Abstract
Objective
To determine perioperative morbidity, survival, and local failure rates in a large group of consecutive patients with rectal cancer undergoing low anterior resection by multiple surgeons on a specialty service. The primary objective was to assess the surgical complications associated with preoperative radiation sequencing.
Summary Background Data
The goals in the treatment of rectal cancer are cure, local control, and preservation of sphincter, sexual, and bladder function. Surgical resection using sharp perimesorectal dissection is important for achieving these goals. The complications and mortality rate of this surgical strategy, particularly in the setting of preoperative chemoradiation, have not been well defined.
Methods
There were 1233 patients with primary rectal cancer treated at the authors’ cancer center from 1987 to 1995. Of these, 681 underwent low anterior resection and/or coloanal anastomosis for primary rectal cancer. The surgical technique used the principles of sharp perimesorectal excision. Morbidity and mortality rates were compared between patients receiving preoperative chemoradiation (Preop RT, n = 150) and those not receiving preoperative chemoradiation (No Preop RT, n = 531). Recurrence and survival data were determined in patients undergoing curative resection (n = 583, 86%) among three groups of patients: those receiving Preop RT (n = 131), those receiving postoperative chemoradiation (Postop RT, n = 110), and those receiving no radiation therapy (No RT, n = 342).
Results
The perioperative mortality rate was 0.6% (4/681). Postoperative complications occurred in 22% (153/681). The operative time, estimated blood loss, and rate of pelvic abscess formation without associated leak were higher in the Preop RT group than the No Preop RT group. However, the overall complication rate, rate of wound infection, anastomotic leak, and length of hospital stay were no different between Preop RT and No Preop RT patients. With a median follow-up of 45.6 months, the overall actuarial 5-year recurrence rate for patients undergoing curative resection (n = 583) was 19%, with 4% having local recurrence only, 12% having distant recurrence, and 3% having both local and distant recurrence, for an overall local recurrence rate of 7%. The actuarial 5-year overall survival rate was 81%; the disease-free survival rate was 75% and the local recurrence rate was 10%. The overall survival rate was similar between Preop RT (85%), Postop RT (72%), and No RT (83%) patients (p = 0.10), whereas the disease-free survival rate was significantly worse for Postop RT (65%) patients compared with Preop RT (79%) and No RT (77%) patients (p = 0.04).
Conclusion
The use of preoperative chemoradiation results in increased operative time, blood loss, and pelvic abscess formation but does not increase the rate of anastomotic leaks or the length of hospital stay after low anterior resection for rectal cancer. The 5-year actuarial overall survival rate for patients undergoing curative resection exceeded 80%, with a local recurrence rate of 10%.
In the past two decades, there has been a significant evolution in the management of rectal cancer. Except for the small subset of patients with very early tumors, most patients have disease beyond the rectal wall, either by direct extension or lymphatic spread. 1 Conventional surgical technique for rectal cancer has often used expeditious blunt pelvic dissection and results in local recurrence rates of 15% to 65%, with survival rates of 35% to 56% for transmural and/or node-positive disease. 2–5
These results prompted the addition of adjuvant or neoadjuvant pelvic irradiation with or without chemotherapy to reduce local recurrence rates and improve survival rates. In the United States, randomized trials have convincingly shown that postoperative combined modality therapy improves local control and increases the overall cancer-related survival rate. 4,5 In Europe, the use of short-course, large-fraction preoperative radiation therapy results in significant improvement in local control 6–8 and survival 7,8 rates when compared with surgery alone. Preoperative radiation therapy results in increased surgical complications, 9 and postoperative radiation therapy produces considerable short-term and long-term complications. Selected centers have also emphasized the use of preoperative combined modality therapy for improved sphincter preservation as well as local control. 10–13 The most effective adjuvant therapy and sequencing strategies remain under investigation.
The initial experience from the University of Chicago 14 and by Heald et al 15 suggested that meticulous pelvic dissection minimized local failure rates. Using this approach of sharp perimesorectal dissection and expanding the concept to total mesorectal excision, both Heald et al 16,17 and Enker et al 18 have reported low local recurrence rates in the hands of single surgeons with or without the use of adjuvant radiation therapy. There has been concern about increased complications associated with this surgical technique, 19 the impact of preoperative chemoradiation in association with mesorectal excision, and the efficacy of these surgical techniques when expanded to a larger group of surgeons.
The aim of this study was to determine the perioperative morbidity, mortality, overall survival, and local failure rates using the technique of mesorectal excision in consecutive patients with rectal cancer undergoing low anterior resection (LAR) by multiple surgeons on a specialty service in a cancer center. The primary objective was to assess the perioperative complications of this surgical technique in association with preoperative radiation sequencing.
METHODS
Between 1987 and 1995, 1233 patients with resected rectal cancer were identified from a prospective colorectal cancer database at Memorial Sloan-Kettering Cancer Center. The following criteria eliminated 552 patients: transanal or posterior excision; abdominoperineal resection or abdominosacral resection; adjuvant therapy received for a prior cancer within the pelvis; resection for recurrent disease. Patients requiring abdominoperineal resection were those with tumors at or below the anorectal ring, as well as women requiring posterior vaginectomy for adequate tumor clearance. The remaining 681 patients underwent LAR for resection of primary rectal cancer and formed the basis of this study. Data were obtained from the prospective database and from detailed chart review and follow-up of the cancer and survival status. Six attending surgeons performed 95% of the surgical procedures in a teaching environment.
Treatments Defined
Surgical Techniques
All patients underwent resection using a sharp perimesorectal excision technique. 18 This involved sharp dissection under direct vision in the areolar plane lateral (peripheral) to the visceral fascia that envelops the rectum and mesorectum. Posteriorly, the plane of dissection was along the parietal fascia overlying the presacral vessels. Laterally, this plane continues between the mesorectum and the parietal fascia overlying the piriformis and levator muscles. The so-called lateral ligaments, or the junction between the mesorectum and the pelvic plexus, was dissected sharply, preserving the autonomic nerve trunks. 18 The anterior dissection in men was performed with scissors and cautery, usually anterior to Denonvillier’s fascia, except for small, posterior tumors, for which the dissection was posterior to Denonvillier’s fascia. In women, the posterior vagina was dissected under direct vision. The entire mesorectum was mobilized to the pelvic floor using sharp perimesorectal dissection. When possible and when appropriate based on the cancer size and penetration, the sympathetic and parasympathetic pelvic autonomic nerve trunks were preserved medial to the parietal fascia. 20. For middle to low rectal cancers, the entire rectal mesentery, including that distal to the tumor, was removed as an intact unit, producing the characteristic smooth, bilobed appearance of the mesorectum covered by intact visceral fascia. For high rectal cancers (above the peritoneal reflection), sharp perimesorectal dissection allowed mobilization to the pelvic floor, after which the mesorectum was divided at right angles to the bowel, 5 to 6 cm distal to the tumor. Anastomoses were performed using an intraluminal circular stapling device or were hand-sewn (primarily for per-anal coloanal reconstruction).
