Abstract
Objective
To compare the long-term functional results of ileorectal anastomosis (IRA) with those of ileal pouch–anal anastomosis (IPAA) in patients with familial adenomatous polyposis (FAP).
Summary Background Data
In patients with FAP, hundreds of colorectal adenomas develop, and the patient will die of colorectal cancer if left untreated. The surgeon must choose between colectomy with IRA and restorative proctocolectomy with IPAA. One factor crucial to decision making is the functional outcome after either procedure. To date, studies on this issue have reported conflicting results and have been based on small series of patients.
Methods
To assess various functional variables, a questionnaire was sent to 323 patients with FAP who underwent either IRA or IPAA and who were registered at the Netherlands Foundation for the Detection of Hereditary Tumors. The overall response rate was 86%; the responders comprised 161 patients who underwent IRA and 118 patients who underwent IPAA.
Results
Patients who underwent IRA scored significantly better for daytime and nighttime stool frequency, soiling, occasional passive incontinence, flatus and feces discrimination, stool consistency, and need for antidiarrheal medication. There was no difference with regard to perianal irritation, episodes of bowel discomfort, or dietary restrictions. The functional results according to the aggregate score of the Gastro-Intestinal Functional Outcome Scale, where the items specified above were integrated (0 indicating a poor and 100 a good overall function), were significantly better in patients with an IRA (74.5) than in patients with an IPAA (66.0) (p < 0.01).
Conclusion
The functional outcome after IRA is significantly better than after IPAA. On the basis of these results, IRA might still be considered in patients with a mild phenotypic expression of the disease in the rectum.
Familial adenomatous polyposis (FAP) is an autosomal dominantly inherited disease caused by mutations at the APC gene on chromosome 5. The disease is characterized by hundreds of adenomatous polyps in the colon and rectum. In most patients, polyps develop during the second and third decade of life. Without timely surgical intervention, colorectal cancer will develop in virtually all patients. 1 Although several studies have shown that chemoprevention has some beneficial effect on the colorectal adenomas, the only curative treatment is still surgical. The two main surgical options are colectomy with an ileorectal anastomosis (IRA) 2 or a restorative proctocolectomy with an ileal pouch–anal anastomosis (IPAA). IRA has the advantage both of a low complication rate and of good functional results with regard to stool frequency, continence, and soiling. However, even after close monitoring, the cumulative risk of cancer evolving in the rectal stump is reported to be 15% after 25 years of follow-up. 3–6 In addition, many patients with an IRA need a secondary proctocolectomy because of uncontrollable polyps. 6
Restorative proctocolectomy and IPAA eradicate virtually all of the colonic mucosa, thereby eliminating the risk for cancer 7–9; it may therefore be the preferred treatment. However, IPAA also has some disadvantages, such as its greater technical complexity and the incidental need to construct a temporary diverting ileostomy, which then requires a second operation for removal. 10 In addition, the complication rate is higher, and there is a not insignificant risk that pouch removal may become necessary due to complication or malfunction. 11,12 Moreover, several short-term studies have reported functional results to be less satisfactory than those after IRA. 13–17 Some reports have also claimed that functional results after an IPAA are decreased if a mucosectomy is performed. 18–20 The fact that many patients need a secondary proctectomy after IRA might constitute a strong argument in favor of performing an IPAA as the primary surgical procedure. Only if the functional outcome of IRA is better than that of IPAA is there still a place for IRA, although this would represent a temporary solution for many patients with FAP.
The aim of the present study, therefore, was to evaluate the possible difference in functional outcome in a large series of patients who had undergone either of the procedures. In addition, the different roles played by comorbidity, age, coexistence of colorectal carcinoma, incidence of relaparotomy, conversion of IRA to IPAA, and anastomotic technique in IPAA on functional outcome were also assessed.
PATIENTS AND METHODS
In 1985, the Netherlands Foundation for the Detection of Hereditary Tumors established a registry of patients with FAP, the Dutch Polyposis Registry. Until 1997, the foundation collected medical and pathology data on 210 families with FAP. The families included 323 patients who had undergone surgery for FAP in various centers between 1961 and 1996. Colectomy and IRA was performed in 183 patients, whereas 140 patients underwent IPAA. From 1984 on, IPAA procedures were performed in patients with FAP in the Netherlands.
