Abstract
Background
Seven years ago, the authors reported on the feasibility and short-term results of minimally invasive surgical methods to treat esophageal achalasia. In this report, they describe the evolution of the surgical technique and the clinical results in a large group of patients with long follow-up.
Patients and Methods
Between January 1991 and October 1998, 168 patients (96 men, 72 women; mean age 45 years, median duration of symptoms 48 months), who fulfilled the clinical, radiographic, endoscopic, and manometric criteria for a diagnosis of achalasia, underwent esophagomyotomy by minimally invasive techniques. Forty-eight patients had marked esophageal dilatation (diameter >6.0 cm). Thirty-five patients had a left thoracoscopic myotomy, and 133 patients had a laparoscopic myotomy plus a partial fundoplication. Follow-up to October 1998 was complete in 145 patients (86%).
Results
Median hospital stay was 72 hours for the thoracoscopic group and 48 hours for the laparoscopic group. Eight patients required a second operation for recurrent or persistent dysphagia, and two patients required an esophagectomy. There were no deaths. Good or excellent relief of dysphagia was obtained in 90% of patients (85% after thoracoscopic and 93% after laparoscopic myotomy). Gastroesophageal reflux developed in 60% of tested patients after thoracoscopic myotomy and in 17% after laparoscopic myotomy plus fundoplication. Laparoscopic myotomy plus fundoplication corrected reflux present before surgery in five of seven patients. Patients with a dilated esophagus had excellent relief of dysphagia after laparoscopic myotomy; none required an esophagectomy.
Conclusions
Minimally invasive techniques provided effective and long-lasting relief of dysphagia in patients with achalasia. The authors prefer the laparoscopic approach for three reasons: it more effectively relieved dysphagia, it was associated with a shorter hospital stay, and it was associated with less postoperative reflux. Laparoscopic Heller myotomy and partial fundoplication should be considered the primary treatment for esophageal achalasia.
In 1992 we described the surgical technique of thoracoscopic Heller myotomy for achalasia, showing that the procedure could be done safely and that if certain surgical details were followed, the clinical results were good. 1 Recovery from surgery was prompt, and the patient’s experience was much smoother and less uncomfortable than with open methods, whether by thoracotomy or laparotomy.
During the ensuing 7 years, the surgical techniques have evolved, and the frequency of unsatisfactory results, which was low to begin with, has dropped still further. This paper summarizes the results of the pre- and postoperative investigations, provides long-term follow-up, and most importantly describes how seemingly minor changes in the operation have continuously improved the results.
PATIENTS AND METHODS
Between January 1991 and October 1998, 168 patients with esophageal achalasia underwent a Heller myotomy by minimally invasive techniques at the University of California San Francisco and the University of Washington, Seattle. There were 96 men and 72 women, whose age ranged from 11 to 96 years (mean, 45 years). The median duration of symptoms was 48 months. Most patients had received nonoperative treatment for achalasia before being referred for surgery. Specifically, 82 patients (49%) had pneumatic dilatations (mean, 2.0/patient; range, 1 to 6), and 33 patients (20%) had intrasphincteric botulinum toxin injections (mean, 1.6/patient; range, 1 to 4), either alone (8 patients) or in conjunction with pneumatic dilatation (25 patients).
Preoperative Evaluation
The severity of dysphagia, regurgitation, chest pain, and heartburn was scored by the patients before and after surgery using a 5-point scale from 0 (no symptom) to 4 (disabling symptom). The ability to swallow was graded before and after surgery as follows: excellent (no dysphagia); good (occasional dysphagia, once a week or less); fair (frequent dysphagia, more than once a week, requiring dietary adjustments); poor (severe dysphagia, preventing ingestion of solid food). Before surgery, all patients had dysphagia (score 3.6 ± 0.6); 105 patients (62%) had regurgitation (score 2.8 ± 1.0); 75 patients (45%) had heartburn (score 2.1 ± 1.0); and 73 patients (43%) had chest pain (score 2.6 ± 1.0).
Esophagrams showed findings typical of achalasia, with narrowing of the distal esophagus (“bird beak” deformity) and esophageal dilatation. The diameter of the esophagus was assessed in 154 patients. It was <4 cm in 73 patients (48%) (grade I, group A); 4 to 6 cm in 33 patients (21%) (grade II, group B); and >6 cm in 48 patients (31%) (grade III, group C). Twenty-one patients in group C had a straight esophagus (group C1), and 27 patients had a sigmoid-shaped esophagus (group C2).
Endoscopy was performed to rule out a peptic or malignant stricture of the esophagus and gastroduodenal disease.
Esophageal manometry was performed after an overnight fast using techniques previously described. 2 Lower esophageal sphincter (LES) resting pressure averaged 27 ± 11 mm Hg, and there was no difference in pressure among patients treated with medications, pneumatic dilatation, or botulinum toxin. Relaxation of the LES in response to swallowing was partial or absent in all patients. Esophageal peristalsis was absent in all patients. The study was repeated 2 months after surgery in 61 patients (36%).