Surgical Characteristics
The type of surgical resection was distinguished between LAR and LAR with coloanal anastomosis (CAA). Tumor location, based on the distance between the lowest edge of the tumor and the anal verge, was divided into three groups. Tumors were classified as low, middle, or high rectal tumors if they were 0 to 5 cm, 5.1 to 10 cm, and 10.1 cm to 15 cm, respectively. All measurements were from the anal verge, using a rigid scope. The resection margin was measured from the nonfixed gross pathologic specimen.
Adjuvant Therapies
Preoperative Chemoradiation Therapy
A multiple field technique was used to deliver 180 cGy per day, 5 days per week, to give a total of 26 fractions over a period of 5 weeks for a total of 4680 cGy. An additional boost dose of 360 cGy was delivered to the primary tumor bed in a field of 10 × 10 cm or 12 × 12 cm in an intent to treat the primary tumor. This provided a total dose of 5040 cGy. Various chemotherapy regimens were used during this time frame, primarily involving various regimens of fluorouracil and leucovorin. The most common protocol consisted of bolus 5-fluorouracil (325 mg/m2/day) and leucovorin (20 mg/m2/day) given for two cycles during 5 consecutive days on week 1 (days 1 to 5) and 5 (days 29 to 3) of the radiation therapy. 11
Surgical resection was performed 4 to 7 weeks after the completion of radiation therapy. Four to 6 weeks after surgery, patients were given bolus 5-fluorouracil and leucovorin for 4 months.
Patients receiving preoperative combined modality therapy were those thought to be at increased risk of local failure on clinical criteria. These included patients with fixed or deeply tethered cancers, men with bulky distal circumferential lesions, and patients with transmural lower-third tumors in which sphincter-preserving surgery was planned.
Postoperative Chemoradiation Therapy
Treatment began 4 to 6 weeks after surgery. Before radiation therapy, in the most common protocol used, bolus 5-fluorouracil and leucovorin were given for 2 months. This was followed by concurrent chemoradiation therapy, as described above. Approximately 1 month after the completion of the radiation therapy, an additional 2 months of chemotherapy was delivered.
Patients with gross transmural penetration and/or nodal metastases were treated with chemoradiation at the discretion of the surgeon; this decision was frequently based on the estimate of the efficacy of the mesorectal excision in the individual patient. In general, patients with transmural tumors and/or nodal metastases were referred for postoperative chemoradiation in compliance with the NIH Consensus guidelines. One attending surgeon (WEE) preferred surgical resection alone in many such patients, particularly those with T3N0M0 disease, based on the published experience with mesorectal excision, and reviewed these issues with all patients as part of his patient management program. 18
Recurrence and Survival
Analysis of recurrence and survival was limited to patients undergoing curative resection (n = 583). Only patients with microscopic positive surgical margins (n = 4) or metastatic disease were excluded. No patients underwent complex analysis of lateral margins. 21 Patients were divided into three groups based on the type of adjuvant treatment received for recurrence and survival analysis: those receiving preoperative chemoradiation (Preop RT, n = 131), those receiving postoperative chemoradiation (Postop RT, n = 110), and those undergoing surgery and receiving no radiation therapy (No RT, n = 342). These data are not randomized. Practice guidelines on the Colorectal Service used preoperative chemoradiation therapy for patients at the highest risk for local recurrence with surgery alone. Clinical criteria were fixed cancers (to pelvis or prostate), or circumferential cancers in men. Clinical judgment was used in selecting additional patients for preoperative chemoradiation based on tethering of the tumor or lower-third cancers.
Recurrences were distinguished as local if they occurred at the suture line or within the confines of the true pelvis and distant if they occurred outside the true pelvis or within a nonregional lymph node (e.g., paraaortic). Overall survival was defined as the time from the primary surgery to the time of death. Disease-free survival was defined as the time from the primary surgery to the time of first recurrence.
Complications of Surgery
To determine the perioperative morbidity rate associated with preoperative radiation sequencing, patients receiving preoperative chemoradiation were compared with those in the other groups (No Pre RT, n = 531). Median operative time, estimated blood loss, number of patients receiving blood transfusions, and length of hospital stay were determined from patients’ hospital records.
All perioperative complications were recorded. A wound was considered infected if cellulitis was observed and antibiotics were administered, or if any wound was opened with purulent drainage or without purulent drainage with antibiotic administration. Anastomotic leaks included those clinically apparent or those determined based on a follow-up Gastrografin enema. Pelvic abscess not associated with a demonstrable leak was reported separately. Perioperative death was defined as a death occurring within 30 days of surgery, or thereafter if clearly related to surgical complications.
Statistical Analysis
Disease-free and overall survival rates were calculated by the Kaplan-Meier actuarial method, and results were compared using log-rank analysis. Paired variables were compared by chi square analysis. Nonparametric data were compared by analysis of variance with the Bonferroni post hoc test.
RESULTS
There were 681 patients in this study. This cohort consisted of 404 (59%) men and 277 (41%) women with a mean age of 61 years (range, 24 to 98). All patients underwent LAR, and 169 (25%) patients had a CAA. One third of the patients undergoing CAA were stapled. Two thirds underwent per-anal sutured anastomoses.
Surgical Characteristics
The median operative time and estimated blood loss associated with LAR in patients who received preoperative chemoradiation was significantly higher than in patients who did not (Table 1). However, this did not result in an increased number of patients who received blood transfusions, nor did it increase the length of stay compared with patients who did not receive preoperative chemoradiation. To assess whether the increased blood loss was associated with stage and extent of disease, a multivariate analysis was performed, and preoperative chemoradiation remained an independent significant variable in regard to blood loss.
