Abstract
Objective
To identify the sources of electromagnetic interference (EMI) that may alter the performance of implanted cardiac devices and develop strategies to minimize their effects on patient hemodynamic status.
Summary Background Data
Since the development of the sensing demand pacemaker, EMI in the clinical setting has concerned physicians treating patients with such devices. Implanted cardiovertor defibrillators (ICDs) and ventricular assist devices (VADs) can also be affected by EMI.
Methods
All known sources of interference to pacemakers, ICDs, and VADs were evaluated and preventative strategies were devised.
Results
All devices should be thoroughly evaluated before and after surgery to make sure that its function has not been permanently damaged or changed. If electrocautery is to be used, pacemakers should be placed in a triggered or asynchronous mode; ICDs should have arrhythmia detection suspended before surgery. If defibrillation is to be used, the current flow between the paddles should be kept as far away from and perpendicular to the lead system as possible. Both pacemakers and ICDs should be properly shielded if magnetic resonance imaging, positron emission tomography, or radiation therapy is to be used. The effect of EMI on VADs depends on the model. Magnetic resonance imaging adversely affects all VADs except the Abiomed VAD, and therefore its use should be avoided in this population of patients.
Conclusions
The patient with an implanted cardiac device can safely undergo surgery as long as certain precautions are taken.
The development of the sensing demand pacemaker in the 1960s brought with it the problem of interference. These new pacemakers were able to sense and react to the changing needs of the heart but could also sense and react to electromagnetic signals that were not cardiac in origin. In the 1970s, as the population of patients with demand pacemakers increased, the interaction between pacemakers and electromagnetic interference (EMI) became a topic of importance within the medical community—indeed, during the 1980s several review articles were written on the subject. The medical community became increasingly aware of the dangers of EMI and the possible responses of pacemakers to it. Physicians were able to counsel pacemaker patients about the dangers of EMI outside the clinical setting and became better prepared to recognize and correct the effects of EMI within the clinical setting. Today’s pacemakers have been engineered to be more resistant to EMI, and few sources of EMI still cause alteration in function. However, in the surgical setting several problems still occur. The present situation is complicated by increasing numbers of surgical patients with implanted cardiovertor defibrillators (ICDs) and ventricular assist devices (VADs), both of which can be affected by EMI. It is the purpose of this article to provide physicians with the practical considerations necessary to perform surgery on patients with implanted cardiac support.
SOURCES OF EMI
Electromagnetic interference occurs in two forms: conducted and radiated. Conducted EMI occurs when an electromagnetic source comes in direct contact with the body. This type of EMI can be generated by electrocautery and defibrillation. Radiated EMI occurs when the body is placed within an electromagnetic field; no contact with the source is necessary. This type of EMI can be generated by magnetic resonance imaging (MRI), positron emission tomography (PET), and radiation therapy. The effects of radiated EMI are usually only temporary, resulting in alteration of device function for the duration the patient spends in the electromagnetic field. However, MRI has been reported to reprogram a pacemaker permanently, causing alteration in function until the device is returned to the appropriate program mode. 1–3 In addition, the ionizing radiation used in radiation therapy and PET has the potential to damage the internal circuitry of the pulse generator, although this is rarely reported. For these reasons, any patient who is to be exposed to these forms of EMI should have his or her device properly shielded during the procedure and thoroughly evaluated afterward.
In the surgical setting, conducted EMI produced by electrocautery and defibrillation can cause several different alterations in device function. Although electrocautery may be avoided in some procedures, other procedures are almost impossible to perform without it. Likewise, in certain situations defibrillation is a necessity. For these reasons it is necessary for surgeons operating on patients who rely on implanted cardiac support to be able to recognize and manage the problems produced by these devices.
Electrocautery involves passing a high-voltage, high-frequency (10,000 Hz) current through tissue to cut or coagulate. This current can be bipolar or monopolar, depending on the type of system being used. Monopolar current begins at the tip of the instrument, travels through the body, and returns to the generator through a dispersing ground plate. Bipolar current does not require a ground plate because both electrodes are built into the tip of the instrument. This means that the current flows only through the area of tissue in direct contact with the instrument. However, bipolar electrocautery units are much less powerful. This can be an advantage if the surgery is delicate but renders them impractical for many procedures. When monopolar electrocautery is used, surgeons may use the electrocautery to pass current through other surgical instruments. This normally poses no problems. However, if the electrocautery is activated before it is in contact with the surgical instrument, the current can arc through the air toward the instrument and demodulate. When the current is demodulated, its frequency fluctuates. Normally the current remains at a frequency of 10,000 Hz, but if it is allowed to demodulate it can dip well into the frequency range that pacemakers and ICDs are designed to sense. These devices might interpret the current as cardiac in origin and respond inappropriately. Normal electrocautery current at 10,000 Hz can also cause pacemaker and ICD malfunction, but it is just much less likely than if the current is demodulated.
