Abstract
Objective
To summarize the 6-month follow-up of a cohort of patients with clinically significant coronary artery disease who received direct myocardial injection of an E1−E3− adenovirus (Ad) gene transfer vector (AdGVVEGF121.10) expressing the human vascular endothelial growth factor (VEGF) 121 cDNA to induce therapeutic angiogenesis.
Background
Therapeutic angiogenesis describes a novel approach to the treatment of vascular occlusive disease that uses the administration of growth factors known to induce neovascularization of ischemic tissues.
Methods
Direct myocardial injection of AdGVVEGF121.10 into an area of reversible ischemia was carried out in 21 patients as an adjunct to conventional coronary artery bypass grafting (group A, n = 15) or as sole therapy using a minithoracotomy (group B, n = 6).
Results
No evidence of systemic or cardiac-related adverse events related to vector administration was observed up to 6 months after therapy. Trends toward improvement in angina class and exercise treadmill testing at 6-month follow-up in the sole therapy group suggest the effects of this therapy are persistent for ≥6 months.
Conclusions
This study suggests that direct myocardial administration of AdGVVEGF121.10 appears to be well tolerated in patients with clinically significant coronary artery disease. Initiation of phase II evaluation of this therapy appears warranted.
Therapeutic angiogenesis is a novel experimental strategy for treating myocardial ischemia in which neovascularization of ischemic tissues is accomplished by the administration of mediators known as “angiogens” that induce the formation of blood vessels. 1,2 This strategy is based on the following observations:
1. The native biologic response to vascular occlusion involves the formation of collateral vessels that serve to bypass these obstructions.
2. Upregulation of the expression of naturally occurring angiogens and their cognate receptors appears to underlie the process of collateral vessel formation.
3. The native process of collateral vessel formation is characteristically incomplete in relieving ischemia secondary to atherosclerotic vascular occlusive disease. 3,4
Percutaneous transluminal coronary angioplasty and coronary artery bypass grafting (CABG), the current interventional therapies for treating atherosclerotic coronary artery disease (CAD), are of limited benefit in patients with severe or diffuse disease and do not adequately address the issue of restenosis. Therapeutic angiogenesis may therefore be a valuable adjunct to conventional interventional strategies for treating CAD.
Of the many polypeptide angiogens implicated as catalysts of angiogenesis, vascular endothelial growth factor (VEGF), a protein coded by a seven-exon gene localized on chromosome 6, appears to be one of the most important. In this context, deletion of the VEGF and VEGF receptor genes in knockout models results in lethal embryonic abnormalities, and VEGF has been demonstrated to induce vigorous collateral vessel formation in several models. 1–8
One approach to therapeutic angiogenesis is gene therapy, a drug-delivery strategy by which the coding sequences for specific angiogens can be delivered to targeted tissues such as the myocardium to enable cells making up the tissue to produce and secrete a selected angiogen such as VEGF. 7–9 Replication-deficient recombinant adenovirus (Ad) gene transfer vectors have been shown in animal models to be particularly advantageous for delivering angiogens such as VEGF to target tissues such as the myocardium, in that Ad vectors provide high levels of localized expression of the angiogen for approximately 1 to 2 weeks, a duration demonstrated in experimental animals to be sufficient to induce angiogenesis but not long enough to evoke abnormal blood vessel formation. 7,10–13
The present study evaluates the administration of AdGVVEGF121.10, an E1−E3− Ad vector expressing the 121-amino-acid form of human VEGF, to patients with clinically significant CAD. We have previously reported the early (30-day) results with this cohort, in which this therapy appeared to be well tolerated. 14 This report summarizes the 6-month results of this phase I trial.
METHODS
AdGVVEGF121.10
The clinical-grade Ad gene transfer vector AdGVVEGF121.10 (GenVec, Inc., Rockville, MD) is made up of an Ad5 serotype backbone, with deletions in the E1 and E3 regions. 7 The expression cassette in the E1 region contains (right to left orientation) the cytomegalovirus early/immediate enhancer/promoter, an artificial splice sequence, the human VEGF121 cDNA, and the SV40 polyA/stop signal. AdGVVEGF121.10 was produced and stored at −70°C as previously described. 14,15 At the time of vector delivery, the vector was thawed, immediately diluted, drawn up as 100 μl in 1-ml insulin syringes with a 27-gauge needle (Becton Dickinson, Franklin Lakes, NJ), and placed into a sterile container for transport to the operating room.
