Abstract
Objective
To study the relation between fibrosis, pancreatic blood flow (PMBF), interstitial pH (pHI), and the effects of pancreaticojejunostomy (PJ) in chronic pancreatitis.
Background
Chronic pancreatitis is associated with low PMBF and pHI, suggesting the existence of underlying ischemia.
Methods
In cats, the main pancreatic duct was partially obstructed and the animals were studied 2, 4, 6, and 8 weeks later. PJ was performed after 2 and 4 weeks of ductal obstruction and studied 4 weeks later. PMBF and pHI were measured before and after stimulation with secretin and cholecystokinin. pHI was measured with microelectrodes, PMBF by hydrogen gas clearance. Histologic analysis of the pancreas with Sirius red (collagen stain) and fast green FCF (noncollagen protein) stains allowed semiquantitative analysis of the ratio between collagen and total protein (C/TP).
Results
With the evolution of chronic pancreatitis, there is a progressive increase in the collagen content and C/TP ratio, a reduction in basal PMBF and pHI, and loss of the normal response to stimulation. Early PJ restores collagen content, C/TP ratio, and basal and stimulated PMBF and pHI to normal. PJ performed in established CP returns the C/TP ratio to normal, improves basal PMBF, and restores the normal hyperemic response to secretion. Basal pHI is improved and the “acid tide” associated with secretin returns, but there is still no response to cholecystokinin.
Conclusions
Pancreaticojejunostomy restores the elevated collagen and C/TP ratio to normal and reverses the ischemia present in CP. The authors speculate that restoration of PMBF and its normal response to stimulation allows “regeneration” and restoration of secretory function.
Pancreaticojejunostomy (PJ) is effective in relieving pain in >80% of patients with chronic pancreatitis (CP), although the mechanism by which this is achieved is not known. 1 Reber et al have suggested that a compartment syndrome with ischemia may exist in CP. 2 In human CP, surgical decompression procedures are associated with a reduction in the elevated pancreatic tissue pressure. 3 This may improve the low pancreatic microvascular blood flow (PMBF) present in CP.
In CP, there is also marked proliferation in the extracellular matrix and loss of secretory function. 4–6 The progressive loss of secretory function is delayed by surgical decompression. 7 Pancreatic secretion is associated with hyperemia and a decrease in pancreatic interstitial pH (pHI) in the normal pancreas. 8 In CP, basal PMBF and pHI are lower than normal. 9 As pancreatic function deteriorates with the evolution of CP, the normal decrease in the low pHI with response to secretory stimulation is lost. 9 PMBF now decreases with secretin stimulation.
The aim of this study was to examine in CP the effects of PJ on the relation among PMBF, pHI, and fibrosis, in particular the effects in early (when fibrosis is first apparent) and established CP.
MATERIALS AND METHODS
All animals used in these experiments were cared for in accordance with humane treatment guidelines established by the Sepulveda (California) VA Medical Center Office of Research and Development.
Experimental Protocol
Mongrel cats (2.0 to 3.0 kg) of either sex were fasted for 16 hours and anesthetized with xylazine hydrochloride (1 mg/kg intramuscularly) and sodium pentobarbital (25 mg/kg intraperitoneally). Anesthesia was maintained by an infusion of sodium pentobarbital (5 mg/kg per hour intravenously). A tracheostomy was performed, and an endotracheal tube (OD 4.5 mm) was inserted. This was connected to external tubing so that a mixture of 3% hydrogen in air could be administered for the blood flow studies. Intravascular volume was maintained with an infusion of isotonic saline (15 ml/kg per hour) through a catheter inserted into the left femoral vein. A catheter was inserted into the left femoral artery to provide blood gas determinations and continuous monitoring of arterial pressure. The arterial catheter was connected to a pressure transducer (P23XL; Statham Instruments Inc., Oxnard, CA). Body temperature was maintained at 37.5 ± 1.5°C.
