Abstract
Objective
To evaluate the Johns Hopkins Hospital experience with 136 thymomas over the past 40 years. This number of patients allowed quantitative estimation of the independent influence of common clinicopathologic risk factors using multivariate analysis.
Summary Background Data
Thymomas vary widely in terms of recurrence and influence on overall survival. Several series have indicated the importance of initial tumor invasion, as well as the extent of surgical resection, as predictors of recurrence and survival after thymoma resection. However, findings have been equivocal when other predictors of prognosis were examined.
Methods
The authors evaluated 136 patients seen at the Johns Hopkins Hospital between 1957 and 1997 with a pathologic diagnosis of thymoma. Demographic information, clinical staging data, surgical and adjuvant treatment details, and patient follow-up data were obtained from the patient record and from detailed patient or family interviews. Microscopic sections of all 136 patients were reviewed by two pathologists blinded to the clinical data. All data were analyzed by multivariate Cox regression analysis, which allowed the quantification of the independent predictive value of 12 putative clinicopathologic prognostic indicators.
Results
Completeness of follow-up was 99%, 99%, and 98% of eligible patients at 5, 10, and 15 years, respectively. Forty percent of the patients had associated myasthenia gravis and 27% had a secondary primary malignancy. Overall patient survival rates were 71%, 56%, 44%, 38%, and 33% at 5, 10, 15, 20, and 25 years, respectively. Overall, the thymoma-related mortality rate was 14%; the nonthymoma-related mortality rate was 26%. Incomplete resection, preoperative absence of myasthenia gravis, and advanced Lattes/Bernatz pathologic class were found to be independent predictors of poorer overall survival.
Conclusions
These findings support a policy of aggressive, complete surgical resection of all thymomas when feasible. Thymoma behaves as a rather indolent tumor, with most deaths from causes unrelated to thymoma or its direct treatment. Clinicians should have an increased awareness of the possibility of second primary malignancies in patients with thymoma.
Thymomas are the most common primary anterior mediastinal tumor and account for 15% to 20% of all mediastinal masses. 1 Thymomas are derived from thymic epithelial cells and are distinguished from thymic lymphomas, germ cell tumors, and carcinoid tumors. 2 Few tumors are associated with as many paraneoplastic syndromes, including myasthenia gravis (MG), pure red cell aplasia, acquired hypogammaglobulinemia, and connective tissue disorders. 1,3 Myasthenia gravis is by far the most common, reported in 25% to 35% of patients with thymoma. 3–8
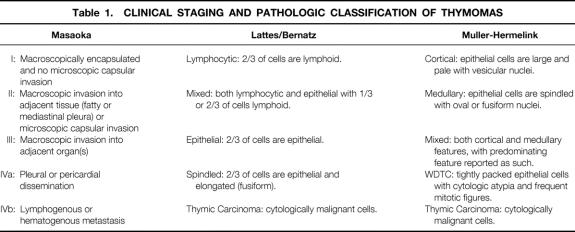
Several studies have indicated the importance of initial tumor invasion and the extent of surgical resection as predictors of recurrence and survival after resection of a thymoma. 5–15 The most widely employed system for clinical staging at initial presentation is that proposed by Masaoka (Table 1). Stage I tumors are completely encapsulated, both grossly and microscopically. This staging system then advances sequentially, with increasing degrees of invasion and spread, to stage IVb (distant [hematogenous] metastatic disease). 7
Table 1. CLINICAL STAGING AND PATHOLOGIC CLASSIFICATION OF THYMOMAS
Rosai and Levine have proposed the simple division of thymomas into benign and malignant, based on the presence or absence of invasion and/or cellular atypia at initial presentation. 16 Malignant thymomas are additionally divided into type I and type II. Type I malignant thymomas are epithelial tumors that show invasion without evidence of cytologic atypia; type II thymomas (thymic carcinomas) display cytologic atypia, with or without evidence of capsular invasion.
Although most authors believe that tumor stage at initial presentation and the extent of surgical resection reliably predict thymoma prognosis, the findings have been equivocal when other predictors of prognosis are examined. Several histologic classifications of thymoma have been proposed (see Table 1), including the traditional (American, or Lattes and Bernatz [L/B]) system proposed by Bernatz and modified by Lattes 2 and Salyer and Eggleston, 17 in which thymomas are divided into predominantly epithelial, predominantly lymphocytic, or mixed lymphoepithelial types. Marino and Müller-Hermelink histologically graded thymomas based on their differentiation toward normal thymic cortical or medullary epithelium. 18–20 Although predominantly epithelial 5,6,8,9,12 and cortical 10,14,15,18,21–27 thymomas have been described as more invasive tumors, the importance of histologic classification as an independent predictor of overall prognosis remains unclear.
Because of the relative rarity of thymomas, most series (all retrospective) contain inadequate numbers of patients to quantitate the independent predictive values of putative clinical and histologic indicators of prognosis. To this end, we evaluated the Johns Hopkins experience with 136 thymomas over the past 40 years. This number of patients allowed us to apply contemporary statistical analysis to estimate the independent predictive values of prominent clinical and histologic features of this disease.
METHODS
A retrospective review of the pathology files and the tumor registry of patients evaluated at the Johns Hopkins Hospital between 1957 and 1997 revealed 154 patients with a histologic diagnosis of primary thymoma. After excluding patients for whom medical records and pathologic slides were unavailable, 136 patients remained for analysis. Of these 136 patients, 4 were patients in whom thymoma was discovered incidentally at autopsy. These patients were included in the analysis. Based on the information available from the clinical records, definitive retrospective clinical staging was possible in 126 of the 136 patients. Clinical staging was based on a combination of preoperative studies, including CT scan, as well as surgical reports and histologic findings. Histologic findings are paramount for precise staging, because Masaoka stages I and II are distinguished by the presence or absence of microscopic capsular invasion. (Ten patients, including those who underwent autopsy, did not undergo surgery.) Patients were staged by a modification of the Masaoka system. Data were obtained also for demographic parameters, the presence or absence and nature of systemic and/or local symptoms at presentation, associated diseases (including paraneoplastic syndromes), results of diagnostic tests, the nature of surgical intervention (if any), the completeness of surgical resection (where applicable), and the use of adjuvant therapy. Microscopic sections of all 136 patients were reviewed by two pathologists blinded to the clinical data and were classified according to the L/B system and the Müller-Hermelink system. 18,19
Patient follow-up data through June 1998 were obtained retrospectively using a meticulous review of hospital records, a review of the Johns Hopkins tumor registry, telephone interviews with patients or their family members and/or physicians, written questionnaires, and death certificates from the Department of Vital Statistics, Baltimore, Maryland. Our review protocol had been prospectively reviewed and approved by the Joint Committee for Clinical Investigation of the Johns Hopkins University School of Medicine and the Maryland Department of Vital Statistics. The patients or their families were sent a letter explaining the study and offering them an opportunity to decline participation by postage prepaid mail before our initial contact for questioning. Patient data were coded to protect confidentiality. By means of the above, we confirmed the initial clinical information and then recorded the presence or absence of thymoma recurrence, patient survival, and (when appropriate) cause of death.
In this series of 136 patients, the follow-up period ranged from 1 month to 40 years. Completeness of patient follow-up was 99%, 99%, and 98% at 5, 10, and 15 years, respectively.