Study Design
Two groups were evaluated. In group A (adjunct group), the AdGVVEGF121.10 vector was administered during conventional CABG surgery by direct intramyocardial injection to an ischemic area that could not be bypassed. In the patients in group B (sole therapy), in whom CABG could not be carried out because there was a lack of suitable bypass graft targets, the vector was administered as a sole therapy by direct intramyocardial injection through a minithoracotomy to an area of ischemic myocardium. 14 The inclusion criteria for groups A and B included men and women ages 18 to 85 with demonstrable reversible left ventricular ischemia, as assessed by rest and stress 99mTc-sestamibi nuclear medicine studies. Twenty-four-hour Holter monitoring was used to exclude patients with life-threatening arrhythmias. Other inclusion criteria included room air PO2 > 60 torr, PCO2 < 50 torr, forced expiratory volume in 1 second > 1.2 L, hematocrit >30%, white blood cell count <10,000, blood urea nitrogen <40 U/L, and creatinine <2.5 g/dl. Exclusion criteria included ejection fraction <25% for group A and <30% for group B.
Ten injections of AdGVVEGF121.10 (100 μl per injection; each site 1 to 1.5 cm apart) were administered by direct myocardial injection to both group A and B patients in a single coronary myocardial territory demonstrating reversible ischemia by 99mTc-sestamibi perfusion scan with or without adenosine stress. For group A, the CABG procedure was performed through a standard median sternotomy, with the vector administered directly to the myocardium during cardiopulmonary bypass, but after rewarming to 36°C after the completion of bypass grafting. Group A total doses were increased in half-log increments from 4 × 108 particle units (pu) to 4 × 1010 pu (n = 3 per dose group). For group B, the myocardium was reached through a small (4- to 5-cm) thoracotomy. The vector (total dose 4 × 109 pu per patient) was injected under direct visualization into the beating heart into the region of reversible ischemia identified by 99mTc-sestamibi scanning.
General Safety Parameters
Routine blood parameters including complete blood count (white blood count, hemoglobin, hematocrit, and platelet count), electrolytes, creatinine, blood urea nitrogen, and lactic dehydrogenase were used as indirect measures of systemic toxicity. With the knowledge that 90% of Ad vectors delivered systemically in animal models are taken up by the liver, liver function tests were serially evaluated, including aspartate transferase, alkaline transferase, bilirubin (total, direct, indirect), alkaline phosphatase, and albumin. Systemic vector-specific parameters included anti-Ad5 neutralizing antibody titers by wild type Ad5, as previously described. 16,17
Cardiac-Specific Parameters
Several parameters were examined at 6 months to assess the cardiac-specific effects of direct myocardial injection of AdGVVEGF121.10 to compare the previously reported preoperative and 1 month posttherapy data. 14 Creatine phosphokinase (CPK; CPK-MB if the total level of CPK was abnormal) was used as a measure of cardiac toxicity. Evidence of myocardial ischemia, infarction, or arrhythmia was assessed by serial electrocardiography. The degree of angina was assessed by direct questioning of the patient, using a 1 to 4 scale according to the Canadian Cardiovascular Society classification. 18 Weekly nitroglycerine intake was similarly determined.
Exercise tolerance testing was performed according to a modified Bruce protocol. 19 Peak heart rate, peak heart rate × peak systolic blood pressure, and ST/HR slope (maximal rate of change of ST depression with respect to heart rate, by linear regression) were determined using conventional methodology. 19
Statistical Analyses
The number of patients at each dose in group A (n = 3) and the total number of patients in group B (n = 6) are too small to provide sufficient statistical power to discriminate within the variability of the various parameters that were assessed. Thus, lack of statistical significance may not necessarily be interpreted as “no difference.” In this context, the results are presented without formal error estimates and are presented in the context of trends suggested by the data.
RESULTS
General Outcome
The demographics and cardiac risk factors of these patients have been reported previously and were typical of the general CABG population. 14 All coronary territories were treated in group A, whereas injections in group B were limited to the left ventricle free wall. Vector administration was well tolerated in both patient groups. 14 There were no complications or late deaths related to vector administration in either cohort. There have been no additional deaths other than the three originally reported. 14 Patients A13 (adjunct group, dose 4 × 1010 pu) and B1 (sole therapy group, dose 4 × 109 pu) underwent subsequent percutaneous transluminal coronary angioplasty, and patient B1 underwent subsequent CABG on postoperative day 130.
No abnormalities were found in any blood tests at the analysis performed 3 or 6 months after surgery (Table 1). Serum anti-Ad5 neutralizing antibody levels had decreased toward baseline by 6 months (Fig. 1).
