Abstract
Objective
To review the results of the authors’ most recent 100 consecutive cases of transcervical thymectomy for myasthenia gravis (MG) in terms of complications and outcome in comparison with other reported techniques.
Summary Background Data
Myasthenia gravis is believed to be an autoimmune disorder characterized by increasing fatigue with exertion. The role of thymectomy in the management of the disease remains unproven, but there is widespread acceptance of the notion that complete thymectomy improves the course of the disease. Complete excision of the thymus is the goal in all cases; however, the best technique to achieve complete thymectomy remains controversial. The authors favor a transcervical approach through a small collar incision aided by a specially designed sternal retractor. Others prefer a transsternal, a combined transcervical and transsternal (“maximal”), or a video-assisted thoracoscopic surgical approach.
Methods
A retrospective review of the authors’ most recent 100 consecutive transcervical thymectomies for nonthymoma-associated MG was performed using medical records and telephone interviews. Patients’ symptoms were graded before surgery and at the most recent (within the last 6 months) postoperative time point, using the modified Osserman classification: 0 = asymptomatic, 1 = ocular signs and symptoms, 2 = mild generalized weakness, 3 = moderate generalized weakness, bulbar dysfunction, or both, and 4 = severe generalized weakness, respiratory dysfunction, or both.
Results
There were 61 female patients and 39 male patients with a mean age of 38 years (range, 14 to 84). The median hospital stay was 1 day. There were no deaths and no significant complications. Seventy-eight patients who had undergone surgery >12 months ago were available for analysis. In these patients, with a mean follow-up time of 5 years (median 5.3; range, 12 months to 10 years), the median preoperative Osserman grade improved from 3.0 (mean 2.73) before surgery to 1.0 after surgery (mean 0.94).
Conclusions
The transcervical approach for thymectomy for the treatment of MG produces results similar to those of other surgical approaches, with the added benefits of shortened hospital stay, decreased complications, reduced cost, and broader physician and patient acceptance of surgical treatment.
Myasthenia gravis (MG) is a rare autoimmune disorder occurring in 0.5 to 1.0 per 100,000 people. For reasons that are not fully elucidated, the thymus is thought to play an integral role in its pathogenesis. The thymus is the site of T-lymphocyte education, with resultant self-tolerance. Any derangement in T-cell education can lead to loss of self-tolerance and autoimmunity. The autoantibodies associated with MG are directed at the acetylcholine receptor in the neuromuscular junction and lead to a functional and numeric decrease in acetylcholine receptors. Myasthenia gravis is characterized clinically by increasing fatigue with exercise. The symptoms can range from isolated ptosis, diplopia, or mild proximal muscle weakness to severe generalized weakness and ventilator dependence. Medical management consists of maintenance anticholinesterase inhibitors, such as pyridostigmine, for symptomatic relief and immunosuppression, such as corticosteroids and azathioprine, to treat the underlying autoimmunity.
One of the earliest reports of thymectomy in a patient with MG was in 1912 by Sauerbruch. 1 In 1939, Blalock performed a transsternal thymectomy in a young woman for a cystic thymoma and MG, which resulted in improvement of her symptoms. 2 He subsequently reported success with thymectomy in a series of patients with MG without thymoma. 3 With increasing experience, thymectomy became a standard treatment adjunct for MG despite the absence of a randomized prospective trial comparing it with medical management alone. Comparisons of surgery and medical management are confounded by the observation that spontaneous remissions occur, different surgical approaches have differing reported results, and some patients take years to demonstrate improvement after thymectomy. The most-cited study to support the beneficial role of thymectomy in the treatment of MG was a retrospective analysis using a computer to match, based on clinical status, patients who underwent thymectomy with similar patients who were managed medically. 4 The conclusion was that patients treated medically had an 8% remission rate (defined as medicine- and symptom-free) and a 43% death rate versus 34% and 14%, respectively, in the surgery cohort.