Comparisons between groups in the distributions of categorical variables were made using the chi square statistic. Differences between groups in the means of continuous variables were compared using either the Student’s t test or analysis of variance. The Kaplan-Meier method 28 was used to estimate time to death from any cause and time to death from a thymoma-related cause. This method calculates the cumulative probability of survival at the time of each event (death), which adjusts for censoring of observations in the form of patients who are either lost to follow-up or whose time since thymoma diagnosis has not exceeded that time point. Survival by groupings of different variables was compared using the log-rank test. 29 The Cox proportional hazards regression model 30 was used to adjust for the simultaneous effects of baseline covariates on time to death from any cause, and time to death from a thymoma-related cause. Adjusted hazard ratios (risk ratios) and associated 95% confidence intervals were calculated for each covariate. Stata statistical software release 5.0 (College Station, TX) was used for all analyses.
RESULTS
Demographics
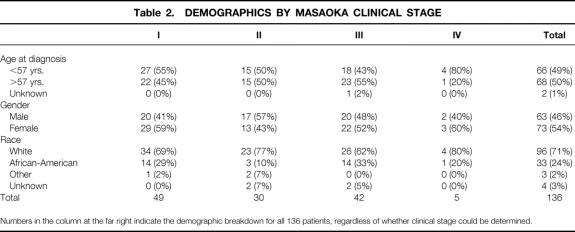
One hundred thirty-six patients with pathologically confirmed thymoma were seen at the Johns Hopkins Hospital between 1957 and 1997. The median age at diagnosis was 57 (range, 8 to 88 years). Overall, 73 (54%) were female and 96 (71%) were white. The results are summarized in Tables 2 through 7 and Figures 1 through 6.
Table 2. DEMOGRAPHICS BY MASAOKA CLINICAL STAGE
Numbers in the column at the far right indicate the demographic breakdown for all 136 patients, regardless of whether clinical stage could be determined.
Table 3. PATHOLOGIC CLASSIFICATION
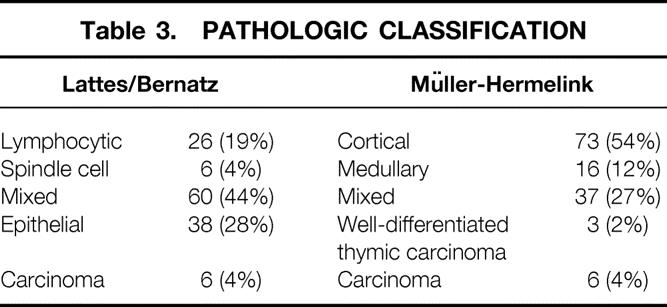
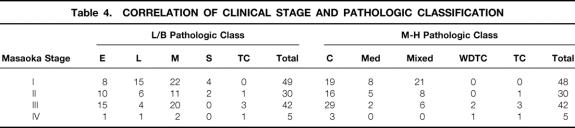
Table 4. CORRELATION OF CLINICAL STAGE AND PATHOLOGIC CLASSIFICATION
Table 5. CAUSES OF DEATH
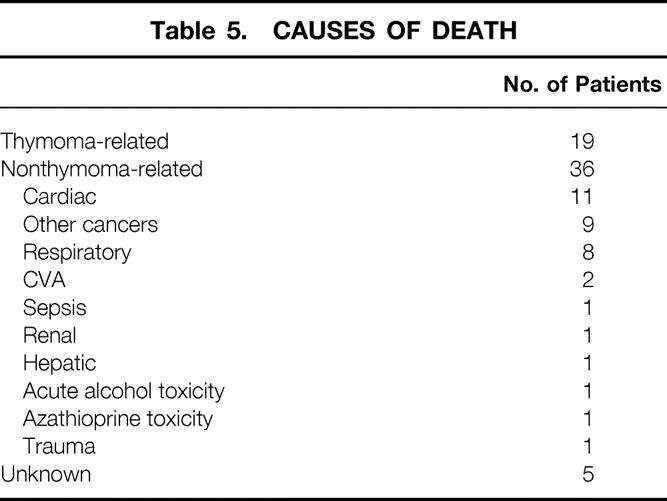
Table 6. ASSOCIATED NEOPLASMS IN THYMOMA PATIENTS
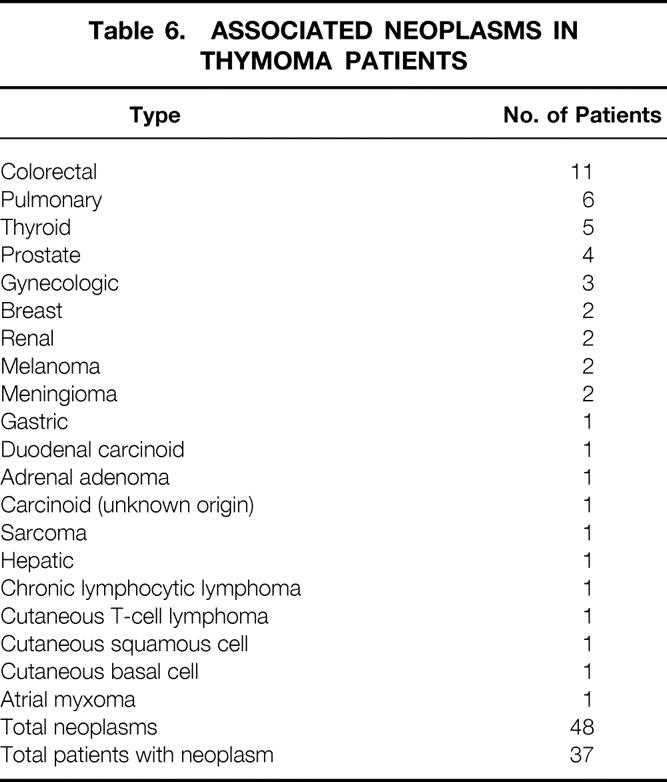
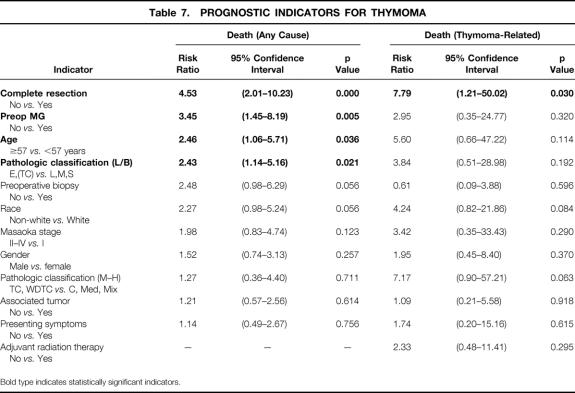
Table 7. PROGNOSTIC INDICATORS FOR THYMOMA
Bold type indicates statistically significant indicators.
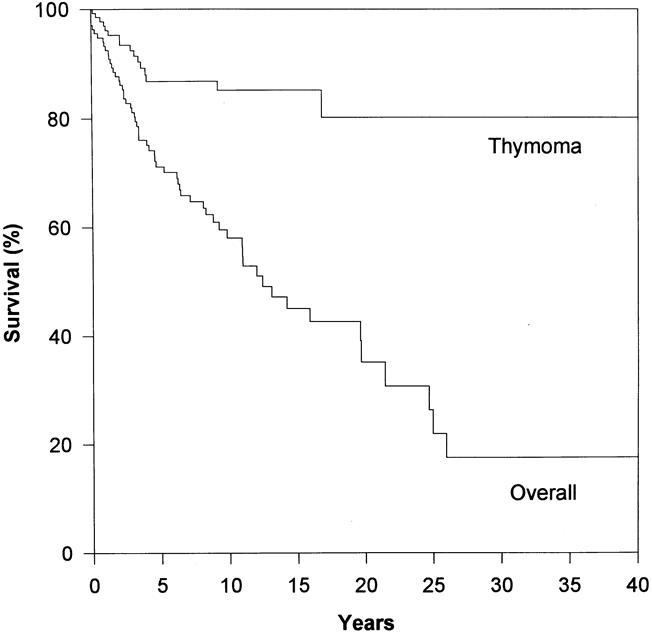
Figure 1. Overall and thymoma-specific Kaplan-Meier survival of 136 thymoma patients. The median overall survival was 12 years. Here, the Kaplan-Meier curve for thymoma-specific survival is shown for comparison. Note that thymoma-specific survival greatly exceeds overall survival.