Table 1. SERUM PARAMETERS COMPARING BASELINE TO 6 MONTHS AFTER SURGERY*
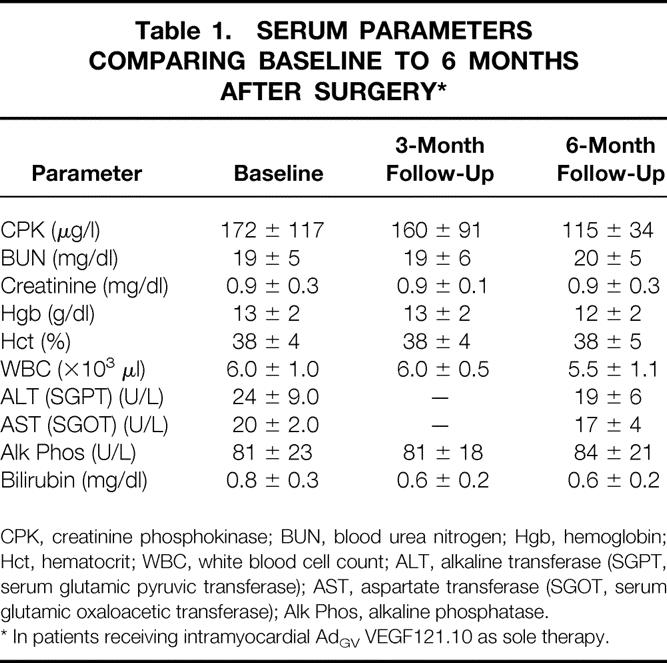
CPK, creatinine phosphokinase; BUN, blood urea nitrogen; Hgb, hemoglobin; Hct, hematocrit; WBC, white blood cell count; ALT, alkaline transferase (SGPT, serum glutamic pyruvic transferase); AST, aspartate transferase (SGOT, serum glutamic oxaloacetic transferase); Alk Phos, alkaline phosphatase.
* In patients receiving intramyocardial AdGV VEGF121.10 as sole therapy.
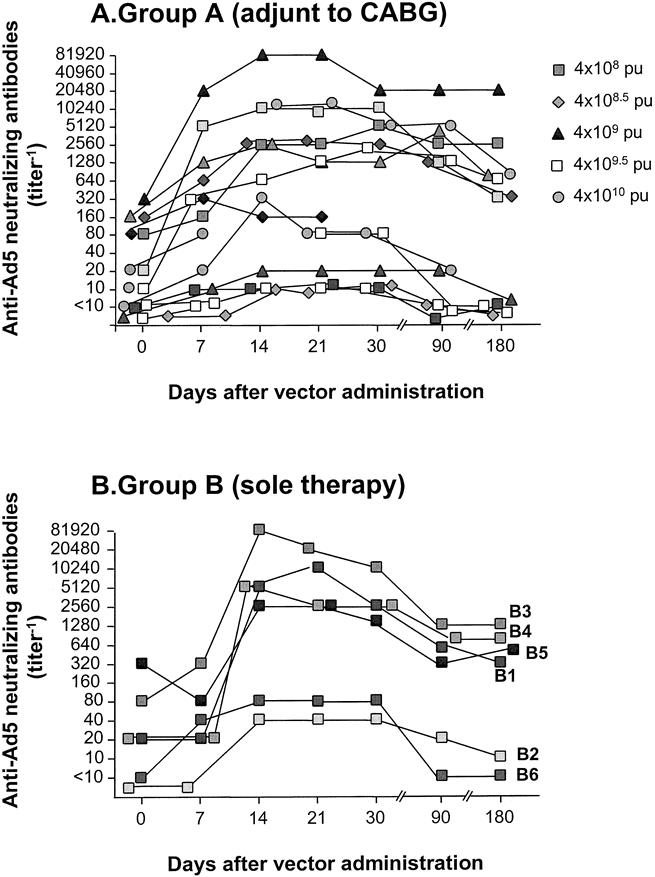
Figure 1. Assessment of anti-Ad5 neutralizing antibody titers−1 before therapy and after intramyocardial administration of AdGVVEGF121.10. (A) Patients receiving AdGVVEGF121.10 as an adjunct to CABG. (B) Patients receiving AdGVVEGF121.10 as sole therapy. Data are presented as titer−1. Data for group A have been previously reported for the 4 × 108 pu dose ≤90 days and for all other doses ≤30 days (Harvey et al 1999 17).
Cardiac-Related Parameters
In both groups, there were no interval changes in the electrocardiogram after the perioperative period (Table 2), and there was no increase in CPK above baseline values at 3 or 6 months of follow-up (see Table 1). Due to the effect of CABG, the relation of angina class in group A patients to the AdGVVEGF121.10 administration cannot be interpreted. However, in all six patients in group B in whom no additional interventional therapies had been performed, there was a decrease in angina classification at 1 and 6 months compared to before therapy (Fig. 2A). A corresponding persistent decrease in sublingual nitroglycerin use was noted in five of the six patients, in the setting of stable dosages of other antianginal medications (Fig. 2B).