Defibrillation involves the delivery of high-voltage current in the immediate vicinity of the heart. Although all pacemakers and ICDs use some form of defibrillation protection (usually zener diodes) to shunt excessive current away from the delicate internal components of the pulse generator, the protection is by no means complete. 4
CONSEQUENCES OF EMI
Variables that determine the effects of a particular source of EMI include the intensity of the field or source, the frequency and waveform of the signal, the distance between the source and the leads of the pacemaker or ICD, and the orientation of the leads with respect to the field or source. 5 Variables that determine the ability of a pacemaker or ICD to pick up EMI include the number of leads and the distance between the anode (positive tip of the lead) and the cathode (negative tip of the lead). This distance is quite small in devices employing bipolar leads because both the anode and the cathode are contained within the tip of the lead. The distance is much greater in devices employing unipolar leads because the anode is the metal case surrounding the pulse generator and the cathode is at the tip of the lead. Therefore, devices that employ bipolar leads are much more resistant to EMI than those that employ unipolar leads. The number of leads is also important. Dual-chamber systems pick up more EMI than single-chamber systems simply because there are more leads acting as antennae within the heart. In addition, because signals can be picked up only if they are traveling parallel to a lead, and the leads in a dual-chamber system are frequently oriented perpendicular to each other, it follows that dual-chamber systems provide a larger area of sensitivity. Unfortunately, few patients have bipolar, single-chamber pacemakers. In fact, most of today’s pacemakers are dual-chamber, unipolar units, many of which employ high sensitivity settings in the atrial component to sense low-amplitude P waves.
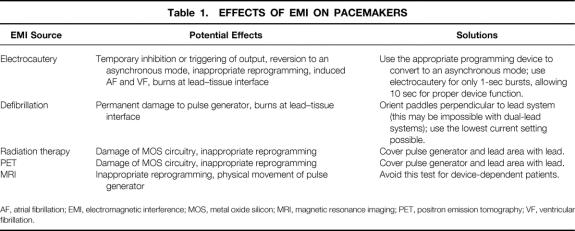
Electromagnetic interference can affect pacing in the following ways (Table 1):
Table 1. EFFECTS OF EMI ON PACEMAKERS
AF, atrial fibrillation; EMI, electromagnetic interference; MOS, metal oxide silicon; MRI, magnetic resonance imaging; PET, positron emission tomography; VF, ventricular fibrillation.
1. The signal might be interpreted as cardiac in origin and temporarily inhibit or trigger output, depending on the pacing mode.
2. The signal might be interpreted as noise and temporarily cause reversion to an asynchronous pacing mode at a rate set by the manufacturer. This can lead to dangerous tachyarrhythmias as a result of an R-on-T phenomenon.
3. The signal might be interpreted by the device as a programming signal, leading to inappropriate reprogramming.
4. A continuous train of electrical impulses, such as that produced by electrocautery, conducted down the lead can induce ventricular or atrial fibrillation.
5. High levels of current can pass through the device, down the lead, and into the endocardium, causing thermal burns at the lead–tissue interface. This can raise the pacing threshold, leading to an inability of the device to stimulate the myocardium.
6. High levels of current can pass from the leads to the pulse generator and cause irreversible loss of battery output.
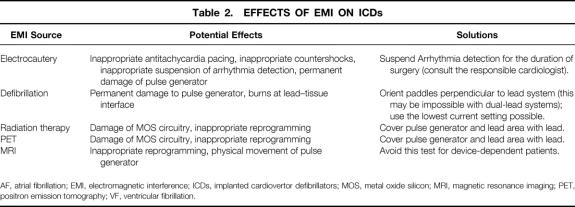
Because many of today’s ICDs also incorporate pacing, all of these effects can be observed in ICDs.