Table 1. IMPACT OF PREOPERATIVE RADIATION THERAPY ON PERIOPERATIVE FACTORS
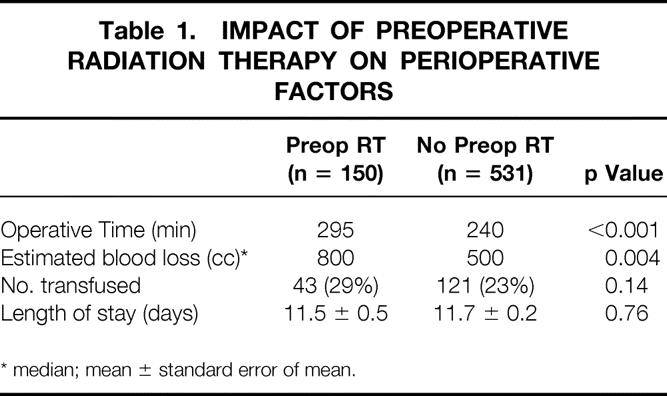
* median; mean ± standard error of mean.
Morbidity and Mortality Rates
The overall 30-day postoperative mortality rate was 0.6% (4/681). Two patients died after having a cardiac arrest on postoperative day 4 and 18, respectively. One patient died of respiratory failure on postoperative day 16. Only one patient died of septic complications related to an anastomotic leak (postoperative day 30).
The overall in-hospital complication rate was 22% (155/681). In 45 (7%) patients, complications developed after hospital discharge that required readmission.
Overall complication rate and the incidence of wound infections, anastomotic leaks, and pelvic abscess formation not associated with a leak were compared between patients who did and did not receive preoperative chemoradiation (Table 2). The incidence of formation of pelvic abscess was significantly higher in those who received preoperative chemoradiation. However, the overall complication rate, the incidence of wound infection, and most importantly the anastomotic leak rate were no different between the two groups.
Table 2. IMPACT OF PREOPERATIVE RADIATION THERAPY ON COMPLICATIONS
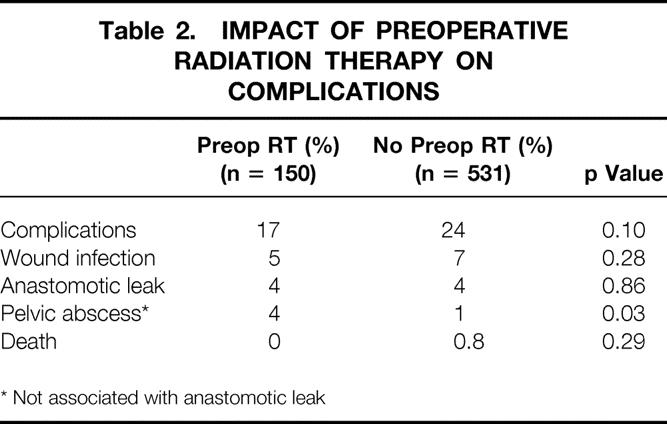
* Not associated with anastomotic leak
Further analysis of anastomotic leaks was performed using various intraoperative and surgical factors (Table 3). The leak rate was significantly higher in patients undergoing LAR than those undergoing CAA. A temporary diverting ileostomy or colostomy was performed in 214 (31%) patients. Of the patients with a diverting stoma, 122 (57%) had a CAA. The leak rate was no different among those with diversion or those without. In addition, a diverting stoma did not reduce the incidence of anastomotic leak among those undergoing LAR without CAA (p = 0.55). An anastomotic leak developed in 2 of the 45 CAA patients without a diverting stoma, whereas no leaks developed in CAA patients with a diversion (p = 0.02). The location of the tumor within the rectum had some impact on the leak rate. The overall leak rates for high, middle, and low rectal cancers were 4%, 5%, and 1%, respectively. The lower leak rate for lower-third cancers was primarily related to the increased use of per-anal reconstruction. However, high stapled anastomoses had a lower leak rate than middle or lower-third stapled anastomoses, but this was not statistically significant. There was a strong trend for increased leak rates in men with stapled anastomoses. With only 44 patients in the lower-third stapled anastomosis group (leak rate 5%), patient numbers were too small to assess the impact of diversion in this group.
Table 3. IMPACT OF SURGICAL VARIABLES ON ANASTOMOTIC LEAK RATE
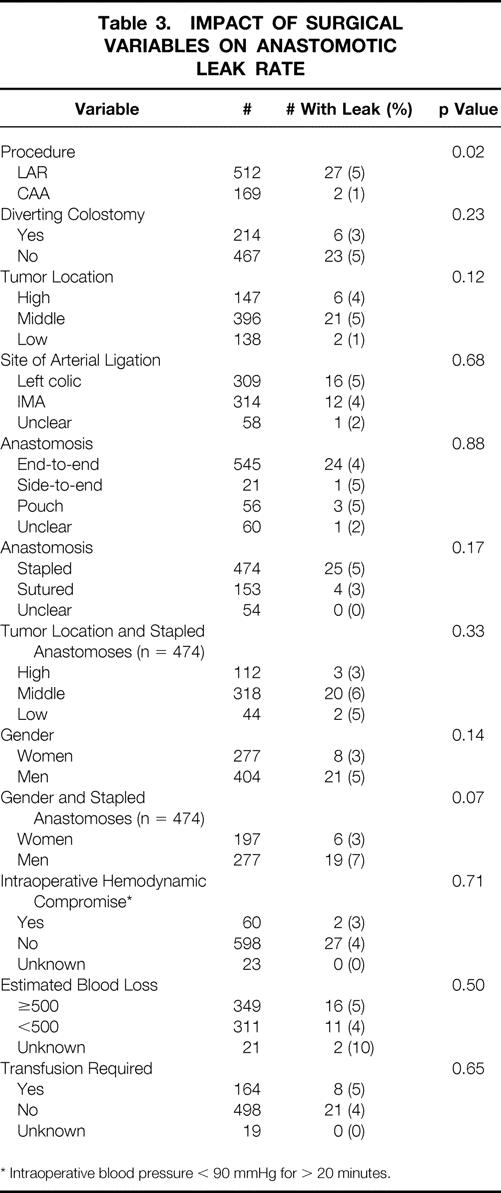
* Intraoperative blood pressure < 90 mmHg for > 20 minutes.