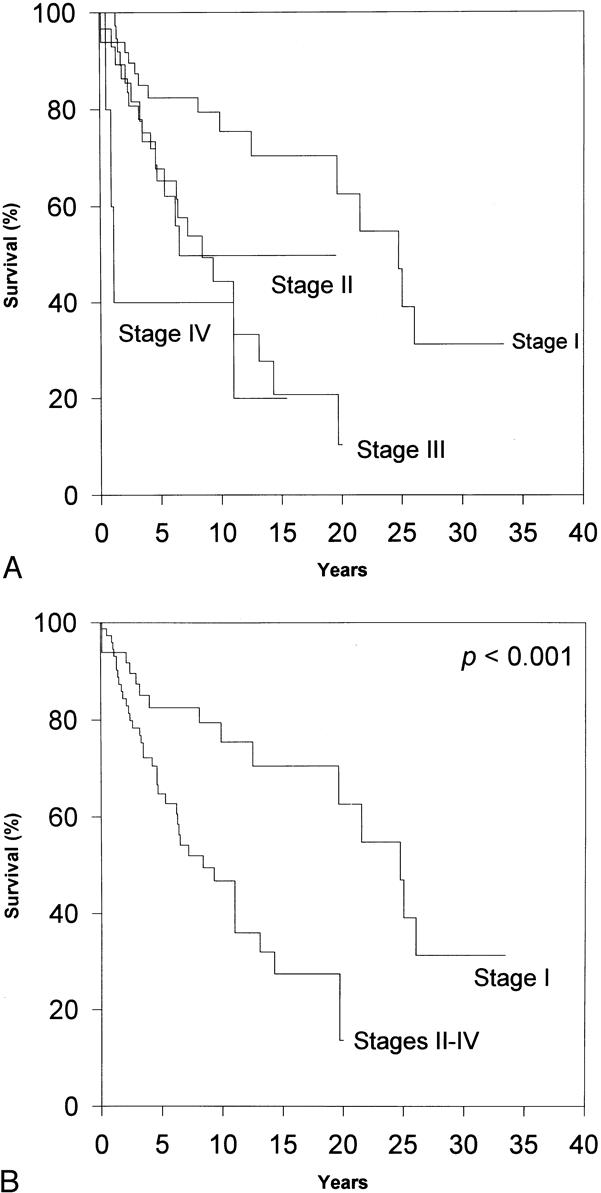
Figure 2. Influence of clinical stage on overall survival. (A) Masaoka stage is a significant prognostic predictor of survival, as evaluated by log-rank analysis (p < 0.003). (B) The discrimination between benign (stage I) and malignant (stages II to IV) disease appeared to be optimal when the Masaoka stages were grouped. However, when Masaoka stage was evaluated by Cox regression, it failed to be a significant independent predictor of overall survival.
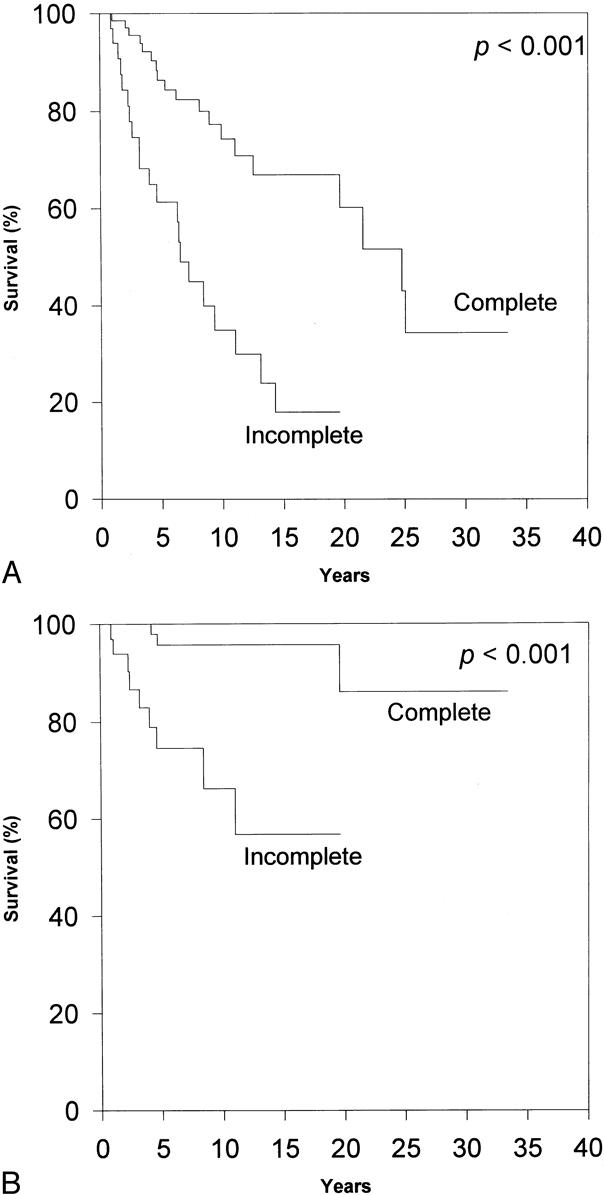
Figure 3. Effect of the extent of surgical resection on survival. (A) Complete resection is a significant prognostic predictor of overall survival, as evaluated by log-rank analysis. (B) Complete resection is a significant prognostic indicator of survival from thymoma, as evaluated by log-rank analysis. Complete resection is the most important independent predictor of overall survival and the only independent predictor of thymoma-related survival, as analyzed by Cox regression.
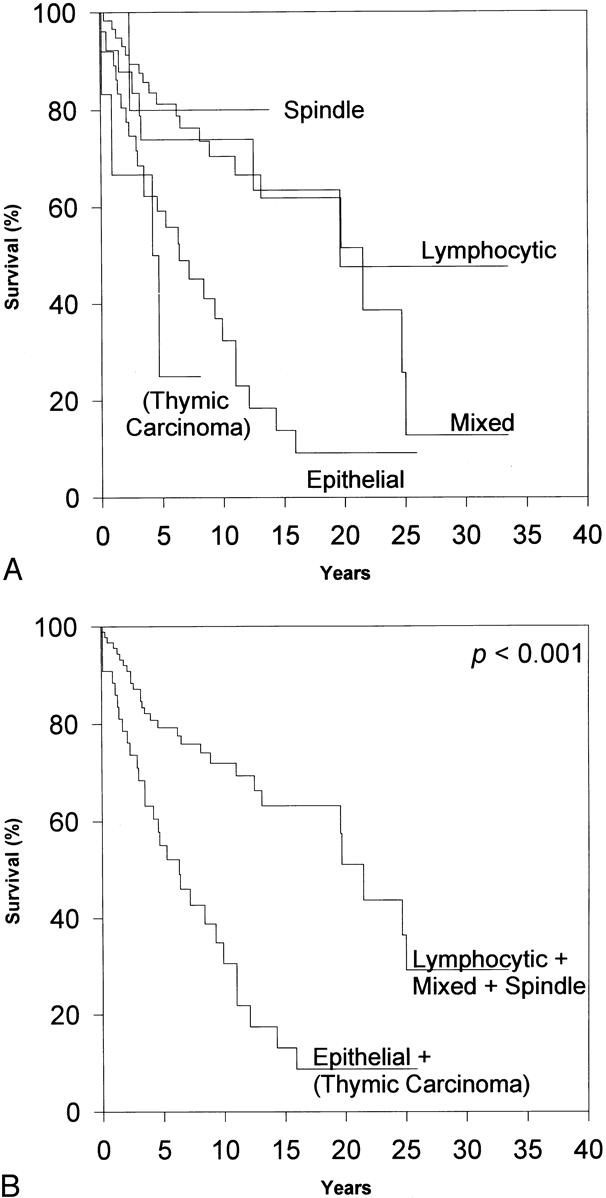
Figure 4. The effect of L/B pathologic classification on overall survival. (A) By log-rank analysis, L/B classification predicts prognosis, with spindle, lymphocytic, and mixed thymomas being associated with fewer overall deaths (p < 0.001). (B) Through selective grouping, the overall differences in survival are emphasized. Indeed, L/B pathologic classification did prove to be an independent predictor of overall survival by Cox regression.