Electromagnetic interference can also alter the functions of cardioversion and defibrillation in the ICD (Table 2). ICDs sense rate (R-R interval), monitor the amount of activity that the ECG spends near the isoelectric line, and may even monitor hemodynamic parameters. Most devices respond to ventricular tachycardia (VT) with either antitachycardia pacing or low-energy countershocks, and to ventricular fibrillation (VF) with high-energy countershocks. 6 However, specific criteria for arrhythmia detection, cardioversion, and defibrillation vary between models and manufacturers. Just as EMI can mimic normal cardiac activity, it can also mimic abnormal cardiac activity. If a signal is interpreted by the ICD as VT or VF, it can respond inappropriately by initiating antitachycardia pacing or delivering a countershock. If the signal is interpreted as noise, the device may respond by suspending arrhythmia detection, leaving the patient unprotected.
Table 2. EFFECTS OF EMI ON ICDs
AF, atrial fibrillation; EMI, electromagnetic interference; ICDs, implanted cardiovertor defibrillators; MOS, metal oxide silicon; MRI, magnetic resonance imaging; PET, positron emission tomography; VF, ventricular fibrillation.
Many strategies have been employed to protect today’s pacemakers and ICDs from EMI. The most important of these advances are electrical filtration systems designed to increase the ability to discriminate between EMI and signals that are cardiac in origin. A band pass filter provides the initial line of defense by prohibiting the entry of signals that are above or below a certain frequency threshold. Once the signal enters the internal circuitry, its frequency and amplitude are evaluated. If the signal is similar in frequency and amplitude to a cardiac signal, then it will be interpreted by the device as cardiac in origin and the appropriate response will be delivered. If the signal is above or below the device’s frequency threshold or continuous in nature, the device will interpret the signal as noise. Most pacemakers respond to noise by reverting to an asynchronous mode; most ICDs respond to noise by suspending arrhythmia detection.
Pacemakers and ICDs also employ zener diodes designed to shunt high levels of current flow away from the delicate internal circuitry. However, repeated exposure to high levels of current, such as occurs during repeated attempts at defibrillation, can overwhelm these protection circuits, resulting in permanent damage to the pulse generator. Also, the current shunted away from the pacemaker or ICD can lead to myocardial burns at the lead–tissue interface.
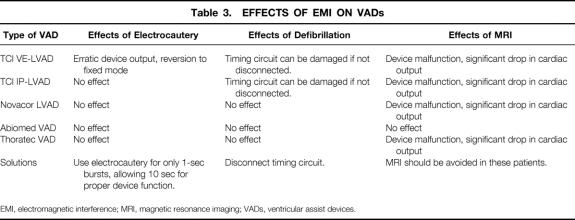
Ventricular assist devices can also be affected by EMI (Table 3). Five devices are currently in widespread use. The Novacor LVAD, TCI VE-LVAD, and TCI IP-LVAD are implantable; the Abiomed VAD and Thoratec VAD are extracorporeal. VADs are implantable pumps used for circulatory support in patients with congestive heart failure. Blood fills the device through a cannulation site in the ventricle or atrium. Within the device, a diaphragm is actuated pneumatically (TCI IP-LVAD, Abiomed VAD, Thoratec VAD), electrically (TCI VE-LVAD), or magnetically (Novacor LVAD), pumping blood into the aorta or pulmonary artery. The rate of operation of these units can be set at a predetermined rate (fixed mode) or a mode in which the rate changes in response to venous return (automatic mode). In automatic mode, the device rate accelerates when filling is adequate and decelerates when filling is inadequate. The Abiomed VAD monitors filling by sensing the pressure in the pneumatic drive line. The other VADs monitor filling using a circuit that relays diaphragm position information to the external controller. In the TCI VE-LVAD, this information comes from a circuit that monitors the angular position of the motor, which correlates with the linear position of the diaphragm. The TCI IP-LVAD and Thoratec VAD use a slightly different circuit. The diaphragm of each device is magnetic, and its position is monitored by a sensor that measures the strength of the magnetic field generated by the diaphragm. The distance between the sensor and the diaphragm correlates with the strength of the magnetic field. The Novacor LVAD monitors diaphragm position in a similar way. 7 In the TCI VE-LVAD, the circuits for both timing and motor current are housed within a single drive line; this means that the timing circuit cannot be disconnected without interrupting the current that drives the pump. The timing circuit of the TCI IP-LVAD can be disconnected separately, and the device will function normally in a fixed rate mode. 8,9
Table 3. EFFECTS OF EMI ON VADs
EMI, electromagnetic interference; MRI, magnetic resonance imaging; VADs, ventricular assist devices.
