Abstract
Objective
The need for esophagectomy in patients with Barrett’s esophagus, with no endoscopically visible lesion, and a biopsy showing high-grade dysplasia or adenocarcinoma has been questioned. Recently, endoscopic techniques to ablate the neoplastic mucosa have been encouraged. The aim of this study was to determine the extent of disease present in patients with clinically occult esophageal adenocarcinoma to define the magnitude of therapy required to achieve cure.
Methods
Thirty-three patients with high-grade dysplasia (23 patients) or adenocarcinoma (10 patients) and no endoscopically visible lesion underwent repeat endoscopy and systematic biopsy followed by esophagectomy. The surgical specimens were analyzed to determine the biopsy error rate in detecting occult adenocarcinoma. In those with cancer, the depth of wall penetration and the presence of lymph node metastases on conventional histology and immunohistochemistry staining was determined. The findings were compared with those in 12 patients (1 with high-grade dysplasia, 11 with adenocarcinoma) who had visible lesions on endoscopy.
Results
The biopsy error rate for detecting occult adenocarcinoma was 43%. Of 25 patients with cancer and no visible lesion, the cancer was limited to the mucosa in 22 (88%) and to the submucosa in 3 (12%). After en bloc esophagectomy, one patient without a visible lesion had a single node metastasis on conventional histology. No additional node metastases were identified on immunohistochemistry. The 5-year survival rate after esophagectomy was 90%. Patients with endoscopically visible lesions were significantly more likely to have invasion beyond the mucosa (9/12 vs. 3/25, p = 0.01) and involvement of lymph nodes (5/9 vs. 1/10, p = 0.057).
Conclusions
Endoscopy with systematic biopsy cannot reliably exclude the presence of occult adenocarcinoma in Barrett’s esophagus. The lack of an endoscopically visible lesion does not preclude cancer invasion beyond the muscularis mucosae, cautioning against the use of mucosal ablative procedures. The rarity of lymph node metastases in these patients encourages a more limited resection with greater emphasis on improved alimentary function (esophageal stripping with vagal nerve preservation) to provide a quality of life compatible with the excellent 5-year survival rate of 90%.
The past two decades have brought about a dramatic change in the epidemiology of esophageal cancer. 1 Adenocarcinoma has replaced squamous cell carcinoma as the most common esophageal malignancy, and the incidence of adenocarcinoma has risen faster than that of any other malignancy in the United States. 2 Barrett’s esophagus, a known complication of gastroesophageal reflux disease, has been shown to give rise to adenocarcinoma of the distal esophagus and gastroesophageal junction through a metaplasia–dysplasia–carcinoma sequence. 3 Recognition of this relation has led to the implementation of surveillance programs and the detection of a growing number of occult esophageal adenocarcinomas in patients who have no endoscopically visible tumor. This has raised questions as to the extent of operation required to cure these patients and has prompted the use of nonsurgical therapeutic techniques to ablate the neoplastic mucosa.
Before these alternative therapies are accepted, the extent of the disease in such patients must be known so as not to compromise the benefit of early detection by incomplete removal. Our prior policy of performing an en bloc esophagectomy with systematic mediastinal and upper abdominal lymphadenectomy for early esophageal adenocarcinoma 4 provides a unique opportunity to study the extent of disease in these patients. The aim of this study was to define the extent of disease present in patients with clinically occult esophageal adenocarcinoma to define the magnitude of therapy required to achieve cure.
METHODS
Patient Population
The study population comprised two groups of patients who underwent esophagectomy by the surgical authors between Jan. 1, 1978, and Feb. 1, 1998, for adenocarcinoma of the esophagus. The first group consisted of 33 patients with Barrett’s esophagus who underwent resection for high-grade dysplasia (n = 23) or adenocarcinoma (n = 10) with no endoscopically visible lesion in the Barrett’s segment. There were 25 men and 8 women, with a median age of 70 years (range 49 to 80 years). A comparison group consisted of 12 patients who underwent resection for high-grade dysplasia (n = 1) or intramucosal adenocarcinoma (n = 11) in the absence of a grossly evident tumor mass but in the presence of a visible lesion such as a mucosal nodule or area of ulceration in the Barrett’s segment. There were 10 men and 2 women, with a median age of 64 years (range 56 to 76 years). None of the patients in the study population had a previous esophageal or gastric resection, or preoperative chemotherapy and/or radiation therapy.