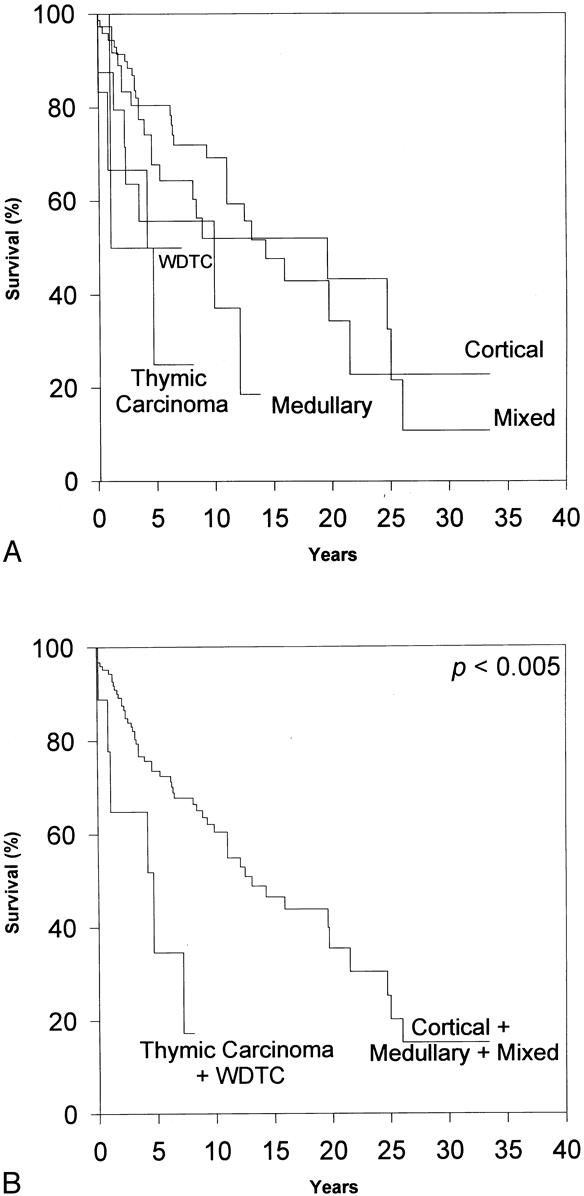
Figure 5. The effect of Müller-Hermelink pathologic grade on overall survival. (A) By log-rank analysis, substantial differences in overall survival as a function of Müller-Hermelink pathologic grade are apparent (p < 0.02). (B) By subsequent grouping of the pathologic grades, the importance of Müller-Hermelink pathologic grade is emphasized. However, Cox regression analysis failed to find Müller-Hermelink grade to be an independent predictor of overall survival.
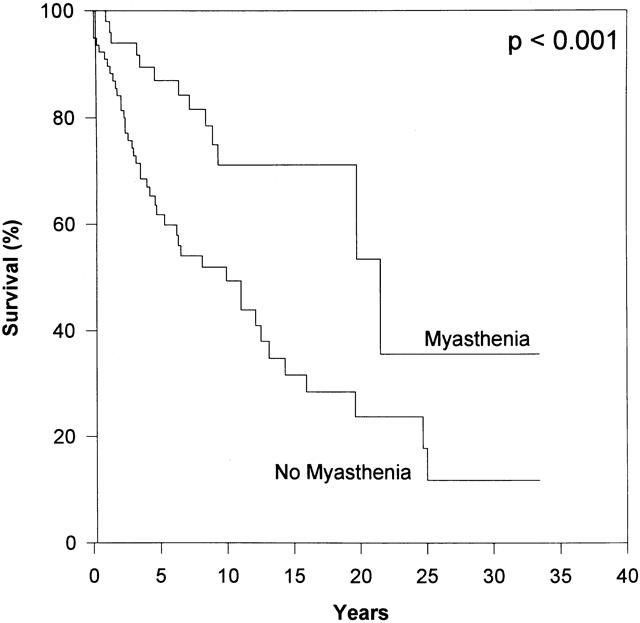
Figure 6. The presence of preoperative MG had a strikingly positive prognostic effect on overall survival. Multivariate analysis by the Cox regression model also confirmed preoperative MG as an independent predictor of overall survival.
Clinical Presentation
Thirty-seven (27%) patients were asymptomatic at the time of diagnosis when thymoma was discovered incidentally during routine chest radiographs, CT scans, or cardiac surgery, or at autopsy. Ninety-three (68%) patients were symptomatic: 32 had local symptoms (24%), including chest pain, cough, pneumonia, or hoarseness; 48 had systemic symptoms (36%), including weight loss, MG, or primary red cell aplasia; and 13 had a combination of both (10%). The mode of presentation was unknown in six patients (4%). Fifty-four patients (40%) had associated MG. Four patients (3%) had associated primary red cell aplasia. Thirty-seven patients (27%) had an independent second primary malignancy at some point.
Diagnostic Studies
The initial diagnostic study was a chest radiograph in 74 patients (54%) and a chest CT scan in 37 (27%). In only four patients (3%), thymoma was discovered incidentally at autopsy. In five patients (4%), the thymoma was discovered unexpectedly at the time of cardiac surgery. Mode of initial diagnosis was unknown in 15 patients (11%). Thirty-seven patients (28%) had undergone preoperative biopsy, either by open (parasternal or mediastinoscopic) biopsy or fine-needle aspiration. Ninety patients (66%) did not have a pathologic diagnosis available before surgery (or before death).
Surgical Intervention
Of the 136 patients, 126 (95%) underwent some form of surgery. Six patients were not considered candidates for surgery because of advanced tumor stage or patient or physician preference; four thymomas were discovered at autopsy. Detailed surgical reports were available for 108 of the 126 patients. Based on these reports and corresponding pathologic confirmation, 74 patients (68%) had undergone complete resection and 34 (32%) had had partial resection. (Included in this group of 34 patients with partial resection are 7 patients who had exploration with open biopsy and only limited debulking.) Five patients with stage III disease had intentional resection of the left phrenic nerve. Four of these patients were alive at the time of last follow-up. The single surgical death in this series was from complications of coronary artery bypass in a patient whose thymoma had been discovered incidentally during cardiac surgery.