A questionnaire designed for self-completion that focused on functional outcome and comorbidity was mailed to all patients. To investigate test/retest reliability, a second, identical questionnaire was mailed within 9 months; the purpose of this was to investigate the extent to which subjects gave the same responses to the same questions in the absence of any intervention.
Functional Outcome
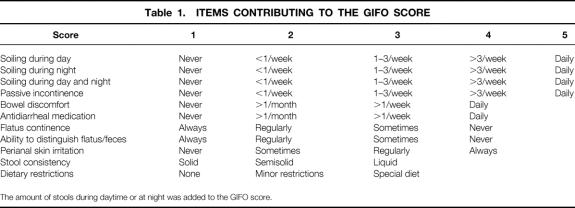
Functional outcome was assessed by questions on various aspects of bowel function—the number of stools during the day and at night, stool consistency (solid, semisolid, liquid), soiling or incontinence during the day or at night, incontinence for gas, ability to distinguish between flatus and feces, need for antidiarrheal medication, dietary restrictions, and incidence of perianal skin irritation. The items were formulated as three-option to five-option multiple-choice questions (Table 1). To obtain a more manageable estimate of the functional outcome, the Gastro-Intestinal Functional Outcome (GIFO) score was created from these items by both reliability analysis and factor analysis; the items were derived from incontinence scoring systems reported earlier. 19,21–25 These scores were adjusted to create a composite score that did not focus exclusively on incontinence but that covered complete bowel function.
Table 1. ITEMS CONTRIBUTING TO THE GIFO SCORE
The amount of stools during daytime or at night was added to the GIFO score.
The separate items inquiring about bowel function and the GIFO score were compared between the groups of patients with IRA and those with IPAA. To correct for the possible confounding effects on functional status of comorbid chronic conditions, respondents were asked whether their doctor had told them they had diabetes, renal and cardiovascular diseases, chronic obstructive pulmonary disease, low back problems, arthritis, obesity, or malignancies other than colorectal cancer. The effect of comorbidity as such (a summation of the different types) and the effect of the specific types of comorbidity on functional outcome were examined. Subsequent analyses were performed to investigate the impact on the GIFO score of age and the length of follow-up, coexistence of colorectal carcinoma at the time of surgery, relaparotomy, conversion of IRA to IPAA, and the anastomotic technique used in IPAA.
Questionnaire Analysis
Reliability and factor analyses were used to investigate the internal structure of the questionnaire. The internal consistency reliability of the GIFO scale (the extent to which the variables making up the aggregate score all measure the same construct) was tested with Cronbach’s α coefficient. As recommended, 26 internal estimates of a magnitude of at least 0.80 were considered as good. The test/retest reliability of the GIFO scale was tested with Pearson’s correlation r.
Factor analysis examines the intercorrelations among all variables and explains these intercorrelations in terms of a reduced number of variables, called factors. 27 In the present study, factor analysis, with maximum likelihood estimation based on a covariance matrix, was used to test the hypothesis of a single underlying factor. This hypothesis implies that the item responses can be aggregated to a single functional outcome score. The hypothesis is evaluated by means of a chi square statistic and two goodness-of-fit measures (the goodness-of-fit index and the adjusted goodness-of-fit index). The chi square should not be larger than two times the degrees of freedom; both the goodness-of-fit index and the adjusted goodness-of-fit index should exceed 0.90. 28
Statistical Analysis
For the factor analysis, LISREL 7.18 29 was used; for all other analyses, SPSS 7.5 was used. Student’s t tests were used for group comparisons. Proportions of events were compared by chi square tests; p < 0.05 was considered statistically significant. Logistic regression analysis was used to explore the meaning of the differences found on the GIFO score.
RESULTS
Responders
After two mailings, 279 questionnaires were returned from the 323 patients, with 161 from the 183 IRA patients (88%) and 118 from the 140 IPAA patients (84%). Table 2 outlines the patient characteristics. The mean age in IRA patients, 41 years, was significantly higher than in IPAA patients (37 years, p = 0.01). The age at the time of surgery did not differ between the groups (29 years vs. 30 years, p = 0.48). The mean follow-up of IRA patients was longer (12 years) than in IPAA patients (6.8 years, p = 0.001).
Table 2. PATIENT CHARACTERISTICS
NR, nonresponders.
Results are expressed as means ± SD; t test, *p < 0.05, IRA vs. IPAA.
The number of control endoscopies per year differed between the various centers where the patients were surveyed. The median number in IRA patients was one endoscopy per 6 months, ranging from six times per year to once per 3 years. The median number in IPAA patients was one endoscopy per year, ranging from three times per year to once per 5 years.