Endoscopic Evaluation
The presence or absence of an endoscopically visible lesion (i.e., a mucosal nodule or area of ulceration) was confirmed on a repeat endoscopy by the operating surgeon in our unit. Patients referred with high-grade dysplasia underwent a repeat biopsy according to a prescribed protocol of four-quadrant biopsies every 2 cm over the length of the Barrett’s mucosa. Biopsy specimens were fixed in a 10% buffered formaldehyde solution and embedded in paraffin, sectioned, and mounted using standard techniques. Slides were stained routinely with hematoxylin and eosin and examined for the presence of intestinal metaplasia, dysplasia, and invasive cancer. Dysplasia was defined according to the criteria of Riddell et al. 5
Surgical Approach
Ten patients without a visible lesion and with a preoperative diagnosis of carcinoma who were physiologically fit underwent en bloc esophagogastrectomy with systematic mediastinal and abdominal lymphadenectomy. 4,6 Nine patients in the comparison group with an endoscopically visible lesion and a diagnosis of carcinoma also underwent en bloc resection. The en bloc dissection was performed through a right thoracotomy and an upper midline abdominal incision. The thoracic dissection included resection of the azygos vein, the thoracic duct, and the low paratracheal, subcarinal, paraesophageal, and parahiatal lymph nodes in continuity with the esophagus. The block of tissue removed was bounded laterally by the mediastinal pleura; anteriorly by the membranous wall of the trachea, the mainstem bronchi, the pericardium, and the diaphragm; and posteriorly by the spine and the aorta.
The abdominal dissection included removal of the proximal two thirds of the stomach, the greater omentum, the spleen, the splenic artery with its surrounding fibroareolar tissue, and the nodes in the porta hepatis, along the hepatic artery, around the celiac axis, and in the retroperitoneal tissue. In most cases, gastrointestinal continuity was established by isoperistaltic colon interposition.
The remaining 23 patients without visible lesions and with biopsy results showing high-grade dysplasia or intramucosal cancer underwent a transhiatal esophagectomy 7 (n = 20) or an esophageal stripping procedure with preservation of the vagal nerves performed through a cervical and abdominal approach without entering the thorax (n = 3). 8,9 The remaining three patients in the comparison group with endoscopically visible lesions underwent transhiatal resections because of compromised cardiopulmonary physiology or an age older than 75 years. The transhiatal operation included an abdominal lymph node dissection similar to the en bloc technique, with the exception that the spleen and the splenic artery were not resected. As many mediastinal lymph nodes were removed as this approach would allow. No lymphadenectomy was performed in patients who underwent esophageal stripping with preservation of the vagal nerves.
Analysis of Resected Specimens
Two experienced pathologists reviewed the endoscopic biopsy specimens, and in all patients they agreed on the diagnosis of high-grade dysplasia or cancer. The resected esophagus was inspected, and suspicious areas were sectioned. In addition, the complete specimen was serially sectioned at 1-cm intervals to search for occult carcinoma. When a carcinoma was found, the depth of penetration into the esophageal wall was recorded. All lymph nodes removed were identified according to the lymph node stations shown in Figure 1. The lymph nodes were fixed in formalin and cut into two 5-μm sections for staining with hematoxylin and eosin.
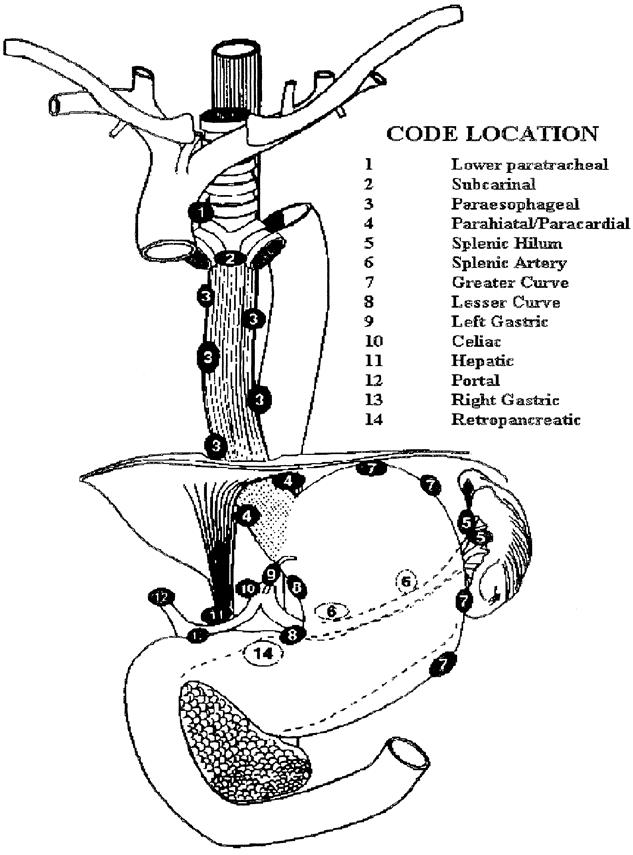
Figure 1. Lymph node staging map used to standardize node locations.
The lymph nodes from patients who underwent en bloc esophagectomy and were free of involvement on routine histology were submitted for immunohistochemical staining. Lymph nodes were cut into two 5-μm sections and stained with a cocktail of antibodies to cytokeratin AE1 and Cam 5.2 to identify the presence of micrometastatic disease. Sections from the patient’s primary tumor served as the positive control.
Follow-Up
Patients with carcinoma were followed by the operating surgeon at 3-month intervals for the first 3 years and every 6 months thereafter. The median duration of follow-up in patients with no visible lesion was 32 months (range 4 to 118 months). The median duration of follow-up in the comparison group with a visible lesion was 44 months (range 13 to 101 months). Each follow-up visit included a physical examination, a complete blood count, serum and liver chemistry panels, a carcinoembryonic antigen level, and computed tomography scans of the chest and abdomen. All surviving patients were either seen in person or contacted by telephone within 3 months of the preparation of this manuscript.