Prolonged pH monitoring was performed before surgery in 70 patients (42%) using techniques previously described. 2 The reflux score was positive (i.e., >14.8 3) in 20 patients (29%). However, after examination of the individual tracings, 10 patients were found to have false reflux, presumably as a result of stasis and fermentation of the food in the esophagus. 4,5 The remaining 10 patients were thought to have real gastroesophageal reflux; of these, 8 patients had been treated with pneumatic dilatation (mean, 2 dilatations/patient; range, 1 to 4) and 3 patients with botulinum toxin. Prolonged pH monitoring was repeated after surgery in 45 patients.
Surgical Technique
Thirty-five patients (21%) were treated by a left thoracoscopic Heller myotomy and 133 patients (79%) by a laparoscopic Heller myotomy and partial fundoplication (Dor fundoplication, 125 patients; Toupet fundoplication, 8 patients).
We have described the technique of thoracoscopic myotomy elsewhere. 1 In brief, the procedure was carried out through four or five ports arranged in a diamond-shaped pattern over the lower left chest. The lung was collapsed and retracted, the esophagus was exposed, and a myotomy was carried out from the inferior pulmonary vein onto the stomach, about 5 mm below the gastroesophageal junction. No fundoplication was done. A 28F chest tube was placed through the lowest trocar site. Intraoperative endoscopy was used in 29 (83%) of the 35 patients to help identify the esophagus and gauge the distal extent of the myotomy on the gastric wall.
The technique of laparoscopic Heller myotomy and partial fundoplication has also been described elsewhere. 6,7 Except in the few patients undergoing a partial posterior fundoplication, mobilization of the abdominal esophagus was limited to the lateral and anterior aspects, leaving the posterior attachments intact. The myotomy was 7 cm long and extended 10 to 15 mm onto the gastric wall. The short gastric vessels were divided to eliminate lateral tension on the fundoplication. An anterior 180° Dor fundoplication was used in 125 patients (94%), a posterior 220° Toupet fundoplication in 8 patients (6%).
Follow-Up
The patients were examined 2 and 6 weeks after surgery and were subsequently seen in the office or contacted by telephone every 3 to 4 months. Follow-up to October 1998 was complete in 145 patients (86%) at a median of 28 months. Specifically, follow-up was complete in 33 (94%) of 35 patients who had a thoracoscopic myotomy, at a median of 72 months, and in 112 (84%) of 133 patients who had a laparoscopic Heller myotomy and partial fundoplication, at a median of 23 months.
Statistical Analysis
The analysis of variance test, the Student’s t test, the Wilcoxon signed rank sum test, and the chi square test were used for statistical evaluation of the data. Results are expressed as medians and as means ± SD. Differences were considered significant at p < 0.05.
RESULTS
Operation and Hospital Course
Thoracoscopic Myotomy
Intraoperative endoscopy was used in 29 (83%) of 35 patients. Thirty-three of the 35 operations were completed thoracoscopically; the operation in two patients was converted to a thoracotomy. The duration of the operation was 159 ± 75 minutes (range, 75 to 480). Three mucosal lacerations occurred; one was repaired thoracoscopically and two by thoracotomy. In a fourth patient, a perforation developed 24 hours after surgery, probably as a result of thermal damage to the mucosa from electrocautery. This patient’s esophagus had a leathery consistency as a result of six aggressive pneumatic dilatations. The esophageal musculature was thin and fibrotic throughout, and the submucosal plane was indistinct. When the perforation appeared, a transhiatal esophagectomy was performed because the prospects seemed slight that esophageal function would ever be satisfactory with a lesser procedure.
In the 32 patients who had a thoracoscopic myotomy only, the chest tube was discontinued after 48 hours; they were fed after 48 hours and left the hospital after 72 hours. Five patients (16%) were discharged within 48 hours of surgery. Five of the 32 patients had a hospital stay >96 hours (average 163 ± 71; range, 120 to 288). One patient had a cardiac arrhythmia, and one patient had angina pectoris. One 86-year-old patient suffered a stroke. With the exception of this man, all patients went home and resumed regular activity within 10 to 14 days. The two patients who had a thoracotomy and the patient who required an esophagectomy remained in the hospital for 10 days.
Laparoscopic Myotomy
Intraoperative endoscopy was used in 74 patients (56%), principally early in our laparoscopic experience. All but one of the operations were completed laparoscopically; in one patient, a laparotomy was performed to evaluate a retroperitoneal hematoma caused by a Veress needle puncture of the inferior vena cava. Additional procedures were performed in four patients: cholecystectomy in three and liver biopsy in one. The length of the operation averaged 173 ± 42 minutes. Mucosal perforations in six patients were all repaired laparoscopically. Three of these six patients had been treated with pneumatic dilatation (1, 2, and 27 times, respectively); a transmural stricture was present at the gastroesophageal junction in two of them. In three patients who had been treated with intrasphincteric injections of botulinum toxin, an inflammatory reaction in the muscular layers hindered development of the submucosal plane. In one patient, a pneumothorax developed, requiring a chest tube.