There were 52% women in the IRA group and 43% in the IPAA group.
Six IRA patients and eight IPAA patients had a permanent ileostomy. A continent ileostomy was constructed in two IRA patients after proctectomy for recurrence of polyps in the rectal remnant. An ileostomy was performed in two other IRA patients because of recurrent distal obstruction due to unresectable desmoid tumors. Two IRA and five IPAA patients had a permanent ileostomy constructed because of anastomotic complications. Therapy-resistant pouch dysfunction developed in two IPAA patients; they needed an ileostomy. In one IPAA patient, the mesenteric vessels were too short to allow an ileoanal anastomosis. These fourteen patients were excluded from this study.
Nonresponders
There were 44 nonresponders, 22 IRA patients and 22 IPAA patients. The mean age was 37.7 years for the IRA group and 36.6 years for the IPAA group. The mean age at the time of surgery was lower for the IRA group (24.6 years) than for the IPAA group (26.4 years). Four patients in the IPAA group and none in the IRA group had a coexistent colorectal malignancy. Two patients in the IRA group had to undergo a relaparotomy, one because of anastomotic leakage requiring a temporary ileostomy and the other because of persisting intestinal obstruction. Three patients in the IPAA group had a relaparotomy due to anastomotic leakage. Nine patients had a subsequent proctectomy after an initial colectomy with IRA because of recurrent polyps, and one patient because of rectal carcinoma.
Questionnaire Analysis
Internal consistency reliability measured with Cronbach’s α coefficient for this composite score was 0.80. The reproducibility of the GIFO scale was estimated in the sample of 184 subjects who answered the questionnaire twice. Pearson’s correlation was 0.83, indicating an adequate test/retest variability. The factor analysis also supported the computations of a single GIFO score: all factor loadings were positive and none were near zero, justifying summing the variables to create a single score (χ2 = 60.7, df = 35, GFI = 0.95, AGFI = 0.93). 30 The total fraction of variance accounted for by the latent variable was 39%. These analyses revealed large differences in item variances, especially for the items inquiring about the number of stools during night and day. This means that a simple sum score of the items would result in a function score that would reflect mainly the number of stools during the day and at night. This problem was solved by applying a scoring rule that takes this difference in standard deviation into account. 29–31 The resulting GIFO score was transformed to a 0-to-100 scale, with 0 indicating poor overall function and 100 good overall function.
Functional Outcome
The mean frequency of bowel movements during the day was 4.7 in IRA patients and 6.0 in IPAA patients (p < 0.0001). Bowel movements during the night were less frequent in IRA patients (1.4) than in IPAA patients (2.0, p = 0.01). There were also significant differences in the items regarding stool consistency (p = 0.009) and the need for antidiarrheal medication (p = 0.04). On the items assessing incontinence (e.g., soiling, particularly at night, incidental passive incontinence, perianal skin irritation, ability to distinguish between flatus and feces), IRA patients had significantly better results. Between the groups, there were no differences regarding dietary restrictions and episodes of bowel discomfort. The functional result measured by the GIFO score was significantly better for IRA patients than for IPAA patients (74.5 vs. 66.0, p = 0.0001) (Table 3). To illustrate the impact of such a difference, a logistic regression analysis of the GIFO score on soiling resulted in an odds ratio of 1.11. Therefore, an eight-point score difference on the GIFO resulted in an odds ratio of 2.33 for the incidence of soiling. The GIFO score could be computed for only 145 of the 161 IRA patients and for 106 of the 118 IPAA patients due to missing values.
