilation and an intensive care unit stay are now entirely avoided in our THE patients. The incidence of postoperative respiratory complications (atelectasis and/or pneumonia) prolonging the hospital stay beyond 10 days has been <2% in our series. Although transient intraoperative hypotension may occur during the mediastinal dissection through the diaphragmatic hiatus, a serious intraoperative “cardiac event” during THE is rare. Postoperative supraventricular arrhythmias, particularly atrial fibrillation, may occur as after any thoracic surgical procedure. The nasogastric tube is removed on the third postoperative day, after which oral intake is advanced sequentially each day from clear liquids to a puréed diet. A barium swallow is obtained on the sixth or seventh postoperative day; if the results are satisfactory, the patient is discharged. Of our most recent 91 THE patients with an uncomplicated course after a side-to-side stapled anastomosis, 79 (86.8%) have been discharged in 8 days or less after surgery (32 in 8 days, 36 in 7 days, 9 in 6 days, and 2 in 5 days).
Patients undergoing an esophagectomy and cervical esophagogastric anastomosis should be counseled regarding the probability of changes in their eating habits and digestive function. Although 50% to 60% may require early postoperative anastomotic dilatations, approximately 90% of patients undergoing THE and cervical esophagogastric anastomosis eventually achieve comfortable swallowing without the need for ongoing esophageal dilatations. For those in whom anastomotic strictures develop, this may require initial vigorous and frequent dilatation therapy, at times teaching the technique of self-dilatation, until healing of the anastomosis in a stable patent configuration occurs. Clinically significant posturally related regurgitation requiring that the patient sleep upright at night or resulting in aspiration pneumonia occurs in <5%. Varying degrees of postvagotomy dumping syndrome (postprandial cramping, diarrhea, and diaphoresis) are experienced by approximately 25% of patients but are almost always controllable with antidiarrheal medication, antispasmodics, and an antidumping high-fiber diet and subside with time.
The appropriateness of THE in patients with carcinoma still engenders some controversy on the part of the minority who believe that an aggressive mediastinal lymphadenectomy is an important aspect of the surgical treatment of esophageal cancer. The survival statistics for our patients with cancer treated with THE remain comparable with those reported after transthoracic esophagectomy: the overall survival rates were 67% at 1 year, 47% at 2 years, and 23% at 5 years. Radical en bloc esophagectomy with “complete” lymphadenectomy continues to have its advocates, 16–18 but the survival statistics after THE for carcinoma reported by several investigators are not significantly different than those after these much more invasive procedures, which are associated with considerably greater morbidity rates. 19–25 For a number of malignancies, including breast cancer and melanoma, extensive radical resections have not proven to be more beneficial than adequate local resection and lymph node sampling. It has been argued that breast cancer, a hormonally sensitive tumor, and melanoma, a cutaneous malignancy, have nothing to do with esophageal cancer, a biologically different, visceral malignancy. However, in a recent prospective randomized trial evaluating extended lymph node dissection for gastric cancer, with >300 patients in each arm of the study, there was no survival benefit for the more radical operation. 26 The finding of occult cervical nodal metastases in 35% of patients undergoing a three-field lymph node dissection for carcinoma of the thoracic esophagus deemed potentially “curable” 27 only further attests to the aggressiveness of this malignancy, in which systemic spread is so often present at the time of diagnosis. Luketich et al 28 have used a reverse transcriptase–polymerase chain reaction assay to identify micrometastases in esophageal cancer and have found that 49% of resected histologically negative lymph nodes contain micrometastases. This type of information only reinforces our belief that it is the stage of the tumor and its biologic behavior when esophageal cancer is diagnosed, not the size of the specimen or the number of lymph nodes resected, that determine survival in the vast majority of these patients.
Our group was among several to suggest that combined chemotherapy and radiation therapy before esophagectomy may alter the natural history of esophageal carcinoma. 29 Of our patients undergoing THE for carcinoma, 27% have had chemoradiation before surgery, either elsewhere or as part of our University of Michigan protocols. The 2-year survival rate of 86% and the 5-year survival rate of 48% of the 49 (23%) complete responders (T0N0 tumors) compares favorably with that of our patients treated with THE alone. Some reported phase III trials appear to be validating this policy of treating with systemic therapy a disease that is basically systemic when it is diagnosed. 30
Acknowledgments
The authors thank M. Anthony Schork, Professor of Biostatistics, and Niko Kaciroti, Research Assistant, Department of Biostatistics, School of Public Health, University of Michigan, for their assistance in the statistical analysis of these data.