Table 2. EVALUATION OF ELECTROCARDIOGRAM CHANGES COMPARING BASELINE TO 6 MONTHS AFTER SURGERY*
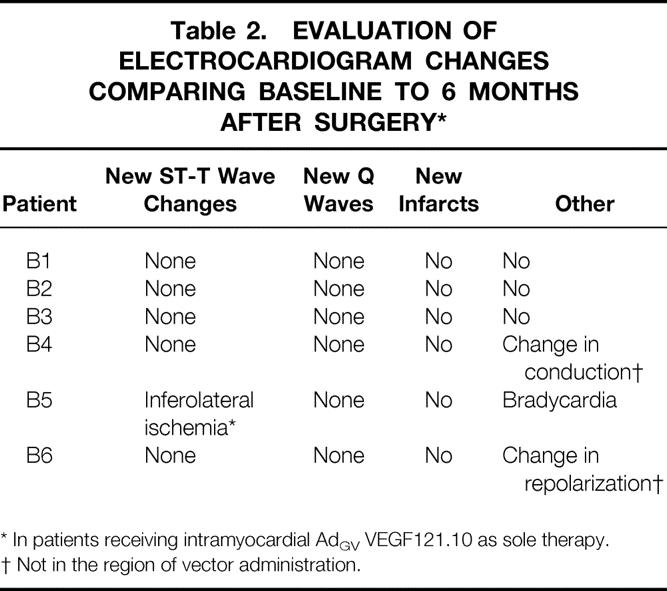
* In patients receiving intramyocardial AdGV VEGF121.10 as sole therapy.
† Not in the region of vector administration.
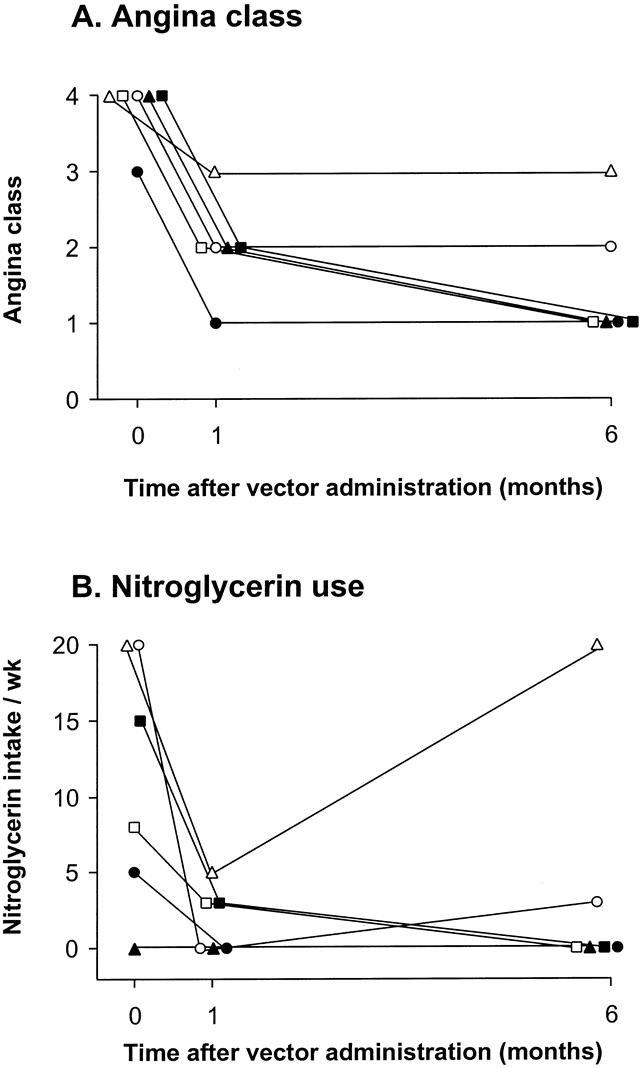
Figure 2. Assessment of angina before therapy and 1 and 6 months after intramyocardial administration of AdGVVEGF121.10 in the sole therapy/minithoracotomy group. (A) Canadian Cardiovascular Society angina classifications before and 1 and 6 months after AdGVVEGF121.10 therapy. (B) Sublingual nitroglycerin use assessed on a weekly basis. Pretherapy and 30-day data have been previously reported (Rosengart et al 1999 14).
Assessment of treadmill exercise in the patients in group B demonstrated a trend toward a decrease in ST/HR slope, suggesting decreased myocardial ischemia at 6 months compared with baseline (Table 3). The extent of exercise and BP × HR remained stable.