Recurrence and Survival
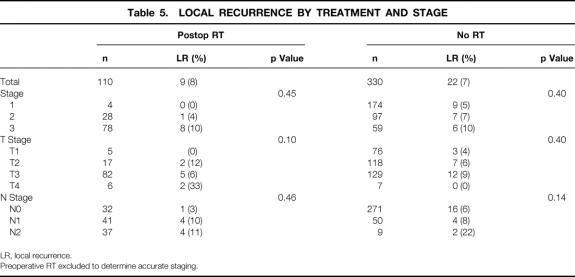
The median follow-up time was 45.6 months. Recurrence data are shown in Tables 4, 5, and 6. The overall 5-year actuarial recurrence rate for patients undergoing curative resection (negative margins, no distant metastases) was 19%, with the local recurrence rate 10% (Fig. 1). There was no difference in the risk of local recurrence between patients receiving preoperative chemoradiation, postoperative chemoradiation, or no chemoradiation (p = 0.49, Fig. 2). Because pathologic stage after preoperative chemoradiation is not comparable with initial resection patients, Table 5 shows local recurrence rates for the patients who underwent resection with or without postoperative adjuvant therapy. Various clinical and pathologic risk factors were compared to determine if any were prognostic for local recurrence (see Table 6). Interestingly, in our series of patients undergoing curative resection for rectal cancer using sharp perimesorectal resection, none of the usual risk factors were significant for determining local recurrence, including adverse pathologic features and even stage of tumor. TABLE 4
Table 4. INFLUENCE OF RADIATION THERAPY ON RECURRENCE RATES
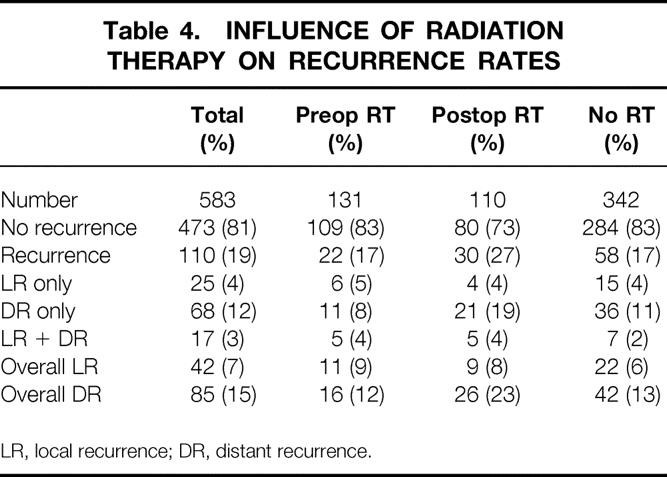
LR, local recurrence; DR, distant recurrence.
Table 5. LOCAL RECURRENCE BY TREATMENT AND STAGE
LR, local recurrence.
Preoperative RT excluded to determine accurate staging.
Table 6. LOCAL RECURRENCE BY DEMOGRAPHIC FACTORS
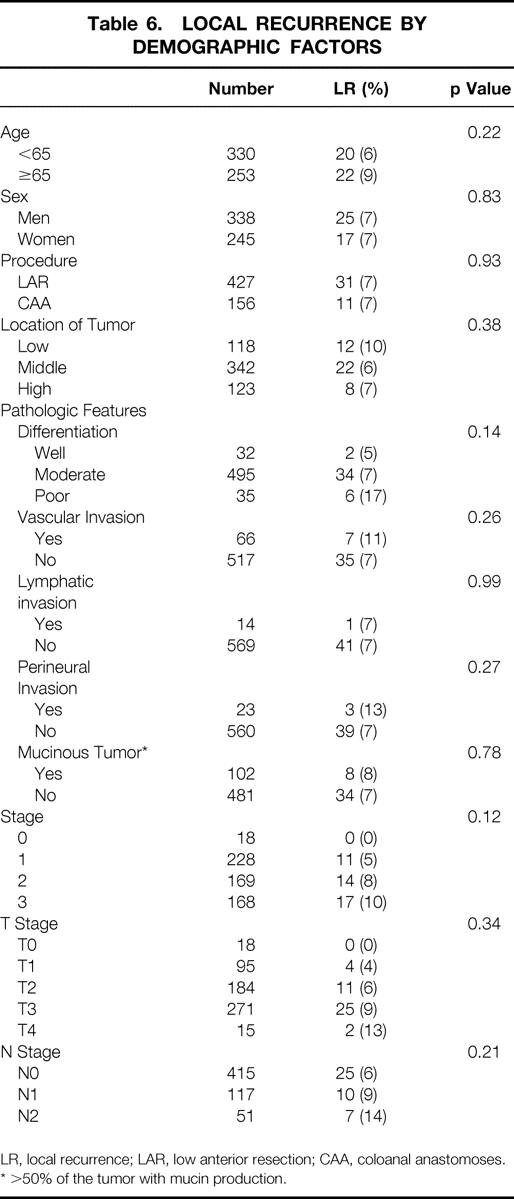
LR, local recurrence; LAR, low anterior resection; CAA, coloanal anastomoses.
* >50% of the tumor with mucin production.
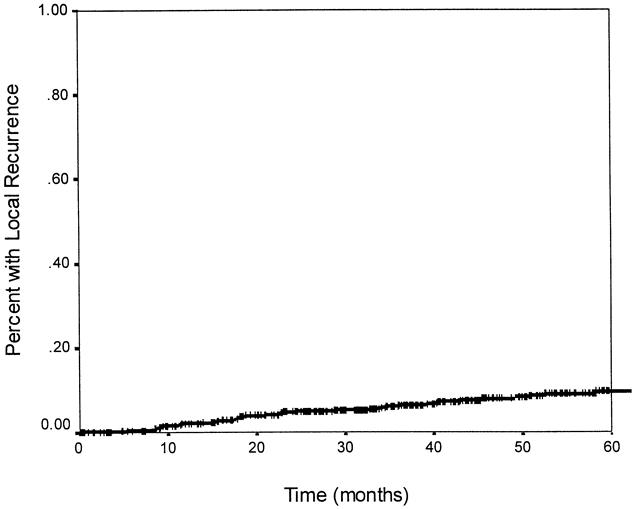
Figure 1. Actuarial local recurrence rate. The probability of having a local recurrence at 5 years was 10%.