Because all of the electronics for timing and driving the Novacor LVAD are housed within the external controller, which is shielded from EMI, it is not affected by defibrillation or electrocautery. 7 The same is true for the Thoratec and Abiomed VADs, which are extracorporeal and therefore electromagnetically isolated from the patient. In the case of the TCI devices, some of the electronics used to monitor the position of the diaphragm are located within the device, which is not completely shielded from EMI. Defibrillation can damage these electronics. TCI recommends that the timing circuit of the TCI IP-LVAD be disconnected from the external controller before any defibrillation attempt. Because its timing circuit cannot be disconnected separately, TCI recommends that the TCI VE-LVAD be turned off and disconnected from the external controller before defibrillation. During this period, device function can be maintained using a pneumatic external controller or a hand pump. 10 Although damage to these timing circuits secondary to defibrillation has occurred, it is rare. However, loss of the automatic mode capabilities of these devices can have a profound impact on a patient’s ability to rehabilitate before transplantation. Therefore, every effort should be made to protect these timing circuits.
Most patients with LVADs are completely supported by the device. VT and VF do not affect cardiac output to the same extent as they would in a patient without support. The cardiac output may decrease slightly as a result of the loss of right ventricular function, but Fontan physiology will usually allow the LVAD to fill and maintain systemic perfusion. This is not to say that these types of arrhythmias are not malignant in this population of patients. However, once the arrhythmia is recognized, the support team can take the few minutes it takes to protect the device before defibrillation is attempted.
The function of the TCI devices can also be affected by electrocautery. Theoretically, the timing circuit of the TCI IP-LVAD can be disrupted by electrocautery, but this is rarely reported. However, if the timing circuit appears to be affected, the device can be put in fixed mode and the timing circuit disconnected. The TCI IP-LVAD, which uses air to drive the pump, is otherwise unaffected. The TCI VE-LVAD uses electricity to drive the pump, and it exhibits an erratic pattern of current output during the use of electrocautery. This results in a significant drop in device output. Electrocautery also causes reversion of the device to fixed mode; automatic mode must then be reentered manually. To avoid constantly changing modes, it is recommended that the TCI VE-LVAD be placed in fixed mode for the duration of the surgery. 9,10
Magnetic resonance imaging adversely affects all VADs except the Abiomed VAD. Its use as a diagnostic tool should be avoided in this population of patients. The magnetic field created by MRI alters the function of the magnets involved in the motor mechanisms of the TCI VE-LVAD and Novacor LVAD. This leads to serious malfunction and a drop in device output. Device malfunction can be both temporary and permanent in both of these models. In the case of the TCI IP-LVAD and Thoratec VAD, the magnetic field generated by MRI disrupts the movement of the magnetic diaphragm, leading to a decrease in the efficiency of the device and a drop in device output. These effects are only temporary, lasting for the duration the test. None of these VADs are adversely effected during computed tomography, PET, or multigated acquisition. 7,10
PREOPERATIVE EVALUATION
Before any planned surgery, the patient with a pacemaker or ICD should be thoroughly evaluated. The patient’s cardiologist or cardiothoracic surgeon should be notified and consulted. In addition, the pacemaker or ICD programming device should be obtained so that device function can be evaluated and appropriately modified during the perioperative period. Someone familiar with the operation of the device should perform any programming and should be available if the device malfunctions. The phone number of the manufacturer of the device should be readily available so that technical support can be obtained during times of uncertainty (Table 4). It is important that the patient be checked for any progression of symptoms of the underlying cardiovascular disease, as well as for any sort of electrical instability. Factors that may predispose to electrical instability, such as electrolyte imbalance, active myocardial ischemia, or hypoxemia, should be actively sought. If identified, they should be stabilized before surgery. If these factors are allowed to persist, they may facilitate the induction of VF on exposure to electrocautery. 11
Table 4. TELEPHONE NUMBERS OF TECHNICAL SERVICE DEPARTMENTS

It is also a good idea to obtain a chest x-ray to ascertain the position and integrity of the lead(s). The chest x-ray can also be helpful in identifying the make and model of the pacemaker or ICD. Each device has a serial number and a unique silhouette that can be used for device identification. A complete workup of the functional status of the pacemaker or ICD and the patient’s dependence on it should be performed. The type of system, the time since implantation, and the settings at the time of the most recent evaluation should be determined. Because all manufacturers provide each patient with an identification card that includes this information, these data are usually simple to obtain. Many patients also have monitoring services that periodically check the device and record these data. Those who do not use a monitoring service are followed by their cardiologist or cardiothoracic surgeon. Precise measurements of the demand and magnet rates, duration and amplitude of the stimulus, and capture and sensing thresholds are also useful, especially for comparing preoperative and postoperative values. In patients with ICDs, it is important to obtain information on the recent frequency of countershocks, because this will be a key factor in determining how safe it is to turn the device off during surgery.
