Abstract
Objective
To review the outcome data for colectomy performed for patients with slow transit constipation (STC).
Background
The outcome of surgical intervention in patients with STC is unpredictable. This may be a consequence of the lack of effectiveness of such interventions or may reflect heterogeneity within this group of patients.
Methods
The authors reviewed the data of all series in the English language that document the outcome of colectomy in ≥10 patients in the treatment of STC.
Results
Thirty-two series fulfilled the entry criteria. There was widespread variability in patient satisfaction rates after colectomy (39% to 100%), reflecting large differences in the incidence of postoperative complications and in long-term functional results. Outcome was dependent on several clinical and pathophysiologic findings and on the type of study, the population studied, and the surgical procedure used.
Conclusions
It may be possible to predict outcome on the basis of preoperative clinical and pathophysiologic findings. This review suggests a rationale for the selection of patients for colectomy.
Constipation is the second most commonly self-reported gastrointestinal symptom, affecting 2% to 34% of populations studied. 1,2 After routine investigations to exclude an organic etiology as a cause of symptoms, a subgroup of patients with intractable symptoms are offered specialist referral for further investigation. Such patients, who usually have a long history of severe constipation, can be further divided on the basis of anorectal physiologic investigations and transit studies into those who have a reduction in the propulsive capacity of all or part of the colon, or slow transit constipation (STC), those who have an isolated disorder of rectal evacuation, and those who have both. A further, larger proportion of patients in this group have no major abnormality on these investigations; these constitute a group with “normal transit constipation” or “constipation-predominant irritable bowel syndrome.” A small number have the separate distinct condition of megacolon or megarectum, which is characterized radiologically. Some of these patients are managed medically, but a proportion will seek a surgical opinion for amelioration of their symptoms.
Surgery for STC has been loosely based on a concept of the pathology (colonic stasis) since the beginning of the century, 3,4 and little seems to have changed to date. Colectomy and ileorectal anastomosis has remained the treatment of choice for STC in preference to other surgical options, such as limited resection. 5 The outcome from such surgery is highly variable, with widespread variability in both satisfaction rates and the incidence of postoperative complications.
The aim of this article is to review the outcome of colectomy in the treatment of STC. On the basis of preoperative clinical and pathophysiologic findings, this review makes observations with respect to predictive factors in the success or failure after surgery and discusses a rationale for selection of candidates for colectomy.
METHODS
Inclusion Criteria
All publications that fulfilled the following criteria were included:
1. Publication in the English language
2. Patients documented in the manuscript as having slow colonic transit of any cause
3. Outcome described in ≥10 patients
4. Surgery included excision of all or part of the colon and restored the continuity of the bowel by primary anastomosis (i.e., subtotal or segmental colectomy).
Studies were not excluded on the basis of design (prospective or retrospective) or length of follow-up; however, one study was excluded on the basis of data repetition. 6
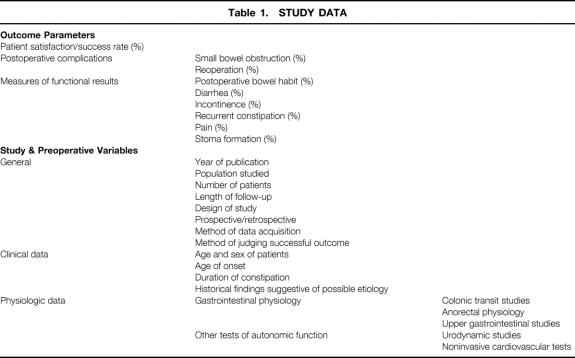
Data were collected for several outcome parameters to allow comparison between studies (Table 1). Other data variables collected included general study data (e.g., study design, outcome measures used, length of follow-up) and preoperative clinical and physiologic data.
Table 1. STUDY DATA
RESULTS
Overall Outcome Results
Thirty-two studies, published between 1981 and 1988, fulfilled the entry criteria. 5,7–37 Overall patient success or satisfaction rates, documented in 31 studies, varied from 39% to 100% (Table 2).
Table 2. SATISFACTION OR SUCCESS RATES AFTER COLECTOMY
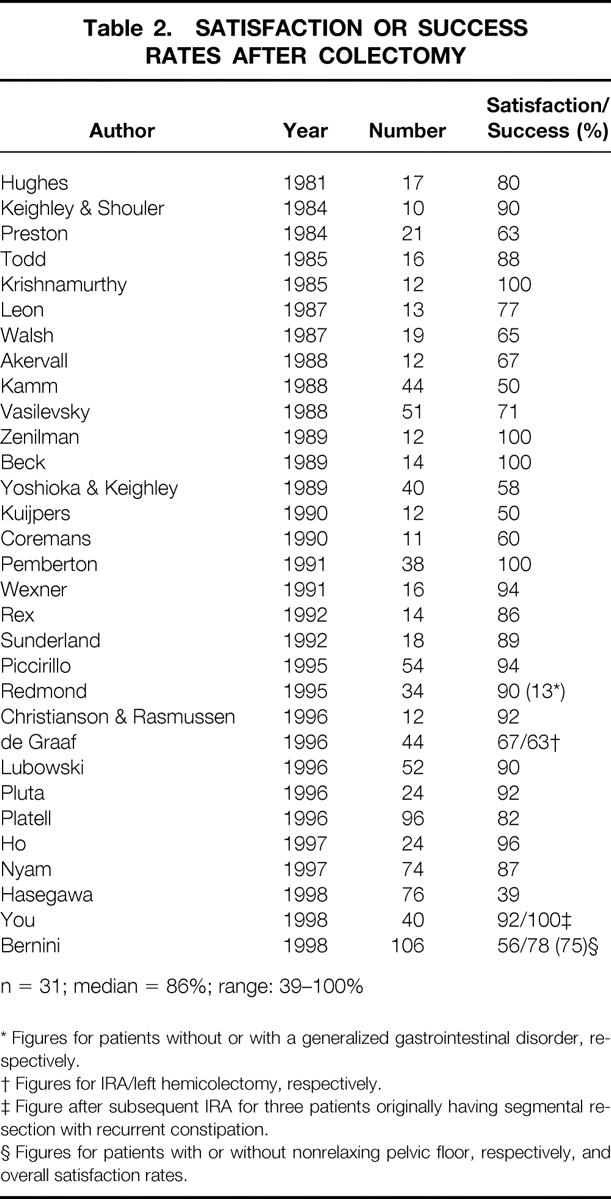
* Figures for patients without or with a generalized gastrointestinal disorder, respectively.