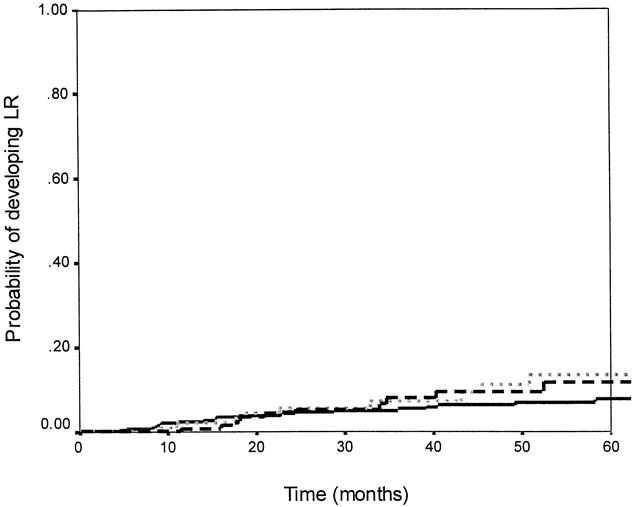
Figure 2. Local recurrence rates and adjuvant therapy. There was no significant difference in the probability of having a local recurrence between patients receiving preoperative chemoradiation, postoperative chemoradiation, or no radiation (p = 0.49). The dashed line indicates preoperative chemoradiation (n = 131); the solid line indicates no radiation (n = 342); the dotted line indicates postoperative chemoradiation (n = 110).
The overall actuarial 5-year survival rate for the entire group was 81% (Fig. 3). The 5-year overall survival rates for patients receiving preoperative chemoradiation, postoperative chemoradiation, and no chemoradiation were 85%, 72%, and 83%, respectively. When stratified by treatment received, there was a trend toward a worse overall survival rate in patients receiving postoperative chemoradiation compared with those receiving preoperative chemoradiation or no chemoradiation; however, this was not statistically significant (p = 0.10, Fig. 4).
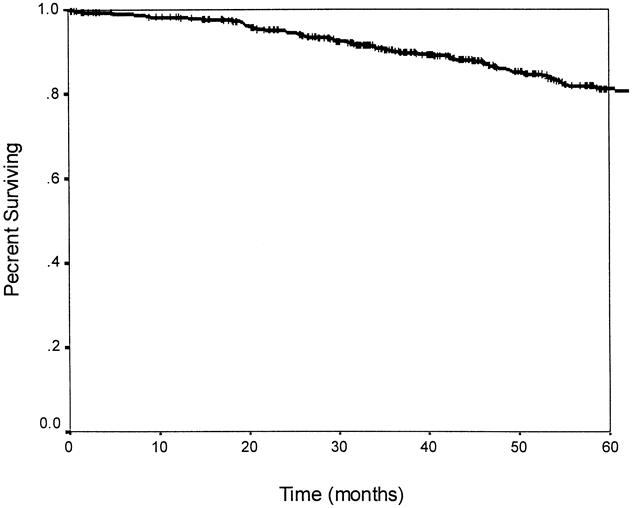
Figure 3. Actuarial 5-year overall survival. The 5-year survival rate is 81%.
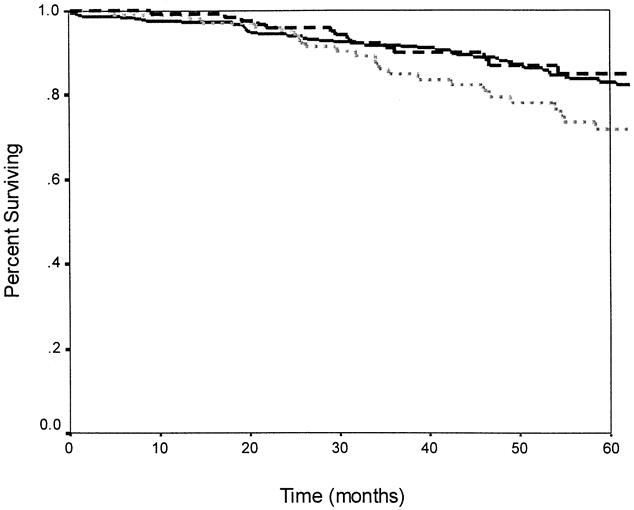
Figure 4. Overall survival and adjuvant therapy. There was no significant difference in overall survival between patients receiving preoperative chemoradiation, postoperative chemoradiation, or no radiation (p = 0.10). The dashed line indicates preoperative chemoradiation (n = 131); the solid line indicates no radiation (n = 342); the dotted line indicates postoperative chemoradiation therapy (n = 110).
Twelve patients receiving preoperative chemoradiation had complete pathologic responses in the resected specimen. There were no local or distant recurrences in this subset.
DISCUSSION
The results of the present study indicate that surgical resection is more complex after preoperative chemoradiation, as evidenced by the significant increase in operative time and estimated blood loss in these patients compared with patients receiving no preoperative chemoradiation. This increased complexity of surgery does not, however, translate into an increase in the postsurgical complication rate, anastomotic leak rate, or length of stay. The incidence of pelvic abscess not associated with an anastomotic leak after preoperative chemoradiation was 4% in our study. Although this rate was significantly higher than in patients who did not receive preoperative chemoradiation, it may reflect the inordinately low rate (1%) of pelvic abscess seen in those patients. Because LAR is a clean-contaminated procedure, localized sepsis in a contaminated radiated field is not surprising. Our prophylactic antibiotic regimen was a single preoperative dose. It would be of interest to evaluate the potential efficacy of a more prolonged antibiotic course in patients receiving preoperative radiation.
The most devastating surgical complication after rectal resection remains an anastomotic leak. Reports indicate that the clinical leak rate ranges from 2% to 17%, 19,22–24 depending on the level of anastomosis, the method of reconstruction, and surgical expertise. An anastomotic leak can be fatal in 2% to 25% of patients. 19,22,23
The technique of total mesorectal excision has resulted in low recurrence rates without the routine use of adjuvant therapy. 16–18,25–28 However, concerns have been raised over the high anastomotic leak rates reported by Heald’s group using this technique. 19 His initial description of the technique included removal of the entire mesorectum, which is then drawn up posteriorly, leaving behind a “muscle tube” 2 to 3 cm off the pelvic floor. This is described as a “rectal reservoir … completely empty of visceral tissue.” 15,25 Clearly, concern is raised regarding the vascularity of this rectal reservoir. On additional analysis, Heald’s group also noted that if the sigmoid colon was used for anastomosis without a splenic flexure mobilization, the leak rate was 22%, compared with only 9% if a complete splenic flexure mobilization was performed. 19 This would also suggest a component of tension at the anastomosis.