Table 3. FUNCTIONAL OUTCOME
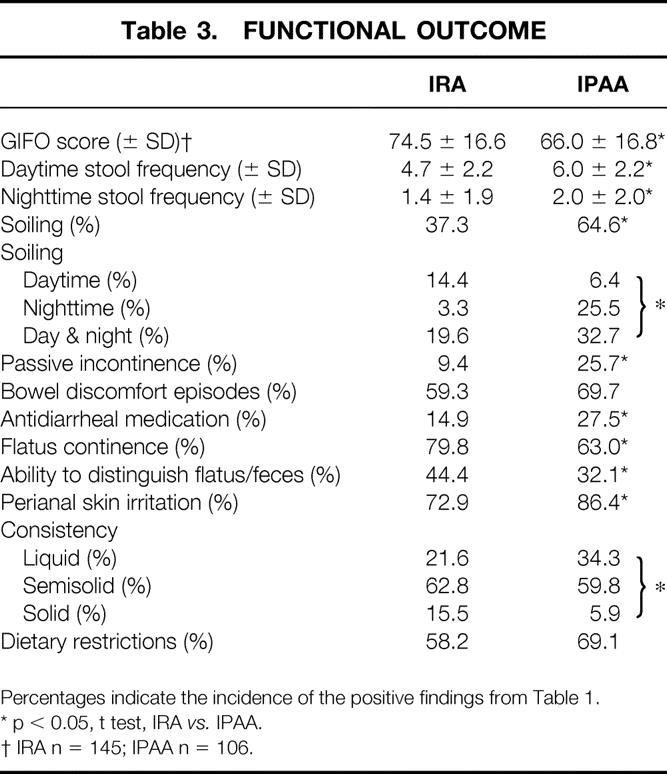
Percentages indicate the incidence of the positive findings from Table 1.
* p < 0.05, t test, IRA vs. IPAA.
† IRA n = 145; IPAA n = 106.
The subanalysis for age showed that there was a significant but relatively small correlation between age and GIFO score (r = 0.16, p = 0.01).
In a subsequent analysis, the effect of the difference in length of follow-up between the groups was estimated. It was shown that a subset of IRA patients, with the same length of follow-up as the IPAA patients, had a GIFO score that was no different from that of the IRA patients with a longer follow-up (75.8 and 72.4, respectively; p = 0.22).
Comorbidity
Comorbidity as such was present in 57% of the IRA group and 62% of the IPAA group (Table 4). Patients from the IRA group with comorbidity had a significantly lower GIFO score than those without comorbidity (70.7 vs. 77.3; p = 0.02). Patients from the IPAA group with comorbidity also had a significant lower GIFO score than those without comorbidity (61.7 vs. 68.5; p = 0.04). Comorbidity as such showed no significant interaction with the type of operation and the subsequent GIFO score, indicating that comorbidity resulted in a lower GIFO score for both surgical procedures. Some of the separate coexisting diseases (i.e., chronic skin disorders, low back problems, restricted limb function) also generated an impaired score for both procedures on the GIFO score. Two other separate coexisting diseases had a significantly lower score in one of the groups: these were diabetes in the IRA group (in the IPAA group there was only one patient with diabetes) and chronic obstructive pulmonary disease in the IPAA group.
Table 4. IMPACT OF COMORBIDITY ON GIFO SCORE
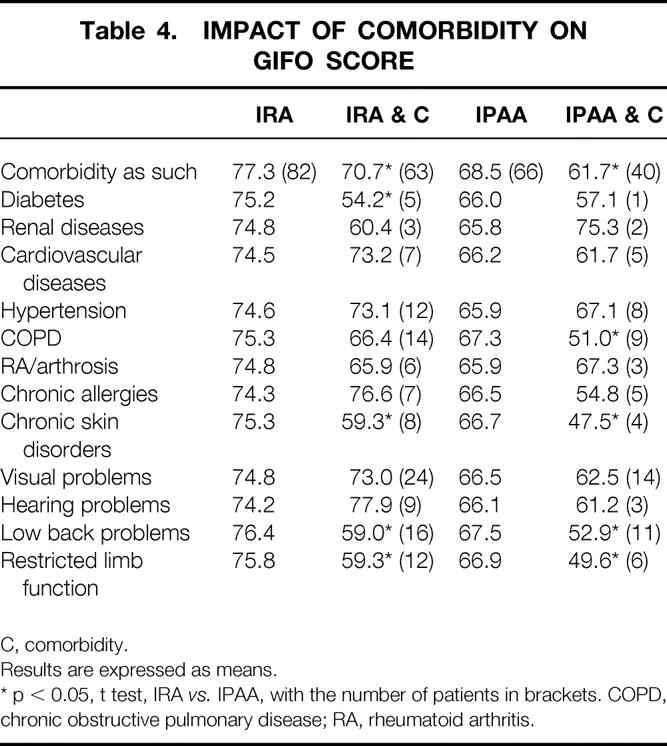
C, comorbidity.
Results are expressed as means.
* p < 0.05, t test, IRA vs. IPAA, with the number of patients in brackets. COPD, chronic obstructive pulmonary disease; RA, rheumatoid arthritis.