† Figures for IRA/left hemicolectomy, respectively.
‡ Figure after subsequent IRA for three patients originally having segmental resection with recurrent constipation.
§ Figures for patients with or without nonrelaxing pelvic floor, respectively, and overall satisfaction rates.
Methods Used in Outcome Assessment
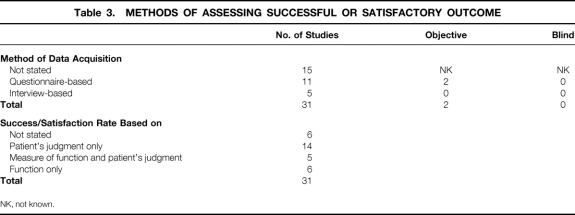
The methods used to assess overall patient success or satisfaction rates varied between studies. Only half of the 31 studies that documented success or satisfaction rates stated the method of data acquisition (Table 3). Of the studies where it was documented, most used questionnaire-based methodology with or without interview and/or clinical examination. Only two studies assessed outcome objectively using personnel not involved in the surgical care of the patient. In all others where stated, such data collection was led by the clinician. Data were not collected blindly in any study. The method by which patient success or satisfaction rates had been calculated was documented in 25 studies. In 14 series, a satisfactory outcome was based on the patient’s judgment alone, although data regarding functional outcome were collected for all these studies. In a further five series, the patient was encouraged to include a measure of bowel function in the assessment of the success of the operation, and a further six studies used functional measures alone. No study was controlled with respect to the outcome from other surgical or medical interventions, or no treatment.
Table 3. METHODS OF ASSESSING SUCCESSFUL OR SATISFACTORY OUTCOME
NK, not known.
Postoperative Morbidity Rates and Functional Outcome
Postoperative morbidity rates of small bowel obstruction (with or without reoperation), documented in 22 series, varied from 2% to 71% (median 18%); this resulted in reoperation in 0% to 50% of patients (median 14%) (Table 4).
Table 4. SMALL BOWEL OBSTRUCTION AFTER COLECTOMY FOR SLOW TRANSIT CONSTIPATION
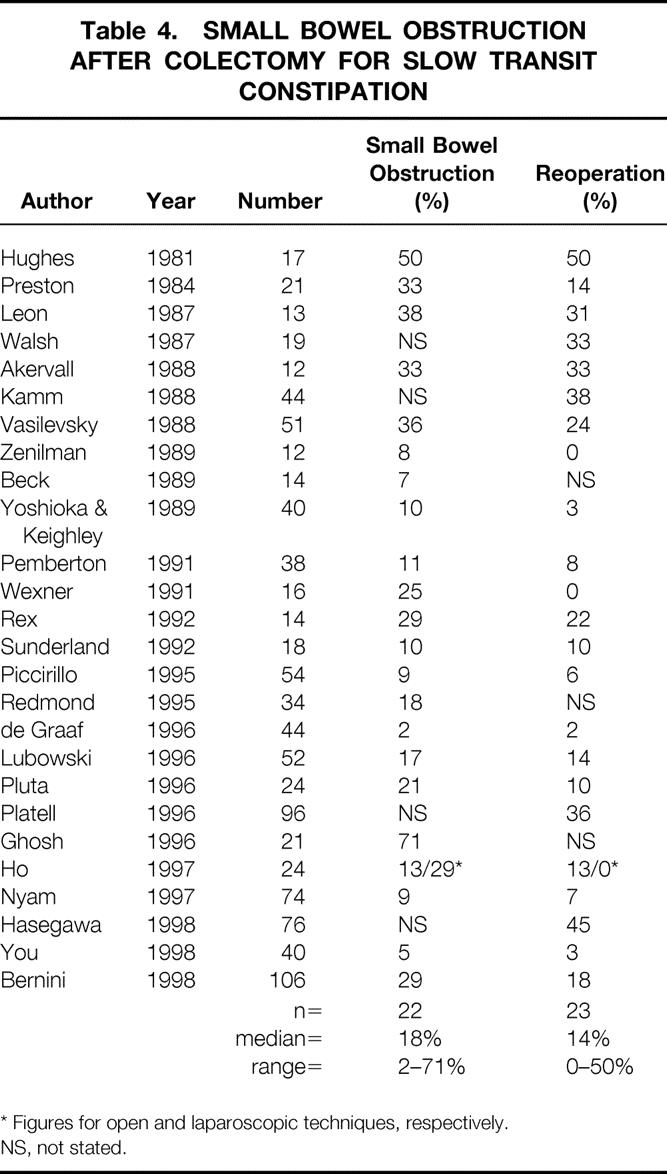
* Figures for open and laparoscopic techniques, respectively.
NS, not stated.
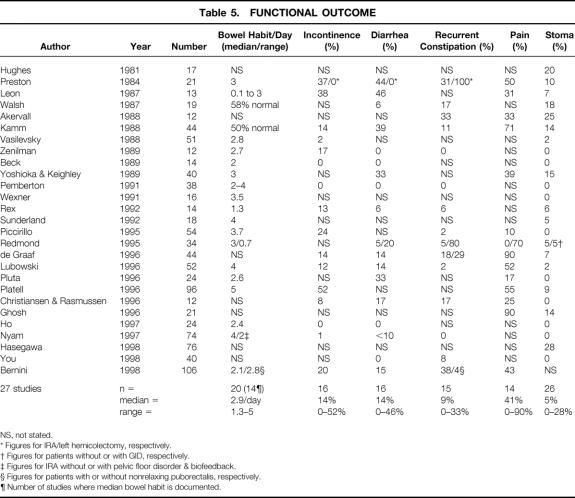
Functional outcome measures are shown in Table 5 and were often poorly documented. Postoperative bowel habit was numerically quantified in only 20 series, with median or mean bowel habit figures available in only 14 series (range of medians/means: 1.3 to 5 times per day, median 2.9). The percentage incidence of diarrhea was documented in 16 series (range 0% to 46%, median 14%). Methods varied markedly in the definition of diarrhea. Numeric frequency criteria were used in three studies (>3 bowel movements per day 14 or >5 movements per day 11,37), consistency (loose or watery stools) was used in three studies, 30,33,35 and the need to use antidiarrheal medications was used in a further three studies. 21,23,29 In all others, diarrhea was defined by the patient, or the criteria used were not stated (seven studies). The percentage incidence of incontinence was documented in 16 series (range 0% to 52%, median 14%) but was numerically scored in only 1 study. 29 Rates of recurrent constipation were recorded in 15 series (range 0% to 33%, median 9%). Objective criteria were documented in two studies (≤2 bowel movements per week). 14,28 In others, constipation was reported by patients as “persistent defecatory difficulty,” defined by the need for continued laxative use, or the definition was not stated.
