Abstract
Objective
To determine rates and mechanisms of failure in 857 consecutive patients undergoing laparoscopic fundoplication for gastroesophageal reflux disease or paraesophageal hernia (1991–1998), and compare this population with 100 consecutive patients undergoing fundoplication revision (laparoscopic and open) at the authors’ institution during the same period.
Summary Background Data
Gastroesophageal fundoplication performed through a laparotomy or thoracotomy has a failure rate of 9% to 30% and requires revision in most of the patients who have recurrent or new foregut symptoms. The frequency and patterns of failure of laparoscopic fundoplication have not been well studied.
Methods
All patients undergoing fundoplication revision were included in this study. Symptom severity was scored before and after surgery by patients on a 4-point scale. Evaluation of patients included esophagogastroscopy, barium swallow, esophageal motility, 24-hour ambulatory pH, and gastric emptying studies. Statistical analysis was performed with multiple chi-square analyses, Fisher exact test, and analysis of variance.
Results
Laparoscopic fundoplication was performed in 758 patients for gastroesophageal reflux disease and in 99 for paraesophageal hernia. Median follow-up was 2.5 years. Thirty-one patients (3.5%) have undergone revision for fundoplication failure. The mechanism of failure was transdiaphragmatic herniation of the fundoplication in 26 patients (84%). In 40 patients referred from other institutions, after laparoscopic fundoplication, only 10 (25%) had transdiaphragmatic migration (p < 0.01); a slipped or misplaced fundoplication occurred in 13 patients (32%), and a twisted fundoplication in 12 patients (30%). The failure mechanisms of open fundoplication (29 patients) followed patterns previously described. Fundoplication revision procedures were initiated laparoscopically in 65 patients, with six conversions (8%). The morbidity rate was 4% in laparoscopic procedures and 9% in open ones. There was one death, from aspiration and adult respiratory distress syndrome after open fundoplication. A year or more after revision operation, heartburn, chest pain, and dysphagia were rare or absent in 88%, 78%, and 91%, respectively, after laparoscopic revision, and were rare or absent in 91%, 83%, and 70%, respectively, after open revision, but 11 patients ultimately required additional operations for continued or recurrent symptoms, 3 after open revision (17%), and 8 after laparoscopic fundoplication (11%).
Conclusions
Laparoscopic fundoplication failure is infrequent in experienced hands; the rate may be further reduced by extensive esophageal mobilization, secure diaphragmatic closure, esophageal lengthening (applied selectively), and avoidance of events leading to increased intraabdominal pressure. When revision is required, laparoscopic access may be used successfully by the laparoscopically experienced esophageal surgeon.
Laparoscopic Nissen fundoplication is changing the way chronic heartburn is managed around the world. Data from New York State and elsewhere have demonstrated a three- to fivefold increase in the number of fundoplications performed for gastroesophageal reflux disorder (GERD) over the last decade. 1 The CDC estimates that 12,000 such procedures were performed in the United States in 1987 and 48,000 in 1998. 2,3
Failure of open fundoplication occurs in 9% to 30% of patients, depending on how failure is defined and how long until follow-up. 4–6 Published failure rates of laparoscopic Nissen fundoplication are 2% to 17%, 7–11 depending on the definition of failure and the experience of the surgeons. The lower rate published for laparoscopic surgery may reflect the shorter follow-up possible for this new procedure.
Four failure patterns after open fundoplication have been described 12 : the slipped or misplaced fundoplication, the disrupted fundoplication, the herniated fundoplication (Fig. 1), and the fundoplication that is too tight or too long. Since the introduction of laparoscopic fundoplication, two additional failure patterns have emerged: the twisted fundoplication and the two-compartment stomach (Figs. 2 and 3). 10,13

Figure 1. Endoscopic view of center of herniated fundoplication.
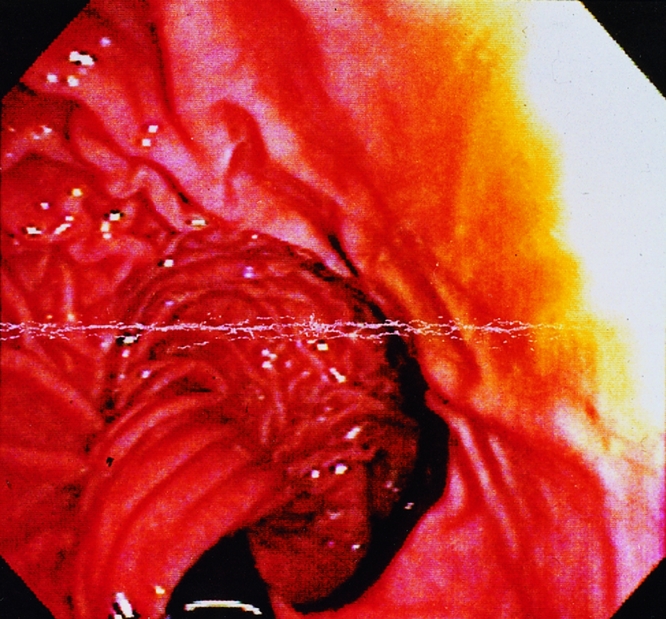
Figure 2. A twisted valve seen after Rossetti fundoplication.

Figure 3. A two-compartment stomach is an extreme variant of the twisted valve. The proximal pouch (fundus) is seen as the dark cavity in the center for the photo.
The aim of this study was to determine the incidence and mechanism of symptomatic fundoplication failures after laparoscopic fundoplication for GERD or paraesophageal hernia, and to compare these findings to those in patients referred from other institutions with symptomatic failure of laparoscopic or open fundoplication. A second aim was to determine whether laparoscopic revision surgery is safe and as effective as open revision surgery.