Table 3. EVALUATION OF EXERCISE TOLERANCE TESTS COMPARING BASELINE TO 6 MONTHS AFTER SURGERY*
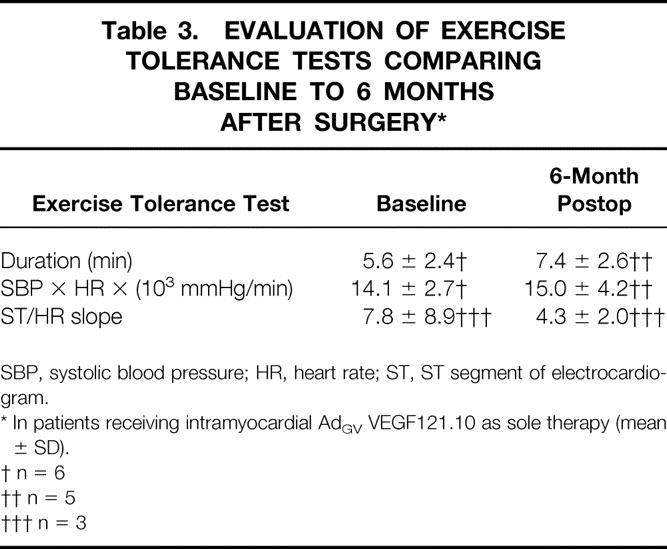
SBP, systolic blood pressure; HR, heart rate; ST, ST segment of electrocardiogram.
* In patients receiving intramyocardial AdGV VEGF121.10 as sole therapy (mean ± SD).
† n = 6
†† n = 5
††† n = 3
DISCUSSION
The present study demonstrates that the administration of an Ad vector delivering the coding sequence of the 121-amino-acid form of human VEGF to the myocardium of patients with clinically significant CAD is well tolerated during a 6-month period. Based on the known biologic activity of VEGF and the limited persistence of transgene expression produced by this vector, it would appear to be reasonable to conclude, at least based on this small cohort, that no adverse effects can be anticipated with this therapy. Although the number of patients in this study is too small to ensure a similar outcome in larger studies or in general use, and these early results are too preliminary to substantiate efficacy, the trends in this study of the persistence of apparently beneficial effects without apparent toxicity 6 months after therapy are encouraging. We conclude that direct myocardial administration of AdGVVEGF121.10 appears to be well tolerated in patients with clinically significant CAD, and initiation of phase II evaluation of this therapy appears warranted.
Cardiac-Specific Parameters
On a theoretical basis, chronic inflammation in response to the Ad gene transfer vector represents the primary long-term risk to the heart after direct myocardial administration of AdGVVEGF121.10. However, there was no evidence of either chronic myocardial inflammation or necrosis after therapy, as evidenced by the absence of increases in CPK at 3 and 6 months, and arrhythmias or other abnormalities assessed by electrocardiography.
Because it is impossible to exclude the possibility of CABG-related “watershed” perfusion affecting the region of vector administration in group A patients, analysis of the persistence of cardiac-related efficacy after direct myocardial administration of the AdGVVEGF121.10 vector in the present study was limited to group B. Although the group B cohort is too small to analyze statistically, group B patients demonstrated a trend in improvement in their angina classification, nitroglycerin use, and some exercise parameters; these changes were persistent at 6 months. Should similar observations be made in large-scale controlled trials, given the 1- to 2-week period of transgene expression characteristic of Ad-mediated gene transfer, these findings suggest that the putative angiogenic effects of this therapy could persist well beyond the expected interval of VEGF angiogen expression.
Systemic Parameters
There was no evidence of deterioration of liver function or any other systemic abnormalities at 6-month follow-up in any of the treated patients in either group A or group B. This observation is important because it is theoretically possible, although inconsistent with the known biology of the Ad vector, that the activity of the AdGVVEGF121.10 vector could somehow be delayed or persistent beyond known expression profiles of the vector. Although administration of the AdGVVEGF121.10 vector to the heart did induce an increase in anti-Ad neutralizing antibodies in most of the study population, as previously reported, 17 these antibody levels returned toward baseline at 6 months in most patients, suggesting that repeat administration of this therapy might be possible.
Acknowledgments
The authors thank M.L. Lessor, North Shore University Hospital, Manhasset, New York, and N. Mohamed for help in preparing this manuscript.
Discussion
Dr. M. Judah Folkman\E (Boston, Massachusetts): I think Dr. Rosengart and his colleagues should be congratulated for a path-breaking story. They have introduced a new modality for the treatment of ischemic heart disease by engineering the heart muscle to continually produce its own private angiogenic protein. The key point in the manuscript is that the angiogenic protein does not appear to spill over into the main circulation, the procedure is safe, with few if any side effects, and myocardial function is improved.