Table 5. FUNCTIONAL OUTCOME
NS, not stated.
* Figures for IRA/left hemicolectomy, respectively.
† Figures for patients without or with GID, respectively.
‡ Figures for IRA without or with pelvic floor disorder & biofeedback.
§ Figures for patients with or without nonrelaxing puborectalis, respectively.
¶ Number of studies where median bowel habit is documented.
The percentage of patients who still had abdominal pain was documented in 14 series (range 0% to 90%, median 41%). As a result of poor functional outcome—in particular, diarrhea and incontinence or recurrent constipation—a permanent ileostomy was performed in up to 28% of patients 34 (median 5%, range 0% to 28%). Mortality rates, documented in 23 series, varied from 0% to 6%. 12
Effect of General Study Variables on Outcomes
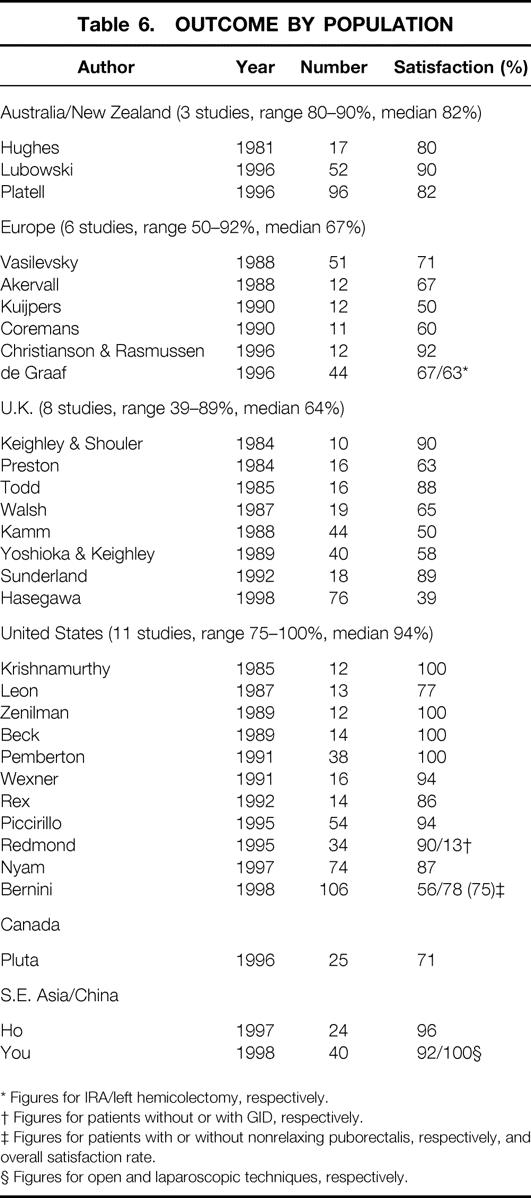
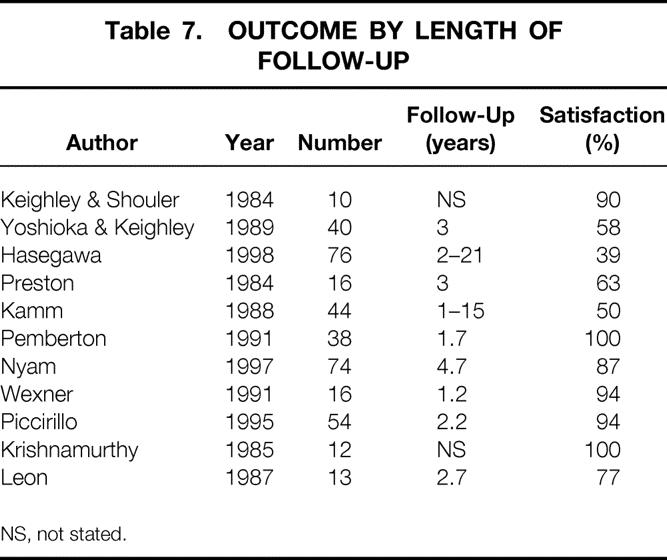
There was no difference in outcome with respect to year of publication, but the population studied appeared to have a marked effect on results. Studies performed in the United States (n = 11) demonstrated success rates of 75% to 100% (median 94%). Studies in the United Kingdom and Europe had median success rates of 64% and 67%, respectively (Table 6). Although there was no overall direct correlation between length of follow-up and success rates, for study groups that had published results at two or more time points, the percentage of successful outcomes appeared to decrease with time (Table 7).
Table 6. OUTCOME BY POPULATION
* Figures for IRA/left hemicolectomy, respectively.
† Figures for patients without or with GID, respectively.
‡ Figures for patients with or without nonrelaxing puborectalis, respectively, and overall satisfaction rate.
§ Figures for open and laparoscopic techniques, respectively.
Table 7. OUTCOME BY LENGTH OF FOLLOW-UP
NS, not stated.