Pancreatic microvascular blood flow was measured by the H2 gas clearance method with an intraductal platinum electrode. The technique had been validated previously. 2,10 At laparotomy, the spleen and the left lobe of the pancreas were delivered into the wound. The main pancreatic duct was located 5 mm distal to the site of ductal ligation; it was opened and cannulated with a platinum electrode, sheathed in a PE10 plastic cannula (external diameter 0.61 mm). The platinum tip of the electrode was placed in the main duct in the middle of the left lobe of the pancreas. The electrode was ligated in position and the abdominal viscera returned with care into the abdominal cavity. The reference electrode was placed in the subcutaneous tissue and secured into position. Using insulated copper wire, both electrodes were connected to a polarographic and amplifying unit. The current from the electrode was displayed graphically with time on a Gilson chart recorder. The preamplified output was fed into the input of an ADA-LAB analog-digital converter (VAL-TECH Electronics, Thousand Oaks, CA). Digital output was sampled every 6 seconds by an Apple IIe computer and stored in labeled sequential ASCII files on floppy discs. The monoexponential desaturation curves were analyzed using a software program that employed a modified nonlinear regression algorithm to provide an estimate of PMBF in ml/min per 100 g tissue. 11
Interstitial pH was measured, as described and validated previously, using a pH-sensitive glass electrode built into the beveled tip of a 21-gauge needle (MI-408; Microelectrodes, Inc., Londonderry, NH). 8 An Ag/AgCl electrode with a flexible barrel was used as a reference electrode (MI-402; Microelectrodes). pH was determined using an electrometer with automatic logarithmic conversion (Orion Research Digital pH/Millivolt Meter 611; Orion Research, Inc., Boston, MA) so that pH could be recorded directly. The electrodes were calibrated before insertion using two-point calibration in buffers of pH 6.0 and 8.0 at 37°C. The calibration was verified by inserting the electrodes into a pH 7.4 buffer at 37°C. The pancreas was exposed through an abdominal incision, and a <1-mm incision was made in its capsule with microscissors. Using a micromanipulator, the electrode was advanced 5 to 10 mm into the parenchyma through the incision and fixed in place. This technique avoided damage to blood vessels and ductal structures; the needle tip was too large to enter parenchymal cells. The reference electrode was placed in the subcutaneous tissue of the right groin.
Interstitial pH and PMBF electrodes were positioned within 45 minutes after the administration of anesthesia, and the animal was allowed to stabilize for 30 minutes before the start of the experiment. Baseline determinations of pHI and PMBF were then performed. Secretin (2 U/kg intravenously) or cholecystokinin (CCK-8; 0.08 μg/kg intravenously) were given in random order at 65 and 125 minutes. Three percent hydrogen in air was administered for 10 minutes via the tracheostomy and then removed. Over the subsequent 10 minutes, the animal was allowed to breathe room air while the desaturation curves were recorded. They were analyzed later to determine PMBF. Desaturation curves were obtained 10 minutes after the start of the experiment and 15 minutes before and 5 minutes after hormonal manipulation. pHI and arterial pressure were recorded at 5-minute intervals throughout the experiment. After the measurements were completed, pancreatic tissue was removed for histologic examination.
At the end of each experiment, the pancreatic duct was cannulated (PE10 cannula, external diameter 0.61 mm) and trypan blue (Sigma, St. Louis, MO) was injected after an enterotomy had been performed just distal to the PJ. If trypan blue was not seen through the small bowel enterotomy, the experiment would have been rejected. Patency of the PJ was demonstrated in all experiments.
At the end of the experiment, the middle of the left lobe of the pancreas was excised and placed in 4% formaldehyde solution and then embedded in paraffin. Blocks were sectioned 5 μm thick and stained with 1% Sirius red (Gurr BDH, London, UK) and 0.1% fast green FCF (Fluka, Buchs, Switzerland), which selectively stain collagen and noncollagen protein, respectively. 5,12 An Olympus light microscope (with a ×10 Nikon objective) attached to a Optronics 470 color camera (McBain Instruments, Chatsworth, CA) was used to view the histology specimens. The camera was directly linked to a Quantamat color image analysis system (Leica, McBain). Digitized images of each slide were obtained and analyzed. Analysis consisted of applying a computer-generated digital mask over the Sirius red stain (collagen protein) and calculating the area stained. The process was repeated with the mask applied to the area stained by both Sirius red and fast green (collagen and noncollagen protein, respectively), and the ratio between collagen and total protein (C/TP) was calculated. Similarly, collagen content (collagen/total area), noncollagen protein content (noncollagen protein/total area), and noncollagen and total protein ratio (NC/TP) were calculated. Each slide was analyzed blindly by three people, with the average of three values used in the final analysis.