Dr. Rosengart has more than a year’s experience with this very new principle. And this puts him in a small club of pioneers, which includes, for example, Jeffrey Isner at St. Elizabeth Hospital in Boston, who has treated 20 patients with injection into the myocardium of naked DNA for an angiogenic protein VEGF, as used here, and these patients had no other surgical options. Yet a year later, their scans still show persistent new collateral vessels, and half have become angina-free. A third pioneer in this club is Michael Simons at Beth Israel Hospital in Boston, who injects an angiogenic protein which is different, FGF, into the myocardium through a cardiac catheter in the arm, using also sustained release polymers.
It is too early to say whether therapeutic angiogenesis of the heart will be added to coronary artery bypass surgery or will be added to angioplasty or will ever be used as first-line therapy. And Dr. Rosengart has been very understated and guarded in his presentation of this nice data.
I think what can be said is that this advance emerged unpredicted from a broad field of angiogenesis research which began 30 years ago in a pediatric surgery laboratory in a children’s hospital in an attempt to study tumor angiogenesis, a grant supported by the Cancer Institute.
From this field has come another potential therapy for ischemic heart disease reported just this week in the journal Circulation—the demonstration in aortas of mice that atherosclerotic plaque growth is angiogenesis-dependent, and that treatment with a naturally occurring angiogenesis inhibitor endostatin suppresses plaque growth by 85%. This is the work of Karen Molton, a cardiologist in our lab.
So this new finding is not yet ready for clinical application. But if and when it is, it suggests the possibility of a two-pronged attack on ischemic heart disease. In addition to Dr. Rosengart’s elegant work, there may be not only a way to stimulate blood vessels as he has shown in the heart, but also to turn off angiogenesis in plaque growth.
Presenter Dr. Todd K. Rosengart (New York, New York): Thank you, Dr. Folkman. And please allow me to thank you for your pioneering work that literally has allowed the creation of this entire new field.
Dr. Thomas C. Moore (Torrance, California): Mr. President, when I approached you early this morning about discussing this important paper and you directed me to our Recorder, Dr. Brennan, it did not occur to me that I would not be the first-listed discussant. When the first-listed discussant arose and turned out to be Judah Folkman, father of angiogenesis, I was delighted—nonetheless, he is a difficult act to follow. In his acceptance last year of the 1998 Flance-Karl Award of this Association, he spoke of some of the interesting, new, and expanding observations relating to “therapeutic” and induced “good” angiogenesis which had been stimulated by his pioneering and most impressive work over the years on “bad” (cancer-related) angiogenesis.
At a national meeting in October 1995, I had the opportunity of discussing with Judah the possible, indeed likely, involvement of a “spontaneous” hypoxia-triggered good angiogenesis relating to up-regulation the VEGF family of molecules, genes, and receptors (including the BB dimer of PDGF) to account for the remarkable (almost miraculous) salvage of gut and life associated with the use of the laparotomy “patch, drain, and wait” (PD&W) approach to the surgical management of perforated necrotizing enterocolitis (NEC) and midgut volvulus (MGV) with extensive ischemia/necrosis in the newborn, an approach which I initiated in 1982 and which I have used and reported upon multiple times since (Pediatr Surg Int 1989;4:110–3, 1991;6:313–7, 1997;12:208–10).
Early in this experience, in the 7- to 10-day period following the PD&W operation, reoperation in two cases encountered an unforgettable massive florid hypervascularity “angiogenesis” which led to exsanguination in one case and almost in the other—good angiogenesis if the surgeon can be persuaded not to mess with it in this most critical of periods.
This type of good angiogenesis is spontaneous and hypoxia-associated rather than induced and therapeutic as in the authors’ report here. Have the authors looked at hypoxia-associated levels of VEGF family of molecules in their clinical and experimental models of myocardial ischemia in comparison with normal and nonischemic myocardium, as well as after their therapeutic and induced VEGF-mediated good angiogenesis?
The laparotomy PD&W approach to severely ischemia/necrotic gut which I have initiated and which appears to be associated with a marked hypoxia-triggered elevation in the level of spontaneous good angiogenesis/hypervascularity involves a quick midline linea alba in-and-out laparotomy with extensive Penrose drain drainage of the entire peritoneal cavity, with drains from the undersurfaces of both diaphragms running down in a serpentine manner to stab wound exit sites in both lower quadrants of the abdomen and with loops into the pelvis. A monitoring gastronomy, TPN, and broad-spectrum antibiotics complete the initial undertaking. Stoma bags at Penrose drain exit sites capture fecal fistulas and function as de facto enterostomies. The peritoneal cavity is rapidly obliterated by adhesions and spontaneous good angiogenesis—no peritoneal cavity, no peritonitis! Spontaneous autoanastomosis occurs in approximately 70% of the cases and no second operation is needed as the Penrose drainage dries up and anal passage of fecal material resumes. With adherence to the simple but effective formula of resect no gut and do no enterostomies, both life and gut may be saved—and likely with many thanks to the good angiogenesis of the basic and hypoxia-triggered spontaneous type, which the authors have described today for the salvation of ischemia-threatened myocardium.