METHODS
Patients
Between October 1, 1991, and November 1, 1998, 857 patients underwent laparoscopic fundoplication for GERD (758 patients) or paraesophageal hernia (99 patients) at the University of Utah (14 patients) or Emory University (843 patients). Thirty-one of these patients (3.6%) underwent fundoplication revision for anatomic failure of the fundoplication or intractable postoperative symptoms. These 31 patients constitute the first study group.
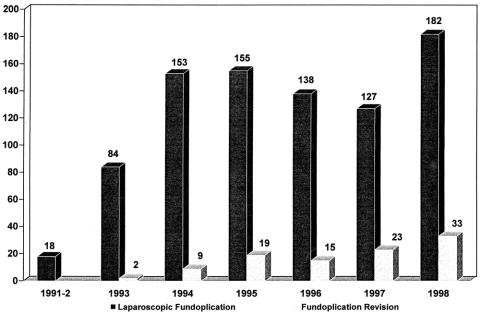
During this same period, 69 patients referred from other institutions underwent fundoplication revision. Forty of these patients had previously undergone only laparoscopic fundoplication, 24 had undergone at least one previous fundoplication by laparotomy, and five patients had undergone a Belsey fundoplication through a left thoracotomy. In total, 100 consecutive patients undergoing fundoplication revision were included in this study (Fig. 4).
Figure 4. Volume of laparoscopic fundoplication and fundoplication revision procedures at Emory Swallowing Center over a 7-year period.
Patients seen during the same period but excluded from this study included those requiring esophagectomy for end-stage reflux disease (two patients) or antrectomy and Roux-en-Y reconstruction for bile reflux (six patients), and patients with reflux and/or dysphagia after previous Heller myotomy for achalasia (nine patients). Also, patients in whom postoperative symptoms could be adequately managed without reoperation were excluded from preoperative evaluation.
Evaluation and Surgical Indications
The Emory Swallowing Center was established in 1993 as a combined effort of the divisions of gastrointestinal surgery and gastroenterology for the evaluation and treatment of patients with esophageal dysfunction. Patients referred to the swallowing center with persistent or recurrent foregut symptoms after fundoplication for GERD or paraesophageal hernia were screened with a barium swallow (Fig. 5). Further preoperative evaluation in the elective setting included esophagogastroduodenoscopy in 96 patients, esophageal motility study in 77 patients, gastric emptying study in 46 patients, and 24-hour ambulatory pH study in 33 patients.
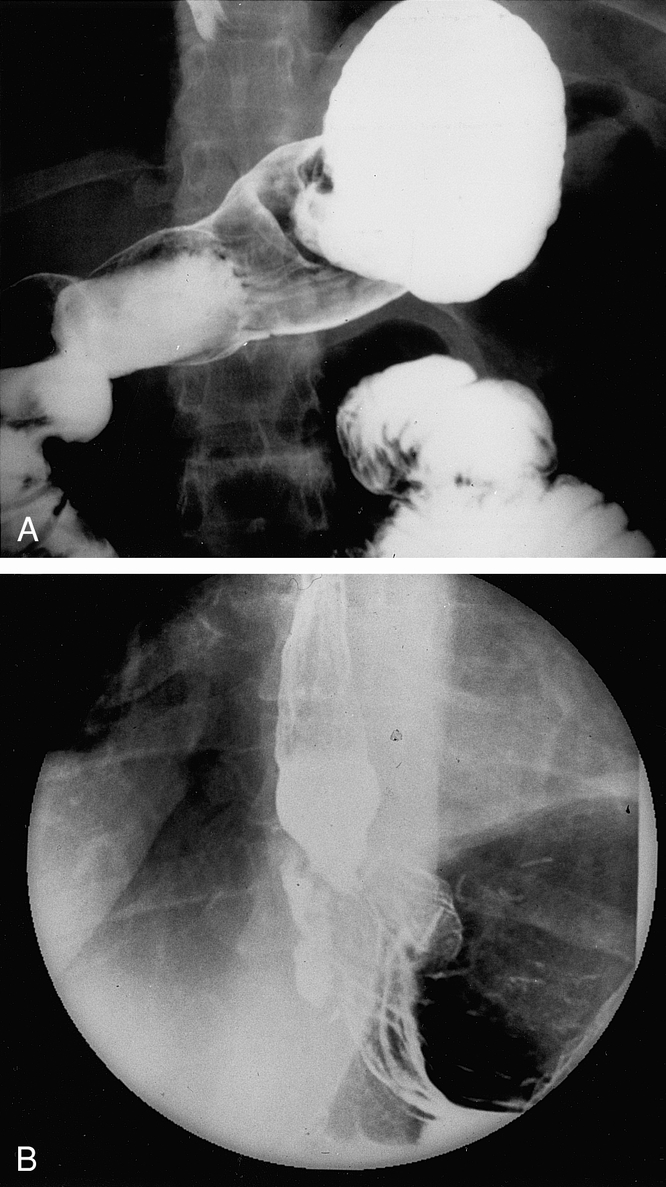
Figure 5. Barium swallow of (A) a patient with a two-compartment stomach (see Fig. 3), and (B) a herniated fundoplication (see Fig. 1).
Fundoplication revision was recommended when the preoperative evaluation revealed a surgically correctable anatomic or functional disorder that corresponded with the patient’s symptoms. Fundoplication takedown without reconstruction was not performed in this series.