Thymectomy as an adjunctive treatment for MG is now considered the standard of care, with the best results achieved generally in younger patients with a short duration of symptoms. There remains controversy regarding the optimal surgical approach to accomplish complete excision of the thymus. We believe, and have previously reported, 5 that a complete thymectomy can be accomplished through a transcervical incision with the use of a specially designed right-angle retractor to lift the sternum anteriorly and a headlight for better visualization. Other reported techniques include a video-assisted thoracoscopic surgery (VATS) approach, 6,7 a transsternal approach, 8 an extended transsternal approach, 9,10 or an extended transcervical, transsternal (“maximal”) approach. 11,12 Herein, we report our results using the transcervical approach in 100 consecutive nonthymoma thymectomies for MG and compare them to those of other published reports.
METHODS
Our technique of transcervical thymectomy has been previously described. 5 In all cases, the chest is prepared for possible median sternotomy and the patient is informed that a conversion to a full sternotomy might be necessary. However, no patient required intraoperative conversion to a sternotomy approach during the period for which this review was conducted.
A retrospective medical record review was conducted on 100 consecutive transcervical thymectomies performed for nonthymoma MG between 1989 and 1998 at Barnes-Jewish Hospital, St. Louis, Missouri. Preoperative Osserman grades were determined from the chart review of the referring neurologist’s notes using the classification system of 0 = asymptomatic, 1 = ocular signs and symptoms, 2 = mild generalized weakness, 3 = moderate generalized weakness, bulbar dysfunction, or both, and 4 = severe generalized weakness, respiratory dysfunction, or both. Postoperative Osserman grades were determined by a telephone interview of the patients. All other data were determined from medical record review and our thoracic surgery database.
Of the 100 patients, 88 had undergone surgery >12 months ago. Of these, six were lost to follow-up and two had late deaths; in two an additional neuromuscular disease had developed, and these patients were removed from the follow-up analysis. Thus, 78 patients were >12 months after surgery and were available for recent (within the past 6 months from data collection) postoperative Osserman classification assignment and medication history. These patients were used in the analysis of functional outcome. The mean follow-up was 5.0 years (range, 12 months to 10 years; median 5.3 years).
Pathologic study of all resected specimens was reviewed by a pathologist blinded to the patient’s clinical status. The pathologic specimens were interpreted as hyperplastic, involuted, or normal (± cyst). Probability values were generated using both the Kruskal-Wallis analysis of variance and Wilcoxon signed-rank nonparametric tests. The two-sample t test and Fisher’s exact test were applied where appropriate. Probability values ≤0.05 were considered to be statistically significant. The remission rate was defined as the number of patients who were both symptom- and medication-free divided by the total number of patients. The palliation rate was defined as the number of patients who had clinical improvement of one or more grades divided by the number of patients.
RESULTS
One hundred consecutive transcervical thymectomies performed at Barnes-Jewish Hospital for nonthymoma-associated MG from 1989 to 1998 were analyzed. There were 61 female patients and 39 male patients with a mean age of 38 years (range, 14 to 84). The mean time interval between diagnosis and surgery was 1.7 years (median 0.72; range, 4 days to 16 years). The mean operative time was 104 minutes (median 95; range, 70 to 185). The mean postoperative length of stay was 1.22 days (median 1.05; range, 0.6 to 4.1). Eighty-five percent of the patients were discharged on the first postoperative day, 96% by the second postoperative day. There were no blood transfusions, no postoperative ventilator requirements, and no recurrent laryngeal or phrenic nerve injuries.
There were eight complications and no deaths within 30 days from surgery. Seizures developed in one patient, who was transferred to neurology. Myasthenic crisis developed in one patient, who was given intravenous pyridostigmine and high-dose corticosteroids with resolution. A left leg deep venous thrombosis developed in one patient, who was given systemic anticoagulation. Five patients had apical pneumothoraces noted on postoperative chest x-rays. Three of the five were observed, and the other two underwent aspirations of the air with prompt resolution.
There were two late deaths: one patient died of cardiac arrest 6.6 years after surgery and one patient died of a cerebrovascular attack 4.2 years after surgery.