Experimental Groups
The animal model of obstructive CP has been described in detail elsewhere. 2 Briefly, cats were anesthetized with ketamine (20 mg/kg intramuscularly) and xylazine hydrochloride (1 mg/kg intramuscularly). A laparotomy was performed and the main pancreatic duct was identified at the junction of the left lobe and the body of the pancreas. The duct was narrowed by 75% of its original diameter using a 4-0 silk ligature. The incision was closed and the animal was allowed to recover.
Early Chronic Pancreatitis Studies
The control groups of cats had PMBF and pHI studies performed at 2 (CP2) and 6 weeks (CP6) after partial pancreatic duct ligation (see Table 1). The surgical decompression group had PJ performed 2 weeks after partial duct ligation and then had PMBF and pHI studies performed 4 weeks later (PJ2/4).
Table 1. EFFECT OF PANCREATICOJEJUNOSTOMY IN CHRONIC PANCREATITIS: EXPERIMENTAL GROUPS
Surgical decompression was achieved by a Roux-en-Y distal PJ. Cats were anesthetized with xylazine hydrochloride (1 mg/kg intramuscularly) and ketamine (20 mg/kg intramuscularly), with repeated injections given to maintain anesthesia. A laparotomy was performed and the pancreas was exposed. The previous site of partial duct ligation was identified at the junction of the left lobe and the body of the pancreas. The main pancreatic duct was identified in the distal 1 to 1.5 cm of the left lobe of the pancreas. The dilated duct was exposed for 5 to 8 mm and a 2- to 3-mm segment of the duct was excised to ensure decompression. The jejunum was then transected 10 cm distal to the duodenojejunal flexure, and a two-layer anastomosis was performed using 6.0 proline between the distal jejunum and pancreas. The proximal jejunum was then anastomosed to the distal jejunum PJ using 5.0 vicryl. The incision was closed and the animal was allowed to recover. After surgery, the animals required intravenous fluids for 4 to 5 days and subcutaneous buprenorphine for 48 to 72 hours.
Established Chronic Pancreatitis Studies
The control groups of cats had PMBF and pHI studies performed at 4 (CP4), 6 (CP6), and 8 (CP8) weeks after partial pancreatic duct ligation (see Table 1). The surgical decompression groups had PJ performed 4 weeks after partial duct ligation, then PMBF and pHI studies were performed at 2 weeks in one group (PJ4/2) and 4 weeks later in another group (PJ4/4).
Data Analysis
Results are expressed as mean ± SEM. Differences among different groups of means were tested by one-way analysis of variance. If this showed that the means within an experiment were significantly different from one another, analysis of variance with multiple contrasts (t tests) was performed with a significance level of 1%. Values of PMBF and pHI were compared at different time points before and after hormonal stimulation with either CCK or secretin, using paired t tests with a significance level of 5%. Relations between variables was assessed using Pearson’s simple linear regression analysis, with a significance level of 5%.
RESULTS
Evolution of Chronic Pancreatitis
In the normal pancreas, PMBF increased as a result of secretory stimulation with secretin (114 ± 27 to 275 ± 50 ml/min per 100 g, p < 0.05) and CCK (128 ± 8 to 160 ± 7 ml/min per 100 g, p < 0.05), and pHI fell (7.41 ± 0.01 to 7.38 ± 0.01, p < 0.05, and 7.40 ± 0.01 to 7.38 ± 0.01, p < 0.05, respectively) (Figs. 1 through 4). Two weeks after partial duct obstruction, basal PMBF decreased to 61 ± 5 ml/min per 100 g (p < 0.01) and pHI to 7.26 ± 0.03 (p < 0.01). Secretory stimulation with secretin and CCK continued to result in a fall in PMBF (44 ± 5 to 33 ± 4 ml/min per 100 g, p < 0.05, and 61 ± 5 to 44 ± 7 ml/min per 100 g, p < 0.05, respectively) and pHI (7.21 ± 0.04 to 7.17 ± 0.04, p < 0.05, and 7.40 ± 0.01 to 7.38 ± 0.01, p < 0.05, respectively). Between 4 weeks and 8 weeks of ductal obstruction, basal PMBF and pHI remained lower than normal (Figs. 5 through 8), and secretory stimulation continued to cause PMBF to reduce further with secretin but not with CCK stimulation (see Figs. 5 and 6). pHI was not altered (see Figs. 7 and 8).