Discussion
Dr. Carlos A. Pellegrini (Seattle, Washington): This paper represents the largest series reported to date on transhiatal esophagectomy by a single individual. It sets a real benchmark against which most of us can now compare results. We are, indeed, grateful to Dr. Orringer and his group for developing and for promoting this approach.
The paper shows that outcomes of this operation are directly related to volume and to experience. In fact, blood loss, pulmonary complications, laryngeal nerve injury, and anastomotic complications, as well as mortality, decreased significantly in each phase of this experience. In fact, Patti and Way (J Gastrointest Surg 1998; 2:186–192) showed that mortality for esophagectomy varies from 5% to 20% depending on the hospital volume. Thus, my first question to you, Dr. Orringer: What is a reasonable number of operations per year that a center should perform to be within a safe range of operative morbidity and mortality?
Secondly, the paper also shows that the stomach is an excellent substitute for the esophagus in patients with benign disease, as side effects were relatively rare. However, you noted that 30% of the patients complained of regurgitation when they recline. I noted that you always use a pyloromyotomy, whereas others use a pyloroplasty. Have you measured gastric emptying? Do you think that a defective emptying of the stomach may play a role in these patients? Or is there a way to make a more continent esophagogastric anastomosis at the neck?
Lastly, 30% of the patients that you operated on had undergone chemoradiation therapy preoperatively. Several other groups have reported no increases in mortality or morbidity of esophagectomy following this kind of treatment. I wonder if you have compared that in your patients.
My last question has to do with laparoscopy. Since you have advanced this field by minimizing the operative trauma involved in esophageal resection, and since you now have shown that it is so important to be delicate with all the structures, would you care to speculate on the role that laparoscopy may play in the future in the field of esophageal resection?
Presenter Dr. Mark B. Orringer (Ann Arbor, Michigan): Thank you, Dr. Pellegrini, for your very insightful questions from somebody who is obviously doing this work.
The question of the number of operations that it takes to be competent to do a transhiatal esophagectomy has no precise answer. It is clear from complications referred to us that the occasional esophageal surgeon, just as the occasional any-other-type surgeon, is more prone to the pitfalls of this operation. I would say that groups that are in the 50 esophagectomies or more per year range have the very best results.
I want to emphasize that the 30% incidence of postoperative regurgitation is not clinically significant regurgitation. We query our patients very carefully in follow-up. They are all instructed to go home and sleep with the head of the bed elevated, since the anastomosis is in the neck, and one intuitively thinks that there will be regurgitation in the recumbent position. When the patients come back to see us, they almost uniformly say that posturally related regurgitation is an uncommon problem for them. Those who had reflux preoperatively and were accustomed to sleeping with the head of their bed elevated on blocks are now sleeping on one or two pillows. If they “cheat” and eat right before they lie down at night, they may experience some regurgitation, particularly in the prone position. But for most of them, the degree of regurgitation that we record is not clinically significant, and less than 2% of our patients have had pulmonary complications of aspiration. So gastroesophageal reflux after transhiatal esophagectomy is usually very well tolerated and managed by having the patients avoid eating before bedtime and sleeping on one or two pillows. The occasional patient requires a nighttime dose of an H2 blocker before bedtime, and this eliminates the majority of any reflux symptoms that may be present.
In constructing the cervical esophagogastric anastomosis, I would like to emphasize what I believe is an important technical detail. For reasons that were not planned, we have always done the anastomosis on the anterior wall of the stomach in the neck, creating an acute angle of entry of the esophagus into the stomach and leaving some retroesophageal stomach to distend with air. I think that this provides some type of antireflux mechanism. Those who have constructed direct end-to-end anastomoses between the tip of the stomach and the esophagus have reported to me many more problems with reflux. Dr. Spencer Payne, who just passed away several months ago, talked to me a number of years ago about this problem, and after converting to an end-to-side anastomosis, he noted that his patients had far less regurgitation and reflux.
Preoperative radiation therapy: I had the same trepidation years ago just expressed by Dr. Pellegrini. It is clear that if you operate within that 3- to 5-week window of completing treatment, there is no increased morbidity and mortality. It takes cooperation between the team administering the chemotherapy and radiation therapy and the surgeons, because the oncologists are not accustomed to getting patients to the next phase of treatment—an operation. During treatment, these patients must be watched closely and adequately hydrated, using a nasogastric feeding tube when necessary if radiation esophagitis is severe. They have not tended to have any higher incidence of anastomotic leak than unradiated patients, and for the most part there has been no increased morbidity in this pretreated group.