Coexistence of Colorectal Carcinoma
A coexisting colorectal carcinoma at the time of surgery was present in 27 of the 279 patients (14 IPAA patients and 13 IRA patients). For six patients, no data were available at the registry. There was no difference in GIFO score between IRA patients with or without a coexisting colorectal carcinoma at the time of surgery (74.2 and 74.6, respectively; p = 0.93), nor was there a difference in the IPAA group (61.0 and 66.6, respectively; p = 0.28).
Relaparotomy
The incidence and the type of relaparotomy are reported in Table 5. The incidence of relaparotomy for the entire group of responding patients was 24%. The incidence of relaparotomy for anastomotic leakage was significantly higher in the IPAA group than in the IRA group. However, there was no difference in GIFO score between IRA patients with or without relaparotomy (69.3 and 75.1, respectively; p = 0.18) and between the IPAA group with or without relaparotomy (63.2 and 67.9, respectively; p = 0.16).
Table 5. POSTOPERATIVE COMPLICATIONS REQUIRING A RELAPAROTOMY
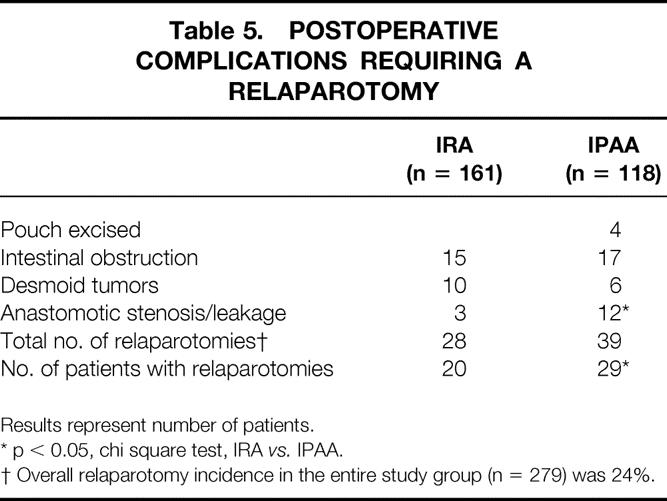
Results represent number of patients.
* p < 0.05, chi square test, IRA vs. IPAA.
† Overall relaparotomy incidence in the entire study group (n = 279) was 24%.
Conversion of IRA to IPAA
A conversion from IRA to IPAA was performed in 32 of the 140 IPAA patients. There were nine nonresponders, and in 3 of the 23 responders a permanent ileostomy was constructed. Two patients had a permanent ileostomy constructed because of anastomotic complications, and in one patient therapy-resistant pouch dysfunction developed. The reason for a conversion was an adenocarcinoma in the rectal remnant in 4 patients, anastomotic complications in 1 patient, and multiple adenomas in the other 27 patients. There was no difference in GIFO score between IPAA patients with or without a history of IRA (61.4 and 67.1, respectively; p = 0.17). Patients who had a IPAA done at the first operation still had worse functional results, as measured by the GIFO score, than patients with an IRA (67.1 vs. 74.5; p = 0.002).
Anastomotic Technique in IPAA Patients
Of the 118 patients who underwent an IPAA procedure, 71 had a hand-sewn anastomosis and 43 had a double-stapled anastomosis. The technique used for four patients was not known. The functional outcome as measured by the GIFO score did not differ between these groups of patients (64.8 hand-sewn and 67.7 stapled; p = 0.39). However, the incidences of soiling (78.1% and 44.2%; p = 0.03) and soiling at night (35.9% and 11.6%; p = 0.03) were significantly higher in patients with a hand-sewn anastomosis.
DISCUSSION
There is an ongoing controversy about the type of surgery that should be performed in patients with FAP. If the rectum contains polyps with severe dysplasia or a carcinoma, if the rectum is carpeted with polyps, or if the patient is unlikely to comply with follow-up, there is a good case for IPAA. If the rectum is relatively free of adenomas (e.g., <5 protruding polyps without severe dysplasia 10), IRA might be the more attractive surgical procedure because of its satisfactory functional results. A major drawback of IRA is the substantial risk of cancer developing in the residual rectum; moreover, many patients with FAP need rectal excision because of uncontrollable polyps. For this reason and also in view of the declining complication rate due to improved techniques of IPAA, 32 an increasing number of surgeons consider IPAA to be the treatment of choice. Only if the functional outcome of IRA were substantially better than that of IPAA would there still be a good case for IRA in selected patients.