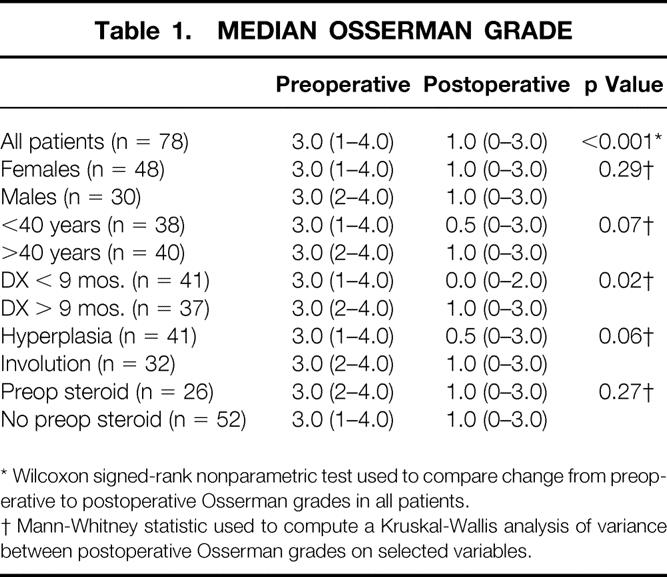
Table 1 demonstrates the change in Osserman grade from before surgery to most recent follow-up. The median Osserman grade of all patients analyzed improved from 3.0 before surgery to 1.0 after surgery (p < 0.001). Eighty-five percent (66/78) of the patients improved one or more Osserman grades; 63% (49/78) improved two or more Osserman grades. Thirty-five percent (27/78) were in remission (symptom- and medication-free), and 71% (55/78) had no generalized symptoms. One patient (1.3%) deteriorated one Osserman grade, and 14% of the patients (11/78) had no change in Osserman grade after thymectomy, but almost all of these required less medication than before surgery. Patients less than 40 years of age (n = 38) had a greater overall net decrease in their Osserman grade at the most recent postoperative follow-up compared with those older than 40 years (n = 40) (see Table 1), but the difference was not statistically significant (p = 0.07).
Table 1. MEDIAN OSSERMAN GRADE
* Wilcoxon signed-rank nonparametric test used to compare change from preoperative to postoperative Osserman grades in all patients.
† Mann-Whitney statistic used to compute a Kruskal-Wallis analysis of variance between postoperative Osserman grades on selected variables.
Ninety percent (70/78) of the patients were taking pyridostigmine before surgery, with a mean daily dose of 365 mg/patient; after surgery, 46% were taking pyridostigmine, with a mean daily dose of 219 mg/patient (p < 0.001). Thus, 54% of the follow-up patients required no pyridostigmine for symptom control. Thirty-three percent (26/78) of the patients were taking prednisone before surgery, with a mean daily dose of 27 mg/patient; after surgery, 27% (21/78) of the patients were taking prednisone, with a mean daily dose of 16 mg/patient (p = 0.03) at most recent follow-up.
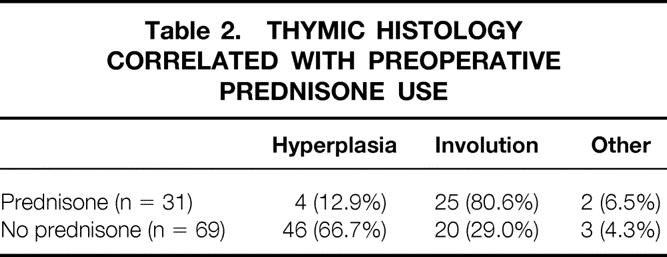
Histology was determined on all 100 resected specimens by a pathologist blinded to each patient’s clinical status. Follicular hyperplasia was the diagnosis in 50 specimens, normal thymus (± cyst) in 5, and involution in 45. Table 2 shows that patients who were taking prednisone before surgery had a much higher incidence of thymic involution at the time of surgery (80.6%) than patients not taking prednisone (29%; p < 0.001). We compared the effect of thymectomy in patients with and without thymic involution (see Table 1) and found that the difference did not quite reach statistical significance (p = 0.06). As also illustrated in Table 1, there was no difference in results when comparing those who received preoperative prednisone and those who did not. This is similar to results reported by Masaoka et al. 10
Table 2. THYMIC HISTOLOGY CORRELATED WITH PREOPERATIVE PREDNISONE USE
To determine whether thymectomy early in the course of disease resulted in a significantly greater clinical improvement, potential points along the time line from diagnosis of MG to thymectomy were evaluated. Table 1 shows that patients operated on within 9 months after the diagnosis of MG had a statistically significantly greater improvement in their postoperative Osserman grade than the patients operated on more than 9 months after the diagnosis (0.0 vs. 1.0; p = 0.02).