Regarding the use of laparoscopy for transhiatal esophagectomy, Dr. Pellegrini is the pro here and I must defer to his expertise. Suffice to say, these operations now take about 3.5 hours. I don’t know if the average time can be shortened even more. Perhaps a more complete lymph node dissection and sampling might be possible with more precise visualization of the mediastinum with video-assisted techniques. There have certainly been some reported early efforts to take out the esophagus transhiatally laparoscopically, and I think that these will have to continue until we see if it really does make a difference so far as operative morbidity is concerned.
Dr. Joseph B. Zwischenberger (Galveston, Texas): I rise to congratulate Dr. Orringer for his outstanding series of over 1,000 transhiatal esophagectomies, a procedure which has become the operation of choice for many of us. I also want to acknowledge Dr. Orringer for his accomplishments as an educator and mentor in the field of general thoracic surgery and thank him personally for serving as a role model for this young surgeon. I have two comments.
First, your 3% incidence of recurrent laryngeal nerve injury is remarkably low. Please comment specifically how you avoid this usually temporary but disheartening complication.
Secondly, please comment on the role of new diagnostic modalities in the staging of esophageal cancer and how you select your patients—specifically, transesophageal echo with fine-needle aspiration, CT guided mediastinal fine-needle aspiration, and/or PET scanning.
Dr. Orringer: Recurrent laryngeal nerve injury may occur with any cervical operation, but when it happens in a patient undergoing an esophageal resection, it is not a minor problem. And it causes more than just hoarseness. The recurrent laryngeal nerve, as you know, provides partial innervation to the upper esophageal sphincter, and the incapacitating dysphagia and aspiration which some patients may experience with recurrent laryngeal nerve injury may be the most significant life-threatening complication that they will encounter after the procedure. There is no question, as you have seen with the sequential experience that we have had with recurrent laryngeal nerve injury during transhiatal esophagectomy, that if one compulsively avoids the tracheoesophageal grove during the cervical portions of the operation, the incidence of injury to the nerve is very low.
Several years after performing my first transhiatal esophagectomy, it was at an American College of Surgeons meeting that I watched a movie of me doing the operation and saw an Army-Navy retractor placed against the recurrent laryngeal nerve in the process of retracting the trachea and thyroid gland to the right. I realized that we had not been injuring the nerve in the chest beneath the aortic arch as an unavoidable consequence of the transhiatal dissection, but rather that recurrent nerve injury was occurring during the neck dissection. And since adopting a policy that only a finger may be used to retract the trachea and thyroid medially, using metal retractors only to retract the sternocleidomastoid muscle and carotid sheath laterally, the incidence of recurrent nerve injury associated with transhiatal esophagectomy is consistently under 3%.
New diagnostic modalities for staging esophageal carcinoma are coming as quickly as we can present papers on them! I think that esophageal ultrasonography may prove to be perhaps the best way to document which patients have T4 disease—that is, disease that is invading adjacent contiguous structures, the aorta and tracheobronchial tree. And for these patients, we might do well to think hard before proceeding with esophageal resection, because the survival is generally so poor—6 to 12 months at best.
The use of CT-guided fine-needle aspiration biopsy of enlarged lymph nodes constituting distant metastatic disease is a valuable preoperative staging technique. In our patients with such documented Stage IV disease, I do not proceed with an esophagectomy. This also applies to the patient with a solitary hepatic metastasis in whom the emotional argument is to proceed with esophageal resection, but the objective survival data show that such an approach is unwarranted.
The PET scan is providing a very exciting means of detecting metastatic disease that until now we could never identify preoperatively. As these staging modalities improve, I think that our results of resection will also improve because of better patient selection.
Dr. Richard J. Finley (Vancouver, Canada): Dr. Orringer, you are to be congratulated on validating the use of this operation. I have one question for you that relates to cancers occurring in the midesophagus between the level of the inferior pulmonary vein and the azygous vein. This area is difficult to visualize during a transhiatal esophagectomy in order to get a good cancer operation. With transhiatal esophagectomy, do you have an increased tracheobronchial injury rate for midesophageal cancers, and do you have an increased local recurrence rate for midesophageal cancers? What indications do you have for opening the chest or carrying out a thoracoscopic mobilization in patients with midesophageal cancers?
Dr. Orringer: Without any question, application of this technique to midesophageal carcinomas requires experience and selectivity. A careful evaluation of the tracheobronchial tree is imperative. If there is any sign of invasion of the airway by the esophageal cancer, transhiatal esophagectomy is contraindicated.