Surgical Procedure and Access
One hundred eleven revision procedures were performed in the 100 patients in this series (Table 1). The type of operation performed was determined by preoperative esophagogastric motility findings and intraoperative assessment of esophageal length. The default operation was a short (2- to 2.5-cm) Nissen fundoplication performed loosely over a 56F to 60F dilator. Subtotal fundoplication (modified Toupet procedure) was performed in patients for severe dysphagia in the absence of an easily recognized anatomic problem, or esophageal dysmotility, defined as an esophageal body amplitude of contraction <30 mm Hg or failed peristalsis in >30% of wet swallows. Heller myotomy was performed in addition to an antireflux procedure in patients for aperistalsis of the esophageal body (primary or acquired achalasia) and in patients with a nonrelaxing lower esophageal sphincter, when an anatomic problem with the previous fundoplication could not be identified before or during surgery. Pyloroplasty was added to the fundoplication in four patients when gastric half-emptying time was more than twice the top limits of normal. Collis gastroplasty was added to two procedures when intraoperative maneuvers were insufficient to return the gastroesophageal junction and 2 cm of distal esophagus to the abdomen without tension.
Table 1. FUNDOPLICATION REVISION, 1991–1998
Laparoscopic access was offered for the majority of first revisions if all previous procedures had been performed laparoscopically or by thoracotomy. Open access was recommended when at least one repair by laparotomy had been previously performed, when a revision performed at Emory had failed, and when two laparoscopic procedures performed at other institutions had failed. As experience was gained with laparoscopic revision, indications for laparoscopic access were extended to those who had previously undergone laparotomy and those who had had several failed laparoscopic repairs.
Laparoscopic fundoplication revision was performed with five trocars. The stomach was sharply taken down from the liver and diaphragm. The stomach was unwrapped from the esophagus, preserving both vagi if possible. The crural closure was reinforced and a three-suture Nissen (or Toupet) fundoplication was created. In sum, 75 revision procedures in 72 patients were initiated with laparoscopic access, and 35 revision procedures in 33 patients were initiated with laparotomy. One procedure was performed through a left thoracotomy.
Data Management
Data on preoperative symptoms, barium swallow, esophagogastroduodenoscopy, esophageal motility study, 24-hour pH monitoring, and gastric emptying study were collected and entered prospectively into a Microsoft Access (Microsoft Corp., Seattle, WA) database. One of the six categories of failure was assigned, as described above. When more than one failure mechanism was identified (e.g., when fundoplication herniation lead to displacement or disruption of the fundoplication), the category of the most prominent defect was chosen. Similarly, surgical and postoperative data were entered prospectively. Postoperative symptom assessment was performed 1 month after surgery and each year thereafter. Effectiveness of fundoplication revision was determined by comparing preoperative with postoperative symptom scores. Statistical comparisons of preoperative findings predicting failure and failure mechanisms between groups were performed with chi square and Fisher’s exact tests (when frequencies in any one cell were <5). Continuous data were compared with analysis of variance.
RESULTS
Failure Patterns of Laparoscopic Fundoplication (Initial Operation at Emory)
Thirty-one of 857 patients (3.6%) undergoing laparoscopic fundoplication for GERD or paraesophageal hernia over a 7-year period required fundoplication revision. The median follow-up of this group was 34 months (range, 1 month to 5 years). Of the 758 patients who underwent laparoscopic fundoplication for GERD, 28 patients (3.6%) required revision (Table 2). Four of these patients (0.5%) required urgent revision for early postoperative transdiaphragmatic herniation of the fundoplication. In three of these four patients, when the problem was identified within the first 24 hours with a water-soluble contrast swallow, laparoscopic revision was possible. Of the 99 patients with paraesophageal hernia, revision was required in three, not significantly different from patients undergoing fundoplication for GERD. However, of the 167 patients (22%) with advanced GERD (esophageal stricture or Barrett’s esophagus), laparoscopic fundoplication required revision in 13 (8%) (p < 0.05 vs. uncomplicated GERD).
Table 2. PREOPERATIVE INDICATORS OF LAPAROSCOPIC FUNDOPLICATION FAILURE
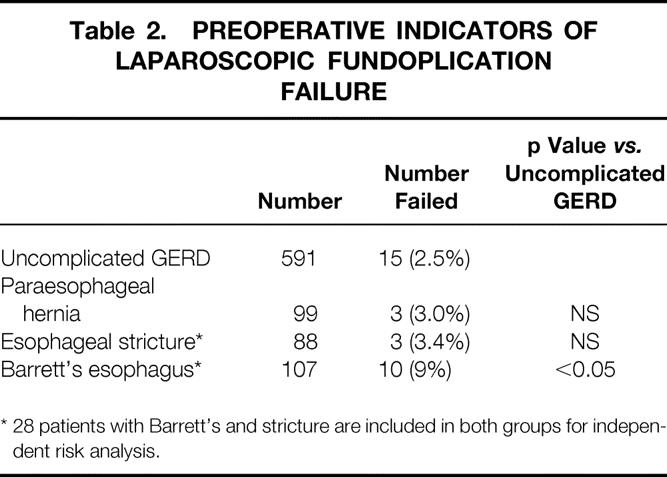
* 28 patients with Barrett’s and stricture are included in both groups for independent risk analysis.
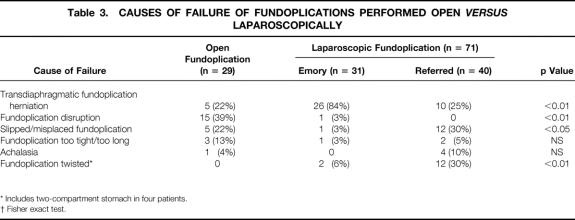
The failure mechanism was transdiaphragmatic migration of the fundoplication in 26 of the 31 patients (84%) (Fig. 5B). The remaining five patients had a disruption (one patient), a twisted fundoplication (two patients), a slipped fundoplication (one patient), and a fundoplication that was too tight (one patient). In patients with transdiaphragmatic migration, an acute event causing wrap migration could be identified in 19 (59%). This event was retching in 10, coughing in 4, and straining to lift or urinate in 5. In seven patients, no event could be identified, but six of these patients had advanced esophageal disease before surgery (Barrett’s esophagus, four patients; esophageal stricture, two patients) (Table 3).