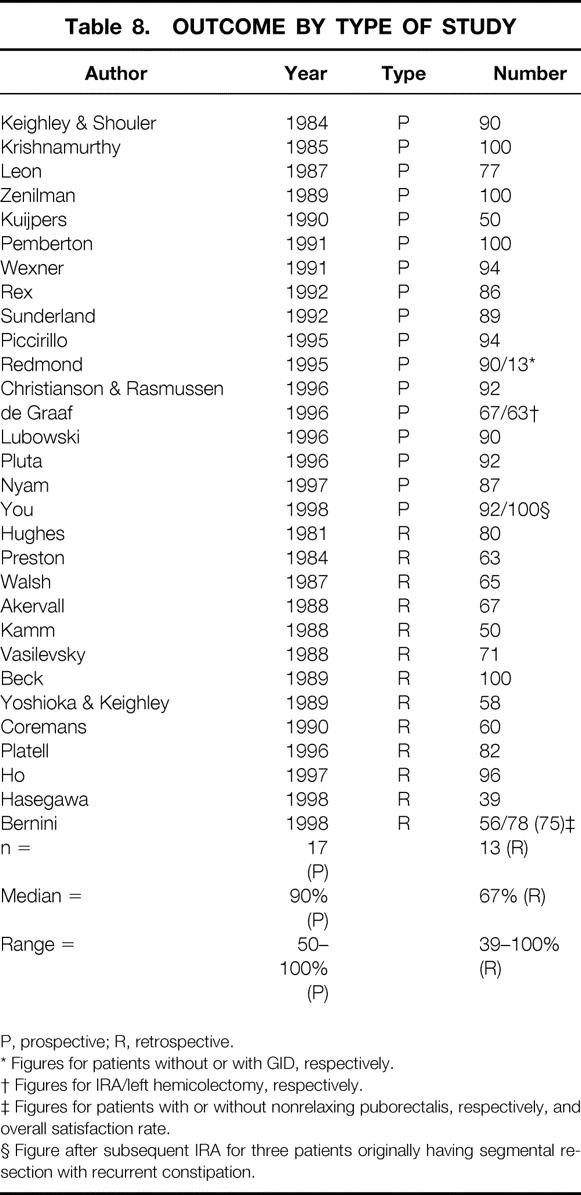
The type of study design had a marked effect on satisfaction or success rates (Table 8) . Prospective studies were superior (n = 16, median 90%, range 50% to 100%) to retrospective studies (n = 13, median 67%, range 39% to 100%).
Table 8. OUTCOME BY TYPE OF STUDY
P, prospective; R, retrospective.
* Figures for patients without or with GID, respectively.
† Figures for IRA/left hemicolectomy, respectively.
‡ Figures for patients with or without nonrelaxing puborectalis, respectively, and overall satisfaction rate.
§ Figure after subsequent IRA for three patients originally having segmental resection with recurrent constipation.
Effect of Clinical Findings on Outcomes
It was difficult to compare outcome studies because of lack of clinical detail regarding patient selection. The term constipation includes both subjective and objective aspects, and in practice patients present when their personal situation is unsatisfactory. Attempts have been made to define constipation by objective criteria, 2 but few studies prospectively 29,35 or retrospectively 32 included only patients who fulfilled such criteria. Other studies, although they did not document such inclusion criteria, nevertheless included patients who would have fulfilled these criteria based on documented preoperative findings. 11,18,21–23
It is evident that patients with slow colonic transit fall into a number of clinical subgroups based on history. In the few previous studies in which such clinical information was documented, there was often a mixture of patients from the various clinically defined groups (e.g., chronic idiopathic, or onset after pelvic surgery or neurologic disease 36). In other studies in which comment was made regarding etiology, this mixture may be inferred from the range of symptom duration (e.g., 1 to 70 years 28) and the age of patients (e.g., 17 to 78 years 25). Some studies included patients with radiologic evidence of idiopathic megabowel and even Hirschsprung’s disease without subsequent stratification of the results of surgery for these distinct conditions. 18 Preston et al 5 documented substantial detail with respect to patient history. Patients who first presented in adulthood and who had undergone a previous hysterectomy had a comparatively worse outcome than those whose disease arose de novo in childhood. This was also the case in the study by Akervall et al 13 but was not borne out by the study by Walsh et al. 12 In the latter study, the onset of symptoms in six patients coincided with pelvic surgery, and the results of surgery were documented as satisfactory in four of the five available for follow-up. In the only two studies to document a “lifelong” history of symptoms in all patients (reflected in one study by the younger age range of patients [17 to 40 years]24), the outcomes were good (89% and 100%). 17,24
The coexistence of psychiatric problems had a generally bad influence on long-term outcome. 14,30,34
Preoperative Physiologic Tests
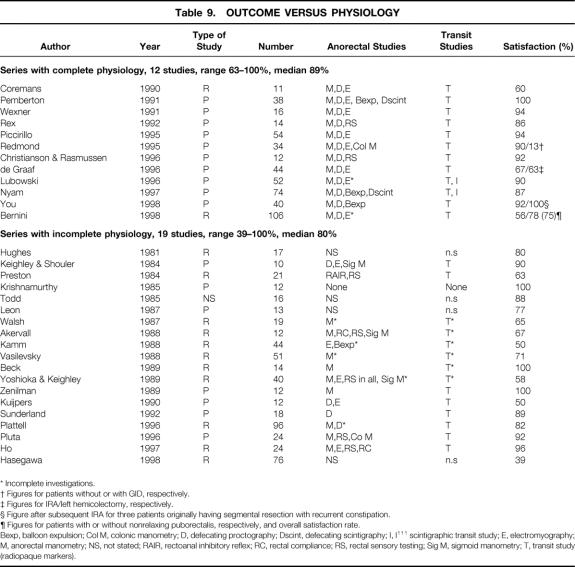
The extent of reported preoperative physiologic assessment was highly variable, both with respect to anorectal physiologic testing (i.e., anorectal manometry, evacuation proctography, rectal sensory testing) and also, surprisingly, to testing of colonic transit. This may have allowed some series to contain patients who in fact had normal transit. Table 9 demonstrates that studies can be divided into two groups according to whether basic physiology was complete (essential tests were regarded to be a minimum of anorectal manometry, defecography, and transit study performed in all patients) or incomplete. There was wider variability in outcome in the group with incomplete physiology (median satisfaction rate 80% [range 39% to 100%]) than in the group with complete physiology (median satisfaction rate 89% [range 63% to 100%]).
Table 9. OUTCOME VERSUS PHYSIOLOGY
* Incomplete investigations.
† Figures for patients without or with GID, respectively.
‡ Figures for IRA/left hemicolectomy, respectively.
§ Figure after subsequent IRA for three patients originally having segmental resection with recurrent constipation.
¶ Figures for patients with or without nonrelaxing puborectalis, respectively, and overall satisfaction rate.
Bexp, balloon expulsion; Col M, colonic manometry; D, defecating proctography; Dscint, defecating scintigraphy; I, I111 scintigraphic transit study; E, electromyography; M, anorectal manometry; NS, not stated; RAIR, rectoanal inhibitory reflex; RC, rectal compliance; RS, rectal sensory testing; Sig M, sigmoid manometry; T, transit study (radiopaque markers).