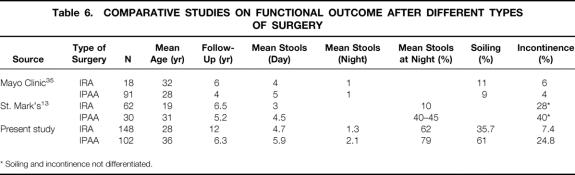
Although several reports have been published on the functional outcome of IRA or IPAA, 14–17,33,34 only two studies, one from the Mayo Clinic 35 and the other from the St. Mark’s Hospital, 13 have provided a comparison of the functional results of patients with an IRA and those with an IPAA (Table 6). The Mayo Clinic found no difference between the functional results of the two procedures, whereas the St. Mark’s study reported a much more satisfactory functional outcome for IRA.
Table 6. COMPARATIVE STUDIES ON FUNCTIONAL OUTCOME AFTER DIFFERENT TYPES OF SURGERY
* Soiling and incontinence not differentiated.
In the present study, which contains the largest sample of patients with the longest follow-up, we found a substantial difference in functional outcome between the two procedures. Compared with IPAA patients, IRA patients had a significantly better functional outcome with regard to several single items of bowel function, such as daytime and nighttime stool frequency, soiling, passive incontinence, flatus and feces discrimination, stool consistency, and need for antidiarrheal medication. Moreover, the functional result according to the aggregate GIFO score in IRA patients was significantly better.
These results cannot, as suggested by others, 35 be explained by the longer follow-up period and the higher age of the IRA patients, because a subset of the IRA group, with the same follow-up period and age distribution as the IPAA group, had a GIFO score that was similar to that of the entire IRA group. Bias by nonresponders seems unlikely, because their number was relatively small and the incidences of relaparotomy and of coexisting colorectal carcinomas did not differ from the responders, especially for the IRA group.
Comorbidity is reported to interfere with postoperative recovery and might therefore be a confounder of functional outcome. 36 Comorbidity has not been taken into account in published data on functional outcome after surgery in patients with FAP. In the present study, functional outcome was found to be impaired by the existence of comorbidity as such. Because the prevalence of comorbidity did not differ between the IRA and the IPAA groups, the differences in functional outcome cannot be explained by this confounder.
Synchronous colorectal carcinoma did not influence the functional outcome in either group. However, there is a tendency in patients with an IPAA for functional outcome to be compromised when colorectal carcinoma is present. This is consistent with results reported by Penna et al 16 and is thought to be related to the wider excision made for oncologic reasons, with the concomitantly higher risk for nerve damage.
Postoperative complications are also thought to have a significant impact on the functional outcome. Although we would like to take this into account, there were reliable data only on the number of relaparotomies. In the present study, the prevalence of relaparotomy after IPAA was higher than in the IRA group; there was, however, no difference in GIFO score between IRA patients with or without relaparotomy, and between IPAA patients with or without relaparotomy.
Patients who undergo IRA and have a carpeting of rectal polyps, severe dysplasia, or rectal cancer during follow-up must undergo a conversion to an IPAA. Bowel function after conversion from an IRA to an IPAA can be compromised due to postoperative complications. The data presented in this paper as well as the data reported by Penna et al 17,37 show that there is no difference in bowel function between IPAA patients who undergo a conversion compared with patients who undergo an IPAA at the first operation.
There is ongoing debate about the most appropriate technique of ileoanal anastomosis—a mucosectomy and a conventional anastomosis at the level of the dentate line (i.e., a hand-sewn anastomosis) or a stapled anastomosis between the pouch and the anal canal at the level of the anorectal junction (i.e., a double-stapled anastomosis). A recognized disadvantage of the double-stapled technique is the fact that it may leave some rectal mucosa. 38 There are conflicting reports in the literature about the functional outcome of either technique, ranging from no difference between the anastomotic techniques to fecal incontinence and nocturnal soiling after a mucosectomy and a hand-sewn anastomosis. 18–20,39,40 Although in the present study the overall functional outcome did not differ significantly, a higher incidence of soiling and soiling at night was observed in patients with a hand-sewn anastomosis; this could constitute an argument in favor of a double-stapled anastomosis.
In conclusion, the present study showed that the functional outcome after IRA is significantly better than that after IPAA. Future studies should evaluate the impact of the less satisfactory functional results on the quality of life. Until such studies are available, IRA might be considered in patients who have few and controllable rectal polyps.