On average, the patients were fed after 23 hours and left the hospital after 48 hours. Forty-six patients (35%) were discharged within 24 hours and 49 patients (37%) within 48 hours. Twelve patients had a hospital stay >96 hours, all caused by minor problems, such as ileus (nine patients). All patients were discharged home and resumed regular activity within 10 to 14 days.
Clinical Outcome
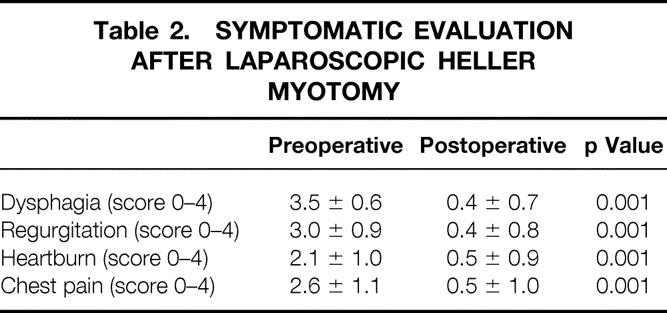
Good or excellent results were obtained in 131 (90%) of the 145 patients for whom late follow-up information was available. Among these patients, the dysphagia score dropped from 3.6 ± 0.6 before surgery to 0.5 ± 0.8 after surgery (p < 0.01). Twenty-four (73%) of the 33 patients treated by thoracoscopic myotomy had excellent or good relief of dysphagia that has continued to date. Including any additional treatment, 28 (85%) of these patients now swallow at the excellent or good level. One hundred of the 112 patients (89%) who underwent a laparoscopic Heller myotomy and partial fundoplication and for whom follow-up was complete had excellent or good results. Including any additional treatment, 104 (93%) of these patients consider their swallowing to be excellent or good. Tables 1 and 2 show the pre- and postoperative symptom scores.
Table 1. SYMPTOMATIC EVALUATION AFTER THORACOSCOPIC HELLER MYOTOMY
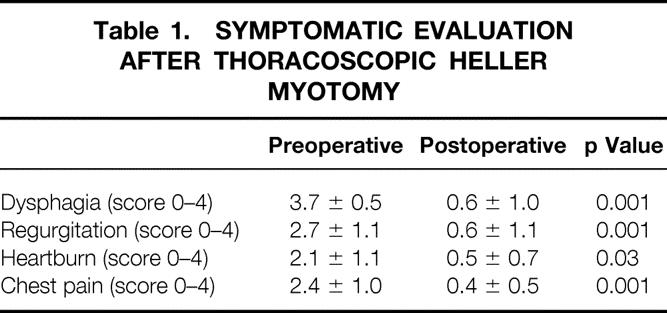
Table 2. SYMPTOMATIC EVALUATION AFTER LAPAROSCOPIC HELLER MYOTOMY
Surgical Failures
Dysphagia was still present after the initial operation in 19 (11%) patients (i.e., persistent dysphagia), and dysphagia returned after an interval of 1 year in 2 (1%) patients (i.e., recurrent dysphagia). The cause of persistent dysphagia in five patients was transmural stricture formation (involving the mucosa as well as the muscle layers), a lesion intractable to treatment by a Heller myotomy. Persistent dysphagia was the consequence of technical factors in the remaining 14 patients. Ten of these cases occurred during 1991 to 1994, when a total of 43 myotomies were performed, and 4 occurred during 1995 to 1999, when a total of 125 myotomies were performed. Thus, the incidence of theoretically avoidable persistent dysphagia from technical factors was 23% during the earlier period and 4% during the later one.
Thoracoscopic Myotomy
Nine patients (27%) had persistent dysphagia secondary to an incomplete myotomy. Four of them had laparoscopic operations to lengthen the myotomy, and a Dor fundoplication was added in two. All four patients are now swallowing well. Four patients were treated after surgery with pneumatic dilatations, but their swallowing status remained fair or poor, and they refused further surgical treatment. One patient had a transabdominal myotomy without improvement, which was followed by a transhiatal esophagectomy. He is now swallowing well.
Laparoscopic Myotomy
Twelve patients (11%) had persistent or recurrent dysphagia.
Four patients had persistent dysphagia from distortion of the gastroesophageal junction by an imperfectly formed Dor fundoplication. The fundoplication was taken down laparoscopically (a posterior fundoplication was constructed in one patient), and the dysphagia resolved in all four. Five patients were found at surgery to have a fibrotic transmural stricture at the gastroesophageal junction, probably as a result of previous treatment (multiple pneumatic dilatations, two patients; botulinum toxin injection, two patients; Nissen fundoplication, one patient). The dysphagia was not improved by the myotomy. Dysphagia improved in one such patient after two pneumatic dilatations. Two patients had incomplete myotomies. Their dysphagia did not respond to pneumatic dilatations, and they refused further treatment.