Colonic Transit
Studies in which all patients had proven slow transit generally had superior outcomes to those with incomplete investigation of transit (median outcome 90%vs. 67%, respectively). The influence of the pattern of colonic motility disturbance (i.e., generalized vs. left segmental) on outcome after surgical intervention remained unclear, but this may affect surgical decision making.
Anorectal Physiology
When anorectal physiology had been assessed, some of the studies demonstrated a deleterious effect of untreated disorders of rectal evacuation 19,34 or rectal hyposensation 13,30; others did not. 14 Some groups had treated coexistent abnormalities of the pelvic floor by preoperative retraining 33 or rectopexy at the time of colectomy 25 with excellent results. However, a recent study of 106 patients demonstrated that despite preoperative biofeedback training, patients with a nonrelaxing pelvic floor (n = 16) had significantly higher rates of recurrent defecatory difficulty and lower satisfaction rates after colectomy. 37
Upper Gastrointestinal Studies
It is generally accepted that patients with a generalized gastrointestinal disorder (GID) rather than an isolated disorder of colorectal dysmotility have poorer outcomes than other patients after colectomy. 19,26,36 A fall in the long-term success rate (as a result of recurrent constipation or intractable diarrhea) was demonstrated by a long-term prospective study by Redmond et al (successful outcome 90% no GID vs. 13% GID). 26 Likewise, Ghosh et al 36 showed a high postoperative morbidity rate from recurrent small bowel obstruction (70%) in patients with GID. This study, which also included urodynamics and autonomic function tests, showed a strong but not statistically significant trend toward increased postoperative morbidity rates in patients with these more widespread abnormalities of autonomic function.
Type of Resection
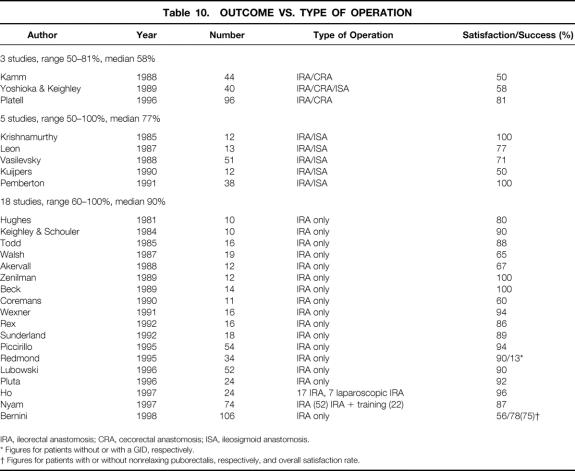
Where selection for extent of colectomy was not based on segmental transit studies, results for limited subtotal resections, either subtotal colectomy with cecorectal anastomosis 5,38 or ileosigmoid anastomosis, 21 proved generally inferior to those for subtotal colectomy with ileorectal anastomosis (Table 10). Similarly, the results of segmental resection (hemicolectomy) for colonic inertia were disappointing. In the few patients who underwent left hemicolectomy, two studies had 100% failure rates, 5,39 although the two patients in a study by Kamm et al 14 who subsequently underwent distal colonic and rectal excision remained well 2 and 3 years after surgery.
Table 10. OUTCOME VS. TYPE OF OPERATION
IRA, ileorectal anastomosis; CRA, cecorectal anastomosis; ISA, ileosigmoid anastomosis.
* Figures for patients without or with a GID, respectively.
† Figures for patients with or without nonrelaxing puborectalis, respectively, and overall satisfaction rate.
Degraaf et al 28 used the segmental transit methodology described by Arhan et al 40 to select patients for partial left colectomy or subtotal colectomy. Although the results as a whole were disappointing, the study concluded that in terms of complications and functional outcome, there was little difference between procedures and that a more limited resection was therefore a reasonable option in this selected group. More recently, You et al 35 reported the use of left, right, or subtotal colectomy based on segmental transit time measurements with excellent results. In the three patients in whom constipation recurred after segmental resection, a subtotal colectomy was undertaken successfully at a later date.
Although it is a more radical procedure, ileoanal pouch surgery as a second operation for patients in whom constipation persisted after subtotal colectomy has had some success in previous studies with small patient numbers. 18,41,42 However, in a larger, more recent study, half of the eight patients required pouch excision for persistent symptoms. 43 In the only study that used ileoanal pouch anastomosis as a primary treatment for six patients with slow transit, the results were not stratified from the other 97 patients undergoing pouch surgery for other reasons. 44
DISCUSSION
Assessing Outcome
The increasing incidence of complications and recurrent symptoms with time after surgery suggests that studies should be prospective and should have long-term follow-up. We have not found any previous study that compares outcomes with those of other surgical treatments (e.g., permanent ileostomy) or with those when no surgery is undertaken; this would obviously be desirable in a future study. Outcome measures should include postoperative complications and functional outcome measures, ideally using accepted questionnaire-based protocols that assess quality of life and even psychiatric morbidity (e.g., the Hospital Anxiety Depression Scale 45) as well as gastrointestinal function (e.g., the Wexner incontinence score 46). Objectivity could be maximized by using external assessment by a separate unit.
It is clear that several different etiologic groups can be defined on the basis of the clinical history, and that patients undergoing colectomy are therefore likely to be heterogeneous in terms of etiology. Most cases of slow transit arise de novo in early childhood and are labeled chronic and idiopathic. 47 The etiology of such idiopathic cases remains unclear and is probably itself heterogeneous. However, a proportion of patients with intractable STC present in later life. Some of these patients have no obvious trigger for their complaints; other cases follow events such as hysterectomy 48 or childbirth. 49 An additional group includes patients whose constipation appears to follow a recognized acute or chronic neural injury. This subgroup includes myenteric damage (diabetes, Chagas’, laxative abuse), spinal injury (trauma, tumor), and central nervous system disease (Parkinson’s, cerebrovascular accident, demyelination [e.g., multiple sclerosis]).