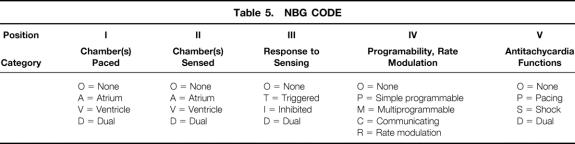
Because modern pacemakers employ a variety of different pacing modes, it is important to be familiar with the NBG (N orth American Society of Pacing and Electrophysiology/ B ritish Pacing and Electrophysiology Group G eneric Pacemaker Code) Code (Table 5). The first three letters of this code describe the basic antibradycardia functions, and the last two letters describe the programmability and antitachycardia functions:
Table 5. NBG CODE
• Letter I, the chamber(s) being paced
• Letter II, the chamber(s) being sensed
• Letter III, the response to sensing
• Letter IV, programmability and rate modulation
• Letter V, antitachycardia functions. 12
The information contained within this code makes it much easier to predict the pacemaker’s response to EMI. For example, inhibited pulse generators respond to EMI by inhibiting output, whereas triggered pulse generators respond to EMI by triggering output. This is, of course, if the signal manages to fool the pacemaker’s protection circuitry and is interpreted as cardiac in origin. If it is interpreted as noise, it will cause reversion to an asynchronous mode.
There is also a code used to identify ICDs (Table 6). The NBD Code (N orth American Society of Pacing and Electrophysiology/ B ritish Pacing and Electrophysiology Group D efibrillator Code) has four letters:
Table 6. NBD CODE
• Letter I, shock chamber(s)
• Letter II, antitachycardia pacing chamber(s)
• Letter III, tachycardia detection
• Letter IV, antibradycardia pacing chamber(s). 13
Because letter IV describes pacemaker activity (many ICDs also employ antibradycardia pacing, which may be needed for a short time after the delivery of a countershock), it is sometimes replaced by a hyphen and the NBG code.
It is also necessary to establish whether the patient is device-dependent, because this will determine whether any clinically significant effect might occur if the device’s function is altered during surgery. For example, if a patient whose pacemaker is in any sort of inhibited mode has an innate rhythm that will sustain an acceptable cardiac output, then EMI that inhibits the pulse generator will probably not be noticed. However, if the patient is pacemaker-dependent, EMI that inhibits the pulse generator will lead to asystole and the patient will be unprotected. To determine whether a patient is pacemaker-dependent, it is necessary to conduct a careful review of the preoperative ECG. If pacing spikes are not seen or if they are intermittent, then it is likely that the patient’s own rhythm will maintain an acceptable heart rate if the pacemaker malfunctions during surgery. If pacing spikes are seen before almost every beat, then the patient is likely to be pacemaker-dependent. However, if this is the case, it is important to determine whether the patient has an innate rhythm. This can be done by temporarily inhibiting the device with the appropriate programmer. If there is any doubt as to whether the pulse generator is capable of stimulating the heart, it may be placed into an asynchronous mode with the programmer or by placing a magnet over the generator. 14
In the past, magnets have been used during surgery to convert devices to an asynchronous mode, counteracting the effects of EMI by eliminating the sensing component of the device. However, magnet application readies many pacemakers for reprogramming. Intraoperative EMI such as defibrillation and electrocautery can mimic programming signals and cause the device to be erroneously reprogrammed. In addition, certain pacemakers revert to an asynchronous mode only for a certain number of beats and then return to the previous pacing mode. In certain patients, especially those with ICDs, asynchronous pacing can lead to VT and even VF as a result of an R-on-T phenomenon. Therefore, asynchronous pacing should be avoided in patients prone to VT and VF. Today, surgeons still go into the operating room armed only with a magnet, confident in its ability to manage the device complications produced by EMI. This can be a dangerous practice: magnet application cannot adequately address all the possible problems one might encounter.
The patient with a VAD should also be thoroughly evaluated. The present situation is such that most surgical procedures involving patients with VADs take place at institutions that can provide technical and surgical support for these devices. This allows for a thorough evaluation of device function, as well as technical support during surgery. If it is not possible to have a device expert in the operating room, someone should be trained to operate the device and given a way to contact technical support.