When the CAT scan shows loss of the fat plane between the airway and the esophagus or displacement of the airway anteriorly by the tumor, we anticipate that there may be a difficult transhiatal dissection. If the esophagus feels mobile at the time of palpation of the area of the tumor through the diaphragmatic hiatus, I will proceed with the transhiatal dissection. Even if the tumor is somewhat adherent to the tracheobrachial tree or the spine, it may be mobilized transhiatally with finger fracture of the involved soft tissue. I have difficulty justifying a transthoracic resection in most of these cases, because “carving” the tumor off the spine or airway and leaving several millimeters of tumor behind is no better a “cancer operation” than the transhiatal approach.
Of our relatively few patients undergoing THE who have had major intraoperative bleeding, half have in fact had middle-third tumors. So this is not a minor consideration. These tumors are technically more difficult to resect transhiatally, but with experience, this approach can be used for most.
It is also of note that our 5-year survival in patients with middle-third tumors is nearly half that in those with upper or distal third tumors. Perhaps this reflects the fact that these tumors have more unrecognized disseminated disease when we operated on them, or the fact that we are not achieving as good local tumor clearance, as is possible with a transthoracic resection under direct vision. However, local tumor recurrence has not been the cause of death in the majority of these patients; for the most part, they have died of distant metastatic disease.
The decision as to the best approach for resecting a middle-third tumor requires surgical judgment. If palpation through the hiatus reveals a mobile tumor, the transhiatal route will most often be quite adequate. Alternatively, if the surgeon feels that it is unsafe to proceed, then the transthoracic approach remains well tried and proven. In either event, a cervical esophagogastric anastomosis is still the safest option for reconstruction.
Dr. G. Alexander Patterson (St. Louis, Missouri): Thank you for asking me to discuss the paper. It is an outstanding manuscript and a marvelous contribution.
I do have one question. I don’t think this is the standard operation that most esophageal surgeons use to conduct an esophagectomy. Many are critical of this procedure because of what you just mentioned: Is it as good a cancer operation? There is a concern that the patients aren’t as adequately staged and perhaps not as adequately resected. Furthermore, it is not an easy procedure to learn how to do.
You will remember 20 years ago Dr. F.G. Pearson urged everybody to keep an open mind. Well, do you have an open mind about other procedures to conduct esophagectomy? What should be the procedure of choice? If this is the operation which should be the procedure of choice, how do we make that happen?
Dr. Orringer: I agree with you that keeping an open mind about the appropriate operation for cancer is key. And I would like to think that my mind is still open. Maybe my residents would disagree! They certainly learn how to do this operation. We have the good fortune of having a high volume of patients requiring esophageal resection, and they do a very good job with the operation when they leave our program.
What is lacking are data from good prospective trials to provide an evidence-based rationale for why we choose one operation over another. So often those that advocate, say, a radical node dissection, will present data from a highly selected series of patients, whom they compare with a group who have undergone a transhiatal approach that has been reserved for the worst-risk patients. The groups are not comparable. Or those who, like our group, performs transhiatal resections in 98% of patients requiring an esophageal resection, provide no comparable data from patients undergoing transthoracic resections. Until this is all sorted out with prospective randomized trials, which may never occur, there will not be much rationale from an objective standpoint for choosing one operative approach over another. But yes, if it is determined, for example, that patients with middle-third tumors have a better long-term survival after a transthoracic resection and mediastinal lymph node dissection, I would be more than happy to join that bandwagon!
Footnotes
Correspondence: Mark B. Orringer, MD, Section of General Thoracic Surgery, University of Michigan Medical Center, Taubman Health Care Center 2120, Box 0344, Ann Arbor, MI 48109.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978; 76: 643–654. [PubMed] [Google Scholar]
- 2.Katariya K, Harvey JC, Pina E, Beattie EJ. Complications of transhiatal esophagectomy. J Surg Oncol 1994; 57: 157–163. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi SK, Naunheim KS. Complications of transhiatal esophagectomy. Chest Surg Clin North Am 1997; 7: 601–610. [PubMed] [Google Scholar]
- 4.Orringer MB, Marshall B, Stirling MC. Transhiatal esophagectomy for benign and malignant disease. J Thorac Cardiovasc Surg 1993; 106: 265–277. [PubMed] [Google Scholar]
- 5.Orringer MB, Orringer JS. Transhiatal esophagectomy with thoracotomy: a dangerous operation? J Thorac Cardiovasc Surg 1983; 85: 72–80. [PubMed] [Google Scholar]
- 6.Orringer MB. Technical aids in performing transhiatal esophagectomy without thoracotomy. Ann Thorac Surg 1984; 38: 128–132. [DOI] [PubMed] [Google Scholar]
- 7.Orringer MB. Transhiatal esophagectomy without thoracotomy. In: Orringer MB, Zuidema GD, eds. Shackelford’s surgery of the alimentary tract, 4th ed, vol I. Philadelphia: WB Saunders; 1996: 414–445.