Dysphagia recurred about 12 months after surgery in two patients who had been swallowing well during the preceding months. Workup ruled out peptic stricture formation and suggested that the distal portion of the myotomy had become reapproximated. One patient is scheduled for a second myotomy. These are the only known late failures in this entire series.
Functional Evaluations
Esophageal manometry and 24-hour pH monitoring were repeated after surgery in 45 patients.
Thoracoscopic Myotomy
In the 10 patients who had esophageal function tests after surgery, median LES pressure decreased from 30 mm Hg before surgery to 9 mm Hg after surgery. Peristalsis remained absent in all patients. Gastroesophageal reflux had developed in 6 (60%) of the 10 patients, and the mean reflux score among these six was 40 (normal <15). Five of these six patients were asymptomatic. One asymptomatic patient with a score of 72 was treated with H2 blocking agents for 6 years, at which time a laparoscopic Dor fundoplication was done. This dropped her reflux score to 24. The other five patients are being treated with acid-reducing medications.
Laparoscopic Myotomy
In the 35 patients who underwent esophageal function testing after surgery, median LES pressure decreased from 28 mm Hg before surgery to 10 mm Hg after surgery. Peristalsis remained absent in all patients. Reflux was present in six (17%) patients, whose mean score was 39 (normal <15). Five asymptomatic patients are being treated with acid-reducing medications. Seven of nine patients found to have gastroesophageal reflux on preoperative evaluation were studied after surgery. The reflux had been corrected in five patients (71%).
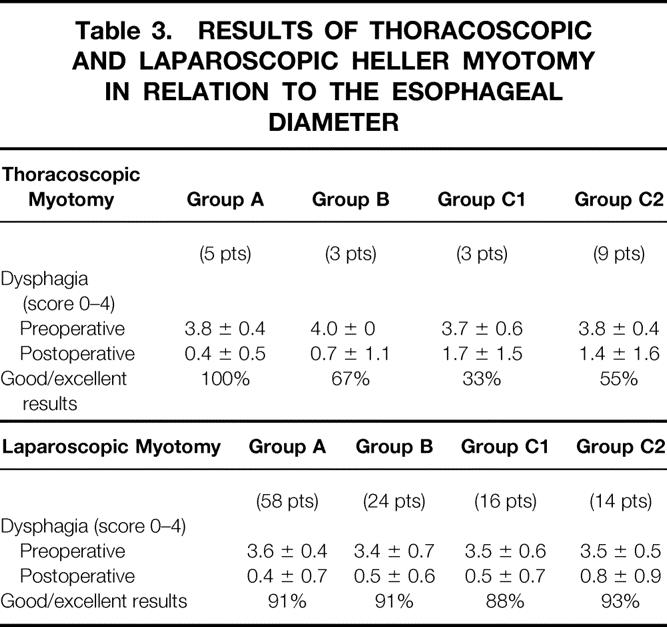
Effect of Esophageal Diameter
Table 3 shows that there were no differences in the results of thoracoscopic and laparoscopic Heller myotomies among patients whose esophageal diameter was <6.0 cm (groups A and B; p = NS). When esophageal diameter exceeded 6.0 cm, however, a laparoscopic myotomy improved swallowing better than did a thoracoscopic myotomy (groups C1 and C2; p < 0.01).
Table 3. RESULTS OF THORACOSCOPIC AND LAPAROSCOPIC HELLER MYOTOMY IN RELATION TO THE ESOPHAGEAL DIAMETER
DISCUSSION
We began using videoscopic techniques for performing Heller myotomies >8 years ago. The operation seemed ideally suited to this new technology because the site of the dissection could be readily exposed once the scope was in the chest, and tissue manipulation was minimal and confined to a small area. We assumed that a thoracoscopic approach would be simpler than a laparoscopic approach because most of the myotomy would be on the thoracic esophagus, and the exposure was anticipated to be easier through the chest.
In open operations, we had used the version of Heller myotomy advocated by Ellis et al, which involved complete division of the LES with a limited (5-mm) extension onto the stomach. 8 The technique was reported to provide complete relief of dysphagia while avoiding gastroesophageal reflux: an antireflux fundoplication was considered unnecessary. This is the method after which the thoracoscopic myotomy was patterned.
Although Heller myotomy had previously been an uncommon operation at our institution, we gained a sizable experience within a year, and at that point reported the early results. 1 The data showed that the procedure was safe, the short-term efficacy was good, and although a chest tube was needed for a couple of days, the patients recovered much faster than if the same operation had been done by thoracotomy. The surgical methods were described in detail.