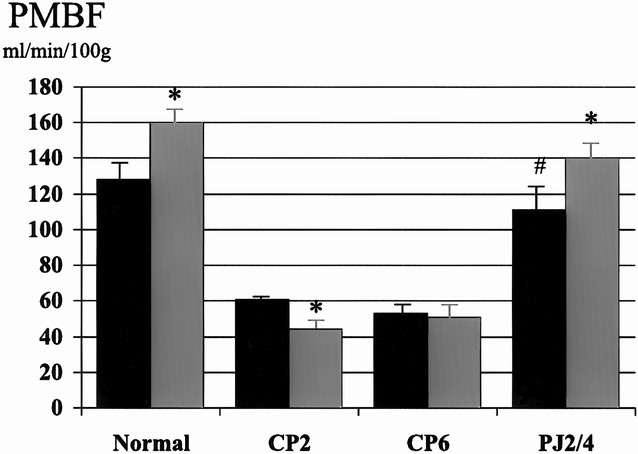
Figure 1. Effect of pancreaticojejunostomy (PJ) on pancreatic blood flow (PMBF) in early chronic pancreatitis. PJ (PJ2/4) returns basal PMBF to normal and restores the normal increase in PMBF associated with cholecystokinin (CCK) stimulation. Black bar, pre-CCK; gray bar, post-CCK. * p < 0.05 vs. basal, # p < 0.01 vs. CP6.
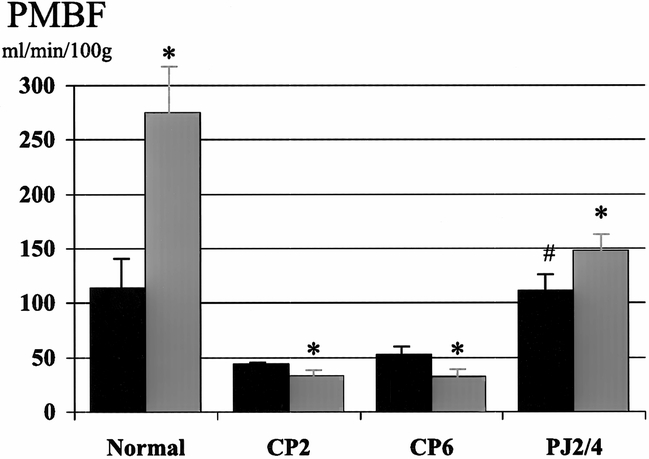
Figure 2. Effect of pancreaticojejunostomy (PJ) on pancreatic blood flow (PMBF) in early chronic pancreatitis. PJ (PJ2/4) returns basal PMBF to normal and restores the normal increase in PMBF associated with secretin stimulation. Black bar, pre-secretin; gray bar, post-secretin. * p < 0.05 vs. basal, # p < 0.01 vs. CP6.
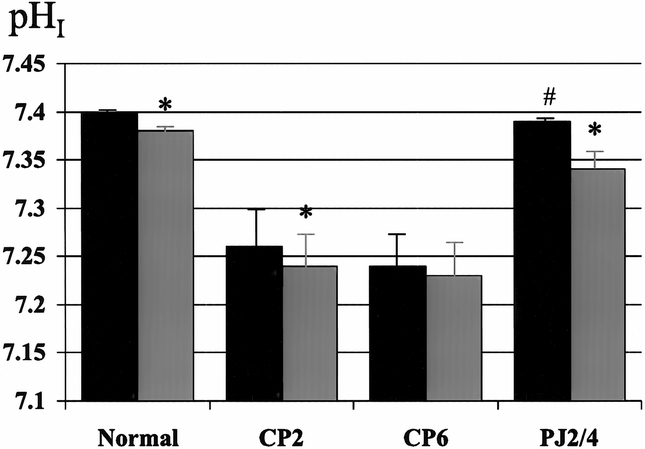
Figure 3. Effect of pancreaticojejunostomy (PJ) on interstitial pH (pHI) in early chronic pancreatitis. PJ (PJ2/4) returns basal pHI to normal and restores the normal decrease in pHI associated with cholecystokinin (CCK) stimulation. Black bar, pre-CCK; gray bar, post-CCK. * p < 0.05 vs. basal, # p < 0.01 vs. CP6.