SURGICAL CONSIDERATIONS
Armed with this information, it is possible to set some guidelines for surgeons operating on patients with implanted cardiac support who may be subject to electrocautery and defibrillation. If the patient has a pacemaker or ICD, the anesthesiologist should be aware that fasciculations associated with the use of depolarizing muscle relaxants such as succinylcholine can create myopotentials. These high-frequency electrical signals can be interpreted as cardiac activity and cause device malfunction. Also, due to the possibility that the pulse generator may be damaged during the surgery, inotropic and chronotropic support should be available in the operating suite in case of emergency. When possible, the pacemaker should be placed in an asynchronous or triggered mode. This will avoid inhibition due to EMI that could lead to asystole in the pacemaker-dependent patient. As discussed earlier, asynchronous pacing should be avoided if possible in patients prone to VT and VF. Placement of a magnet over the pulse generator to convert it to an asynchronous mode is not optimal in pacemakers that require magnet application to access the programming circuit. This is especially true during surgery. Conversion to any mode is best made with the appropriate programming device. Some pacemakers are rate-responsive, meaning they can sense various stimuli and thereby increase the pacing rate. The purpose is to provide a timely increase in heart rate when there is a need for increased cardiac output. Stimuli such as muscle activity, movement, minute ventilation, temperature, and evoked QT interval are all used to assess the need for an increase in rate. 6 These pacemakers should have their rate-responsive modes deactivated with a programmer before surgery. If this cannot be done, then the mode of rate response must be known so that inappropriate changes in heart rate can be avoided. ICDs should be deactivated before surgery, but only after consultation with the responsible cardiologist or cardiothoracic surgeon. If antibradycardia pacing is an integral component of the ICD, it should placed in a triggered or asynchronous mode to avoid the consequences of inappropriate inhibition.
The following steps should be taken if the use of electrocautery is unavoidable:
1. When possible, bipolar electrocautery should be used.
2. If this is not practical, and monopolar electrocautery must be used, the cautery current pathway should be perpendicular to the pacemaker’s lead system when possible. This is done by manipulating the placement of the grounding plate. However, a perpendicular pathway is not always a realistic possibility, especially when operating on a patient with a dual-lead system.
3. The grounding plate should be placed so that the current flows away from the pulse generator, and the distance between it and the active tip of the electrocautery should be as small as possible.
4. Care should be taken not to arc current between the tip of the electrocautery and another surgical instrument, to prevent demodulation of the signal. The instrument and the cautery tip should be in physical contact before the electrocautery is activated.
5. If it is impossible to place the pacemaker in a triggered or asynchronous mode, and it becomes apparent that the electrocautery is adversely affecting the pacemaker, the cautery current should be applied for no more than 1 second at a time, allowing at least 10 seconds for the device to function properly. This will permit the pacemaker enough time to maintain cardiac output.
6. Because the use of electrocautery also interferes with the ability of the ECG to monitor the heart, heart rate and blood pressure should be monitored using an arterial line. When the electrocautery is not in use, the ECG should be checked for arrhythmias or alterations in pacemaker function.
7. If the device appears to have been inappropriately reprogrammed by electrocautery, it is advisable to take the time to return the device to the mode selected before continuing the procedure. This is why it is important to have the programming device in the operating room.
The following steps should be taken if defibrillation is unavoidable:
1. If possible, use the anterior-posterior type of paddles, placing the anterior paddle as far away from the pulse generator as possible. This should allow the current to flow away from the generator.
2. If the anterior type of paddles must be used, place them along a line perpendicular to the lead(s). This may be difficult if the patient has a dual-lead system.
3. Use the lowest defibrillator current setting possible. Inability to defibrillate at low current settings will necessitate an increase, and damage to the pulse generator may become unavoidable. For these reasons, a temporary pacing system must be available.
If at any time during the procedure it becomes apparent that the device has lost its ability to stimulate the heart, due to damage to the pulse generator or the lead–tissue interface, temporary pacing measures must be taken. Inotropic support should also be available and used if necessary. The appropriate cardiology or cardiothoracic surgery department should be informed, and a decision should be made about the repair or replacement of the device.