Approximately 6 months after starting this program, we also began performing laparoscopic Nissen fundoplications for gastroesophageal reflux. At the outset, exposure of the lower esophagus seemed to be more difficult laparoscopically than thoracoscopically, so there was little impetus to switch to a laparoscopic approach for the Heller myotomies. Before long, however, we encountered a patient with achalasia whose previous balloon dilatation therapy had resulted in genuine gastroesophageal reflux while paradoxically failing to relieve the dysphagia. The absolute need in this case for a fundoplication in addition to the myotomy was the indication for our first laparoscopic operation for achalasia.
Meanwhile, we had been carrying out routine postoperative pH monitoring studies after the thoracoscopic myotomies on patients who could be induced to volunteer. Even though their dysphagia was gone and heartburn was absent, an unexpectedly high proportion were revealed on pH testing to have myotomy-induced reflux. 7 The reflux was largely asymptomatic, but this observation challenged our earlier belief that a myotomy could regularly be made that simultaneously eliminated dysphagia and avoided reflux.
Consequently, we converted to a laparoscopic myotomy coupled with a Dor fundoplication as our standard operation, a procedure popularized earlier by Peracchia et al. 9,10 Postoperative pH monitoring in these patients demonstrated much less reflux, and relief of dysphagia was excellent. 7 We concluded from this work that the laparoscopic operation was better and that clinical findings should be supported by the results of pH monitoring tests when assessing the outcome of operations for achalasia.
General Principles of Surgical Technique
Thoracoscopic myotomy is a relatively straightforward procedure. The submucosal plane can usually be found without much difficulty, and the myotomy can then be lengthened proximally and distally. The main problem is in determining precisely how far distally to extend the myotomy. A visual estimate of the end point (i.e., aiming for 5 mm beyond the gastroesophageal junction) was unreliable in our experience; the myotomy too often proved to be short, and dysphagia persisted. Intraoperative esophagoscopy was found to be a better guide. When the myotomy looked complete both externally (i.e., thoracoscopically) and endoscopically, dysphagia was eliminated, but as noted above postoperative pH monitoring recorded the presence of too much reflux. 7 A perfect middle ground between too short and too long a myotomy remained elusive. Another technical drawback of thoracoscopic myotomy is that the area most difficult to expose well, the gastroesophageal junction, is the point where the muscle is most apt to have been damaged by previous treatment and the mucosa is most vulnerable to an enterotomy. Finally, there is no satisfactory method of performing an antireflux procedure thoracoscopically.
Circumstances are the opposite for the laparoscopic operation. Here the most difficult part of the dissection (at the gastroesophageal junction) is the area easiest to expose, whereas the area most difficult to expose (the mediastinal esophagus) is the site of the easiest dissection. We have never made a perforation of the mediastinal portion of the esophageal mucosa while performing a laparoscopic myotomy. Further, although we used it for a while, intraoperative endoscopy is unnecessary in this procedure. Finally, an effective antireflux procedure is feasible.
Effect of Heller Myotomy When the Esophagus is Dilated
It has been said that a Heller myotomy is predictably ineffective when the esophagus is dilated, particularly when it takes a sigmoid shape. Some authors recommend esophagectomy as primary treatment in such cases. 11,12 However, esophagectomy for achalasia still carries a 4% mortality rate, even in expert hands. 11,12 Forty-eight of our 168 patients (29%) had a markedly dilated esophagus. Thoracoscopic myotomy was acceptably good in these patients, but our results are confounded by the early struggles to define the end point of the myotomy and are worse than what could be achieved now. The size and shape of the esophagus had no discernible impact on the results of laparoscopic myotomy, however: excellent and good results were obtained with the same frequency as in patients whose esophagus was not dilated. None of the laparoscopically treated patients with an esophagus >6 cm has required an esophagectomy. The ability during laparoscopic myotomy to mobilize and straighten a sigmoid-shaped esophagus may contribute to this outcome.
In our experience, therefore, esophagectomy should rarely be the initial surgical treatment for achalasia, regardless of the size and shape of the esophagus, but it should be reserved for the occasional patient in whom a laparoscopic Heller myotomy has failed.
Operative and Postoperative Course
The surgical setup was easier for the laparoscopic approach and anesthesia was simpler because single-lung ventilation was not required. The patients were more comfortable without a chest tube, and they left the hospital earlier after laparoscopic myotomy. For instance, only 16% of patients after thoracoscopic myotomy, but 72% of patients after laparoscopic myotomy, were ready to be discharged within 48 hours of surgery. In general, we now expect that the patient who undergoes a laparoscopic myotomy will be able to be discharged from the hospital the day after surgery, taking an unrestricted solid-food diet.
Treatment Failures
In 21 patients (14%), the first myotomy did not give long-lasting relief of dysphagia. The problem was persistent dysphagia in 19 patients and recurrent dysphagia in 2 patients. An understanding of the causes and prevention of these failures is of importance. As is evident, the details of our surgical methods have continued to evolve throughout the period of this study; concomitantly, the frequency of persistent dysphagia from avoidable technical causes has progressively dropped.