Have the authors looked at baseline (spontaneous rather than induced) levels of VEGF and other upregulated molecules, genes, and receptors such as serotonin, substance P, the BB-dimer of PDGF and others in their experimental acute and chronic models of myocardial ischemia, and does their induced therapeutic angiogenesis speed up or augment this baseline level, and to what degree? In other words, the question is, are you with your approach simply augmenting or speeding up a process which is sort of chugging along and not doing the job quite well enough?
In Dr. Folkman’s discussion, he mentioned the exciting work of the Jeffrey Isner group in Boston with induced/therapeutic angiogenesis. In an important 1997 report by this group (Science 1997;175:964–7), putative endothelial cell progenitors or angioblasts (CD34-positive mononuclear blood cells) were isolated from human peripheral blood by means of magnetic beads coated with antibody to CD34. On in vitro culture, these cells differentiated into endothelial cells. They confirmed an endothelial cell-like phenotype of these cells by documenting expression of ecNOS and the two VEGF receptors (Flk-1 and KDR). In animal models of ischemia, these cells homed to areas of ischemia and were incorporated into sites of active angiogenesis. These findings suggested to them that endothelial cell progenitors of this type may be useful for augmenting collateral vessel growth to ischemic tissues (therapeutic angiogenesis). Have the authors today considered this approach or others as an addition to augmenting their exciting clinical findings?
Dr. Rosengart: Thank you very much, Dr. Moore. I would say that as Dr. Folkman has taught us, angiogenesis is really a fundamental biological process. And I think one of the reasons why we are so hopeful that therapeutic angiogenesis holds promise is that we are simply taking the native angiogenic process and augmenting it, just as we do with many of our current therapies for a number of disease states.
Certainly we and a large number of other investigators have seen the up-regulation of growth factors and receptors in the ischemic state. Again I think this highlights the point that what we are doing is simply taking the natural process and augmenting it.
I think there are a number of mediators that can be utilized. We have utilized VEGF. But certainly there are a large number of trials ongoing right now looking at different mediators to induce this strategy.
Dr. Anthony A. Meyer (Chapel Hill, North Carolina): I enjoyed the presentation and have questions about two things. I know you looked at neutralizing antibody against the adenovirus. Did you look at neutralizing antibody to VEGF which may have been induced because of the proximity? Also, viral infections known to introduce an autoimmune response—did you look at any evidence of autoimmune response to myocardium after the injection?
Dr. Rosengart: We have thought about looking at immune responses to the VEGF per se. This is human VEGF. And our thinking going into these studies was that in fact it would not be immunogenic. But that is an analysis we intend to do and do have samples to perform.
In terms of your second question, we did a large number of studies in animal models, but certainly that was not equivalent to the human experience. We were admonished that it was likely that adenovirus administration to the heart was going to be a catastrophic event in terms of myocarditis.
In the animal models, again, although it did not approximate the human, there was no evidence of inflammation or micronecrosis. In the human studies we looked very, very carefully at CPK levels, motion abnormalities by echocardiogram, as well as electrocardiographic changes, and in none of the patients—and now we have treated over 30 patients—in none of these instances have we seen any evidence of myocarditis, either in patients with high preoperative antibody levels or low levels. Thirty patients still remains a small study, but certainly that has been very encouraging to us.
Dr. Larry R. Kaiser (Philadelphia, Pennsylvania): I enjoyed the paper very much. I have a couple of specific questions for you regarding some of the techniques.
You have chosen to use an adenoviral vector. And we know there are some built-in limitations to adenoviral vectors, especially an adenoviral vector that is replication-deficient. This was a phase 1 toxicity trial. Did you ever reach your maximum tolerated dose? Or was that ever the intent?
We have talked a lot about efficacy, but of course, efficacy is not really the endpoint of a phase 1 trial. I noticed that in your sole therapy group, I believe you used 109 pfu. Is that a dose that you think will be efficacious? Or is that the dose that the FDA allowed to you use and have you subsequently used a higher dose of vector?
Also, would you comment on the possibility of using a replication-competent factor, because the efficiency of transduction here likely is not very good. We know that when we try to transduce tumors, at best we get about 10% of the cells transduced. Do you have any idea of the efficiency of transduction with your particular vector? And you might comment on how you might improve that.