Statistical Analysis
A two-tailed Fisher’s exact test was used to compare proportions. Survival probabilities were calculated using the method of Kaplan and Meier. 10 Comparison of survival was performed using the log-rank test.
RESULTS
Accuracy of Diagnosis of Occult Adenocarcinoma
A repeat biopsy of the 23 patients without visible lesions and with an initial diagnosis of high-grade dysplasia revealed adenocarcinoma in 9 patients (39%). High-grade dysplasia was again found in the remaining 14 (Fig. 2). After esophagectomy, adenocarcinoma was found in six. The error rate for detecting early adenocarcinoma using a prescribed protocol of four-quadrant biopsies every 2 cm throughout the length of the Barrett’s segment was 43%.
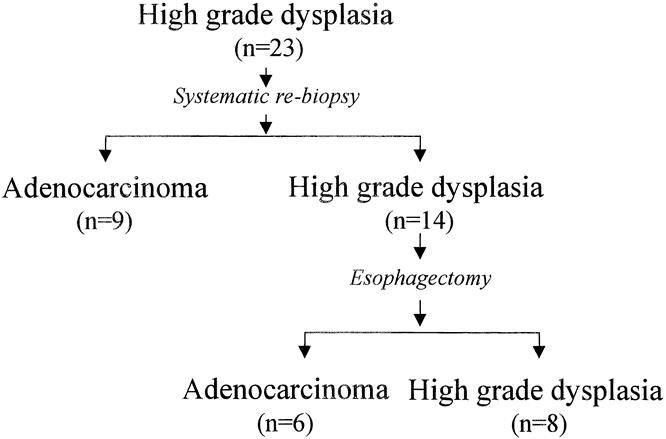
Figure 2. Diagnostic accuracy of the referral endoscopy with biopsy and the repeat endoscopy with systematic biopsy in detecting occult adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia in the absence of a visible lesion.
Depth of Tumor Penetration of Occult Adenocarcinoma
Twenty-five of the 33 patients without an endoscopically visible lesion had adenocarcinoma. The tumor was limited to the lamina propria in 22 (88%) and invaded into the submucosa in 3 (12%) (Fig. 3). In contrast, all the patients in the comparison group with an endoscopically visible nodule or ulceration were found to have adenocarcinoma in the resected esophagus. The tumor was limited to the mucosa in three; it invaded into the submucosa in five and the muscularis propria in two; and it was transmural in two. Patients with an endoscopically visible lesion were significantly more likely to have invasion beyond the lamina propria (9/12 vs. 3/25, p < 0.01).
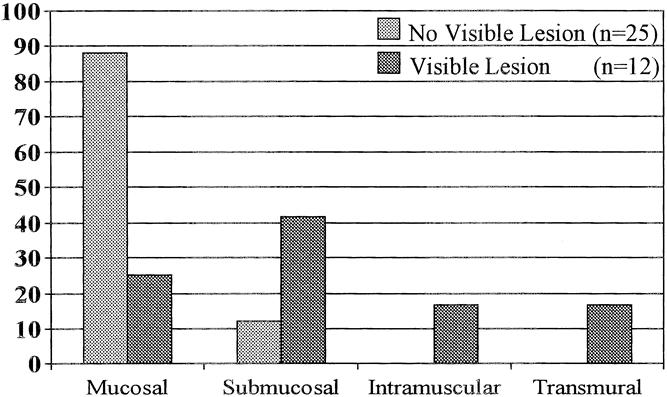
Figure 3. Comparison of depth of tumor penetration in patients with occult adenocarcinoma based on the presence of an endoscopically visible lesion.
Lymph Node Involvement in Occult Adenocarcinoma
Of the 10 patients in the study group without a visible lesion who underwent en bloc esophagectomy with systematic mediastinal and abdominal lymphadenectomy, only 1 patient had a single node metastasis. It was located along the lesser curvature of the stomach. The lymph nodes from all patients who underwent en bloc esophagectomy and were free of node metastases on routine histology were submitted for immunohistochemical analysis. A total of 323 lymph nodes were examined. There was no evidence of lymph node metastases on immunohistochemical analysis in any of the patients. Further, the remaining 46 nodes in the patient with a single histologic node metastasis showed no evidence of metastases on immunohistochemical staining.
In summary, of the 370 lymph nodes analyzed by routine histology and by immunohistochemistry in patients with carcinoma and no visible lesion, there was only one lymph node metastasis. This emphasizes the rarity of this event in patients with occult esophageal adenocarcinoma. This was substantiated by the analysis of the abdominal and lower mediastinal lymph nodes in the 12 patients with carcinoma and no visible lesion who underwent transhiatal resection. (The three patients who underwent an esophageal stripping with vagal preservation were excluded.) In these patients, a total of 109 nodes were evaluated, and all were free of node metastases.