DISCUSSION
Thymectomy for MG as an adjunct to medical management is widely accepted but remains unproven from a strictly scientific standpoint. Controversy still exists regarding the best surgical approach for thymectomy to achieve optimal benefit. We have advocated a transcervical approach and have previously reported on its comparable efficacy relative to the transsternal and “maximal” approaches. The long-term efficacy of the transcervical approach was recently reported with an 8.4-year average follow-up of our original series, demonstrating an improvement of the mean Osserman grade from 2.7 before surgery to 0.4 after surgery, a complete remission rate of 44%, and a palliation rate of 91%. 13 These results compare favorably to the report by Jaretzki et al, 11 which indicate a remission rate of 46% and a palliation rate of 96% at 89 months using the “maximal” approach. Our current data indicate a lower remission rate (35% at 5-year average follow-up) than our previously reported rate of 52%. 5 However, we found it difficult to compare the two series from the standpoint of complete remission because many asymptomatic patients in the current series are routinely maintained on chronic prednisone by the treating neurologist, thus technically eliminating them from the category of “remission” (i.e., no symptoms and no medication). Corticosteroids are used routinely even after remission of symptoms because of the belief that some patients are at risk for recurrence and that maintenance immunosuppression may inhibit reemergence of symptoms. If patients who are asymptomatic and not on pyridostigmine, but are on maintenance prednisone are counted as remissions, the overall remission rate becomes 46%. This is identical to the 5-year remission rate reported by Masaoka et al using their extended transsternal approach. 10 If one includes patients who require no pyridostigmine for symptom control, the “remission” rate becomes 54%.
The difficulty in comparing results from different series was addressed by Bulkley et al, 12 who noted the same confounding variable of continued corticosteroid use after surgery in asymptomatic patients. This report did not specify the remission rate but used a multivariate regression analysis to analyze data using the Drachman classification of signs and symptoms to grade the patients.
In our series, the transcervical approach for thymectomy was associated with a low morbidity rate and zero deaths. This current series of 100 consecutive patients had an 8% rate of minor complications versus a 33% rate (including six nerve injuries) in the recent report by Bulkley et al 12 using a transsternal and cervicomediastinal (“maximal”) approach and a 4.5% incidence of major complications, including sternal wound infections, reported by Jaretzki et al, 11 using the “maximal” approach. Thus, there is a significant reduction in complications with the transcervical approach compared with the more invasive transsternal or combination transsternal and cervical approaches. In addition, the transcervical approach offers several advantages worth noting. It requires a decreased operative time (mean 104 minutes) compared with the more invasive cervicomediastinal approach (mean 163 minutes). The transcervical approach also is associated with a minimal postoperative length of stay (mean 1.22 days).
Because the operative time, length of stay, and morbidity rate of the transcervical approach are all less than with the more radical approaches, we can infer that the cost of the surgery is also significantly less. Further, our patients can return to complete unrestricted activity within 3 to 4 days after surgery. This early return to normal activity, plus the favorable cosmetic result with a small transverse collar incision (compared with a median sternotomy), may make the procedure more acceptable to both patients and referring physicians alike.
Recently, several groups have reported performing thymectomies using a VATS approach with or without a cervical collar incision. 5,6 The short follow-up on the patients treated with VATS thus far, along with the small cohort size, makes it difficult to interpret the early low remission rates of 27% and 25%. 5,6 Video-assisted thoracoscopic surgery is more invasive than the transcervical approach because of the multiple trocar sites, which are prone to cause both short-term and long-term pain from trauma to the intercostal nerves, and the need to collapse one lung during the procedure. The ultimate clinical utility of this approach remains undefined, but the possibility of the VATS approach being more easily mastered by surgeons may make it an appealing minimally invasive approach if a morbidity rate and long-term results equivalent to those of the transcervical approach can be achieved. Of course, one can easily use a videoscope through the transcervical incision if desired, but we have not found this necessary (except to demonstrate the anatomy to observers in the operating room).