Five patients were found at the time of the first operation to have a fibrotic transmural stricture at the gastroesophageal junction that was not suspected from the preoperative studies. A Heller myotomy is not designed to correct such lesions, and all of these patients had persistent dysphagia after surgery. Therefore, in the strict sense, these cases represent a failure of diagnosis rather than a failure of the operation. Whether some other procedure should be performed when a stricture is unexpectedly encountered during a Heller myotomy remains an unanswered question.
Persistent dysphagia was the result of an incomplete myotomy in 11 patients. Ten of these cases occurred in the first 5 years of our experience, and all but two occurred in the group of patients who had thoracoscopic myotomies. Thus, the change to laparoscopic myotomy with a more generous extension onto gastric muscle has sharply reduced this category of failure.
In four patients who had laparoscopic myotomies, persistent dysphagia was discovered to be a consequence of esophageal distortion from a malpositioned Dor fundoplication. All of these cases occurred before 1997, indicating that this problem can largely be avoided by careful attention to technique.
In summary, if those with transmural strictures are excluded, 14 patients had persistent dysphagia as a result of technical factors. Ten of these cases were during 1991 to 1994, when a total of 43 operations were performed. Four cases were during 1995 to 1999, when a total of 125 operations were performed. Thus, the incidence of preventable technical failure was 23% during the early period and 3% during the later period, suggesting that the operations were being improved as we continued to learn the technical nuances.
Only two patients (1%) had recurrent dysphagia, both after a symptom-free interval of 1 year. Workup uncovered no evidence of stricture formation or gastroesophageal reflux. The explanation appeared to be spontaneous closure or healing of the myotomy. One of these patients is scheduled for a second myotomy.
These observations show that short-term success, with few exceptions, indicates that the long-term outcome will also be good.
CONCLUSIONS
Experience to date has led us to the following conclusions. Minimally invasive Heller myotomy is a highly effective treatment for achalasia. If the operation relieves the dysphagia, the good result is durable. For the reasons discussed above, laparoscopic myotomy with a fundoplication is superior to thoracoscopic myotomy. Thoracoscopic myotomy in our practice would be confined to patients with insurmountable abdominal adhesions precluding a laparoscopic approach and patients with diffuse esophageal spasm who do not need complete division of the sphincter.
Heller myotomy should generally be accompanied by an antireflux procedure because reflux, at least on pH monitoring testing, is otherwise common. A Dor fundoplication is both simple and effective, so more complex antireflux measures are unnecessary. Laparoscopic Heller myotomy is effective in patients with a dilated esophagus, even with sigmoid changes, so esophagectomy in this disease should usually be reserved for the occasional failure of myotomy.
Attention to the details of the surgical technique is important to success, and many of the technical refinements are difficult to convey in words. Our rate of persistent dysphagia dropped substantially after approximately 40 operations, but the minimum learning curve is probably less than this, because we were in the process of developing the laparoscopic methods.
Compared with the alternative forms of therapy, balloon dilation and botulinum toxin, laparoscopic Heller myotomy stands out as safe, effective, expeditious, and durable. We believe it should be the primary form of treatment for achalasia, and trends in surgical referrals suggest that the medical community at large is coming to the same conclusion.
Discussion
Dr. John G. Hunter (Atlanta, Georgia): One of the very interesting experiments in evolution—Darwinism at the speed of light, if you will—is watching high-volume endoscopic surgeons converging on a single technique for these new procedures over a period of 10 years.
The technique you have demonstrated to us needs little improvement. It is so good that the dean of medical esophagology, John Dent from Adelaide, has proclaimed in a state-of-the-art address recently that primary therapy of untreated achalasia should be Heller myotomy and fundoplication performed laparoscopically. Balloons and botulinum have been relegated to the bullpen.
This is an important paper. The thoroughness of the physiologic evaluation in these patients offers us the opportunity to learn how patient symptoms do or do not correlate with the pathophysiology of achalasia before and after treatment. My questions center around the evaluation of these patients.
After barium flow and EGD, did you perform motility studies on the large type-3 esophagus? If so, what did you learn that changed your approach? We are more likely to perform a CAT scan looking for causes of pseudoachalasia in esophageal motility studies in these patients where the esophagus is so big that the catheter is likely to get lost. What are the criteria for performing pre- and postop 24-hour pH studies? Did all these patients have heartburn before or after surgery? Before we start citing your data, we must be sure we are looking at an unselected population and not just 33% of the population who had symptoms.
Lastly, focusing on your patients with unsuspected strictures, I have also had the trouble of trying to sort out preoperatively those who have fibrotic strictures from those with hypertensive sphincters. Because discovering intraoperatively that you are not going to help these patients with a myotomy is not optimal. Are there any clues that you can use to determine the patients with fibrotic strictures preoperatively? Is preop motility helpful? And did any of these patients have preop 24-hour pH studies to show that they might be refluxing?