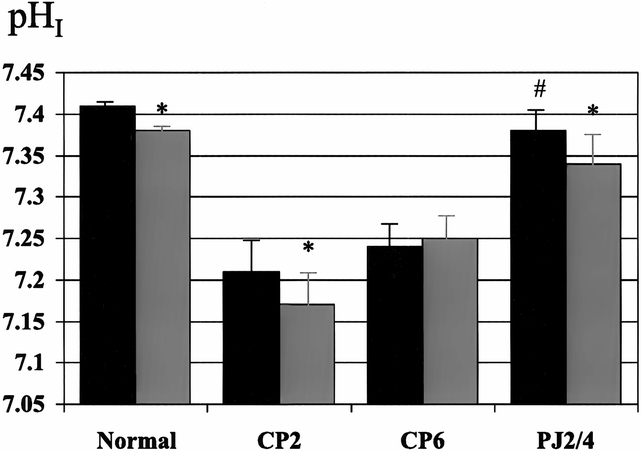
Figure 4. Effect of pancreaticojejunostomy (PJ) on interstitial pH (pHI) in early chronic pancreatitis. PJ (PJ2/4) returns basal pHI to normal and restores the normal decrease in pHI associated with secretin stimulation. Black bar, pre-secretin; gray bar, post-secretin. * p < 0.05 vs. basal, # p < 0.01 vs. CP6.
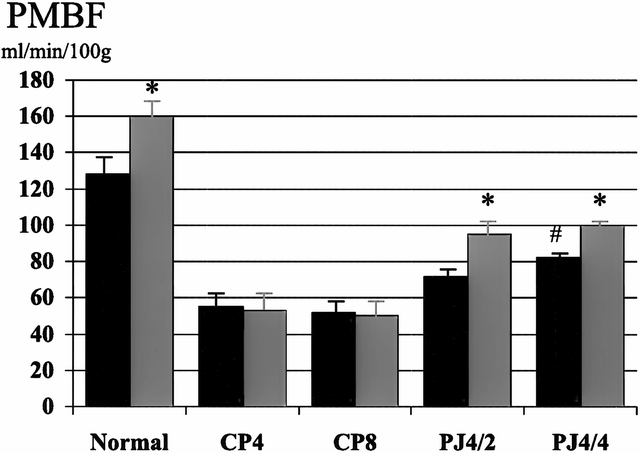
Figure 5. Effect of pancreaticojejunostomy (PJ) on pancreatic blood flow (PMBF) in established chronic pancreatitis. PJ improves basal PMBF and restores the normal increase in PMBF associated with cholecystokinin (CCK) stimulation. There was no difference in PMBF whether the duct remained decompressed for 2 (PJ4/2) or 4 (PJ4/4) weeks. Black bar, pre-CCK; gray bar, post-CCK. * p < 0.05 vs. basal, # p < 0.01 vs. CP8.
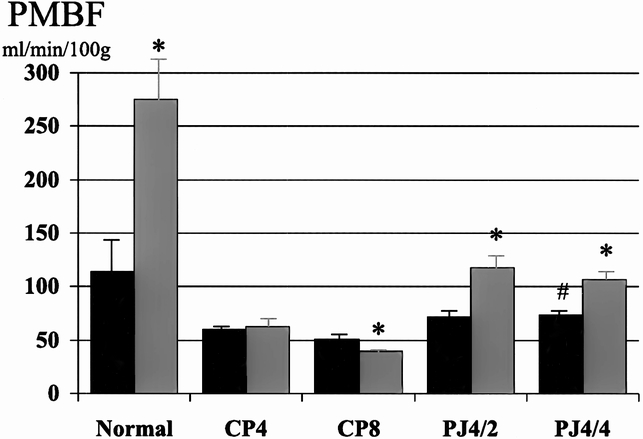
Figure 6. Effect of pancreaticojejunostomy (PJ) on pancreatic blood flow (PMBF) in established chronic pancreatitis. PJ improves basal PMBF and restores the normal increase in PMBF associated with secretin stimulation. There was no difference in PMBF whether the duct remained decompressed for 2 (PJ4/2) or 4 (PJ4/4) weeks. Black bar, pre-secretin; gray bar, post-secretin. * p < 0.05 vs. basal, # p < 0.01 vs. CP8.