Of the nine patients in the comparison group with an endoscopically visible lesion who underwent en bloc esophagectomy with systematic mediastinal and abdominal lymphadenectomy, three had lymph node metastases on routine histology—a single node in one patient and two nodes in the other two. As in the study group, lymph nodes from all patients who were free of node metastases on routine histology were submitted for immunohistochemical analysis. A total of 339 lymph nodes were examined. Two additional patients were identified as having involved nodes, one node in each patient. Consequently, when a visible lesion was present, lymph node involvement either by routine histology or immunohistochemistry analysis was significantly more common (5/9 vs. 1/10, p = 0.057) (Fig. 4).
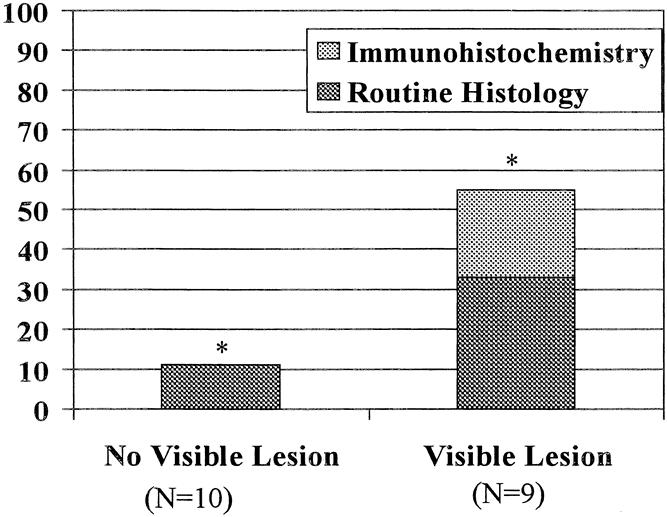
Figure 4. Relation between the presence of an endoscopically visible lesion and the presence of lymph node metastases.
Outcome
In the study group, one patient died from a pulmonary embolism, giving a 30-day surgical mortality rate of 3%. In the single patient with lymph node involvement, recurrent disease developed and the patient died at 14 months. Four additional patients died from noncancer causes. The actuarial survival rate at 5 years for patients without endoscopically visible lesions, excluding noncancer deaths, was 90%. In the comparison group, one patient died after surgery and in two recurrent disease developed, giving an actuarial 5-year survival rate for patients with endoscopically visible lesions of 82% (p = 0.52, log-rank test).
DISCUSSION
History has shown that the two pillars of cure in cancer are early recognition of the cancer and its complete removal. The institution of widespread endoscopic surveillance programs for patients with Barrett’s esophagus has provided an opportunity to achieve the first pillar by identifying those with high-grade dysplasia and those who are likely to harbor an occult carcinoma. 11 Our study emphasizes caution regarding the continued surveillance of patients with high-grade dysplasia, as some authors have suggested. 12 In these patients, we have shown that the error rate of the recommended systematic biopsy in detecting cancer remains high (43%). Consequently, widespread application of the practice of following patients with high-grade dysplasia could compromise one of the pillars of successful cancer therapy: early recognition.
The need for esophagectomy, with its attendant death and complications, in patients with high-grade dysplasia or occult carcinoma has been questioned, and endoscopic techniques to ablate the suspected mucosa have been encouraged. 13,14 Widespread adoption of these alternative treatments would be premature before the extent of neoplastic disease in such patients is known, and the two therapies have been compared. In the present study, we analyzed 25 patients who on preoperative endoscopy did not have a visible lesion, but did have adenocarcinoma in the surgical specimen. Although most had tumors confined to the mucosa, 12% had tumors that penetrated into the submucosa. In those with endoscopically visible lesions, such as mucosal nodules or ulcers, deeper invasion was the rule. Therapy designed with a depth of tissue destruction limited to the mucosa would be inadequate in some of the patients with the former condition and the majority of patients with the latter condition. Consequently, widespread application of these mucosal ablative techniques in either group could compromise the second pillar of cancer therapy: complete removal.
The currently popular therapeutic approach for patients with no visible lesion and a biopsy showing high-grade dysplasia is mucosal ablation by photodynamic therapy. Overholt et al 15 recently reported on a large series of patients treated with photodynamic therapy, including 73 patients with high-grade dysplasia. These results are compared with our results with esophagectomy in Table 1. The authors did not report how thoroughly those who received photodynamic therapy were investigated before therapy, but we assume that none of the patients had endoscopically visible lesions. It is impossible to know how many had occult tumors, because the mucosa was ablated, but it is reasonable to expect a prevalence, based on surgical studies, of 43% to 65%. With short follow-up, two patients have emerged with cancer, and both underwent an esophagectomy. Both had lymph node metastases, suggesting that the tumor had advanced between the time of photodynamic therapy and its detection. The incomplete removal of the Barrett’s mucosa, the frequent persistence of dysplasia, the occurrence of subsquamous persistence of Barrett’s mucosa, and the subsequent development of cancers after photodynamic therapy emphasize the need for restrained enthusiasm for this novel therapy. Esophagectomy, in contrast, removes the abnormal mucosa along with the full thickness of the esophageal wall. Consequently, persistence of Barrett’s mucosa, dysplasia, squamous overgrowth, and subsequent cancer development are not seen.