Adjuvant Therapy
Forty-six patients (37%) received adjuvant radiation therapy. Information on adjuvant therapy was unavailable on 10 (7%) of the patients. Three patients with Masaoka stage III disease received preoperative adjuvant radiation therapy. Only one of these patients also received postoperative adjuvant radiation therapy. Only one patient in this series had received preoperative chemotherapy; nine patients (7%) received postoperative adjuvant chemotherapy. (For the purposes of this study, the use of steroids was not considered a form of chemotherapy.)
Masaoka Clinical Staging
Clinical staging was possible from the material available in 126 patients. If Masaoka stage I is considered benign and stages II to IV are considered malignant, 16 61% of these thymomas could be called malignant.
On initial inspection, clinical stage appeared to correlate well with the overall survival rate and with thymoma-related death (data not shown). This discrimination appeared to be optimal when the patients with stage I disease were compared with all others (p < 0.001). Surprisingly, however, when clinical stage was analyzed by Cox regression, it failed to be an independent predictor of overall survival or of thymoma-related death.
Pathologic Classification
The pathologic classification of the tumors by both the L/B and Müller-Hermelink systems is summarized in Tables 1 and 3. (One of the 136 patients could not be evaluated by the Müller-Hermelink classification because of inadequate tissue availability.) There was a striking correlation between clinical stage and pathologic grade. By L/B classification, lymphocytic, mixed lymphoepithelial, and spindle cell thymomas tended to present at earlier clinical (Masaoka) stages, whereas epithelial and thymic carcinomas appeared in more advanced stages. In the Müller-Hermelink system, cortical thymoma, thymic carcinoma, and well-differentiated thymic carcinoma (WDTC) presented at later clinical stages, whereas medullary and mixed tumors presented at earlier stages. Not surprisingly, all spindle cell thymomas (L/B) were classified as medullary tumors (Müller-Hermelink), and all WDTCs (Müller-Hermelink) were classified as epithelial tumors (L/B).
Preoperative Myasthenia Gravis
Of the paraneoplastic syndromes associated with thymoma, only patients with MG provided numbers sufficient for statistical analysis. Fifty-four (40%) of the patients had been diagnosed with MG before the diagnosis of thymoma. The diagnosis of MG proved to be a significantly positive independent prognostic factor for overall survival and approached significance as a predictor of survival from thymoma per se.
Overall Survival and Predictors of Overall Survival
Overall patient survival rates were 71%, 56%, 44%, 38%, and 33% at 5, 10, 15, 20, and 25 years, respectively. Median survival was 12 years. Only one patient was lost completely to follow-up. Sixty people (44%) had died by the conclusion of the study. In five patients (8%), the cause of death could not be determined. Of the 60 known deaths, 19 (32%) were related directly to thymoma or its treatment and 36 (60%) were not related. Therefore, the overall thymoma-related mortality rate was 14%, and the nonthymoma-related mortality rate was 26%. This means that patients with thymomas appeared much more likely to die from causes unrelated to this tumor or (directly) from its treatment.
Complete resection, the preoperative presence of MG, age younger than 57 years at the time of diagnosis, and advanced L/B pathologic classification proved to be significant independent predictors of survival, as evaluated by Cox regression analysis for prediction of death from any cause. The Müller-Hermelink pathology grading system was not found to be a statistically significant independent predictor of overall survival. Moreover, gender, race, the presence of symptoms at diagnosis, preoperative biopsy, and the presence of an associated tumor each failed to influence survival to a significant degree, although the predictive value of race and preoperative biopsy each approached statistical significance. An advanced Masaoka stage (II to IV) tended to predict a poorer prognosis, but this failed to reach independent statistical significance.
Because there is controversy with respect to the best system for the pathologic classification of thymoma, we rereviewed all of the available slides (for all 136 patients) and classified each patient’s tumor by each of the two major pathologic classification systems, independently. Each system appeared to be useful for predicting prognosis on initial (associative) analysis. The older L/B classification appeared to be the most discriminant (p < 0.001), but the Müller-Hermelink system also appeared to be predictive (p < 0.005). However when the prognostic capability of pathologic grade was analyzed in the multivariate Cox model, only the L/B system proved to be a significant independent predictor of overall survival.
Because the clinical staging and the pathologic gradings of thymoma are correlated, separate Cox regression analyses were performed that incorporated only one system (Masaoka, L/B, or Müller-Hermelink), along with the other covariates. The findings of these analyses were consistent with those of the full model. The direction and magnitude of the risk ratios and associated significance levels were not influenced appreciably.
A Cox regression analysis also was used to investigate the relation between overall survival and adjuvant radiation therapy. There appeared to be a slight interaction effect, with better survival rates seen for patients with Masaoka stage I and no radiation versus patients with Masaoka stage I and adjuvant radiation or patients with Masaoka stages II to IV, regardless of radiation status. This interaction term was not statistically significant when included in the Cox model due to small subgroup sizes; therefore, both the interaction term and adjuvant radiation were omitted from the final model. Too few patients had received adjuvant chemotherapy to afford meaningful statistical analysis.
Thymoma-Related Death and Predictors of Thymoma-Related Death
Overall, 19 (14%) of the patients died from a cause directly related to thymoma or its treatment. Kaplan-Meier estimates of the mortality rate from thymoma and its treatment were strikingly lower than those from nonthymoma-related causes in all patients at each follow-up interval. The thymoma-related survival rate was 87% at 5 years, 85% at 10 years, and 80% at 15 years. The only clinical indicator that proved to be a statistically significant independent predictor of improved survival was complete surgical resection. Neither pathologic classification system proved to be a significant independent predictor of death from thymoma. This was the result, in part, of the relatively small numbers of patients who died of their thymomas, lowering the power of the statistical analysis.
Recurrence
Seventeen (12%) patients were not eligible for recurrence follow-up: those whose thymomas had been discovered at autopsy, those who had never undergone surgery, or those who had undergone only limited debulking procedures (not true resection). Of the 119 (88%) patients eligible for follow-up for recurrence, specific recurrence information was available for only 97 (82%). Of these, 79 (81%) had no documented recurrences, whereas 18 (19%) patients did have recurrence. Time to recurrence could not be determined for one patient with a known recurrence. Of the patients in whom time to recurrence could be calculated, eight patients (47%) had only local or regional recurrence in the mediastinum, and nine patients (53%) had hematogenous, distant metastases. For this group of patients with known recurrence status, the median time to recurrence was 4 years (range, 0.5 to 12 years). Of the 18 patients with recurrence, the extent of initial surgical resection could not be found for 2 patients. Of the remaining 16, 8 (50%) had undergone “complete” resection and 8 (50%) had undergone partial resection. The clinical stage of one patient with a known recurrence could not be determined. Of the remaining 17, 4 (24%) were Masaoka stage I, 3 (18%) were stage II, 10 (59%) were stage III, and 0 were stage IV.