Presenter Dr. Marco G. Patti: Our protocol calls for motility studies to be done in all patients. It is technically more difficult in those with a dilated esophagus, so in them the catheter is placed under fluoroscopy. If we are unable to position the catheter under fluoroscopy, however, we proceed and treat the patient based on the clinical and X-ray findings. We generally do not, in that uncommon situation, resort to endoscopic placement of the catheter.
CT scans and endoscopic ultrasound are occasionally used to rule out pseudoachalasia, for example, when the patient has had dysphagia for less than 6 months, has recently lost weight, or is over age 60.
The patients who had postoperative esophageal manometry and pH monitoring were unselected, which explains why those who were found to have reflux were asymptomatic. We ask all patients to have manometry and pH monitoring studies 2 or 3 months after the operation. The data from these routine studies were important, for they were a major reason why we switched from the thoracoscopic to the laparoscopic approach and added a fundoplication.
The strictures resulting from previous treatment were not detected during the preoperative work-up. In patients who have had multiple dilatations, endoscopic ultrasound might help distinguish normal esophageal wall from fibrosis, but we have not tried this approach. The drawback, of course, is that many procedures would have to be done to find the occasional stricture. The patients with strictures might eventually need to have an esophagectomy, but their symptoms are not yet that severe.
Dr. Philip A. Donahue (Chicago, Illinois): Congratulations to Dr. Patti on another outstanding presentation. He and Drs. Pellegrini, Way, and others have been leaders in the minimally invasive treatment of this interesting condition. We understand it a lot better than we did in ’92 when we were still debating the relative merits of extending myotomy onto proximal stomach. Now we understand clearly that the disease process involves degenerative changes in neurons in proximal stomach as well as in the esophagus. As their experiences suggested, they and most others have adopted the laparoscopic approach.
My first question is about the utility of intraoperative endoscopy. In our series of 55 patients in Chicago, we have often been quite surprised by residual constrictions that were still present when we thought that the myotomy was complete—that is, after 15 to 20 mm extension onto the stomach. I was surprised in your manuscript that you suggested endoscopy is not necessary. Since we know that there is a learning curve in any medical center, don’t you think we should encourage routine use of intraoperative endoscopy until people have at least gotten past 20 or 30 cases?
My second question addresses the etiology of achalasia. If this disease is due to “myenteric plexitis”—and I think it is, as shown by work in Cleveland and from Bologna—how often have you seen active inflammatory reaction in the wall of the esophagus? I have seen it in the younger patients, that is, less than 20 years old, who never had pneumatic dilation or botulinus toxin injections. Have you seen that frequently? Do you concur that that is the likely explanation in the younger patients?
I will close with thanks for sending me a copy of the manuscript, which has a wealth of interesting technical suggestions. I think you have addressed the fibrotic and the late complications extremely well. I congratulate you for your comprehensive evaluation of your patients.
Dr. Patti: Intraoperative esophagoscopy was essential when the operation was done thoracoscopically. Otherwise, the incidence of incomplete myotomy was excessive. Even with endoscopy, however, there was a fine line using the limited thoracoscopic myotomy between relieving dysphagia and causing reflux. We definitely recommend that endoscopy be used routinely when a Heller myotomy is done thoracoscopically.
When we first switched to the laparoscopic approach, we used endoscopy in all patients. Before long, however, we realized that because the myotomy extended 1.5 cm onto the stomach, it regularly turned out to be complete when checked by endoscopy. After a long series of endoscopies added nothing to the operation, we stopped using it as a routine. Thus for a laparoscopic Heller myotomy that entails a long myotomy, the visual estimate of completeness was accurate by itself. We agree that endoscopy might be useful early in a surgeon’s experience with this operation.
We treated just 10 patients younger than age 16. Inflammation was sometimes found in patients who had had balloon dilatation, but the esophageal texture was normal in patients who had had neither balloon dilatation nor Botox injections. There were few such patients, so we may encounter this phenomenon in the future.
Dr. Richard J. Finley (Vancouver, Canada): The purpose of this operation is to obliterate a dysfunctional lower esophageal sphincter and prevent gastroesophageal reflux. Gastroesophageal reflux is prevented by anchoring the esophagus in the abdomen and carrying out a partial fundoplication.
In your series, I understand you took down some fundoplications because of ongoing dysphagia. Did you redo the fundoplication? If not, did those patients develop reflux? Do you believe that you have proven that you require a partial fundoplication to prevent reflux?
My second question is, did any of your patients have so-called vigorous achalasia? Did that have impact on your outcomes?
My third question relates to quality of life. We have heard some wonderful papers about quality of life today. I think the lack of validated quality-of-life tools remains a major problem in the analysis of swallowing in the treatment of achalasia.