It remains unclear what independent effect the variation in etiology of STC has on outcome. However, the response to surgery might be expected to be different for diseases with diverse pathogenesis. For example, if the cause of post-hysterectomy STC is extrinsic anatomic pelvic denervation, it is unlikely to have the same response to the same surgical procedure as chronic idiopathic STC that might be considered to arise from a “congenital,” intrinsic enteric problem, as evidenced by some pathologic studies. 50,51 Likewise, the degree and extent of central denervation seen with spinal transection must be expected to have an effect on outcome. Further, it has been demonstrated that different etiologic subgroups based on clinical history have a different profile of pathophysiologic abnormality. 49,52,53 This is especially true when comparing patients with chronic idiopathic STC and patients whose problems arise after pelvic surgery. Such physiologic differences also influence outcome. Future studies should therefore attempt to stratify results based on etiologic groups. Patients with radiologic evidence of idiopathic megabowel are likely to represent a distinct entity and should not be grouped with STC patients when considering outcome.
Evaluation and Selection of Patients for Colectomy
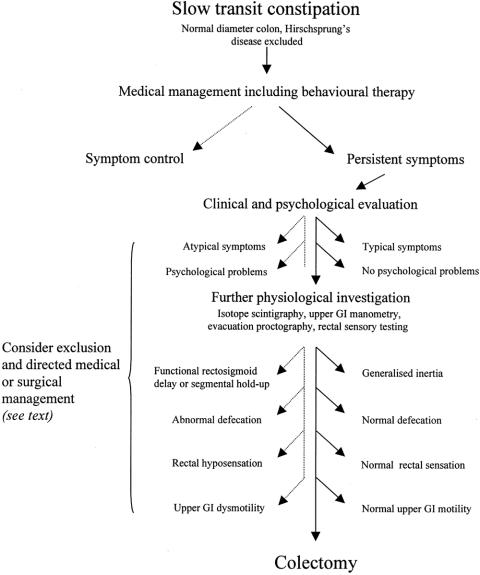
A scheme for the evaluation and selection of patients with STC as candidates for colectomy is presented in Figure 1.
Figure 1. Schematic for the evaluation and selection for colectomy of patients with slow transit constipation, highlighting clinical and physiologic criteria. Dotted line indicates pathways for exclusion; solid line indicates pathways for inclusion.
Clinical and Psychological Evaluation
Because the definition of constipation includes both subjective and objective aspects, there is often variation in the main symptoms reported. If a patient’s reported symptoms do not equate well with those primarily addressed by colectomy for slow transit (e.g., infrequency of bowel habit), the benefit of surgery may be reduced. Indeed, symptoms such as bloating and abdominal pain are not generally improved by surgery. 14 Symptom scoring systems 53 may help to discriminate patients who will benefit from surgery in the future. Kamm et al 14 noted that patients with the greatest psychological problems may have the lowest tolerance for abdominal pain and seek surgical treatment, and it is clear from recent series that the presence of severe psychological problems adversely influences outcome. 14,30,34 Referral for formal psychological investigation 54–56 is therefore recommended, especially in patients where such a disorder is suspected or is known to be present from the patient interview before surgery is considered.
Preoperative Physiologic Evaluation
This review demonstrates that outcome from surgery for STC is influenced by variation in pathophysiology. Because colectomy aims to address the problem of disordered colonic motility, such a motility disturbance should at least be inferred (on the basis that even when prolonged colonic manometry is available, the benefits are not proven 57) by a positive transit study. Although previous studies have shown that radiopaque markers are adequate for screening for STC, they are not considered sufficiently discriminatory in showing different sites of transit delay. 58,59 Preoperative isotope scintigraphy is therefore advised 60 to assess the influence of transit pattern on outcome from subtotal colectomy or selected segmental resections. 28,35 Patients with isolated rectosigmoid retention of radiopaque markers or isolated functional rectosigmoid holdup of isotope 61,62 who have proctographic evidence of significant pelvic outlet obstruction should probably be excluded from colectomy on the basis that they are unlikely to benefit from a procedure directed at correcting slow colonic transit.
Some studies have demonstrated a deleterious effect of untreated disorders of rectal evacuation, 19,34,37 and therefore consideration should be given to excluding this group from colectomy unless these problems are satisfactorily addressed by preoperative retraining 33 (or the patients are treated at the time of surgery 25). Rectal hyposensation is well documented in patients with STC 63 and may also adversely affect outcome. 13,30
The presence of upper gastrointestinal dysmotility is well documented in idiopathic STC 64 and should act as a contraindication to surgical intervention because of both the high risk of postoperative complications and long-term failure rates. 19,26,36 Upper gastrointestinal motility studies should therefore be performed in any patient in whom surgery is being considered. It is generally agreed that the sensitivity and discriminatory value of manometric studies (antroduodenal and esophageal) is superior to that of gastric emptying studies and small bowel transit studies (see review by Camilleri et al 65). Although the presence of urodynamic, 66 autonomic, and other neurologic abnormalities 67 has been documented in subgroups of patients with chronic idiopathic STC, their role in guiding clinical management is not yet established. 36 Attention to refining the selection of patients who might benefit from colectomy for slow transit, on the basis of physiologic investigation, has been shown to improve outcome. 24,33
CONCLUSION
This review documents the high variability of outcomes after colectomy for patients with STC. Surgery for this condition should probably be considered only in a highly selected group of patients who fulfill certain clinical and physiologic criteria. Future studies should be directed at further establishing these criteria using a design that has sufficient stratification of patients to allow reasonable interpretation of outcome results. On such a basis, surgery may be directed in a more evidence-based fashion.
Acknowledgment
The authors thank Professor N.S. Williams for his support and advice in this project.
Footnotes
Supported by the Lillian May Coleman Fellowship in Surgery, The Royal College of Surgeons of England. Correspondence: Peter J. Lunniss, MS, FRCS, Academic Department of Surgery, Royal London Hospital, Whitechapel, London E1 1BB, United Kingdom.
Reprints: Charles H. Knowles, BChir, FRCS, Academic Department of Surgery, Royal London Hospital, Whitechapel, London E1 1BB, United Kingdom.
Accepted for publication May 12, 1999.
References
- 1.Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum 1989; 32:1–8. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead WE, Chaussade S, Corazziari E, Kumar D. Report of an international workshop on management of constipation. Gastroenterol Intl 1991; 4:99–113. [Google Scholar]
- 3.Arbuthnot Lane W. The results of operative treatment of chronic constipation. Br Med J 1908; I:1:26–30. [DOI] [PMC free article] [PubMed]
- 4.Arbuthnot Lane W. Chronic intestinal stasis. Br Med J 1909; I:1:408–411.