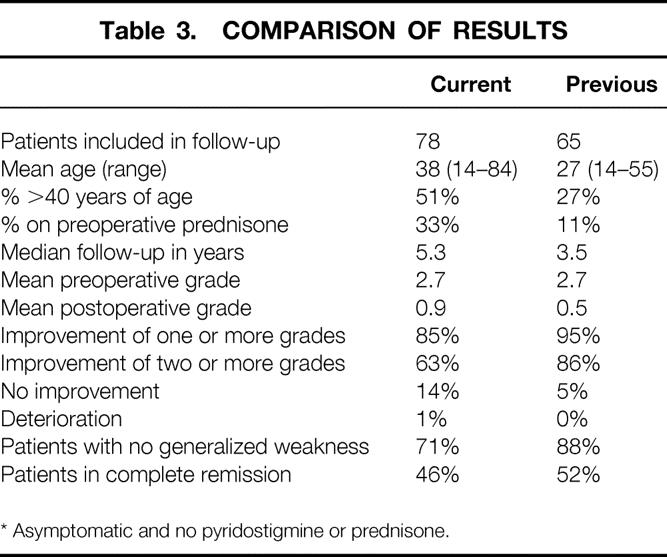
Inherent in this entire analysis is the great difficulty of comparing studies regarding thymectomy and its clinical efficacy. There are so many variables from study to study: surgical approach, mean age of cohort, duration of symptoms, use of preoperative and postoperative corticosteroids, philosophy of neurologist, clinical scoring of signs and symptoms of MG, and others. The difficulty of comparing studies is highlighted in Table 3, where we compare our current data to our previous report. 5 In the current series, the patients were an average of 11 years older, included more patients receiving preoperative corticosteroids, and included postoperative patients in clinical remission who were nonetheless maintained on corticosteroids because of the philosophy of the treating neurologist. Further, different investigators assigned the Osserman grades in the two studies. Although the lesser improvement in older patients did not reach statistical significance in this series, in a larger series by Masaoka et al, 10 patients less than 35 years of age had a significantly greater improvement after thymectomy than those older than 35. If one analyzes the percentage of postoperative patients not requiring pyridostigmine in our two series, the results appear much more comparable (57% in the current series vs. 49% in the previous one). We support the concept of a developing unified classification system to standardize treatment and analysis. Until such a unified system exists to compare data, we submit that the transcervical approach to thymectomy in patients with nonthymoma-associated MG offers comparable efficacy with decreased complications and cost relative to more invasive procedures.
Table 3. COMPARISON OF RESULTS
* Asymptomatic and no pyridostigmine or prednisone.
Discussion
Dr. Gregory B. Bulkley (Baltimore, Maryland): Dr. Cooper, this is an important contribution. Once again, you and your colleagues have provided us with a really thoughtful challenge to our conventional approach to this problem.
As you pointed out, since Jaretski first showed us two important principles of this operation—one, that thymic tissue is widely distributed through the anterior and superior mediastinum, and two, and just as importantly, that one cannot tell whether it is thymic tissue or fat by gross observation—since that time, the consensus approach to this problem has been to do as maximal a thymectomy as possible. Apparently, you agree with those principles. You clearly agree with that. And we have discussed this.
Dr. Osler has very wisely advised us to never be the first nor the last to adopt a new procedure, although rigid adherence to this characteristically conservative advice from an internist would result in no innovation whatsoever. I am trying to decide whether I should follow this advice and be the second to adopt this procedure. You have shown us that the morbidity is less in this operation. The question is, is it as good? In that light I would like to ask you a couple of specific questions.
First, we found when we did our own study that our follow-up data was remarkably sensitive to the intensity of follow-up. (You have acknowledged your own problems with subjectivity.) When we asked people twice, we got more symptoms than when we asked them once. When we called their neurologist, we got more information. There were particular problems with the transient nature of the symptoms. Patients would say, “Gee, I feel great today.” Then you call them back and they say, “Yeah, but last week I had a little ptosis.” You report a relatively low percentage follow-up. How reliable was the follow-up you did obtain? Did this influence your results?