Footnotes
Correspondence: Peter van Duijvendijk, MD, Academic Medical Center, Dept. of Surgery, G4-144, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. E-mail: p.vanduijvendijk@amc.uva.nl
Accepted for publication June 25, 1999.
References
- 1.Bussey HJR. Family studies, histopathology, differential diagnosis and results of treatment. Baltimore: The Johns Hopkins University Press; 1975.
- 2.Lockhart-Mummery JP. The causation and treatment of multiple adenomatosis of the colon. Ann Surg 1934; 99:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper JC, Jones D, Williams NS. Outcome of colectomy and ileorectal anastomosis in Crohn’s disease. Ann R Coll Surg Engl 1986; 68:279–282. [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG, Hill JR, Adson MA. Surgical management of multiple polyposis. The problem of cancer in the retained bowel segment. Arch Surg 1970; 100:521–526. [DOI] [PubMed] [Google Scholar]
- 5.Bess MA, Adson MA, Elveback LR, Moertel CG. Rectal cancer following colectomy for polyposis. Arch Surg 1980; 115:460–467. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HFA, van der Luijt RB, Slors JFM, et al. Molecular genetic tests as a guide to surgical management of familial adenomatous polyposis. Lancet 1996; 348:433–435. [DOI] [PubMed] [Google Scholar]
- 7.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J 1978; 2:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utsunomiya J, Iwama T, Imajo M, et al. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum 1980; 23:459–466. [DOI] [PubMed] [Google Scholar]
- 9.Taylor BM, Beart RW Jr, Dozois RR, Kelly KA, Phillips SF. Straight ileoanal anastomosis vs. ileal pouch–anal anastomosis after colectomy and mucosal proctectomy. Arch Surg 1983; 118:696–701. [DOI] [PubMed] [Google Scholar]
- 10.Slors JFM, den Hartog Jager FC, Trum JW, Taat CW, Brummelkamp WH. Long-term follow-up after colectomy and ileorectal anastomosis in familial adenomatous polyposis coli. Is there still a place for the procedure? Hepatogastroenterology 1989; 36:109–112. [PubMed] [Google Scholar]
- 11.Nyam DC, Brillant PT, Dozois RR, Kelly KA, Pemberton JH, Wolff BG. Ileal pouch–anal canal anastomosis for familial adenomatous polyposis: early and late results. Ann Surg 1997; 226:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dozois RR, Kelly KA, Welling DR, et al. Ileal pouch–anal anastomosis: comparison of results in familial adenomatous polyposis and chronic ulcerative colitis. Ann Surg 1989; 210:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madden MV, Neale KF, Nicholls RJ, et al. Comparison of morbidity and function after colectomy with ileorectal anastomosis or restorative proctocolectomy for familial adenomatous polyposis. Br J Surg 1991; 78:789–792. [DOI] [PubMed] [Google Scholar]
- 14.Tjandra JJ, Fazio VW, Church JM, Oakley JR, Milsom JW, Lavery IC. Similar functional results after restorative proctocolectomy in patients with familial adenomatous polyposis and mucosal ulcerative colitis. Am J Surg 1993; 165:322–325. [DOI] [PubMed] [Google Scholar]
- 15.Church JM, Fazio VW, Lavery IC, Oakley JR, Milsom J, McGannon E. Quality of life after prophylactic colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 1996; 39:1404–1408. [DOI] [PubMed] [Google Scholar]
- 16.Penna C, Tiret E, Daude F, Parc R. Results of ileal J-pouch–anal anastomosis in familial adenomatous polyposis complicated by rectal carcinoma. Dis Colon Rectum 1994; 37:157–160. [DOI] [PubMed] [Google Scholar]
- 17.Penna C, Kartheuser AH, Parc R, et al. Secondary proctectomy and ileal pouch–anal anastomosis after ileorectal anastomosis for familial adenomatous polyposis. Br J Surg 1993; 80:1621–1623. [DOI] [PubMed] [Google Scholar]
- 18.Gozzetti G, Poggioli G, Marchetti F, et al. Functional outcome in handsewn versus stapled ileal pouch–anal anastomosis. Am J Surg 1994; 168:325–329. [DOI] [PubMed] [Google Scholar]