Like you, we had bad results with thoracoscopic myotomy because we didn’t carry our myotomy into the stomach well enough. What are your indications now for thoracoscopic esophageal myotomy?
Dr. Patti: We now reserve thoracoscopic myotomy in achalasia for patients who have had multiple abdominal operations, where adhesions might preclude a successful laparoscopic approach. On the other hand, thoracoscopy is the method of choice in patients with diffuse esophageal spasm who have normal LES function, for the entire sphincter does not have to be divided.
In the few patients with vigorous achalasia, the results were the same as in those with classic achalasia.
We agree that more sophisticated measures of quality of life would be interesting. Overall, the patients considered themselves quite well after the operation. Nearly all were able to eat food they had to avoid before surgery, and many gained weight.
We feel it is important to anchor the fundoplication in the abdomen. In patients who required a second operation, another form of fundoplication was constructed in 75% of them. Our data indicate that a fundoplication should be used routinely to prevent reflux or to correct existing reflux that resulted from balloon dilatation.
Dr. Alan G. Johnson (Sheffield, England): You make the statement that this should be the primary treatment of achalasia. To understand that statement, we need to know how you defined failure of dilatation, because, I think, 40% of your patients had a dilatation. How many patients had dilatation and never needed an operation? My second point is technical. I was a bit concerned with nearly 3 hours as the mean operating time. This has huge cost implications. If you are looking at cost effectiveness of a procedure, why do you divide the short gastric vessels when you are doing an anterior 180° wrap?
Dr. Patti: We routinely divide the short gastric vessels in order to avoid tension on the fundoplication. It takes about 10 minutes to do, and the worldwide experience with fundoplication for GERD suggests that it is important.
The operation now takes about 21/2 hours, less than when we started. The higher OR costs are offset by the fact that most patients nowadays are discharged after a 23-hour hospital stay.
Botulinum toxin has not fulfilled the early expectations—it rarely provides long-lasting relief of dysphagia, and it makes a myotomy more difficult. Dilatation is done safer and better today than in the past, especially using the graduated approach proposed by Dr. Joel Richter. Nevertheless, good results are achieved in only 80% of patients, and about 25% of those whose swallowing improves acquire reflux as a consequence of the dilatation. Compared with the alternatives, we believe that a laparoscopic Heller myotomy and fundoplication is currently the preferred treatment for achalasia.
Footnotes
Correspondence: Marco G. Patti, MD, Dept. of Surgery, University of California, San Francisco, 533 Parnassus Ave., Room U-122, San Francisco, CA 94143-0788.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Pellegrini C, Wetter LA, Patti M, et al. Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg 1992; 216: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcerito M, Patti MG. Esophageal function testing: : indications, methods and interpretation. Progr Chirurg 1995; 6: 33–36. [Google Scholar]
- 3.Jamieson JR, Stein JH, DeMeester TR, et al. Ambulatory 24-hr esophageal pH monitoring:: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992; 87: 1102–1111. [PubMed] [Google Scholar]
- 4.Crookes PF, Corkill S, DeMeester TR. Gastroesophageal reflux in achalasia. When is reflux really reflux? Dig Dis Sci 1997; 42: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 5.Patti MG, Arcerito M, Tong J, et al. Importance of preoperative and postoperative pH monitoring with esophageal achalasia. J Gastrointest Surg 1997; 1: 505–510. [DOI] [PubMed] [Google Scholar]
- 6.Patti MG, Pellegrini CA. Minimally invasive approaches to achalasia. Sem Gastrointest Dis 1994; 5: 108–112. [PubMed] [Google Scholar]
- 7.Patti MG, Arcerito M, De Pinto M, et al. Comparison of thoracoscopic and laparoscopic Heller myotomy for achalasia. J Gastrointest Surg 1998; 2: 561–566. [DOI] [PubMed] [Google Scholar]
- 8.Ellis FH Jr, Gibb SP, Crozier RE. Esophagomyotomy for achalasia of the esophagus. Ann Surg 1980; 192: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonavina L, Nosadini A, Bardini R, Baessato M, Peracchia A. Primary treatment of esophageal achalasia. Arch Surg 1992; 127: 222–227. [DOI] [PubMed] [Google Scholar]
- 10.Rosati R, Fumagalli U, Bonavina L, et al. Laparoscopic approach to esophageal achalasia. Am J Surg 1995; 169: 424–427. [DOI] [PubMed] [Google Scholar]
- 11.Orringer MB, Stirling MC. Esophageal resection for achalasia: : indications and results. Ann Thorac Surg 1989; 47: 340–345. [DOI] [PubMed] [Google Scholar]
- 12.Pinotti HW, Cecconello I, Mariano da Rocha J, Zilberstein B. Resection for achalasia of the esophagus. Hepato-Gastroenterology 1991; 38: 470–473. [PubMed] [Google Scholar]