Secondly, as per Jaretski, we do a very thorough anatomic dissection of all of the soft tissue. This is the way the dissection looks following the operation that we do. (Slide.) On the left, you can see the dissection of the trachea, the innominate artery, and the recurrent nerve, and on the right side, you can see the importance of dissecting out the pulmonary window and the depth behind the nerve. Are you doing the same dissection through that little hole? Or does it not matter whether you do as good a dissection as this?
Related to that is my third question: Since only a single operator can view the field at any one time, how do you teach this operation? Do the residents do these cases with you? If so, how can you then tell if he or she has done an adequate dissection, if one cannot tell by looking at the tissue whether it is thymus or not?
Fourth, I am very concerned about this question about whether your patients have thymomas or not. We have found the MRI and/or CT is only correct about the presence of a thymoma 90% of the time. Can you determine this preoperatively? What do you do when you find a thymoma? There are zero thymomas in this series. Have you taken those patients out of the series? In most series, 20% to 30% of patients have thymomas. In some series, these patients do worse. Is this a selected series without thymomas, or did you just never find any thymomas?
With all the variability in technique, in the operator, in patient selection, in subjective follow-up, obviously this is a question that can only be resolved by a randomized, controlled trial. In your manuscript, you challenged those of us doing the conventional technique to do the trial. Although we could quibble about whether the innovator should do the trial or not, I think that even if we did it and if our old approach worked better, you would just say we didn’t know how to do your operation.
So what I would like to ask you is, with our 20 to 30 patients a year, and your numbers, which are somewhat similar, would you be willing to join with me and subject patients in both of our centers to a randomized trial of this, and with uniform follow-up by some uniform standard, and settle this once and for all?
Your contributions to this field have been important. I have nothing but the greatest regard for your work. And this paper certainly exemplifies that.
Presenter Dr. Joel D. Cooper (St. Louis, Missouri): Thank you very much, Dr. Bulkley. I appreciate your contributions and your generous comments. Let me try to address some of the questions.
First of all, an observation. I was not convinced in the early years that we did accomplish the same operation. And when I was initially taught this operation without the retractor, without the headlight, I hated it. It seemed like an expedition into the Dark Ages. And if someone told me that could you do a complete clean-out of the mediastinum through a neck incision, I would tell you that they are smoking something. But with modification, with the retractor, the headlights and loops, I do believe, and on the basis of our long-term follow-up do believe, that we are accomplishing the same thing.
Now, since the complete remission rate and the improvement rate in all of the major series come out exactly the same, you can only draw two possible conclusions. Either that each of the surgical procedures is accomplishing the same goal, namely, complete thymectomy —and I choose to believe that—or you can believe the operation has nothing whatsoever to do with the outcome and that surgery for myasthenia gravis really has no role and the nature of the operation has in fact nothing to do with the outcome, it is the nature of the disease. And there are those who would argue that. So one of the questions I asked you when you were talking about a randomized trial, are we talking about a trial of different surgical procedures or are we talking about a trial of surgery versus no surgery, as some have called for?
The follow-up, I completely agree—we have not done a good job in the follow-up of these patients. This is the only group of patients—and I regret it—that I do not follow for life. All other patients that I operate on, I see, as I tell them, for life, theirs or mine, whichever comes first. But for some reason, I started referring these patients back to the neurologists. There isn’t much to follow surgically after initial postoperative visits. And we have not therefore maintained our own database, as we have on all other operations that we do. And I regret that. And so we are dependent, as perhaps you are, on follow-ups, phone calls, neurologists, and variable input, which I admit completely confounds the situation.
Are we doing the same dissection that you illustrated? No. Could we? Yes. And in the early days I took out the gland and I took out all the fat and I would send off 20 different specimens to the pathologist as part of a learning process to see, could I or could I not discern by the naked eye which of the fat I ought to take and which not. I believe in fact that I pretty much can, and I take perhaps a little less fat than I used to, having gone through that process.
Now, if some would argue that failure to achieve a good result in myasthenia is solely based upon failure to completely do a thymectomy, I would argue two things.
Dr. Jaretzki and yourself, by doing these radical operations, should accomplish a much better remission rate—in fact, a 100% complete remission rate—if all you needed to do to cure myasthenia was to do a complete thymectomy. But such is not the case. So complete excision of the gland may be necessary but is not sufficient to produce complete remission in the first case.