Table 1. RESULTS OF PHOTODYNAMIC THERAPY AND ESOPHAGECTOMY IN PATIENTS WITH HIGH-GRADE DYSPLASIA
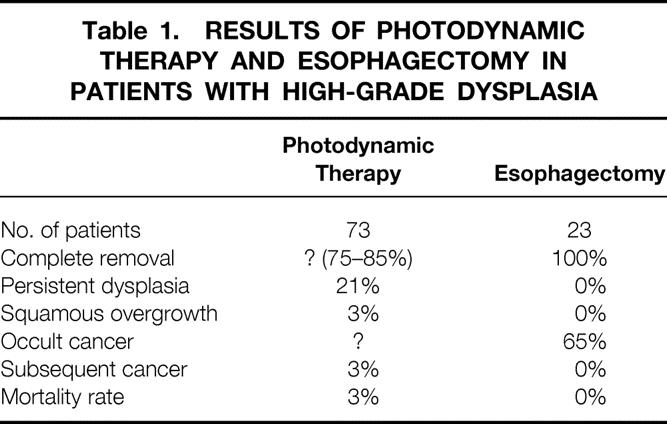
The current study is unique in providing knowledge about the extent of lymph node disease in patients with occult esophageal carcinoma. Detailed analysis of lymph nodes obtained by systematic mediastinal and abdominal lymphadenectomy has shown only one patient with nodal involvement when there was no endoscopically visible lesion present. Further, there was only one positive lymph node, by either routine histology or immunohistochemical staining, out of a total of 370 nodes removed by en bloc resection. This suggests that systematic lymph node dissection is unnecessary in these patients and encourages a more limited resection with greater emphasis on improved postoperative alimentation and a quality of life compatible with the excellent cure rate that can be achieved. An esophageal stripping with vagal nerve preservation and colon interposition achieves this goal. It can be done without manually entering the chest and provides exceptionally good alimentary function.
In contrast, the presence of a visible lesion such as an ulcer or mucosal nodule in the Barrett’s segment and a biopsy showing high-grade dysplasia or intramucosal cancer should prompt more aggressive therapy. Three quarters of these patients will have tumors that penetrate beyond the lamina propria, and more than half will have evidence of metastatic spread to the lymph nodes. In these patients, an en bloc esophagectomy with removal of all potentially involved nodes should be performed.
In conclusion, patients with Barrett’s esophagus and high-grade dysplasia commonly harbor adenocarcinomas that are not endoscopically visible. Endoscopy with systematic biopsy in these patients has an error rate in detecting carcinoma of 43%. The observation that these cancers can invade beyond the muscularis mucosae cautions against the use of mucosal ablative procedures. The rarity of lymph node metastases in these patients encourages a surgical procedure that removes the entire esophageal wall while retaining vagal innervation of the intact stomach and distal gut to preserve alimentary function that is compatible with their excellent life expectancy.
Discussion
Dr. Carlos A. Pellegrini (Seattle, Washington): This is a very timely presentation that addresses the issue of appropriate management of a patient with diagnosis of high-grade dysplasia. I have a little bit of concern about the overall interpretation of your paper by the general surgical community, and that is the essence of what I would like you to address at the end of my comments. If one looks at what is the appropriate management of the patient initially diagnosed with high-grade dysplasia, the surgical literature published over the last 10 years suggests that about 45% of these patients have an occult adenocarcinoma. The biopsy shows only high-grade dysplasia. That is the rationale for a resection.
On the other hand, there is data from our institution that shows that if you take the other approach, and you observe these patients carefully with repeated biopsies over time, only a few develop a tumor, and they are all curable. For example, of 29 patients followed for 18 months, 7 developed a cancer, but 22 eventually went on without having an esophagectomy (Levine et al, Gastroenterology, 1993; 105:40–50). That is supported by another study that showed that in over 40 patients, again diagnosed with high-grade dysplasia and followed over a 7-year period, only six patients developed cancer, and they were operated at what was thought to be a curative time (Gastroenterology 1996; 110:A590).
The concern I have is the risk of the esophagectomy. One is putting 55% of patients through an operation that has somewhere between a 4% and a 20% mortality. And I am concerned with the interpretation of your data in the sense that if now we try to apply the treatment you propose, namely resection and the colonic interposition, the mortality may be even greater.
My personal approach, and I would like you to comment on that, has been as follows: When we get someone with high-grade dysplasia, we rebiopsy. We frequently find that those patients referred with high-grade dysplasia do not have high-grade dysplasia. We then place the patients on an aggressive antireflux regimen for 2 months, and then rebiopsy. Some of those patients fall off at that time. We use endosonoscopy extensively, to determine whether there is any nodularity, or anything that is not quite visible. Eventually, a small group will have high-grade dysplasia. We carefully evaluate them for esophagectomy, but are willing to follow them carefully if they so wish. Sometimes it is a matter of discussing the patient’s preferences with the patient.
Can you tell us how many patients originally referred to you with high-grade dysplasia were eventually found not to have any dysplasia? Do you agree with the management I outlined?