Table 3. CAUSES OF FAILURE OF FUNDOPLICATIONS PERFORMED OPEN VERSUS LAPAROSCOPICALLY
* Includes two-compartment stomach in four patients.
† Fisher exact test.
The interval between the initial operation and reoperation is shown in Figure 6. More than 50% of patients in whom the procedure failed underwent reoperation within a year of the initial procedure, and 90% underwent revision within 2 years. After 2 years of follow-up, only 3 of 548 (0.5%) patients who had laparoscopic fundoplication performed >2 years previously had a newly detected fundoplication failure.
Figure 6. Interval between first operation and fundoplication revision. The total number of Emory patients at risk for fundoplication failure is shown in solid bars.
Failure Patterns of Laparoscopic Fundoplication (Referred to Emory)
Forty patients referred to Emory after a failed laparoscopic fundoplication were considered suitable candidates for laparoscopic fundoplication revision. The incidence of laparoscopic fundoplication failure at other institutions could not be determined without the denominator of total cases performed. Nonetheless, the most common anomalies identified were the twisted fundoplication in 12 patients (30%), the slipped or misplaced fundoplication in 12 patients (32%), and the herniated fundoplication in 10 patients (25%). The remaining six patients had motility and radiographic findings consistent with achalasia (four patients) and a fundoplication that was too tight (two patients). This distribution of failure patterns is statistically different from the patterns observed in patients who had their first operation at our institution (p < 0.05). All but one of the patients with twisted fundoplications had not had the greater curvature of the stomach mobilized on the first operation (Rossetti-Nissen fundoplication). Four of these patients had two-compartment stomachs. The interval between initial operation elsewhere and reoperation at Emory in these patients was similar to that seen in patients who had their initial and second operations at Emory (see Fig. 6).
Failure of Open Fundoplication
Twenty-nine patients with symptomatic failure of an open fundoplication (thoracotomy 5, laparotomy 24) were deemed suitable candidates for fundoplication revision. The mechanism of failure was disruption of previous repair in 10 of the patients who had undergone fundoplication by laparotomy and all 5 of the patients who had undergone Belsey fundoplication (39%). A slipped or misplaced valve was present in five patients (22%), a herniated fundoplication was present in five patients (22%), a fundoplication that was too tight was detected in three (13%), and acquired achalasia was detected in one patient (4%). The interval between the initial operation and reoperation in this group was quite different: 12 patients (41%) underwent revision >5 years after their original operation (see Fig. 6).
Surgical Details
Of the 75 operations initiated with laparoscopic access, 6 were converted to laparotomy (8%). The reason for conversion was adhesions complicating laparoscopic access in four patients, three of whom had previous laparotomy, and lacerations in the stomach requiring repair in two patients. In all patients, regardless of previous access technique, adhesions between the diaphragm, liver, and stomach were encountered but did not pose a significant barrier to fundoplication. Similarly, small lacerations of the stomach were not uncommon and were easily repaired with two layers of suture or endoscopic staples. The median duration of laparoscopic revision was 210 minutes for the first 25 operations, 203 minutes for the second 25 procedures, and 183 minutes for the last 25 operations. The median operative time for revision by laparotomy was 211 minutes. Length of hospital stay was 2.6 days in those undergoing laparoscopic revision and 7.5 days in those undergoing open repair. Complications requiring prolongation of the hospital stay or additional intervention occurred in four patients (5%) undergoing laparoscopic fundoplication revision and three patients (9%) who underwent revision by laparotomy. One elderly patient with chronic pulmonary disease from recurrent aspiration events developed aspiration pneumonia after open revision and ultimately died of overwhelming sepsis (Table 4).
Table 4. DEATH AND COMPLICATIONS AFTER FUNDOPLICATION REVISION

* Hospital stay > 4 days.
Eleven patients required two revisions during this study period. Eight of these patients had laparoscopic revision previously and three had laparotomy previously. In six of these patients (including one patient who had revision by laparotomy), there was a recurrence of transdiaphragmatic herniation of the fundoplication. In two of these six, a Collis gastroplasty was added to the third repair, and in four patients it was necessary only to repair crural defects and redo the fundoplication. Five patients whose dysphagia was unrelieved by the first revision (two open, three laparoscopic) underwent a second revision. In four of these patients, a Heller myotomy was performed because preoperative data showed a nonrelaxing lower esophageal sphincter, without an identifiable anatomic derangement of the fundoplication to explain the dysphagia. In one of these patients, a twisted valve was untwisted and a pyloroplasty was added because of profoundly delayed gastric emptying.
Symptomatic Outcome
Laparoscopic Revision
Of the 71 patients undergoing one or more laparoscopic fundoplication revisions, 66 (93%) responded to the follow-up symptom survey; 37 of these had undergone laparoscopic revision >1 year earlier. Thirty-two (87%) reported good to excellent control of heartburn and regurgitation, with the remainder reporting fair or poor control. Thirty-four patients (91%) had no dysphagia; however, three patients reported occasional dysphagia, and one patient was still significantly bothered by dysphagia (Fig. 7).
Figure 7. Symptomatic outcomes after laparoscopic fundoplication revision (minimum follow-up, 1 year).
Open Revision
Of the 29 patients in the group undergoing open revision, 26 (90%) responded to the follow-up symptom survey; 23 of these had undergone open revision >1 year earlier. Twenty of these patients (91%) reported good to excellent control of heartburn and regurgitation; 9% report fair or poor control. Dysphagia to solids was absent or rare after open fundoplication revision in 70%; it was moderate in 22% and was bothersome daily in 9% (Fig. 8).
Figure 8. Symptomatic outcomes after open fundoplication revision (minimum follow-up, 1 year).