DISCUSSION
In general, the demographics and clinical presentation of the patients with thymoma in this series were quite similar to those of other reports. 5–11,13–15 Although most of the patients had local symptoms, a significant number were asymptomatic or had the thymoma diagnosed during a routine chest x-ray. The 40% incidence of MG seen here, higher than the 25% to 35% reported in most other series, 14,15,26,27,31,32 is probably because of the large number of patients referred to the Johns Hopkins Hospital primarily for the neurologic management and surgical treatment (thymectomy) of MG per se. 4 The 3% of the patients with associated pure red cell aplasia is comparable to the proportions reported in other series. 6,9,11,12,15
The overall survival rate of the patients in this series was similar to that reported in other series. 5–11,13,14,26 Forty-four percent of the patients were dead at the conclusion of the study, but, strikingly, less than a third (32%) of these deaths were thymoma-related. Indeed, the nonthymoma-related death rate exceeded the thymoma-related death rate at every time point evaluated. Most other series have reported only overall survival curves. Verley et al 5 and Lewis et al, 8 however, reported thymoma-related death rates of 20% and 13%, respectively, comparable to the 14% rate in our series. In fact, in our series 39% of the patients had encapsulated thymomas (Masaoka stage I) at initial diagnosis; only 4% had disseminated disease (Masaoka stage IV). The small number of deaths related directly to thymoma supports the view of an indolent nature of thymomas, with few patients developing or dying of disseminated disease. Instead, the disease often spreads locally within the mediastinum or to the adjacent pleural cavity and is frequently manageable with radiation, chemotherapy, and/or surgery for recurrent disease. (Recurrence rates reported in other series vary from 15% to 25% at 10 to 15 years. 5,8,33 In our series, recurrence rate per se was not used as an endpoint because of inadequate recurrence data.) This also indicates that patients must be followed for many years because it is not unusual for patients to present more than 10 years after resection with late recurrence or even death. 5,6,8
Several retrospective studies have attempted to define clinicopathologic variables that can be used to predict prognosis in patients with thymoma. However, many of these series contain inadequate numbers of patients to allow the quantitation of the independent prognostic value of these variables. Our series of 136 patients did allow for such a multivariate analysis. Of all the clinicopathologic indicators we investigated, four factors proved to have an independent influence on overall survival: the extent of surgical resection, the presence of MG, the L/B classification, and age at initial diagnosis.
Most authors have reported that both the clinical stage at presentation and the extent of surgical resection are important predictors of overall survival. In the present study, thymomas were grouped as noninvasive (Masaoka stage I) or invasive (Masaoka stage II to IV). In contrast to other series, 5–11,15,25 although advanced clinical stage did tend to predict a poorer prognosis, the clinical (Masaoka) stage failed to reach statistical significance as an independent predictor of overall survival. The presence of either local or systemic symptoms also failed to reach statistical significance for independent predictive value. Complete surgical resection proved to be the most important predictor of increased survival, independent of Masaoka stage. Other authors have also reported the importance of complete resection. 6,9,13,15 However, several series were unable to detect a difference in outcome between patients who had debulking procedures versus biopsy alone. 9,15,34 Whether or not a preoperative biopsy had been obtained did not prove to be an independent predictor of overall survival in the present series. Taken together, these results support aggressive surgical intervention, even in patients with advanced disease at initial presentation. For example, the patient who has a Masaoka stage III thymoma with local invasion into the lung appears to benefit from an aggressive resection that would include complete thymectomy (anterior-superior cervicomediastinal exenteration) 4 as well as in-continuity wedge resection of the involved lung.
In the current series, patients with MG had a markedly better overall survival rate than those without MG. Earlier series had reported that patients with MG had a decreased overall survival rate. 7,12,35 This was likely secondary to the increased risk of surgery in the patients with MG, as well as less effective medical management of such patients in earlier years. Many subsequent series have reported no difference in survival rates in patients with versus without MG. This has been attributed to better critical care availability as well as generally improved management of patients with MG. 4,8,11,13,14,36,37 Although Monden et al 33 reported fewer thymoma recurrences in patients with MG versus those without MG, the overall effect of MG on survival was not quantitated. Our series is unique in indicating that patients with MG (diagnosed before surgery) had significantly increased survival overall. Because the presence of MG was identified here as an independent predictor of overall survival, it cannot be argued that patients have a better prognosis because their MG symptoms had led to a diagnosis of thymoma at an earlier stage. In our experience and in the literature, 38,39 it has been noted that steroids may cause involution of recurrent thymoma. Although we did not record who was being treated with steroids for MG, it is plausible that steroids could be responsible for the improved overall survival of patients with MG, because small recurrences might have involuted without appearing clinically relevant. One could reason that if this were true, there should be significantly fewer thymoma-related deaths in patients with MG. Our study does not reveal such a finding, but this may be because of the small total number of thymoma-related deaths in this series. Moreover, patients with MG may have had closer medical follow-up, possibly facilitating the earlier detection and better management of other medical problems and thereby prolonging survival.
Histologic classification has inconsistently been reported to be a reliable prognostic indicator. 6,8,10,12–15,17,21,24,25,27,31,40 We found that the L/B pathologic classification is an independent predictor of overall survival, patients with predominantly epithelial thymoma or thymic carcinoma having a significantly decreased overall survival rate. (The epithelial thymomas and thymic carcinomas were grouped together because of the small number of thymic carcinomas in the series. However, our finding remained the same when thymic carcinomas were excluded from analysis.) Other series have also found that predominantly epithelial thymomas portend a worse prognosis, 8,12 but in the other series this effect has not been clearly demonstrated to be independent of the clinical stage at presentation. Surprisingly, the more modern Müller-Hermelink pathologic grade failed to be a significant independent predictor of overall prognosis, in contrast to the trends reported in other series. 10,15,27,31 However, the Müller-Hermelink grade approached statistical significance as an independent predictor of death secondary to thymoma, with WDTC/thymic carcinoma being associated with decreased survival. Although this and other series indicate that cortical thymomas tend to present at more advanced clinical stages, 10,14,15,18,21–27,40 our series does not indicate a worse prognosis in patients with cortical thymomas. Therefore, the true prognostic value of the Müller-Hermelink grading system remains unclear.
Not surprisingly, age 57 or older was an independent significant predictor of an increased mortality rate from any cause, because patients diagnosed with any condition at an advanced age obviously have a greater chance of dying of causes unrelated to that condition. More importantly, age at diagnosis was not an independent predictor of thymoma-related death. Finally, gender and race also failed to reach statistical significance as independent predictors of both overall and thymoma-related survival.
All of these clinicopathologic predictors were also analyzed for their relative importance as independent predictors of death from thymoma-related causes. The only variable that proved to be a significant independent predictor of decreased thymoma-related death was complete surgical resection. It may be that other predictors failed to reach statistical significance because of the relatively small number of patients who actually died of thymoma or its treatment, thereby decreasing the power of the statistical analysis to detect significance. The indolent nature of this disease is particularly evident from these data.
One of the more interesting findings in this study was the significant number of patients (37 [27%]) who had associated, discrete neoplasms at some time in their clinical course. (The presence of an associated tumor was not, however, an independent predictor of overall survival.) McCart et al have reported similar findings. 6 Although it is not altogether surprising that such an association exists, given the role of the thymus in immune function, the precise mechanism of the relation is unclear. If the secondary neoplasm had always arisen after thymectomy, or subsequent to adjuvant therapy for thymoma, then one could argue that removal of the thymus could diminish immune surveillance, or that radiation therapy might have induced the development of second primary neoplasm. However, the fact that the separate neoplasm was often diagnosed either before or at the same time as the thymoma suggests that there might be an underlying predisposition to neoplasia in these patients. The fact that thymomas are associated frequently with secondary neoplasms should heighten the physician’s clinical suspicion and facilitate the earlier diagnosis of other tumors in patients with thymomas.
Our findings are consistent with the approach that all patients with thymoma should undergo an aggressive attempt at complete resection of their tumor, including, if indicated and clinically feasible, the resection of invaded adjacent organs, including pericardium, lung, innominate vein, and/or diaphragm. Patients with MG should be reassured that the presence of this paraneoplastic syndrome actually portends an improved prognosis. Finally, clinicians should have heightened awareness regarding second primary neoplasms in the patient with thymoma.
Acknowledgment
The authors thank Anne Kammer, ART, CTR, Manager, Oncology Medical Information at Johns Hopkins, the Maryland Department of Vital Statistics for access to their data, and Elise Hopkins for technical assistance.