Considerations when operating on a patient with a VAD are much less complicated but just as important. It is recommended that patients with the TCI IP-LVAD have the timing circuit disconnected from the external controller before defibrillation. This will necessitate the use of a fixed rate mode during the period of defibrillation but will eliminate the possibility of damage to this circuit. Patients with the TCI VE-LVAD should have the drive line disconnected from the external controller before defibrillation. This will prevent damage to the timing circuit but requires the pump to run pneumatically or manually for the duration of defibrillation. There are no specific recommendations concerning defibrillation on patients with the Novacor LVAD, Thoratec VAD, or Abiomed VAD. 9
The use of electrocautery in patients with the TCI VE-LVAD leads to the inability of the device to sustain cardiac output, regardless of what mode it is in. Because electrocautery causes reversion to the fixed rate mode every time it is used, the device should be placed in this mode for the duration of surgery. Although there is no permanent damage to the device, there are some important considerations. Because adequate pumping is not possible during the use of electrocautery, it should be applied for no more than 1 second at a time, allowing 10 seconds for the device to function properly. The cardiac output of the device should be monitored during this time. However, the beats after electrocautery will all be at maximal stroke volume because the temporary cessation of pumping has increased the filling of the device. Subsequently, the stroke volume will decrease as the device begins to catch up. Thus, it is necessary to allow time for the cardiac output to stabilize before determining its value. 8,9 The use of electrocautery does not affect pumping in the TCI IP-LVAD, Novacor LVAD, Thoratec VAD, or Abiomed VAD. However, if electrocautery appears to be disrupting the timing of the automatic mode in the TCI IP-LVAD, conversion to fixed mode is recommended, and the timing circuit should be disconnected. Both TCI LVADs come with an external hand pump that allows manual maintenance of device function. This provides an extra level of protection if the device malfunctions for any of the above reasons. This pump should be available during surgery, along with someone familiar with its use.
POSTOPERATIVE EVALUATION
The appropriate cardiology or cardiothoracic surgery department should be informed that electrocautery or defibrillation was used during surgery. An evaluation of pacemaker and ICD function similar to that performed before surgery should be done in the early postoperative period and then again 24 to 48 hours later. This is necessary because failure of the device to capture, due to damage at the lead–tissue interface, may not be apparent until 24 to 48 hours after surgery. If the capture threshold has increased, then endocardial burns should be suspected and the patient should be followed until stability is demonstrated. However, a progressive rise in capture threshold may ultimately exceed the output of the pulse generator, resulting in a loss of capture. Revision of the pacing system before loss of capture requires replacement of the pulse generator with one capable of higher output, replacement of the lead(s), or both. If any of the postoperative measurements of the demand or magnet rates vary from those obtained before surgery, one must suspect that the pacemaker has been inadvertently reprogrammed during surgery or has sustained permanent damage.
Alteration of the TCI VE-LVAD motor current occurs only during exposure to electrocautery and causes no permanent effects on the device. Defibrillation, however, can damage the timing circuit associated with the automatic mode in the TCI VE-LVAD, and in the TCI IP-LVAD if it is left connected during defibrillation. The department responsible for monitoring the device should be informed if this circuit is damaged during surgery, because repair or replacement should be considered.
DISCUSSION
Although pacemakers and ICDs have been engineered to be more resistant to EMI, the protection is by no means complete. The effects of EMI on different models is highly variable and rarely reproducible. However, when something does occur, especially in a device-dependent patient, the effects can be life-threatening. Understanding the possibilities before the patient goes into the operating room can prepare the surgeon to deal with intraoperative complications, possibly saving a life. Due to the nature of pacemakers and ICDs and the necessity to sense subtle changes in cardiac electrical activity, it may be impossible for engineers to eliminate the problem of EMI.
Because VADs do not need to sense cardiac electrical activity, complete protection from EMI is a realistic goal and has been accomplished in the extracorporeal VADs. Protection from EMI in the fully implantable VADs is slightly more difficult. The Novacor LVAD seems to have accomplished this goal. All the electronics involved for timing and driving the pump are housed in the external controller, which is shielded from EMI. Work must be done to eliminate the effects of EMI on the TCI devices. The effects of EMI on VAD function must become a priority for the engineers in charge of designing future generations of VADs. This will become increasingly important as devices become completely implantable. This will require transcutaneous transmission of power as well as diaphragm position information. It is likely that these devices will be even more susceptible to EMI.