DISCUSSION
Laparoscopy has changed the face of general surgery in the past decade, but the diseases and clinical problems we face are no different from those of previous decades. Similarly, the only fundamental difference between a laparoscopic antireflux operation and an open one is the access and the instrumentation. This provides us with a unique opportunity to compare the patterns of failure between the two approaches.
Extensive evaluations of failed antireflux operations and postoperative symptomatology have led to many changes in fundoplication technique over the years. The primary goal is to obtain a short, loose fundoplication around a portion of intraabdominal esophagus. 4 Many early failures are the result of technical errors made during the initial operation. 9 Failures may also result from deteriorating foregut motility or wear and tear on the fundoplication. 12 The indication for surgery, the choice of antireflux procedure, and the technical quality of the operation have all been shown to determine the incidence of subsequent failure. 14–16
Long-term follow-up of 20 years is available for open antireflux procedures. Good outcomes have been reported in 85% to 90% of patients after a Belsey, Nissen, or Hill procedure. Recurrent reflux has been reported in 7% to 10% and dysphagia in 6% to 14%. 12 Long-term follow-up is not yet available for laparoscopic antireflux surgery, but early results are encouraging (91% to 98% relief of symptoms). 7–11
A few studies have specifically addressed the issue of failure after laparoscopy, demonstrating failure rates of 1.5% to 9% requiring revision. 17,18 Our failure rate of 3.6% in a population of nearly 1000 patients with a median follow-up of 34 months is in the middle portion of this range; however, this study includes only patients who have undergone fundoplication revision. Fundoplication revision was performed on all patients with recurrent (or new-onset) esophageal or extraesophageal manifestations of GERD in whom reflux could be demonstrated with endoscopy or 24-hour pH study. Also, patients with new symptoms that were moderate to severe (symptom score 2 to 4) and anatomic findings compatible with their symptoms were offered fundoplication revision. Not included in this 3.6% failure rate are patients who, for one reason or another, resumed taking antisecretory medicines but did not warrant further evaluation because their symptoms were mild and not bothersome. Without further evaluation, it is impossible to determine how many of these patients were truly refluxing or had anatomic fundoplication failure. Our database was not designed to pick up patients in whom the results of postoperative evaluation with endoscopy, pH study, gastric emptying study, barium swallow, or CT scan were normal (i.e., no defect identified) or patients in whom evaluation led to another diagnosis (e.g., gastritis, duodenal ulcer, cancer). Finally, a handful of patients (<10) not included in this study have had a small asymptomatic recurrence of their hiatal hernia detected on surveillance endoscopy for Barrett’s esophagus or an upper gastrointestinal examination.
We found dramatic differences in the patterns of failure between fundoplications performed laparoscopically at our institution and elsewhere and fundoplications performed open. Transdiaphragmatic herniation of the intact fundoplication was the cause of failure in most laparoscopic fundoplication failures performed at Emory but in only 25% of patients referred after surgery, whether open or laparoscopic. The reason for this difference is not clear. Failure studies of open fundoplication have demonstrated fundoplication herniation in only 17% to 20%, similar to the frequency observed in patients referred to Emory after open fundoplication. 14,15 A higher incidence of transdiaphragmatic herniation after laparoscopic fundoplication than after open fundoplication has been reported by others. 18,19 Given the otherwise excellent results, it appears that fundoplication herniation is the Achilles’ heel of laparoscopic fundoplication. Fewer adhesions form between the fundoplication and the diaphragm after a laparoscopic procedure than after an open operation. The few adhesions that form after laparoscopic fundoplication are found on the anterior surface of the fundoplication, tethering the fundoplication to the undersurface of the overlying liver. The herniated portion of the fundoplication generally occurs on the opposite side of the esophagus, posteriorly, where there are few adhesions anchoring the fundoplication to the crura. Without adhesions, the laparoscopic fundoplication is at risk to herniate with increased intraabdominal pressures. Fixation of the fundoplication to the undersurface of the diaphragm seemed not to prevent this complication, but thorough esophageal mobilization and snug crural closure has made this complication less common in the past 2 years.
Retching or straining in the early postoperative period is particularly troublesome after a fundoplication. In this series, acute fundoplication herniation developed in four patients when they retched (3 patients) or strained to urinate (1 patient) in the early postoperative period. We were able to reduce the fundoplication laparoscopically within the first 24 to 48 hours after this complication in two patients but had to convert to laparotomy in one patient in whom the diagnosis was delayed 72 hours. To prevent this complication, nausea should be treated aggressively, and inability to urinate should be treated with a Foley catheter. In this study and elsewhere, we have reported a higher rate of fundoplication herniation in patients with esophageal stricture and Barrett’s esophagus. 20,21 Also, six of seven patients with fundoplication herniation but no inciting events had advanced esophageal disease. Both of these observations suggest that esophageal foreshortening may play a role in herniation after laparoscopic fundoplication. Collis gastroplasty should be seriously considered in all patients with advanced esophageal disease in whom fundoplication herniation develops without an identifiable event causing hiatal disruption.
Two unique fundoplication deformities seen dramatically or subtly in most patients after Rossetti fundoplication undergoing redo procedures for dysphagia were the spiral valve and the two-compartment stomach. The former occurs when the unmobilized fundus is pulled tightly around the esophagus. The fundoplication valve is twisted by the fundus attempting to unwind itself. 13 The two-compartment stomach occurs when a point too low on the greater curvature is pulled up to the gastroesophageal junction, leaving a large portion of the fundus isolated from the antrum and a band around the midstomach. 10 Patients with this deformity have dysphagia and also a great deal of postprandial pain (from filling but not emptying the fundic pouch). Attempts to vomit to empty the fundic pouch usually fail because of the competent Nissen valve.