Discussion
Dr. Joel D. Cooper (St. Louis, Missouri): I want to congratulate Drs. Wilkins and Bulkley for their comprehensive evaluation of a very interesting subject and I appreciate the opportunity of reviewing the manuscript in advance.
I remarked yesterday to Dr. Judah Folkman as I was sitting next to him, “You know, only at the American Surgical Association could you have a thoracic surgeon get up and give a presentation about operating on the neck followed immediately by a presentation by general surgeons operating on the chest.” He recalled that years ago at Grand Rounds at the Massachusetts General Hospital, the illustrious gynecological surgeon Joe Meigs presented a case in which he had ventured into the chest, presumably because of metastases or extension of a pelvic problem. The eminent thoracic surgeon Dr. Richard Sweet, not to be outdone, at the next Grand Rounds presented a case in which he had done a total hysterectomy. That apparently prompted Dr. Pete Churchill to observe that since, at least in those days, surgeons were expected to be masters of everything, your specialty was defined solely by what you chose to busy yourself with.
I agree, as you point out in the manuscript, that most series of thymomas are not large enough to draw some of the interesting conclusions that you have, and there are still some unanswered questions.
You know, originally the diagnosis of benign or malignant, as defined by Dr. Castleman and the AFIP fascicle on thymomas from the Armed Forces Institute of Pathology, was made by the surgeon, not the pathologist, who made the determination of whether it was benign or malignant depending on whether or not the capsule was breached. The pathologist under the microscope couldn’t tell on the basis of histology. So if it invaded the capsule, it was considered malignant and if it didn’t invade the capsule, it was considered benign.
I have never understood—and perhaps you could answer—can all thymomas become malignant? I mean, somehow the ones that we see invading are always large and the ones that are not invading are almost always small. Are we talking about one type of tumor which can develop and become malignant? Or are these tumors either benign and stay benign or start off malignant and stay malignant? I would love to know if you have any insight into that question.
In your series, did you observe, as has been done in the past, patients without myasthenia who had a thymoma excised and became myasthenic? We now think that that was because they were not doing complete excisions of the thymus gland along with the thymoma, because I think that problem is rarely seen these days.
As you have, we have observed that in the last 10 or 15 years that if you have myasthenia gravis and a thymoma, you have a much better prognosis. Twenty years ago it was worse. But if you look at it, you will find the deaths were not due to thymoma, they were due to myasthenia. And although you seem to suggest to the contrary in your manuscript, it is still our impression that the reason myasthenia is such a favorable covariable is that when you have myasthenia, you get a CAT scan, you pick up the thymoma much earlier than you would have picked it otherwise, and that that is a more favorable group of tumors.
In the manuscript, there were very few cases of neoadjuvant therapy. And I would like to ask you what you now do? If you have a CAT scan that suggests that the thymic tumor is in fact penetrating the capsule, we tend to now give chemotherapy first, shrink it, remove it surgically, and then follow with consolidation radiotherapy. I would be interested in your own approach.
Finally, a vexing issue has been the finding by pathologists now of microscopic capsular invasion. In the past if it was grossly uninvaded we just didn’t give follow-up therapy. And since Masaoka has refined this and added a classification for microscopic capsular invasion, what is currently your treatment recommendation for a tumor removed which seems to be grossly contained, but which is pathologically found to have some microscopic capsular invasion? Do you give postoperative radiotherapy?
Presenter Dr. Kirsten Bass Wilkins (Durham, North Carolina): In terms of the treatment of patients with microinvasive disease, at Johns Hopkins these patients receive postoperative adjuvant radiotherapy.
Why are the patients with myasthenia doing better? One of the advantages of our study is that we control for other cofactors. So that when we say that patients with myasthenia gravis did better, we can pretty securely say this was not due to stage alone, because we controlled for that.
We found that people are dying mostly from causes unrelated to thymoma. It may just be that the patients with myasthenia are being followed more closely by their doctors, and perhaps other medical conditions that are going to cause their death are being better controlled.
Secondly, many of the patients with myasthenia gravis are being treated with steroids. And there have been anecdotal reports in the literature of decreased rates of thymoma recurrence and treatment of small recurrences with steroids. It could be that myasthenic patients treated with steroids are having involutional occurrence before it is ever apparent.
You are correct to say that few of the patients had neoadjuvant treatment. In fact, not many of the patients at Hopkins are being treated with neoadjuvant therapy even if there is concern about extracapsular extension on the initial diagnostic test.
In terms of whether or not there is actually a benign thymoma, you are correct to say that over the years there has been very much controversy over this. In this series, we did find that there were patients that did die from thymoma who did have apparent stage 1 disease. So I think that even if you have a tumor that is considered a stage 1 benign disease because it is completely encapsulated, a few of these patients can go on and have a malignant course.
Dr. Carl E. Bredenberg (Portland, Maine): I would like to congratulate the authors and thank them for the opportunity of reviewing the manuscript. The manuscript is as well written as it was presented, and will become a standard reference for this topic in the future. This work achieves a goal for which we all strive and too often fail to reach. It is a large series, well analyzed, with excellent long-term follow-up; 98% at 15 years is truly impressive. I don’t think anyone would disagree with the essential bottom line: completeness of resection counts.
One of the difficulties, however, is that, although for a clinician it is a large series, it is still for a statistician a relatively small number, particularly a small number of thymoma-related deaths. My first question is the question that you have alluded to: What is the proper endpoint to correlate with these predictors, given that only one third of the mortality was related to thymoma? Is it overall mortality or is it mortality from thymoma?
My second, more speculative, question is the impressive 27% of the patients that had another malignancy and maybe half of those had a third malignancy. Colorectal was the most frequent additional malignancy. Given the interest at Hopkins in looking at the sequence of genetic hits that determine malignancy, and particularly using colon malignancy as the initial model, is this a group of patients that Dr. Vogelstein and the molecular geneticists at Hopkins would be particularly interested in looking at?
My final question is a simple practical one. With the mediastinal mass, there seems to be, among thoracic surgeons, a certain reluctance to do a preoperative biopsy. The differential is often thymoma or lymphoma, and the imaging may not provide a clear answer. On the basis of your experience, where is the place for preoperative biopsy?
Again I congratulate you. This will be required reading for all thoracic surgeons and all people who deal with thoracic malignancy.
Dr. Wilkins: In terms of the preoperative biopsy, in our study we did see that there was no difference in overall, or thymoma-related survival. We are not performing preoperative biopsies in these patients routinely, although there is a subgroup of patients who will have fine-needle aspiration performed prior to referral.
In terms of the other malignancies, I think it is a good idea to have Dr. Vogelstein look at this. There is not a simple explanation. The patients in our series developed these neoplasms at all different times, sometimes before the thymoma is detected, sometimes simultaneously, and sometimes afterward.
It could be argued that if the patients always developed the secondary malignancies after thymectomy, this could be because of decreased immune surveillance without the thymus, or that perhaps it was related to adjuvant therapy that had been given for the treatment of thymoma. But this was certainly not the case. So we seem to think that there must be some underlying predisposition to neoplasia in these patients which we have not been able to define.
In terms of the proper endpoint for these patients, I think that it is important to know that these patients do have decreased overall survival. And I think it is important to know that, yes, thymoma is likely not going to kill you, and it is encouraging to tell that to your patients.
Footnotes
Correspondence: Gregory B. Bulkley, MD, Blalock 685, The Johns Hopkins Hospital, 600 N. Wolfe St., Baltimore, MD 21287-4685.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Morgenthaler TI, Brown LR, Colby TV, et al. Thymoma. Mayo Clin Proc 1993; 68: 1110–1123. [DOI] [PubMed] [Google Scholar]
- 2.Rosai J, Levine GD. Tumors of the thymus. Washington DC: Armed Forces Institute of Pathology; 1976.