Second of all, if failure to achieve complete remission were based upon hypertrophy of microscopic foci of remaining thymic remnants missed at the time of surgery, then when a patient with persistent symptoms is reexplored—as all of us have had occasion to do, usually cases done elsewhere—to see if there is any missed thymus, why have I never found a surgeon who has told me that, on such reexplorations, he has ever found anything other than a retained thymic lobe, looking like it should, being where it should, and having the usual appearance. I have never found a surgeon who said, “Yes, the patient had a thymectomy, had recurrent symptoms, we reexplored them, and down near the diaphragm or behind the phrenic nerve, we found a blob of thymic tissue growing in an expanded fashion as if a remnant had hypertrophied and caused the symptoms.” I have never heard of that. So I now believe that removal of the gland and all surrounding and contiguous fat is sufficient to produce the maximum result.
How do you teach it? Another very good question. It is like mediastinoscopy, it is difficult to transmit, only one person can really see. We do occasionally take a thoracoscope for visitors and put it in the neck and show them around. I have the resident now sit on a mobile stool, we sit on a stool with wheels, each with headlights and loops, and we just sort of move back and forth across the field when trying to teach it. But it is one of the deficiencies of this operation. It undoubtedly is more difficult to teach and to master.
How do we know the thymoma is present or not? And I agree the radiologists are often wrong. If we are sure there is a thymoma, we do a sternotomy. But in many cases when there is a question, we will do the neck incision, and if we should happen to encounter thymoma, then we convert to sternotomy. The series that I am presenting to you are those cases in which there was no thymoma. I can recall in this series of 100, I believe only one case in which an uncertainty about thymoma turned out to show that in fact it was a thymoma. We then converted to the sternotomy. That case is not included here. This series is only those without thymoma.
So as far as a trial, it depends on what kind of a trial. I would have a difficult time doing a trial between medicine and surgery. The Academy of Neurology has just concluded that although a randomized trial would scientifically be nice to have, the burden of evidence thus far shows improved outcomes following thymectomy.
Dr. Richard J. Finley (Vancouver, Canada): Dr. Cooper taught me how to do this operation, for which I am very grateful, but there are some problems with it. I would like to address a couple of the problems.
In order to do this operation, you have to be able to extend the neck of the patient. My first question is, can you do this operation in the older patients who cannot extend their necks?
The second question relates to the fact that occasionally the thymus goes behind the innominate vein. How do you manage this situation when you are using the transcervical approach?
My third question is, when do you do a sternotomy other than for thymoma? Are there situations where you had to do a sternotomy to completely remove the gland? How do you manage these patients who do not improve after the operation? Do you get a repeat CAT scan? Do you reoperate on them? How many in your series have been reoperated on?
Finally, with the advent of thoracoscopy, have you used a thoracoscopic-assisted approach to this operation in order to accurately dissect out the lower parts of the thymus gland?
Dr. Cooper: When I examine the patient preoperatively, I do look for mobility of the neck. In fact, the last time I can recall converting to sternotomy, it was a patient with ankylosing spondylitis and I could not get sufficient extension of the neck. That was about 12 to 14 years ago. But I do find that in the older patients—and I admit that in them, this is a trial of surgery, because it is really uncertain as to whether it is going to be beneficial or not—I have not found rigidity of the cervical spine to be a problem and have not had to convert for any reason in the last 15 years that I can recall other than the case of a thymoma.
As you point out, the upper pole sometimes goes behind the innominate vein—that is almost always the left pole which descends behind the innominate vein—and I then dissect out the upper pole completely behind the vein, pass it behind the vein, draw it up in front of the vein, and then continue into the mediastinum.
I have also encountered three cases in which the innominate vein is extremely low and instead of being up in the sternal notch was really about 3 cm below the sternal notch. I wasn’t aware of that anomaly, but fortunately was able to see it and dissect it off completely and clean it off in spite of that particular anomaly. We have not had occasion to open, but are always prepped and draped and consented to do so.