- 8.Orringer MB. Partial median sternotomy: anterior approach to the upper thoracic esophagus. J Thorac Cardiovasc Surg 1984; 87: 124–129. [PubMed] [Google Scholar]
- 9.Fleming ID, Cooper JS, Henson DE, et al, eds. AJCC cancer staging handbook from the American Joint Committee on Cancer Staging Manual, 5th ed. Philadelphia: Lippincott-Raven; 1998: 65–69.
- 10.Orringer MB, Bluett M, Deeb GM. Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery 1988; 104: 720–726. [PubMed] [Google Scholar]
- 11.Orringer MB, Lemmer JH. Early dilatation in the treatment of esophageal disruption. Ann Thorac Surg 1986; 42: 536–539. [DOI] [PubMed] [Google Scholar]
- 12.Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of esophageal carcinoma. Br J Surg 1990; 77: 845–857. [DOI] [PubMed] [Google Scholar]
- 13.Iannettoni MD, Whyte RI, Orringer MB. Catastrophic complications of the cervical esophagogastric anastomosis. J Thorac Cardiovasc Surg 1995; 110: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 14.Gillinov AM, Heitmiller RF. Strategies to reduce pulmonary complications after transhiatal esophagectomy. Dis Esophagus 1998; 11: 43–47. [PubMed] [Google Scholar]
- 15.Patti MG, Wiener-Kronish JP, Way LW, Pelligrini CA. Impact of transhiatal esophagectomy on cardiac and respiratory function. Am J Surg 1991; 162: 563–567. [DOI] [PubMed] [Google Scholar]
- 16.Altorki NK, Girardi L, Skinner DB. En bloc esophagectomy improves survival for stage III esophageal cancer. J Thorac Cardiovasc Surg 1997; 114: 948–955. [DOI] [PubMed] [Google Scholar]
- 17.Hagan JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106: 850–859. [PubMed] [Google Scholar]
- 18.Akiyama H, Tsurumaru M, Udagawa H, Kajiyawa Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994; 220: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolton JS, Sardi A, Bowen JC, Ellis JK. Transhiatal and transthoracic esophagectomy: a comparative study. J Surg Oncol 1992; 51: 249–253. [DOI] [PubMed] [Google Scholar]
- 20.Bolton JS, Ochsner JL, Abdoh AA. Surgical management of esophageal cancer: a decade of change. Ann Surg 1994; 51: 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gertsch P, Vauthey JN, Lustenberger AA, Friedlander-Klar H. Long-term results of transhiatal esophagectomy for esophageal carcinoma: a multivariate analysis of prognostic factors. Cancer 1993; 72: 2312–2319. [DOI] [PubMed] [Google Scholar]
- 22.Horstmann O, Verreet PR, Becker H, et al. Transhiatal esophagectomy compared with transthoracic resection and systemic lymphadenectomy for the treatment of esophageal cancer. Eur J Surg 1995; 161: 557–567. [PubMed] [Google Scholar]
- 23.Moon MR, Schulte WJ, Haasler GB, Condon RE. Transhiatal and transthoracic esophagectomy for adenocarcinoma of the esophagus. Arch Surg 1992; 127: 951–955. [DOI] [PubMed] [Google Scholar]
- 24.Sugarbaker DJ, DeCamp MM. Selecting the surgical approach to cancer of the esophagus. Chest 1993; 103: 410–414. [DOI] [PubMed] [Google Scholar]
- 25.Gluch L, Smith RC, Bambach CP, Brown AR. Comparison of outcomes following transhiatal or Ivor Lewis esophagectomy for esophageal carcinoma. World J Surg 1999; 23: 271–276. [DOI] [PubMed] [Google Scholar]
- 26.Bonenkamp JJ, Hermans J, Sasako M, van de Vekle CJH. Extended lymph node dissection for gastric cancer. N Engl J Med 1999; 340: 908–914. [DOI] [PubMed] [Google Scholar]
- 27.Altorki NK, Skinner DB. Occult cervical modal metastasis in esophageal cancer: preliminary results of three-field lymphadenectomy. J Thorac Cardiovasc Surg 1997; 113: 540–544. [DOI] [PubMed] [Google Scholar]
- 28.Luketich JD, Kassis ES, Shriver SP, et al. Detection of micrometastases in histologically negative lymph nodes in esophageal cancer. Ann Thorac Surg 1998; 66: 1715–1718. [DOI] [PubMed] [Google Scholar]
- 29.Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol 1993; 11: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 30.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335: 462–467. [DOI] [PubMed] [Google Scholar]