Dr. Rosengart: In animal studies, a dose response is demonstrable over a viral particle administration range of 4 to 6 logs. We have not seen a dose response in the human trials, although numbers have been very, very small. Our dosing was based on therapeutic effects in animal studies. Toxicity was demonstrated only in small-animal models at several logs above that needed for efficacy.
In terms of our use of the replication-competent virus, we would be hesitant to do that, certainly. One of our feelings is that many of the preclinical studies in which inflammation was seen may, in fact, be related to the number of replication-competent particles. We think one of the reasons why we do not see inflammation is that we have extremely low titers of replication-competent particles.
In terms of the extent of transfection, at least in myocardium in animals, in the tissues that we have looked at we have high levels of transfection and were able to produce VEGF expression on the level of 1 ng per mg of protein, certainly a level that we think is very adequate to induce angiogenesis.
Dr. Lazar J. Greenfield (Ann Arbor, Michigan): In terms of functional significance of the collateral perfusion, were you able to demonstrate any change on cardiac scans?
Dr. Rosengart: We have looked at perfusion scans, echocardiograms, and actually wall motion on the perfusion scans. There is evidence of improvement of wall motion in these studies. It is difficult to assess these small changes in the clinical setting, so we are hesitant to present that data until further analysis. In animal studies, using an ameroid constrictor in pigs, in fact, we were able to induce very significant improvement in function, regional wall motion with this therapy.
Footnotes
Correspondence: Todd K. Rosengart, MD, Dept. of Cardiothoracic Surgery, Weill Medical College of Cornell University—New York Presbyterian Hospital, New York, NY 10021.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Supported in part by NIH grants R01 HL 57318 and M01RR00047; Jeffry M. and Barbara Picower Foundation, Palm Beach, Florida; Will Rogers Memorial Fund, Los Angeles, California; GenVec, Inc., Rockville, Maryland; and Parke-Davis Pharmaceutical Research, Warner-Lambert Company, Ann Arbor, Michigan.
Accepted for publication April 1999.
References
- 1.Rosengart TK, Patel SR, Crystal RG. Therapeutic angiogenesis:: protein and gene therapy delivery strategies. J Cardiovasc Risk 1999; 8: 29–40. [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Ware JA. Food for starving hearts. Nat Med 1996; 2: 519–520. [DOI] [PubMed] [Google Scholar]
- 3.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia:: implications for coronary angiogenesis. Cardiovasc Res 1994; 28: 1176–1179. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol 1996; 270: H1803–H1811 [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem 1991; 47: 211–218. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature 1980; 288: 551–556. [DOI] [PubMed] [Google Scholar]
- 7.Mack CA, Patel SR, Schwarz EA, et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg 1998; 115: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem 1996; 271: 603–606. [DOI] [PubMed] [Google Scholar]
- 9.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis:: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 1998; 98: 2800–2804. [DOI] [PubMed] [Google Scholar]
- 10.Mack CA, Magovern CJ, Budenbender KT, et al. Salvage angiogenesis induced by adenovirus-mediated gene transfer of vascular endothelial growth factor protects against ischemic vascular occlusion. J Vasc Surg 1998; 27: 699–709. [DOI] [PubMed] [Google Scholar]
- 11.Magovern CJ, Mack CA, Zhang J, et al. Direct in vivo gene transfer to canine myocardium using a replication-deficient adenovirus vector. Ann Thorac Surg 1996; 62: 425–434. [PubMed] [Google Scholar]
- 12.Muhlhauser J, Merrill MJ, Pili R, et al. VEGF165 expressed by a replication-deficient recombinant adenovirus vector induces angiogenesis in vivo. Circ Res 1995; 77: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 13.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: : potential role for vasculogenesis in adults. Mol Cell 1998; 2: 549–558. [DOI] [PubMed] [Google Scholar]
- 14.Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy:: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 1999; 100: 468–474. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld MA, Yoshimura K, Trapnell BC, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 1992; 68: 143–155. [DOI] [PubMed] [Google Scholar]
- 16.Mack CA, Song WR, Carpenter H, et al. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther 1997; 8: 99–109. [DOI] [PubMed] [Google Scholar]
- 17.Harvey B-G, Hackett NR, El-Sawy T, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol 1999; 73: 6729–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campeau L. Grading of angina pectoris [letter]. Circulation 1976; 54: 522–523. [PubMed] [Google Scholar]
- 19.Okin PM, Kligfield P. Heart rate adjustment of ST segment depression and performance of the exercise electrocardiogram:: a critical evaluation. J Am Coll Cardiol 1995; 25: 1726–1735. [DOI] [PubMed] [Google Scholar]