Our technique is to transect the rectum at right angles just above the levator ani muscles after complete removal of the mesorectum for low rectal cancers. A low pelvic anastomosis is then performed after complete splenic flexure mobilization. For midrectal cancers, 2 to 3 cm of rectal muscular tube may be preserved. For high rectal cancers, the mesorectum and rectum are divided at right angles 5 to 6 cm distal to the primary tumor, leaving behind the lowest mesorectum as a vascular pedicle for the rectal remnant and not always fully mobilizing the splenic flexure. Using this technique, we and others have shown equally low recurrence rates (<10%), but with an anastomotic leak rate ≤5%. 18,27 The addition of preoperative chemoradiation did not influence the leak rate, as corroborated by others. 29
Preoperative therapy (most commonly in the United States, radiation therapy combined with systemic chemotherapy) is gaining acceptance as a common adjuvant treatment for locally advanced rectal cancer. Tumor downstaging and enhanced sphincter preservation have been well reported using this approach. 10–13 Its use, however, is also associated with acute grade 3+ toxicity in up to 25% of patients. 11,30 Pelvic irradiation in either the preoperative or postoperative setting may result in significant long-term functional complications. 31,32
Given the consistently reported local recurrence rates of <10% and survival rates of >70% using the technique of mesorectal excision without the use of any adjuvant therapy, 16–18,25–28 the incremental benefit of adjuvant chemoradiation in the setting of mesorectal excision is unclear. Our results also show a local recurrence rate of 6% and a 5-year survival rate of 83% in patients undergoing mesorectal excision without any adjuvant or neoadjuvant therapy. Because patients who received either preoperative or postoperative chemoradiation in this report were nonrandomized, and were in general selected because of clinical or pathologic risk factors, our data should not be interpreted to discount the value of such adjuvant therapy. There is currently an ongoing randomized trial in the Netherlands assessing the role of high-dose preoperative radiation therapy followed by mesorectal excision. Short-course, large-fraction radiation is used. Our data are reassuring that despite full-dose preoperative chemoradiation using 180 rads/fraction, sharp mesorectal excision is remarkably safe.
Discussion
Dr. Fabrizio Michelassi (Chicago, Illinois): We are privileged today to hear the results on safety and efficacy of low anterior resection for rectal cancer by a group of skilled surgeons who are very well-versed in the treatment of rectal cancer. By any standard and definition, they belong to the high-volume surgeon/high-volume hospital category, where we have come to expect the lowest mortality/morbidity and the best long-term results. And indeed this is the case. Their results will be very difficult to improve on, and the main message of the paper sets a standard against which all of us have to measure our results.
The authors have attempted to assess the surgical complication rate associated with preoperative radiation therapy and have concluded that the use of preoperative radiation therapy increases operating time, blood loss, and pelvic abscess formation. I am not sure that these conclusions are justified by the study presented. Rather, the study, which is retrospective in nature although analyzing a prospective database, indicates the kind of optimal results which can be achieved with excellent patient selection.
So I would like to ask, Dr. Cohen, how did you select patients for preoperative chemoradiation therapy? In the manuscript you mentioned that the selection criteria included fixed cancer, circumferential cancers in males, and selected patients with lower third cancers. Were these additional patients selected on your prediction of a more difficult resection because of gender, body weight, or other considerations?
It is easy to imagine a scenario where obese males with lower rectal cancers preferentially receive preoperative radiation therapy. Surgery in these patients is associated with longer operative times, higher blood loss, and pelvic abscess formation independent of preoperative radiation therapy. As a corollary question, did you use preoperative transanal ultrasound to guide you in your selection of patients?
Equally, what criteria did you use for the selection of postoperative radiation therapy? Were these patients selected on additional criteria besides tumor stage, such as an estimate of the adequacy of mesorectal excision?
Further, in your manuscript you refer to high rectal cancers above the peritoneal reflection. This prompts me to ask you for the definition of rectal cancer used in this study. Tumors above the peritoneal reflection are usually considered colon rather than rectal cancers as they lack a lateral pelvic lymphatic drainage. I doubt that any of these patients ever received any postoperative radiation therapy, further adding to the selection criteria to which I alluded earlier.
One last question: Although you did not report specifically on long-term functional results, I wonder whether you could elaborate on the effects of radiation therapy on intestinal continence and frequency. I notice that in only 51 patients the procedure was complemented by a colonic pouch. Did the addition of a pouch make a difference and what are your indications now for a colonic J pouch?
Again, this is a large series from very experience clinicians whose strategy has produced excellent results.
Presenter Dr. Alfred M. Cohen (New York, New York): As I mentioned, these are not randomized data, so they have to be looked at carefully in terms of the selection bias for both preoperative and postoperative adjuvant therapy. So let me combine your two questions.
The selection criteria for preoperative chemoradiation therapy are fixed tumor and then a heterogeneous group of high-risk patients. These would include circumferential tumors, particularly circumferential tumors in the obese male. Additional lower third patients were treated as part of a sphincter preservation program. Those were the major patients who received preoperative radiation.
Postoperative radiation was also done on selected basis. The general criteria were a large number of positive lymph nodes. In Dr. Enker’s personal series, the N2 (4 or more positive nodes) without radiation therapy had a markedly increased local failure rate. So the stage 3 cancers, particularly multiple nodes, received adjuvant therapy. An additional criterion was the efficacy of the operation. We may not do it in a formal way, but when we get done with an operation, we look at the pelvis, we look at the specimen, and then we look at the pathology report, and we have used those not exactly quantitative aspects to select additional patients for postoperative adjuvant therapy.
You asked me whether we use endorectal ultrasound. Our data was quite unreliable because so much of this test is operator-dependent. Dr. Doug Wong was recruited to New York from Minnesota and we solved that problem, and we have now 90% accuracy. It is our standard of care for selection.