You can see the diaphragm from this approach. I won’t tell you that you can easily work around it, but you can visualize down to the diaphragm if you make the effort. And you could put in the thoracoscope. But, as I say, I have only done that through this incision to show guests the anatomy, since otherwise it is almost impossible for anybody but the surgeon to see.
The question of what do you do if they are not improved: As you recall, in Toronto when we worked with the eminent neurologist Dr. John Humphrey, he had a certain sixth sense of patients operated on elsewhere, who didn’t seem to get the anticipated improvement. And Dr. Pearson and I had the occasion to use the transcervical approach on patients who previously had had a full sternotomy, and we went down into the chest from the neck and found a missed lobe of thymus gland in the aorta pulmonary window area or somewhere else, presumably from a surgeon who didn’t know thymic anatomy all that well.
In my own personal experience, there are only two of my cases I am aware of that have been reexplored. One was explored after I left Toronto by Dr. Patterson because the patient didn’t seem to have as good a response as one would anticipate. And one was recently done in New York on a patient whom I had done. And in both cases there was no thymic tissue found on reexploration.
As far as thoracoscopy assistance, I haven’t used it, but could do it through the midline neck incision. Those people who prefer the thoracoscopy approach of course have to put in a double lumen tube, collapse the lung, expose the patient to the problems of video-assisted thoracotomies—namely, chronic pain from levering on the intercostal nerves—and we have not found it an useful adjunct.
But I must say that this is not an easy procedure to learn to do. And if the video-assisted approach is something easier to teach and easier to learn, although it is associated with more morbidity than this, it may be a very reasonable option.
Footnotes
Correspondence: Joel D. Cooper, MD, Division of Cardiothoracic Surgery, Washington University School of Medicine, Suite 3108 Queeny Tower, One Barnes-Jewish Hospital Plaza, St. Louis, MO 63110.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Schumacher R, Roth J. Thymektomie bei cenem fall von morbus basedowii mit myasthenie. Med Chir 1912; 25: 746. [Google Scholar]
- 2.Blalock A, Mason M, Morgan H, Riven S. Myasthenia gravis and tumours of the thymic region. Ann Surg 1939; 110: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blalock A, McGehee HA, Ford FR, et al. The treatment of myasthenia gravis by removal of the thymus gland. JAMA 1941; 117: 1529. [Google Scholar]
- 4.Buckingham J, Howard F Jr, Bernantz P, et al. The value of thymectomy in myasthenia gravis: : a computer-assisted matched study. Ann Surg 1976; 84: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper JD, Al-Jilaihawa A, Pearson FG, Humphrey J, Humphrey H. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988; 45: 242–247. [DOI] [PubMed] [Google Scholar]
- 6.Mack M, Scuggs G. Video-assisted thoracic surgery thymectomy for myasthenia gravis. Chest Surg Clin North Am 1998; 8: 809–825. [PubMed] [Google Scholar]
- 7.Mantegazza R, Confalonieri P, Antozzi C, et al. Video-assisted thoracoscopic extended thymectomy (VATET) in myasthenia gravis. Ann N Y Acad Sci 1998; 841: 749–752. [DOI] [PubMed] [Google Scholar]
- 8.Mulder D, Graves M, Hermann C. Thymectomy for myasthenia gravis: : recent observations and comparisons with past experience. Ann Thorac Surg 1989; 48: 551–555. [DOI] [PubMed] [Google Scholar]
- 9.Olanow C, Wechsler A, Sirotkin M, Stajch J, Roses A. Thymectomy as primary therapy in myasthenia gravis. Ann NY Acad Sci 1986; 505: 595. [DOI] [PubMed] [Google Scholar]
- 10.Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: : a 20-year review. Ann Thorac Surg 1996; 67: 853–859. [DOI] [PubMed] [Google Scholar]
- 11.Jaretzki A II, Penn A, Younger D, et al. “Maximal” thymectomy for myasthenia gravis. J Thorac Cardiovasc Surg 1988; 95: 747–757. [PubMed] [Google Scholar]
- 12.Bulkley G, Bass K, Stephenson R, et al. Extended cervico-mediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997; 226: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bril V, Kojic J, Ilse W, Cooper J. Long-term clinical outcome after transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1998; 65: 1520–1522. [DOI] [PubMed] [Google Scholar]