- 5.Preston DM, Hawley PR, Lennard-Jones JE, Todd IP. Results of colectomy for severe idiopathic constipation in women (Arbuthnot Lane’s Disease). Br J Surg 1984; 71:547–552. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Fitzgerald SD, Pemberton JH. Evaluation and treatment of constipation. Rev Gastroenterol Mex 1994; 59:133–138. [PubMed] [Google Scholar]
- 7.Hughes ES, McDermott FT, Johnson WR, Poglase AC. Surgery for constipation. Aust NZ J Surg 1981; 51:144–148. [DOI] [PubMed] [Google Scholar]
- 8.Keighley MRB, Shouler P. Outlet syndrome: is there a surgical option? J Royal Soc Med 1984; 77:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todd IP. Constipation: results of surgical treatment. Br J Surg 1985; 72:S1:2–3. [DOI] [PubMed]
- 10.Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE II. Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology 1985; 88:26–34. [DOI] [PubMed] [Google Scholar]
- 11.Leon SH, Krishnamurthy S, Schuffler MD. Subtotal colectomy for severe idiopathic constipation. Dig Dis Sci 1987; 32:1249–1254. [DOI] [PubMed] [Google Scholar]
- 12.Walsh PV, Peebles-Brown DA, Watkinson G. Colectomy for slow transit constipation. Ann R Coll Surg Engl 1987; 69:71–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Akervall S, Fasth S, Nordgren S, Oresland T, Hulten L. The functional results after colectomy and ileorectal anastomosis for severe constipation (Arbuthnot Lane’s disease) as related to rectal sensory function. Int J Colorectal Dis 1988; 3:96–101. [DOI] [PubMed] [Google Scholar]
- 14.Kamm MA, Hawley PR, Lennard-Jones JE. Outcome of colectomy for severe idiopathic constipation. Gut 1988; 29:969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasilevsky CA, Nemer FD, Balcos EG, et al. Is subtotal colectomy a viable option in the management of chronic constipation? Dis Colon Rectum 1988; 31:679–681. [DOI] [PubMed] [Google Scholar]
- 16.Zenilman ME, Dunnegan DL, Soper NJ, Becker JM. Successful surgical treatment of idiopathic colonic dysmotility. The role of preoperative evaluation of coloanal motor function. Arch Surg 1989; 124:947–951. [DOI] [PubMed] [Google Scholar]
- 17.Beck DE, Fazio VW, Jagelman DG, Lavery IC. Surgical management of colonic inertia. South Med J 1989; 82:305–309. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka K, Keighley MRB. Clinical results of colectomy for severe constipation. Br J Surg 1989; 76:600–604. [DOI] [PubMed] [Google Scholar]
- 19.Kuijpers HC. Application of the colorectal laboratory in diagnosis and treatment of functional constipation. Dis Colon Rectum 1990; 33:35–39. [DOI] [PubMed] [Google Scholar]
- 20.Coremans GE. Surgical aspects of severe chronic non-Hirschsprung constipation. Hepatogastroenterology 1990; 37:588–595. [PubMed] [Google Scholar]
- 21.Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg 1991; 214:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wexner SD, Daniel N, Jagelman DG. Colectomy for constipation: physiological investigation is the key to success. Dis Colon Rectum 1991; 34:851–856. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Lappas JC, Goulet RC, Madura JA. Selection of constipated patients as subtotal colectomy candidates. J Clin Gastroenterol 1992; 15:212–217. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland GT, Poon FW, Lauder J, Finlay IG. Videoproctography in selecting patients with constipation for colectomy. Dis Colon Rectum 1992; 35:235–237. [DOI] [PubMed] [Google Scholar]
- 25.Piccirillo MF, Reissman P, Wexner SD. Colectomy as treatment for constipation in selected patients. Br J Surg 1995; 82:898–901. [DOI] [PubMed] [Google Scholar]
- 26.Redmond JM, Smith GW, Barofsky I, et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol 1995; 90:748–753. [PubMed] [Google Scholar]
- 27.Christiansen J, Rasmussen OO. Colectomy for severe slow-transit constipation in strictly selected patients. Scand J Gastroenterol 1996; 31:770–773. [DOI] [PubMed] [Google Scholar]
- 28.de Graaf EJ, Gilberts EC, Schouten WR. Role of segmental colonic transit time studies to select patients with slow transit constipation for partial left-sided or subtotal colectomy. Br J Surg 1996; 83:648–651. [DOI] [PubMed] [Google Scholar]
- 29.Lubowski DZ, Chen FC, Kennedy ML, King DW. Results of Colectomy for severe slow transit constipation. Dis Colon Rectum 1996; 39:23–29. [DOI] [PubMed] [Google Scholar]
- 30.Pluta H, Bowes KL, Jewell LD. Long-term results of total abdominal colectomy for chronic idiopathic constipation. Value of preoperative assessment. Dis Colon Rectum 1996; 39:160–166. [DOI] [PubMed] [Google Scholar]
- 31.Platell C, Scache D, Mumme G, Stitz R. A long-term follow-up of patients undergoing colectomy for chronic idiopathic constipation. Aust NZ J Surg 1996; 66:525–529. [DOI] [PubMed] [Google Scholar]
- 32.Ho YH, Tam M, Eu KW, Choen FS. Laparoscopic-assisted compared with open total colectomy in treating slow transit constipation. Aust NZ J Surg 1997; 67:562–565. [DOI] [PubMed] [Google Scholar]
- 33.Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997; 40:273–279. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa H, Radley S, Keighley MRB. Long-term results of colorectal resection for chronic constipation [abstract]. Association of Coloproctology of Great Britain and Ireland conference proceedings 1998. Oxford: Blackwell Science; 1998:3. [DOI] [PubMed]
- 35.You YT, Wang JY, Changchien CR, et al. Segmental colectomy in the management of colonic inertia. Am Surg 1998; 64:775–777. [PubMed] [Google Scholar]