The definition of rectal cancer, first of all, is measured from the anal verge with a rigid scope, and it includes up to 15 cm. The mid- and lower rectum are below the peritoneal reflection. The upper rectum includes tumors whose inferior margin is just above the peritoneal reflection but does not include rectosigmoid.
Function is of great concern in regard to postoperative treatment. Dr. Paty in our group reported our experience with coloanal reconstruction and late bowel function and postoperative radiation was the primary determinant of poor function. These data were confirmed by Kollmorgen from the Mayo Clinic. We try to avoid postoperative radiation if at all appropriate.
Pouch selection is not routine. In the husky male with a very narrow pelvis and a capacious colon, technically it is not possible to insert a pouch. However, in a patient with a very small colon, wide pelvis, very low reconstruction, that is, coloanal or very low colorectal, we will preferentially use a pouch.
Dr. David A. Rothenberger (St. Paul, Minnesota): Congratulations to Drs. Cohen, Enker, and colleagues, who have presented a large single-institution experience of elective low anterior resection for rectal cancer. The manuscript really addresses three interrelated subjects: safety, efficacy, and the role of adjuvant chemoradiation.
With regard to safety, this large series along with several other recent reports collectively can establish what I think should be a modern benchmark of safety for this type of surgery, i.e., we should be able to do this operation with a mortality of less than 2% and a clinically apparent leak rate of 5% or less when GI continuity is restored.
Somewhat surprisingly, the leak rates for high, middle, and low rectal cancers in this series were 4%, 5%, and 1% respectively, which prompted the authors to conclude that the anastomotic leak rate was not related to the location of the tumor or the type of anastomosis. I am not convinced that that is a valid conclusion when you consider that your data suggests that 72% of the 169 coloanal anastomoses had a temporary partial diversion versus only 18% of the 512 other anastomoses which were diverted.
So my first question for you is whether you truly believe lower anastomoses without diversion would leak no more frequently than more proximal anastomoses? What are your current criteria for diversion?
With regard to efficacy, your data showed an actuarial local recurrence rate of 10% and a 5-year disease-free survival of 75%. These results are outstanding. But I think the audience should keep this in context. This series, as noted, has excluded about 45% of the patients who are treated by other operations, including abdominoperitoneal resection. Most series would show that patients with distal rectal cancers requiring an abdominoperitoneal resection would fare less well, with higher rates of local recurrence and lower survivorship. How many abdominoperitoneal resections were performed in your overall series of 1,233 patients? Were you able to achieve the same sorts of local recurrence rates and excellent survival in that group of patients? Did more such patients receive chemoradiation?
With regard to adjuvant chemoradiation, the first question that I have relates to the interval between completion of your preoperative chemoradiation therapy and the surgery. How long did you wait? Did you follow a uniform policy? If not, did you see any kind of correlation between complication rates and interval to surgery? What would you consider as the optimal timing of this intervention?
Finally, I want to emphasize the point that Dr. Michelassi made. You suggest that there is no difference in risk of local recurrence between the 131 patients who received chemoradiation, the 110 patients who received postop chemoradiation, and the 342 patients who received no adjuvant therapy. The implication in the manuscript as I read it was that perhaps chemoradiation was of little or no value. I wonder if you really believe that or whether you believe, as I do and as I think Dr. Michelassi does, that this probably reflects your team’s experience and ability to select, almost by some sort of intuition, which patients would benefit from such adjuvant therapy preoperatively. Isn’t it time that we finally get on to better defining which patients would really benefit by conducting a controlled trial?
Dr. Cohen: The lower leak rate with lower third tumors is related to the fact that many of these were coloanal anastomoses.
I provided some data in regard to our stapled reconstructions. In those patients, it does not appear to make any difference as to height—quite different from the Bill Heald data. I think that has to do with our policy of making sure that the proximal bowel is not under tension and is well vascularized. We mobilize the splenic flexure in most patients.
The criteria for diversion varied. Almost all of our coloanal reconstructions were diverted. We use an individual policy. If we are uncomfortable with the anastomosis, if the bowel prep was poor, if we have had some intraoperative bleeding, if we are unhappy with the “donuts,” if we have diverticulosis, then we will divert those patients. I think most of us would agree that diversion per se does not impact on the leak rate—it just impacts on the morbidity of a subsequent leak. I think this is an important message. But having said that, if the patient is postoperative day 5 and has a fever, he will be in the CAT scanner within the hour. I think if you have a policy of nonroutine diversion, then you have to be extremely aggressive about following up the patients.
Abdominal perineal resection is, unfortunately, still a common operation. Of our 1,200 or so patients, about 350 of them have had APR. And along with Mr. Heald, the local failure and survival rates are worse in the APR group. The indications for APR are not just limited to tumor locations, but histology and the extent of disease.
In the manuscript we talk about a 4- to 7-week wait—generally we prefer a 6-week wait following completion of preoperative therapy.
To focus in on, to me, the most important issue that you related in regard to the selection for adjuvant therapy, we have traditionally used clinical and pathological criteria. I think we are all waiting for a good biological determinant. I think that we need—whether it is a different imaging modality, whether it is a PET scan, or more likely a molecular biological correlate—to help us with the selection process.
I think the fact that our data show no difference in local control and overall survival whether you have had adjuvant therapy or not is an indicator that adjuvant therapy works, since we only picked our highest risk patients for adjuvant therapy.
Dr. Victor M. Fazio (Cleveland, Ohio): I would like to congratulate Dr. Cohen and his colleagues on this presentation with its uniquely low mortality rate. This is clearly the best presentation I have ever seen for mortality rate reduction of any serious extent.
We have published our results in a similar number of cases and reported very similar local recurrence rate of 7.2% and have noticed that there has been an inverse relationship between the height of the lesion from the dentate line and recurrence rates. Indeed, upper third rectal cancers recur in exactly the same way as sigmoid cancers in our series, thus possibly negating any need or role for TME for upper third rectal cancers.
In a way, rectal cancer has now become substratified much the way it had been subset out from colorectal cancer and colon cancer in the past. So my first question, Dr. Cohen, is: Have you analyzed oncologic outcome by the size of the primary in the rectum, i.e., its intraperitoneal or extraperitoneal location? Our data would suggest that recurrences are much higher for the lower third rectal cancers. Secondly, who now does or does not get adjuvant preoperative chemoradiation therapy in your group? For example, does a T3N0 or T2N0 if it is in the low rectum, would that qualify in your present guidelines?