Presenter Dr. Jeffrey A. Hagen (Los Angeles, California): Your comments about the suggestion that repeat endoscopy and systematic biopsy can allow these patients to be safely followed are timely. This is an alternative approach, advocated by some, to the routine policy of resecting these patients that we have employed. Addressing this issue was one of our major points in looking at this population of patients. We found that even with an aggressive systematic biopsy protocol, performed by the operating surgeons, there are substantial numbers of patients who have cancer that remains occult. We have not followed these patients with high-grade dysplasia as you propose, so I cannot specifically comment on the number who will ultimately develop visible cancers over time.
Following patients with biopsy proven high-grade dysplasia, as you propose, might be a dangerous approach. In our experience, in the absence of a visible lesion at endoscopy, nearly half already have cancer in spite of two sets of detailed biopsies that show only high-grade dysplasia. The risk of occult cancer appears to be even higher in the presence of visible abnormalities such as mucosal nodules or areas of ulceration. These patients, who have progressed to the next step on the pathway from Barrett’s esophagus to invasive cancer, commonly have tumors that penetrate deeply into the esophageal wall, often with lymph node metastases. The issue then is not so much how many will ultimately develop cancer, but rather, how do we effectively deal with those we know are likely to harbor cancer that we simply cannot prove is present prior to resection. Following these patients until the cancer becomes visible at endoscopy, as they surely will, has the potential of converting a situation where a simple procedure of removal of the esophagus can be turned into a situation where you are dealing with frankly invasive cancer and lymph node spread. For this reason, it remains our policy to approach all of these patients surgically.
The mortality in our series of patients operated on for high-grade dysplasia was 3%. This figure includes the 19 patients who underwent resection and colon interposition, in addition to those resected by the transhiatal approach and those who underwent the esophageal stripping procedure. We have not found an increase in mortality in association with the addition of a colon interposition. We do acknowledge, though, that there is going to be some variability in mortality figures across the country. However, I think approaching these patients surgically is justifiable, if resection can be done with mortality figures such as these.
In this regard, the esophageal stripping procedure with vagal nerve preservation is exciting. We have performed this new procedure in three patients in this series, none of whom have died. Of course, this is a very small sample, early in our experience with this new approach. The point is, though, that it does not appear to be a very invasive operation. It is done through the neck and the abdomen without entering the chest. In terms of the physiologic insult, it is basically an extended colon resection. We are optimistic that with larger numbers of patients, the advantages over conventional transthoracic or even transhiatal operations will become evident.
The approach you have outlined places emphasis on endoscopic ultrasound to aid in the detection of occult tumors in patients with high-grade dysplasia. While not all patients in this series had endoscopic ultrasound, we did not find it to be helpful in those who had EUS performed. Higher frequency ultrasound may allow us to do this better in the future, but for now it is usually difficult to find any evidence of tumor on ultrasound when no tumor is evident on endoscopy.
Dr. John Hunter (Atlanta, Georgia): This provides us accurate cancer staging in patients with endoscopically invisible adenocarcinoma. We can’t see the cancer but the experienced pathologist says there is high-grade dysplasia, we now know we must consider the cancer to be present. We now know that the likelihood of finding a positive lymph node is one in 370. And interestingly, of course, that patient died of disseminated disease despite an en bloc esophagectomy. Most importantly, we now know that the risk of finding muscular invasion is about 12% in endoscopically invisible cancer.
The first frequency is one quarter of 1%, is probably inconsequential, and radical surgery may not make a difference anyway. The second frequency, that of muscularis invasion, is a great concern for those advocating mucosal ablation techniques to treat those with high-grade dysplasia. So the first question really echoes on what you have already partially answered, and that is, will endoscopic ultrasound pick up those 12%? Because if it doesn’t, then any mucosal ablated techniques shouldn’t be pursued because they are bound to fail.
Secondly, your group has evolved toward less extirpative procedures for high-grade dysplasia through en bloc esophagectomy to transhiatal, and now the vein-stripping esophagectomy. What are your indications for performing the vein-stripping esophagectomy? Is it substantially better than THE in terms of your observations of alimentary function?
I would also like to know, because I know that you use USC or you are interested in pursuing ablation of Barrett’s esophagus: Do you perform this procedure in high-risk patients? If so, what method do you use for ablation and what are your results?
Lastly, this study suggests that 88% of patients might be cured with mucosal ablation if those T2 lesions can be identified preoperatively and if all mucosal ablation or mucosal ablation techniques remove all the metaplastic and dysplastic epithelium. So the only way to answer this question as far as I know is to perform ablation in patients with high-grade dysplasia, then examine the entire specimen by performing esophagectomy. I am afraid that we will find the current methods of ablation are woefully inadequate.
Dr. Hagen: I agree that it would be very interesting to look at patients who have had an ablation procedure followed by an esophagectomy. I am sure that we would find a lot of mucosa left behind, and I have concerns about the depth of ablation by presently available techniques.