Recently, there has been interest in using an ultrasonically activated scalpel (Harmonic scalpel; Ultracision Inc., Smithfield, RI) as an alternative to electrocautery in patients with implanted cardiac devices. The scalpel thermally transfers heat to the tissue without electrical current passing through the patient. This scalpel has been used safely and effectively in laparoscopic as well as open abdominal and pelvic surgical procedures. 15–17 More recently it has been used to assist in pacemaker replacements and explants. 18 Although the experience with this instrument is limited, the concept of an instrument that can cut and coagulate like electrocautery without generating EMI is attractive to surgeons operating on patients with implanted cardiac devices. EMI is a problem in this cohort of patients, and solutions like this one should be sought.
Footnotes
Correspondence: John D. Madigan, c/o Niloo Edwards, MD, Columbia Presbyterian Medical Center, Milstein Hospital Building 7-435, 177 Fort Washington Ave., New York, NY 10032.
Accepted for publication May 10, 1999.
References
- 1.Erlebacher JA, Cahill PT, Pannizzo F, Knowles RJR. Effect of magnetic resonance imaging on DDD pacemakers. Am J Cardiol 1986; 57:437. [DOI] [PubMed] [Google Scholar]
- 2.Fetter J, Aram G, Holmes DR, Gray JE, Hayes DL. The effects of nuclear magnetic resonance imagers on external and internal pulse generators. PACE 1984; 7:720. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman BH, Faul DD. Artifacts and hazards in nuclear magnetic resonance imaging due to metal implants and cardiac pacemakers. Diagn Imag Clin Med 1984; 53:53. [PubMed] [Google Scholar]
- 4.Warnowicz MA. The pacemaker patient and the electromagnetic environment. Clin Prog Pac Electr 1983; 11:66–176. [Google Scholar]
- 5.Sager DP. Current facts on pacemaker electromagnetic interference and their application to clinical care. Heart & Lung 1987; 16:211–221. [PubMed] [Google Scholar]
- 6.Bourke ME. The patient with a pacemaker or related device. Can J Anesth 1996; 43:R24–R32. [DOI] [PubMed] [Google Scholar]
- 7.Written communication, Robert Kormos, MD, Director of Mechanical Assist Program, University of Pittsburgh, March 24, 1998.
- 8.Poirier VL. The TCI HeartMate Blood Pump. In: Lewis T, Graham T, eds. Mechanical circulatory support. London: Edward Arnold; 1995: 229–236.
- 9.Choudhri AF, Michelman PC, Tsitlik JE, Oz MC, Levin HR. LVAD Motor Current Waveform Analysis. ASAIO J (in press).
- 10.Thermo Cardiosystems Inc. HeartMate LVAS Clinical Training Manual. Woburn, MA: Thermo Cardiosystems Inc.; revised 1998.
- 11.Levine PA, Baladay GJ, Lazar HL, Belott PH, Roberts AJ. Electrocautery and pacemakers: management of the paced patient subject to electrocautery. Ann Thorac Surg 1986; 41:313–317. [DOI] [PubMed] [Google Scholar]
- 12.Bernstien AD, Camm AJ, Fletcher RD, et al. The NASPE/BPEG generic pacemaker code for antibradyarrhythmia and adaptive-rate pacing and antitachycardia devices. Pacing Clin Electrophysiol 1987; 10:794–798. [DOI] [PubMed] [Google Scholar]
- 13.Bernstien AD, Camm AJ, Fletcher RD, et al. North American Society of Pacing and Electrophysiology policy statement: the NASPE/BPEG defibrillator code. Pacing Clin Electrophysiol 1993; 16:1776–1780. [DOI] [PubMed] [Google Scholar]
- 14.Bloomfield P, Bowler GMR. Anesthetic management of the patient with a permanent pacemaker. Anesthesiology 1989; 44:42–46. [DOI] [PubMed] [Google Scholar]
- 15.Amaral JF. Ultrasonic dissection. Endosc Surg 1994; 2:181–185. [PubMed] [Google Scholar]
- 16.Amaral JF. Laparoscopic application of an ultrasonically activated scalpel. Gastrointest Clin North Am 1993; 33:81–92. [Google Scholar]
- 17.Amaral JF. Laparoscopic cholecystectomy in 200 consecutive patients using an ultrasonically activated scalpel. Surg Laparosc Endosc 1995; 5:255–262. [PubMed] [Google Scholar]
- 18.Epstein MR, Mayer JE Jr, Duncan BW. Use of an ultrasonic scalpel as an alternative to electrocautery in patients with pacemakers. Ann Thorac Surg 1998; 65:1802–1804. [DOI] [PubMed] [Google Scholar]