Dr. Cohen: Our practice standards separate cancers of the intraperitoneal rectum, that is defined that the lower edge is above the peritoneal reflection. It is extremely important to pass this information along to the medical oncologists and the radiation therapists because the pathology report will indicate rectal cancer, and they have their standard chemoradiation paradigm. Patients with intraperitoneal rectal cancer are treated as colon cancer; if they are node-positive, they get chemotherapy and don’t get radiation therapy.
Dr. Jerome J. Decosse (New York, New York): Dr. Cohen, an excellent report which provides a model for quality in early results. My question is directed toward what happens after 5 years. You have an extraordinary database covering 12 years, and I gather radiation therapy has been administered for a decade. After 5 years, is there any disparity or change with respect to increasing local recurrence in the radiated group, as compared to untreated controls, or is there any increase in incontinence in the group who had radiation for low rectal cancer? Are there long-term consequences of radiation therapy and chemotherapy that we have yet to fully appreciate?
Dr. Cohen: A very interesting, very important, provocative question to which I unfortunately don’t have the answer. Our median follow-up is approximately 4 years. I think we are going to need another 3 years of maturation of the data to sort out whether we have finally flattened out the actuarial local recurrence curve in the patients that received adjuvant therapy. In many other sites, adjuvant therapy has delayed but not eliminated recurrence.
Footnotes
Correspondence: Alfred M. Cohen, MD, Dept. of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Cohen A, Minsky B, Schlisky RL. Cancer of the rectum. In: DeVita V, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology, 5th ed. Philadelphia: Lippincott-Raven; 1997.
- 2.Pilipshen SJ, Heilweil M, Quan SH, Sternberg SS, Enker WE. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer 1984; 53: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT. Resectable adenocarcinoma of the rectosigmoid and rectum. I. Patterns of failure and survival. Cancer 1988; 61: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 4.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 1985; 312: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 5.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–715. [DOI] [PubMed] [Google Scholar]
- 6.Stockholm Rectal Cancer Study Group. Preoperative short-term radiation therapy in operable rectal carcinoma. A prospective randomized trial. Cancer 1990; 66: 49–55. [DOI] [PubMed] [Google Scholar]
- 7.Stockholm Colorectal Cancer Study Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol 1996; 3: 423–430. [DOI] [PubMed] [Google Scholar]
- 8.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997; 336: 980–987. [DOI] [PubMed] [Google Scholar]
- 9.Pahlman L, Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg 1990; 211: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis:: long-term follow-up. Int J Radiat Oncol Biol Phys 1998; 42: 51–57. [DOI] [PubMed] [Google Scholar]
- 11.Grann A, Minsky BD, Cohen AM, et al. Preliminary results of preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for clinically resectable T3 rectal cancer. Dis Colon Rectum 1997; 40: 515–522. [DOI] [PubMed] [Google Scholar]
- 12.Rouanet P, Fabre JM, Dubois JB, et al. Conservative surgery for low rectal carcinoma after high-dose radiation. Functional and oncologic results. Ann Surg 1995; 221: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyams DM, Mamounas EP, Petrelli N, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: : a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum 1997; 40: 131–139. [DOI] [PubMed] [Google Scholar]
- 14.Enker WE, Laffer UT, Block GE. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann Surg 1979; 190: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982; 69: 613–616. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341: 457–460. [DOI] [PubMed] [Google Scholar]
- 17.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer:: the Basingstoke experience of total mesorectal excision, 1:978–1997. Arch Surg 1998; 133: 894–899. [DOI] [PubMed] [Google Scholar]
- 18.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995; 181: 335–346. [PubMed] [Google Scholar]
- 19.Karanjia ND, Corder AP, Bearn P, Heald RJ. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg 1994; 81: 1224–1226. [DOI] [PubMed] [Google Scholar]
- 20.Havenga K, Enker WE, McDermott K, Cohen AM, Minsky BD, Guillem J. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg 1996; 182: 495–502. [PubMed] [Google Scholar]
- 21.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986; 2: 996–999. [DOI] [PubMed] [Google Scholar]
- 22.MacRae HM, McLeod RS. Handsewn vs. stapled anastomoses in colon and rectal surgery:: a meta-analysis. Dis Colon Rectum 1998; 41: 180–189. [DOI] [PubMed] [Google Scholar]
- 23.Vignali A, Fazio VW, Lavery IC, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: : a review of 1,014 patients. J Am Coll Surg 1997; 185: 105–113. [DOI] [PubMed] [Google Scholar]
- 24.Rullier E, Laurent C, Carles J, Saric J, Michel P, Parneix M. Local recurrence of low rectal cancer after abdominoperineal and anterior resection. Br J Surg 1997; 84: 525–528. [PubMed] [Google Scholar]
- 25.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 26.Arbman G, Nilsson E, Hallbook O, Sjodahl R. Local recurrence following total mesorectal excision for rectal cancer. Br J Surg 1996; 83: 375–379. [DOI] [PubMed] [Google Scholar]
- 27.Zaheer S, Pemberton JH, Farouk R, Dozois RR, Wolff BG, Ilstrup D. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998; 227: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenas RB, Fichera A, Mhoon D, Michelassi F. Total mesenteric excision in the surgical treatment of rectal cancer: : a prospective study. Arch Surg 1998; 133: 608–611. [DOI] [PubMed] [Google Scholar]
- 29.Friedmann P, Garb JL, McCabe DP, et al. Intestinal anastomosis after preoperative radiation therapy for carcinoma of the rectum. Surg Gynecol Obstet 1987; 164: 257–260. [PubMed] [Google Scholar]
- 30.Stryker SJ, Kiel KD, Rademaker A, Shaw JM, Ujiki GT, Poticha SM. Preoperative “chemoradiation” for stages II and III rectal carcinoma. Arch Surg 1996; 131: 514–518. [DOI] [PubMed] [Google Scholar]
- 31.Kollmorgen CF, Meagher AP, Wolff BG, Pemberton JH, Martenson JA, Ilstrup DM. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg 1994; 220: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paty PB, Enker WE, Cohen AM, Minsky BD, Friedlander-Klar H. Long-term functional results of coloanal anastomosis for rectal cancer. Am J Surg 1994; 167: 90–94. [DOI] [PubMed] [Google Scholar]