It has been our experience that endoscopic ultrasound is of no benefit in identifying patients with tumors that invade beyond the mucosa. We have not been able to find tumors in most of these patients when ultrasound was done, and have not reliably been able to determine when submucosal invasion was present. Many of these are very small microscopic foci of tumor. Again, these are patients with no endoscopically visible lesion, no ulcers, no nodules, and no raised areas. They have perfectly normal bland-looking Barrett’s esophagus. While we have not used ultrasound systematically in all patients with high-grade dysplasia, I am not at all optimistic that present ultrasound techniques will be very helpful in identifying those with early cancers.
Our indications for the vagal-sparing esophageal stripping procedure include patients who have Barrett’s esophagus with biopsy-proven high-grade dysplasia or intramucosal cancer with no endoscopically visible lesion. I think it would be a mistake to perform such a limited procedure in patients who have any suggestion of a lesion in the Barrett’s segment, including ulcers or small mucosal nodules, because these patients have been shown to have a relatively high frequency of deeper tumor invasion and lymph node metastases. This type of technique may incompletely resect these patients, with tumors that extend beyond the esophageal mucosa.
It has been our experience, in patients who have undergone the esophageal stripping procedure thus far, that alimentary function is significantly better on short-term follow-up. These patients come back to the office a few weeks or a month after surgery eating pretty much normally. This, of course, is not the case with most esophageal resection and reconstructions. At present, the number of patients operated on with this technique is still quite small, but we intend to study these patients further with a detailed analysis of alimentary function.
Our main interest in mucosal ablation at USC is with the CUSA technique. We limit this approach, however, to patients with nondysplastic Barrett’s. We have not applied this procedure, and are hesitant to do so, in patients who are identified with high-grade dysplasia, because of the high frequency of cancer in these patients: tumors that can invade beyond the mucosa and potentially involve local lymph nodes.
Dr. Hiroshi Akiyama (Tokyo, Japan): I think Dr. Hagen’s presentation was super because the strategy was constructed based on clinical evidence. In order to evaluate depth of wall penetration, endoscopic ultrasonography is most desirable. Therefore, we routinely perform endoscopic ultrasonography and, according to the findings, endoscopic mucosal resection or en bloc esophagectomy is chosen for therapy. My first question is if you use endoscopic ultrasonography preoperatively for analysis of wall penetration—I mean for visible lesions.
Secondly, in comparison with squamous cell carcinoma, lymphatic spread is much more frequent than that seen in adenocarcinoma, particularly in submucosa cancers. My second question is if the case with positive nodes was in mucosal cancer or submucosal cancer?
I am glad to hear that you are making such strategies as similar to ours in squamous cell carcinoma, namely, en bloc esophagectomy with nodal dissection was done in order to find tumor extension. Then we can select cases for limited esophageal resection; finally, we can improve postoperative function.
My philosophy is that anything we can preserve should be preserved. Preservation of vagal nerves in early cancer is one such example. This type of esophageal reconstruction in terms of a QOL is, I believe, stomach in situ, in position—and vagal preservation. This type of reconstructive surgery has excellent quality of life, with no diarrhea. I am glad that you agree with this reconstructive surgery.
Dr. Hagen: Thank you, Dr. Akiyama, for your comments. You have led the way in performance of the vagal-sparing esophagectomy in patients with early cancers, and have a lot more to say about it in terms of long-term function than we do. Again, I appreciate your comments.
Endoscopic mucosal resection is something that has been done for early squamous cancers, but as far as using this technique in Barrett’s with high-grade dysplasia or occult cancer, we do not as yet have any experience with this technique. As a consequence, I can’t really comment any further regarding the potential value of this technique in patients with early Barrett’s cancers.
Endoscopic ultrasonography was nearly always done in this series of patients when a lesion was visible endoscopically. It has been our experience in these patients, just as it is for patients with bulky tumors, that the sensitivity and specificity leaves something to be desired for early cancers. We have found it very difficult to differentiate between mucosal and submucosal disease with the presently available ultrasound equipment. The reliability of ultrasound is much better in differentiating patients with transmural tumors from those confined to the esophageal wall, but separation among the subgroups of T1 disease is very difficult.
Finally, you asked about the one patient who had a single node metastasis at the time of resection. She had a tumor that extended into the submucosa, in spite of the fact that no lesion was visible endoscopically.
Dr. Jeffrey L. Ponsky (Cleveland, Ohio): Thank you for a very provocative paper. I think you have convinced most of us that there is no place for mucosal ablation in high-grade dysplasia.
Reviewing the techniques of mucosal ablation available presently, what we found is that there is spotty ablation, and indeed when esophagectomy is done we sometimes actually cover up areas of dysplastic Barrett’s esophagus and put it underneath a layer of squamous mucosa, making it harder to find.
My question for you is, in the future if we are able to develop better techniques of mucosal ablation, how do you perceive them being used, perhaps in earlier forms of dysplasia before waiting for the late dysplasia—the severe dysplasia—which seems to indicate esophagectomy?