The interval between initial laparoscopic fundoplication and reoperation is quite short (90% <2 years). Similar to open fundoplication, most laparoscopic fundoplications fail early. 12 The large percentage of open fundoplication failures presenting >5 years after the initial procedure in this series may reflect the fact that few open fundoplications were performed in our region in the past 5 years. It is encouraging to note that <1% of patients who had a fundoplication >2 years previously needed reoperation, and no patient with a laparoscopic fundoplication >5 years previously has returned for revision. Although complete follow-up is <80% after 5 years, we are aware of only one of our patients who underwent revision elsewhere.
A favorable outcome can be achieved after fundoplication revision in most patients. It appears that laparoscopic revision is as successful as open fundoplication revision and can be accomplished with fewer complications than open fundoplication. However, the rate of symptomatic success after fundoplication revision does not duplicate that seen after initial antireflux surgery. In an earlier study of 300 patients undergoing laparoscopic fundoplication for the first time, 93% were free of reflux symptoms after surgery. 8 Although laparoscopic fundoplication revision is an acceptable option for the patient with recurrent symptoms, a properly performed initial antireflux procedure is the key to achieving the best surgical outcomes.
Discussion
Dr. Carlos A. Pellegrini (Seattle, Washington): I wish to congratulate John Hunter on another excellent presentation. The data presented here today emphasize the high success rate of laparoscopic fundoplication and the relationship between technique and outcomes. It also underscores two principles of fundoplication: the importance of a good esophagogastric mobilization and the importance of a systematic closure of the crura and fixation of the fundoplication to the undersurface of the diaphragm.
We analyzed the results of our own series earlier this year (Arch Surg 1999; in press). Our findings strongly support those presented here today. Herniation of the hiatus was, indeed, the most common anatomic malformation. I would like to ask two related but separate questions.
First, I would like you to comment on patient selection. What determines the need for operation on a patient that comes in with foregut symptoms after an initial laparoscopic operation? After very thorough work-up of our patients, we found that about 35% of them, that is, one third of the patients referred to us with foregut symptoms after first operations, could be managed very well medically and did not require an operation. I think it would be important to stress when you indicate surgery.
Specifically, how do you react to an abnormal upper gut intestinal series after an operation, i.e., a small herniation of the fundus on an otherwise asymptomatic patient? What do you do when you find an abnormal 24-hour pH monitoring in a patient who is asymptomatic, i.e., the test was done as part of your routine postop follow-up?
The second question refers to esophageal shortening. I think your series, as well as ours, shows that the great majority of patients who recur after initial operation do not have a short esophagus. I think it is important to emphasize that because there is a tendency to believe that the short esophagus is a relatively common occurrence and people may go on with the idea of doing Collis gastroplasty and other lengthening procedures, all of which have been shown to be less effective in the ultimate control of reflux.
Presenter Dr. John G. Hunter (Atlanta, Georgia): The first question asks about patient selection for revision. Our surgical database didn’t capture the number of patients who were referred but could be managed conservatively, but one third is a rough estimate.
How do you determine who gets an operation and who doesn’t? Prokinetic agents and antisecretory agents are used empirically to see whether you can manage without recurrent reflux symptoms without an operation. The second thing is making sure that the symptoms are compatible with the preoperative anatomic evaluation. That is, a patient with a herniated fundoplication usually has dysphagia, heartburn, chest pain, or all three of those symptoms. If the symptoms are compatible with the anatomic finding, then I think a revision is appropriate. On the other hand, if patients are asymptomatic and have an abnormal upper GI exam or an abnormal 24-hour pH exam, we would not entertain a reoperation.
As for esophageal foreshortening, I agree that this is present. I think it did contribute to failure in our patients with advanced esophageal disease, such as Barrett’s esophagus. The identification of esophageal shortening may be difficult. If one cannot mobilize at least 2 cm of esophagus back to the abdomen in a tension-free fashion, a lengthening procedure is indicated. That happens in about 2% or 3% of cases. And only two patients in this series had a Collis gastroplasty.
Dr. Nathaniel J. Soper (St. Louis, Missouri): I reported earlier our results with anatomic fundoplication failure following laparoscopic Nissen fundoplication. We demonstrated many of the same things that Dr. Hunter reported today. In our first 50 patients, we selectively divided the short gastric vessels, selectively closed the crura, etcetera, and we had a 19% failure rate with wraps migrating and disrupting. Subsequently, we now routinely divide the short gastric vessels and approximate the crura, and our rate of fundoplication failure is 4%, almost identical to the Emory group’s failure rate of 3.6%. Factors associated with fundoplication failure were diaphragmatic stressors such as coughing, straining, weightlifting, vomiting, retching, and high-speed motor vehicle accidents, as well as the size of the hiatal hernia as measured preoperatively.
Dr. Hunter, what is the true denominator of failure? That is, have other of the operations failed and not required operative therapy?
Conversely, have there been any esophagogastrectomies that have been required for patients who have had difficult problems or prostheses that have been placed migrating into the esophagus and that sort of thing? Did you try to assess the size of the hiatal hernias or the esophageal length preoperatively and see whether that was associated with your incidence of failure?
What about the time course of these failures? Have you reached the plateau, do you think, in your own series, where it is well defined, or are these things going to continue to accrue over time?
One group that had a higher incidence of failure was the patients with Barrett’s esophagus. Why did these patients fail? Is it because of a short esophagus? Is it because there is worse inflammation?
Last, I would like to comment on what Dr. Pellegrini talked about in terms of fixing the wrap to the undersurface of the diaphragm. Do you do that? If so, why? If not, why not? My own concern is, if you fix the fundus or the wrap to the diaphragm, these two structures ordinarily move in different planes; with violent motion of the diaphragm, such a point of attachment could conceivably contribute to wrap disruption.
Dr. Hunter: We haven’t had as much disruption by automobile accidents in Atlanta. I think the traffic is always gridlocked and doesn’t move fast enough for there to be such accidents.