- 19.Gemlo BT, Belmonte C, Wiltz O, Madoff RD. Functional assessment of ileal pouch–anal anastomotic techniques. Am J Surg 1995; 169:137–142. [DOI] [PubMed] [Google Scholar]
- 20.Tuckson WB, Lavery IC, Fazio VW, Oakley J, Church JM, Milsom J. Manometric and functional comparison of ileal pouch anal anastomosis with and without anal manipulation. Am J Surg 1991; 161:90–96. [DOI] [PubMed] [Google Scholar]
- 21.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993; 36:77–97. [DOI] [PubMed] [Google Scholar]
- 22.Oresland T, Fasth S, Nordgren S, Hulten L. The clinical and functional outcome after restorative proctocolectomy. A prospective study in 100 patients. Int J Colorectal Dis 1989; 4:50–56. [DOI] [PubMed] [Google Scholar]
- 23.Miller R, Bartolo DC, Locke-Edmunds JC, Mortensen NJ. Prospective study of conservative and operative treatment for faecal incontinence. Br J Surg 1988; 75:101–105. [DOI] [PubMed] [Google Scholar]
- 24.Browning GG, Parks AG. Postanal repair for neuropathic faecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg 1983; 70:101–104. [DOI] [PubMed] [Google Scholar]
- 25.Hallbook O, Sjodahl R. Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg 1996; 83:60–62. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Cronbach’s alpha. Br Med J 1997; 314:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunnally JC, Bernstein IH. Confirmatory factor analysis. In Vaicunas J, Belser JR, eds. Psychometric theory. New York: McGraw-Hill; 1994: 542–594.
- 28.Marsh HW, Balla JR, McDonald RP. Goodness-of-fit indexes in confirmatory factor analysis: the effect of sample size. Psychol Bull 1988; 103:391–410. [Google Scholar]
- 29.Joreskog KG, Sorbom D. LISREL 7, A guide to the program and applications. Chicago: SPSS; 1989:93.
- 30.Johnson RA, Wichern DW. Applied multivariate statistical analysis. Englewood Cliffs, NJ: Prentice-Hall; 1982:436.
- 31.Norusis MJ. SPSS/PC+ Professional statistics V5.0. Chicago: SPSS; 1992:142.
- 32.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch–anal anastomoses: complications and function in 1005 patients. Ann Surg 1995; 222:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horai T, Kusunoki M, Shoji Y, Yamamura T, Utsunomiya J. Clinicopathological study of anorectal mucosa in total colectomy with mucosal proctectomy and ileoanal anastomosis. Eur J Surg 1994; 160:233–238. [PubMed] [Google Scholar]
- 34.Braun J, Treutner KH, Schumpelick V. Stapled ileal pouch–anal anastomosis with resection of the anal transition zone. Int J Colorectal Dis 1995; 10:142–147. [DOI] [PubMed] [Google Scholar]
- 35.Ambroze WL Jr, Dozois RR, Pemberton JH, Beart RW Jr. Familial adenomatous polyposis: results following ileal pouch–anal anastomosis and ileorectostomy. Dis Colon Rectum 1992; 35:12–15. [DOI] [PubMed] [Google Scholar]
- 36.Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Medical Care 1993; 31:141–154. [DOI] [PubMed] [Google Scholar]
- 37.Penna C, Tiret E, Kartheuser AH, et al. Comparison of functional results of ileorectal and ileo-anal anastomoses in familial adenomatous polyposis. Conversions of ileorectal anastomoses into ileo-anal anastomoses. Gastroenterol Clin Biol 1992; 16:401–405. [PubMed] [Google Scholar]
- 38.Slors JFM, Ponson AE, Taat CW, Bosma A. Risk of residual rectal mucosa after proctocolectomy and ileal pouch–anal reconstruction with the double-stapling technique. Postoperative endoscopic follow-up study. Dis Colon Rectum 1995; 38:207–210. [DOI] [PubMed] [Google Scholar]
- 39.Seow-Choen F, Tsunoda A, Nicholls RJ. Prospective randomized trial comparing anal function after handsewn ileoanal anastomosis with mucosectomy versus stapled ileoanal anastomosis without mucosectomy in restorative proctocolectomy. Br J Surg 1991; 78:430–434. [DOI] [PubMed] [Google Scholar]
- 40.Luukkonen P, Järvinen H. Stapled vs. hand-sutured ileoanal anastomosis in restorative proctocolectomy. A prospective, randomized study. Arch Surg 1993; 128:437–440. [DOI] [PubMed] [Google Scholar]