Dr. Hagen: The issue of subsquamous persistence of Barrett’s esophagus or squamous overgrowth is a very important question as we discuss the outcome of mucosal ablative techniques. That, of course, is not an issue if you take the esophagus out. For mucosal ablation to be acceptable treatment for patients with high-grade dysplasia, we need both a reliable means of removing all of the mucosa and a reliable way to detect invasion into the submucosa or beyond. Perhaps vital staining techniques or higher frequency ultrasound may be of benefit here in the future. At the present time, however, concerns regarding the deeper tumor penetration are quite real, and our ability to detect them is limited.
Patients with nondysplastic Barrett’s, I think, are the optimal candidates for mucosal ablation. If you can get rid of all of the nondysplastic Barrett’s mucosa, and effectively control all of the reflux—perhaps with an antireflux operation—this may be the ideal approach, the goal being the prevention of the development of high-grade dysplasia in the first place.
Dr. Alan G. Johnson (Sheffield, England): In the Sheffield prospective randomized trial of photodynamic therapy in moderate dysplasia, although there was a significant decrease in the amount of area of Barrett’s esophagus, the pathologic finding was that the dysplasia actually disappeared, even in those areas where there was still abnormal mucosa. In an open study, high-grade dysplasia was also reversed. There is a real prospect of using PDT in mild and moderate dysplasia.
Have you any experience with resecting the esophagus of patients who have had PDT in the past for moderate or severe dysplasia? Have you seen what happened to the dysplasia in those patients?
Dr. Hagen: We have not had any experience resecting patients for less than high-grade dysplasia, so I can’t really comment on that. In addition, we haven’t, as of this date, resected any patients who have had prior photodynamic therapy. These remain open questions in our experience.
The only additional comment I would make is to emphasize that we do not object to the efficacy of photodynamic therapy, or some of the other ablative techniques, on the mucosa itself in patients with high-grade dysplasia. It may indeed be proven in time that such techniques as PDT are quite effective in treating the dysplastic Barrett’s epithelium. Rather, it is the finding of occult cancers in nearly half of these patients that concerns us. As we have shown, without any evidence of a visible lesion, 12% will have tumors that invade beyond the mucosa. When there is an ulcer or nodularity present, more than half will have tumor that invades deeper into the esophageal wall. In many cases, there may also be involved lymph nodes. Therein lies our main objection to mucosal ablation, not necessarily whether or not these techniques are effective in removing the dysplastic mucosa itself. Rather, we are concerned that when tumor is present, it not uncommonly invades beyond the point where therapy focusing on the mucosa alone can ensure adequate treatment for the disease present.
Footnotes
Correspondence: Jeffrey A. Hagen, MD, University of Southern California, Dept. of Cardiothoracic Surgery, 1441 Eastlake Ave., Suite 7418, Los Angeles, CA 90033-4612.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Wang HH, Antonioli DA, Goldman H. Comparative features of esophageal and gastric adenocarcinomas: recent changes in type and frequency. Hum Pathol 1986; 17: 482–487. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Clapp RW, Doos WG, Spechler SJ. The increasing frequency of adenocarcinoma of the esophagus. Cancer 1989; 64: 526–530. [DOI] [PubMed] [Google Scholar]
- 3.Hameeteman W, Tytgat, GN, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma [see comments]. Gastroenterology 1989; 96 (5 Pt 1): 1249–1256. [DOI] [PubMed] [Google Scholar]
- 4.Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Sur 1993; 106: 850–859. [PubMed] [Google Scholar]
- 5.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983; 14 (11): 931–968. [DOI] [PubMed] [Google Scholar]
- 6.Hagen JA, DeMeester TR. En bloc oesophagectomy for cancer of the distal oesophagus, cardia and proximal stomach. In: Jamieson GG, Debas HT, eds. Surgery of the upper gastrointestinal tract. London: Chapman & Hall; 1994: 214–229.
- 7.Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Sur 1978; 76: 643–654. [PubMed] [Google Scholar]
- 8.Akiyama H, Tsurumaru M, Kawamura T, Ono Y. Esophageal stripping with preservation of the vagus nerve. Intl Surg 1982; 67 (2): 125–128. [PubMed] [Google Scholar]
- 9.Akiyama H, Tsurumaru M, Ono Y, Udagawa H, Kajiyama Y. Esophagectomy without thoracotomy with vagal preservation. J Am Coll Surg 1994; 178 (1): 83–85. [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–462. [Google Scholar]
- 11.Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Sur 1994; 108: 813–822. [PubMed] [Google Scholar]
- 12.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus [see comments]. Gastroenterology 1993; 105 (1): 40–50. [DOI] [PubMed] [Google Scholar]
- 13.Overholt BF, Panjehpour M. Barrett’s esophagus: photodynamic therapy for ablation of dysplasia, reduction of specialized mucosa, and treatment of superficial esophageal cancer [see comments]. Gastrointest Endosc 1995; 42: 64–70. [DOI] [PubMed] [Google Scholar]
- 14.Sampliner RE, Jaffe P. Malignant degeneration of Barrett’s esophagus: the role of laser ablation and photodynamic therapy. Dis Esoph 1995; 8: 104–108. [Google Scholar]
- 15.Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett’s esophagus: follow-up in 100 patients [see comments]. Gastrointest Endosc 1999; 49 (1): 1–7. [DOI] [PubMed] [Google Scholar]