In general, failure in these patients is probably underestimated. We have very rarely had to do esophagogastrectomies. In every situation where that has been necessary, a prosthetic material has been placed in the hiatus by surgeons previously. This is not really the topic of this presentation, but the use of prosthetic mesh to close the diaphragm needs to be better evaluated. When one has to redo these procedures after mesh placement, very frequently portions of the stomach or esophagus have to be removed.
The preop assessment of esophageal length is difficult. Certainly patients with strictures, with Barrett’s, with paraesophageal hernias, all have a risk factor for esophageal foreshortening. We discuss Collis gastroplasty with all these patients preoperatively. Having done that, only one in five, or 20%, of patients are judged to have esophageal foreshortening intraoperatively. Although the predictors of shortening are helpful, they certainly don’t tell you absolutely who is going to have a short esophagus.
Is this failure frequency a steady-state phenomenon? Is this evolving? I think it is clearly evolving. You see the numbers of redos continue to climb. I tell my patients that there is going to be about a 10% failure rate in the long term. That is certainly the best results we see with open fundoplication. It overshoots by a factor of two what we are seeing so far, but I think that might be a realistic estimate in the long term of failure.
Why Barrett’s esophagus patients are more prone to failure, I am not sure, but Tom DeMeester has shown us that patients with Barrett’s have the most advanced esophageal disease, more strictures, more shortening of the esophagus. And I think that is probably the reason why 9% of our Barrett’s patients failed.
Fixation of the fundoplication to the diaphragm does not help. We did that for a while. We fixed it with two or three sutures. In fact, wherever we didn’t fix it with a suture, that was where the herniation occurred. So we substituted fixation of the fundoplication to the diaphragm with a snug crural closure which circumferentially limits the transdiaphragmatic migration of the wrap.
Dr. Lawrence W. Way (San Francisco, California): This important paper shows the mechanisms of failure of laparoscopic fundoplication, information that theoretically could be translated into prevention through changes in operative techniques. Furthermore, the data show that with few exceptions, operations that have failed for technical reasons can be corrected by a second operation, in most cases laparoscopically.
Herniation of the wrap, the most common form of failure, was attributed in some instances to retching or vomiting in the postoperative period. Although the authors said it is unimportant to fix the wrap in the abdomen, there is considerable debate on this question, and many surgeons believe that fixation is important. I think this is still an open issue, very much in need of further investigation.
My question is as follows. From the preoperative upper GI series, videotapes of the operations, and functional tests, did the patients whose wraps herniated stand out in any way by comparison with the rest?—for example, were their hernias larger, or did the videotapes show faults in the hiatal closure? Or were there peculiarities in other aspects of the fundoplication itself? Did the motility studies provide clues? Or was retching/vomiting the only identifiable factor?
The good news is that failure of laparoscopic fundoplication is uncommon. And it may also be good news that the few failures are usually explainable in mechanical terms. On the other hand, we do not fully understand the variables involved, although this work serves as a milestone on the way to better understanding.
Dr. Hunter: Dr. Way, thank you very much. If I can summarize, what I think you are saying is we shouldn’t really take a nihilistic view towards recurrence and say it is going to happen, and can’t be prevented. There are a lot of things we can do technically to prevent it. To answer your questions specifically:
In one patient, it was clear that we hadn’t adequately closed the crura and the herniation was posterior to the crural closure. In other words, the stitches were too far anteriorly, we had not completely mobilized the sac posteriorly, and she recurred through that posterior sac. This is a technical failure that can be avoided. We really didn’t find anything in motility testing that struck me that would help us predict who was going to fail.
One of the very difficult groups to deal with, and our experience has tempered my approach toward them, are the patients with coughs who are on steroids. Chronic coughers stress their diaphragm all day, every day. If they are on steroids, it is very difficult to get a secure crural closure that will not tear out.
As far as the gastropexy, we have not been doing a posterior gastropexy. Dr. Moody taught me that any operation that ended in “pexy” should be mistrusted. I am not sure that is entirely true, but I think there is a lot to be said for that. I think it really does deserve looking at, however, to see whether posterior gastropexy might make a difference. I don’t think fixation of the fundoplication to the rim of the crural closure anteriorly makes any difference in preventing recurrence. It certainly didn’t in our series.
Dr. Philip A. Donahue (Chicago, Illinois): This is a pretty convincing manuscript that will convince a lot of people to approach these recurrences laparoscopically, and I thank you for informing us about that. I am going to skip by my first comment about fixation of the wrap because I think Dr. Way addressed that with the idea of posterior fixation, which I think is a good idea.
This idea of learning curve has surfaced again; every medical center sees a marked decrease in the incidence of migration of the wrap, and there is a difference between the first 25 cases and the next 100 cases. The way we have avoided migration is with circumferential fixation of the wrap, with special attention to posterior stitches.
My question for you is about advanced reflux disease and esophageal shortening. We are not seeing the tight fibrous strictures or really serious shortening that we saw 20 or even 15 years ago; we do see occasional patients, even young ones, with lots of periesophagitis. Is this a consequence of alkaline reflux and is that alone capable of causing shortening? Again, we found very few patients who need a Collis.
Again I thank you for an outstanding effort. You have taught us a lot about reflux.
Dr. Hunter: I think your comments really do underscore the fact that even without a stricture, many patients with Barrett’s who have had prolonged erosive esophagitis do develop a transmural fibrosis of the esophagus and periesophagitis that limits your ability to mobilize the esophagus down into the abdomen. And it is the pliability and flexibility of the esophagus, I think, that allows you to get additional length so that you don’t need to do a Collis procedure. In fact, in performing these operations on Barrett’s patients you can almost always tell when you are getting above the Barrett’s segment because the esophagus softens up, becomes pliable, the fat wrapping goes away, and you can see the longitudinal muscle fibers appearing again.