- 3.Soudajian JV, Enriquez P, Silverstein MN, Pepin J-M. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Arch Intern Med 1974; 134: 374–379. [PubMed] [Google Scholar]
- 4.Bulkley GB, Bass KN, Stephenson GR, et al. Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997; 226: 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verley JM, Hollmann KH. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985; 55: 1074–1086. [DOI] [PubMed] [Google Scholar]
- 6.McCart JA, Gaspar L, Inculet R, Casson AG. Predictors of survival following surgical resection of thymoma. J Surg Oncol 1993; 54: 233–238. [DOI] [PubMed] [Google Scholar]
- 7.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981; 48: 2485–2492. [DOI] [PubMed] [Google Scholar]
- 8.Lewis JE, Wick MR, Scheithauer BW, et al. Thymoma. A clinicopathologic review. Cancer 1987; 60: 2727–2743. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg D, Port JL, Weksler B, et al. Thymoma: : a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995; 60: 908–914. [DOI] [PubMed] [Google Scholar]
- 10.Quintanilla-Martinez L, Wilkins EW Jr, Choi N, et al. Thymoma. Histologic subclassification is an independent prognostic factor. Cancer 1994; 74: 606–617. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins EW Jr, Grillo HC, Scannell JG, et al. Role of staging in prognosis and management of thymoma. Ann Thorac Surg 1991; 51: 888–892. [DOI] [PubMed] [Google Scholar]
- 12.Batata MA, Martini N, Huvos AG, et al. Thymomas:: clinicopathologic features, therapy, and prognosis. Cancer 1974; 34: 389–396. [DOI] [PubMed] [Google Scholar]
- 13.Maggi G, Giaccone G, Donadio M, et al. Thymomas. A review of 169 cases, with particular reference to results of surgical treatment. Cancer 1986; 58: 765–776. [DOI] [PubMed] [Google Scholar]
- 14.Pescarmona E, Rendina EA, Venuta F, et al. Analysis of prognostic factors and clinicopathological staging of thymoma. Ann Thorac Surg 1990; 50: 534–538. [DOI] [PubMed] [Google Scholar]
- 15.Schneider PM, Fellbaum C, Fink U, et al. Prognostic importance of histomorphologic subclassification for epithelial thymic tumors. Ann Surg Oncol 1997; 4: 46–56. [DOI] [PubMed] [Google Scholar]
- 16.Levine GD, Rosai J. Thymic hyperplasia and neoplasia: : a review of current concepts. Hum Pathol 1978; 9: 495–515. [DOI] [PubMed] [Google Scholar]
- 17.Salyer WR, Eggleston JC. Thymoma. A clinical and pathological study of 65 cases. Cancer 1976; 37: 229–249. [DOI] [PubMed] [Google Scholar]
- 18.Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch (Pathol Anat) 1985; 407: 119–149. [DOI] [PubMed] [Google Scholar]
- 19.Shimosato Y, Mukai K. Tumors of the Mediastinum. Washington DC: Armed Forces Institute of Pathology; 1997.
- 20.Müller-Hermelink HK, Marino M, Palestro G, et al. Immunohistological evidences of cortical and medullary differentiation in thymoma. Virchows Arch A Pathol Anat Histopathol 1985; 408: 143–161. [DOI] [PubMed] [Google Scholar]
- 21.Kornstein MJ, Curran WJ Jr, Turrisi AT, III, Brooks JJ. Cortical versus medullary thymomas: : a useful morphologic distinction? Hum Pathol 1988; 19: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Hermelink HK, Marino M, Palestro G. Pathology of thymic epithelial tumors. In: Müller-Hermelink HK, ed. The human thymus. Histophysiology and pathology. Berlin: Springer-Verlag; 1986: 208–268.
- 23.Kuo T-T, Lo S-K. Thymoma:: a study of the pathologic classification of 71 cases with evaluation of the Müller-Hermelink system. Hum Pathol 1993; 24: 766–771. [DOI] [PubMed] [Google Scholar]
- 24.Quintanilla-Martinez L, Wilkins EW Jr, Ferry JA, Harris NL. Thymoma—morphologic subclassification correlates with invasiveness and immunohistologic features:: a study of 122 cases. Hum Pathol 1993; 24: 958–969. [DOI] [PubMed] [Google Scholar]
- 25.Pan C-C, Wu H-P, Yang C-F, et al. The clinicopathological correlation of epithelial subtyping in thymoma: : a study of 112 consecutive cases. Hum Pathol 1994; 25: 893–899. [DOI] [PubMed] [Google Scholar]
- 26.Ho FCS, Fu KH, Lam SY, et al. Evaluation of a histogenetic classification for thymic epithelial tumours. Histopathology 1994; 25: 21–29. [DOI] [PubMed] [Google Scholar]
- 27.Ricci C, Rendina EA, Pescarmona EO, et al. Correlations between histological type, clinical behaviour, and prognosis in thymoma. Thorax 1989; 44: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 29.Peto R, Peto J. Asymptotically efficient rank invariant procedures. J R Stat Soc A 1972; 135(pt 2):1:85–206.
- 30.Cox DR. Regression models and life-tables. J R Stat Soc B 1972; 134(pt 2):1:87–220.
- 31.Egea AM, Albasini JLA, Paricio PP, et al. Prognostic factors of thymomas. Eur J Surg Oncol 1995; 21: 482–485. [DOI] [PubMed] [Google Scholar]
- 32.Bergh NP, Gatzinsky P, Larsson S, et al. Tumors of the thymus and thymic region:: I. Clinicopathological studies on thymomas. Ann Thorac Surg 1978; 25: 91–98. [DOI] [PubMed] [Google Scholar]
- 33.Monden Y, Nakahara K, Iioka S, et al. Recurrence of thymoma:: clinicopathological features, therapy, and prognosis. Ann Thorac Surg 1985; 39: 165–169. [DOI] [PubMed] [Google Scholar]
- 34.Ciernik IF, Meier U, Lutolf UM. Prognostic factors and outcome of incompletely resected invasive thymoma following radiation therapy. J Clin Oncol 1994; 12: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 35.Wilkins EW Jr, Edmunds LH, Castleman B. Cases of thymoma at the Massachusetts General Hospital. J Thorac Cardiovasc Surg 1966; 52: 322–330. [PubMed] [Google Scholar]
- 36.Shamji F, Pearson FG, Todd TRJ, et al. Results of surgical treatment for thymoma. J Thorac Cardiovasc Surg 1984; 87: 43–47. [PubMed] [Google Scholar]
- 37.Maggi G, Casadio C, Cavallo A, et al. Thymoma:: results of 241 operated cases. Ann Thorac Surg 1991; 51: 152–156. [DOI] [PubMed] [Google Scholar]
- 38.Tandan R, Taylor R, DiCostanzo DP, et al. Metastasizing thymoma and myasthenia gravis. Favorable response to glucocorticoids after failed chemotherapy and radiation therapy. Cancer 1990; 65: 1286–1290. [DOI] [PubMed] [Google Scholar]
- 39.Hu E, Levine J. Chemotherapy of malignant thymoma. Case report and review of the literature. Cancer 1986; 57: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 40.Pescarmona E, Rendina EA, Venuta F, et al. The prognostic implication of thymoma histologic subtyping. A study of 80 consecutive cases. Am J Clin Pathol 1990; 93: 190–195. [DOI] [PubMed] [Google Scholar]