- 36.Ghosh S, Papachrysostomou M, Batool M, Eastwood MA. Long-term results of sub-total colectomy and evidence of noncolonic involvement in patients with idiopathic slow-transit constipation. Scand J Gastroenterol 1996; 31:1083–1091. [DOI] [PubMed] [Google Scholar]
- 37.Bernini A, Madoff RD, Lowry AC, et al. Should patients with combined colonic inertia and nonrelaxing pelvic floor undergo subtotal colectomy? Dis Colon Rectum 1998; 41:1363–1366. [DOI] [PubMed] [Google Scholar]
- 38.Fasth S, Hedlund H, Svaninger G, et al. Functional results after subtotal colectomy and cecorectal anastomosis. Acta Chir Scand 1983; 149:623–627. [PubMed] [Google Scholar]
- 39.Gray EJ, Marteinsson TH. Dolichocolon: indications for operations. Am Surg 1971; 37:509–511. [PubMed] [Google Scholar]
- 40.Arhan P, Devroede G, Jehannin B, et al. Segmental colonic transit time. Dis Colon Rectum 1981; 24:625–629. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls RJ, Kamm MA. Proctocolectomy with restorative ileoanal reservoir for severe idiopathic constipation: report of 2 cases. Dis Colon Rectum 1988; 31:968–969. [DOI] [PubMed] [Google Scholar]
- 42.Hosie KB, Kmiot WA, Keighley MR. Constipation: another indication for restorative proctocolectomy. Br J Surg 1990; 77:801–802. [DOI] [PubMed] [Google Scholar]
- 43.Keighley MRB, Grobler S, Bain I. An audit of restorative proctocolectomy. Gut 1993; 34:680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCourtney JS, Finlay IG. Totally stapled restorative proctocolectomy. Br J Surg 1997; 84:808–812. [PubMed] [Google Scholar]
- 45.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983; 67:361–370. [DOI] [PubMed] [Google Scholar]
- 46.Johansen OB, Wexner SD, Daniel N, et al. Perineal rectosigmoidectomy in the elderly. Dis Colon Rectum 1993; 36:767–772. [DOI] [PubMed] [Google Scholar]
- 47.Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: idiopathic slow transit constipation. Gut 1986; 27:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vierhout ME, Schreuder HW, Veen HF. Severe slow-transit constipation following radical hysterectomy. Gynecol Oncol 1993; 51:401–403. [DOI] [PubMed] [Google Scholar]
- 49.McDonald A, Baxter JN, Bessent RG, et al. Gastric emptying in patients with constipation following childbirth and due to idiopathic slow transit. Br J Surg 1997; 84:1141–1143. [PubMed] [Google Scholar]
- 50.Lincoln J, Crowe R, Kamm MA, et al. Serotonin and 5-hydroxyindoleacetic acid are increased in the sigmoid colon in severe idiopathic constipation. Gastroenterology 1990; 98:1219–1225. [DOI] [PubMed] [Google Scholar]
- 51.Schouten WR, ten Kate FJ, de Graaf EJ, et al. Visceral neuropathy in slow transit constipation: an immunohistochemical investigation with monoclonal antibodies against neurofilament. Dis Colon Rectum 1993; 36:1112–1117. [DOI] [PubMed] [Google Scholar]
- 52.Roe AM, Bartolo DCC, Mortenson NJ McC. Slow transit constipation. Comparison between patients with or without previous hysterectomy. Dig Dis Sci 1988; 3:1159–1163. [DOI] [PubMed] [Google Scholar]
- 53.Smith AN, Varma JS, Binnie NR. Disordered colorectal motility in intractable constipation following hysterectomy. Br J Surg 1990; 77:1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agachan F, Chen T, Pfeifer J, et al. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 1996; 39:681–685. [DOI] [PubMed] [Google Scholar]
- 55.Devroede G, Girard G, Bouchoucha M, et al. Idiopathic constipation by colonic dysfunction. Relationship with personality and anxiety. Dig Dis Sci 1989; 34:1428–1433. [DOI] [PubMed] [Google Scholar]
- 56.Heyman S, Wexner SD, Gulledge AD. MMPI assessment of patients with functional bowel disorders. Dis Colon Rectum 1993; 36:593–596. [DOI] [PubMed] [Google Scholar]
- 57.Bassotti G, Gaburri M, Imbimbo BP, et al. Colonic mass movements in idiopathic chronic constipation. Gut 1988; 29:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Sjip JRM, Kamm MA, Nightingale JMD, et al. Radioisotope determination of regional colonic transit in severe constipation: comparison with radioopaque markers. Gut 1993; 34:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamm MA. Surgery for idiopathic megarectum and megacolon. In: Kamm MA, Lennard-Jones JE, eds. Constipation. Petersfield, UK: Wrightson Biomedical Publishing Ltd.; 1994: 233–238.
- 60.Roberts JP, Newell MS, Deeks JJ, et al. Oral [111In] DTPA scintigraphic assessment of colonic transit in constipated subjects. Dig Dis Sci 1993; 8:1032–1039. [DOI] [PubMed] [Google Scholar]
- 61.Mclean R, Smart R, Barbagallo S, et al. Colonic transit scintigraphy using oral indium-111-labeled DTPA. Can scan predict the final diagnosis? Dig Dis Sci 1995; 40:2660–2668. [DOI] [PubMed] [Google Scholar]
- 62.Krevsky B, Maurer AH, Fisher RS. Patterns of colonic transit in chronic idiopathic constipation. Am J Gastroenterol 1989; 84:127–132. [PubMed] [Google Scholar]
- 63.Kamm MA, Lennard-Jones JE. Rectal mucosal electrosensory testing: evidence for a rectal sensory neuropathy in idiopathic constipation. Dis Colon Rectum 1990; 33:419–423. [DOI] [PubMed] [Google Scholar]
- 64.Bassotti G, Stanghellini V, Chiarioni G, et al. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci 1996; 41:1999–2005. [DOI] [PubMed] [Google Scholar]
- 65.Camilleri M, Hasler WL, Parkman HP, et al. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology 1998; 115:747–762. [DOI] [PubMed] [Google Scholar]
- 66.Abdel-Rahman M, Toppercer A, Duguay C, et al. Urorectodynamics in patients with colonic inertia. Urology 1981; 18:428–432. [DOI] [PubMed] [Google Scholar]
- 67.Knowles CH, Scott SM, Wellmer AC, et al. Sensory and autonomic neuropathy in patients with idiopathic slow transit constipation. Br J Surg 1999; 86:54–60. [DOI] [PubMed] [Google Scholar]