I am sort of dodging your question because I don’t know the answer to it, and that is: What is the role of alkaline reflux? I think the issue is injury. And if the injury is alkaline injury or if the injury is low pH or acid injury, I think the damage will probably be reasonably equivalent. But that is purely conjecture.
Dr. Bruce M. Wolfe (Sacramento, California): This is clearly a benchmark series done by what many of us consider to be the outstanding group in the country in the performance of this operation. And yet there is approximately a 2% incidence of failure requiring reoperation due to superior migration of the fundoplication into the mediastinum.
Perhaps extensive mobilization of the esophagus to achieve an adequate intraabdominal esophagus to satisfy oneself that short esophagus is not present is not the answer. A short esophagus may be contributing substantially to the superior migration of these fundoplications such that a Collis gastroplasty should be applied more liberally.
Dr. Hunter: I think we are all still learning how to do this.
Of the 26 patients who had the transdiaphragmatic migration, we identified diaphragmatic stressors, as Dr. Soper calls them, in all but eight patients. And of those eight patients, six had either Barrett’s esophagus or an esophageal stricture. So I think there is no doubt that the advanced esophageal disease in those patients is playing a role.
But if you accept that those patients, some of which may have needed Collis gastroplasty, would fail or could fail, there is still a large group of patients in whom if you can remove the stressors you may actually prevent recurrence. And I think you can. I have been much more cautious in these later days of telling patients that they are free to go back to doing whatever they want. We have seen failure in a power lifter, and an architect lifting a large stone, who felt something pop and herniated his fundoplication recently.
So I don’t think this will ever be a perfect operation. But I think we should continue to strive to make the improvements necessary to decrease fundoplication failure.
Footnotes
Correspondence: John G. Hunter, MD, FACS, Dept. of Surgery, Emory University Hospital, Rm. H122, 1364 Clifton Rd., NE, Atlanta, GA 30322.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Sparcs Discharge Database, New York State Bureau of Health Statistics, 1999.
- 2.Centers for Disease Control Bureau of Health Statistics, Rockville, MD, 1987.
- 3.Synergy Healthcare, Inc., Waltham, MA, 1999.
- 4.DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesophageal reflux disease: : evaluation of primary repair in 100 consecutive patients. Ann Surg 1986; 204 (1): 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiebert CA, O’Mara CS. The Belsey operation for hiatal hernia: : a twenty-year experience. Am J Surg 1979; 137: 532. [DOI] [PubMed] [Google Scholar]
- 6.Shirazzi SS, Schulze K, Soper RT. Long-term follow-up for treatment of complicated chronic reflux oesophagitis. Arch Surg 1987; 122: 548–552. [DOI] [PubMed] [Google Scholar]
- 7.Hinder RA, Filipi CJ, Wetscher G, Neary P, DeMeester TR, Perdikis G. Laparoscopic Nissen fundoplication is an effective treatment for gastroesophageal reflux disease. Ann Surg 1994; 220: 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter JG, Trus TL, Branum GD, Waring JP, Wood WC. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg 1996; 223: 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters JH, Heimbucher J, Kauer WK, Incarbone R, Bremner CG, DeMeester TR. Clinical and physiologic comparison of laparoscopic and open Nissen fundoplication. J Am Coll Surg 1995; 180: 385–393. [PubMed] [Google Scholar]
- 10.Jamieson GG, Watson DI, Britten-Jones R, Mitchell PC, Anvari M. Laparoscopic Nissen fundoplication. Ann Surg 1994; 220: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushieri A, Hunter JG, Wolfe B, Swanstrom LL, Hutson W. Multicenter prospective evaluation of laparoscopic antireflux surgery. Surg Endosc 1995; 7: 505–510. [DOI] [PubMed] [Google Scholar]
- 12.Hinder RA, Klingler PJ, Perdikis G, Smith SL. Management of the failed antireflux operation. Surg Clin North Am 1997; 77 (5): 1083–1098. [DOI] [PubMed] [Google Scholar]
- 13.Hunter JG, Swanstrom L, Waring JP. Dysphagia after laparoscopic antireflux surgery. Ann Surg 1996; 224 (1): 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein HJ, Feussner H, Siewert JR. Failure of antireflux surgery: : causes and management strategies. Am J Surg 1996; 171: 36–40. [DOI] [PubMed] [Google Scholar]
- 15.DeMeester TR, Stein HJ. Surgical treatment of gastroesophageal reflux disease. In: Castell CO, ed. The esophagus. Boston: Little, Brown and Company; 1992: 579–625.
- 16.Rafferty EA, Rattner DW. Gastroesophageal reflux disease: : indications for surgery, preoperative evaluation, and choice of operation. Prob Gen Surg 1996; 13: 29–37. [Google Scholar]
- 17.Dallemagne B, Weerts JM, Jehaes C, Markiewicz S. Causes of failures of laparoscopic antireflux operations. Surg Endosc 1996; 10: 305–310. [DOI] [PubMed] [Google Scholar]
- 18.Soper NJ, Dunnegan D. Anatomic fundoplication failure after laparoscopic antireflux surgery. Ann Surg 1999; 229: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson DI, Jamieson GG, Devitt PG, Mitchell PC, Game PA. Paraesophageal hiatus hernia: : an important complication of laparoscopic Nissen fundoplication. Br J Surg 1995; 82: 521–523. [DOI] [PubMed] [Google Scholar]
- 20.Spivak H, Trus T, Waring JP, Branum G, Hunter J. Laparoscopic fundoplication for peptic esophageal strictures and dysphagia. Gastroenterology 1997; 112 (4): A1475. [Google Scholar]
- 21.Farrell TM, Smith CD, Metreveli RE, Johnson AB, Galloway KD, Hunter JG. Fundoplication provides effective and durable symptom relief in patient with Barrett’s esophagus. Am J Surg (in press). [DOI] [PubMed]