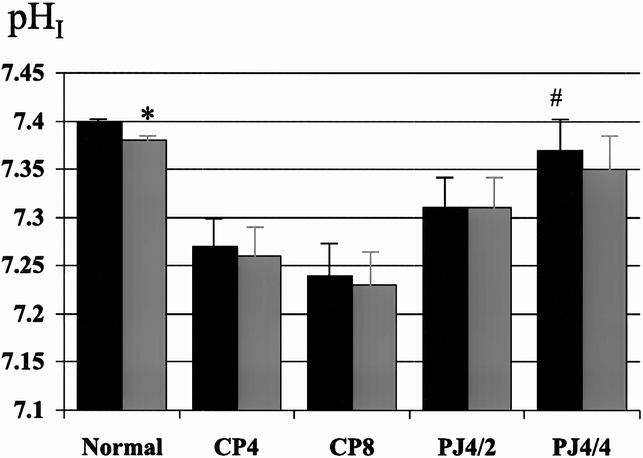
Figure 7. Effect of pancreaticojejunostomy (PJ) on interstitial pH (pHI) in established chronic pancreatitis. PJ improves basal pHI, but cholecystokinin (CCK) stimulation has no effect on pHI, either 2 (PJ4/2) or 4 (PJ4/4) weeks after decompression. Black bar, pre-CCK; gray bar, post-CCK. * p < 0.05 vs. basal, # p < 0.01 vs. control.
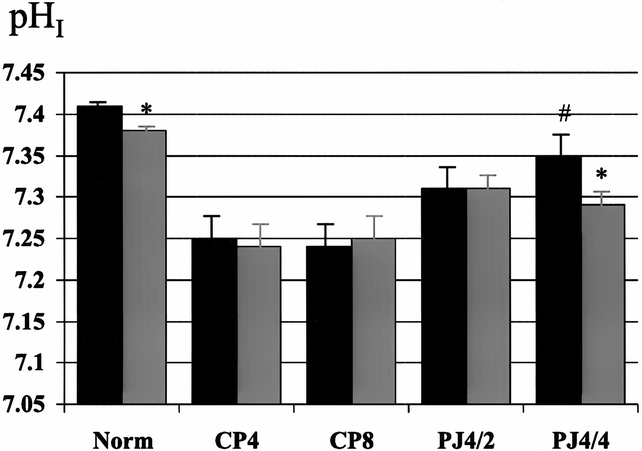
Figure 8. Effect of pancreaticojejunostomy (PJ) on interstitial pH (pHI) in established chronic pancreatitis. PJ improves basal pHI. pHI response to secretin stimulation is present after 4 week of ductal decompression (PJ4/4). Black bar, pre-secretin; gray bar, post-secretin. * p < 0.05 vs. basal, # p < 0.01 vs. CP8.
Two weeks after partial duct obstruction, parenchymal fibrosis was evident; collagen content and C/TP increased (0.31 ± 0.02 to 0.45 ± 0.02, p < 0.01, and 0.4 ± 0.04 to 0.56 ± 0.04, p < 0.01, respectively). The C/TP ratio increased progressively with the increasing period of pancreatic duct obstruction (Fig. 9). There was good correlation between collagen content and C/TP (n = 9, r = 0.95, p < 0.001). The NC/TP decreased with the increasing period of duct obstruction (Fig. 10). The NC/TP correlated well with the noncollagen protein content (n = 9, r = 0.99, p < 0.001).
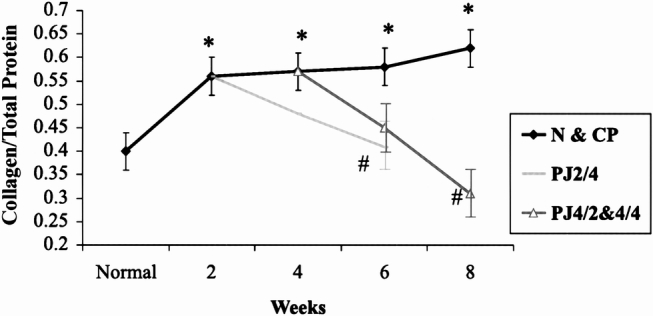
Figure 9. Effect of pancreaticojejunostomy (PJ) on collagen and total protein (C/TP) in chronic pancreatitis. The progressive increase in C/TP after partial pancreatic duct ligation returns to normal 4 weeks after PJ. * p < 0.007 vs. normal, # p < 0.007 vs. CP.
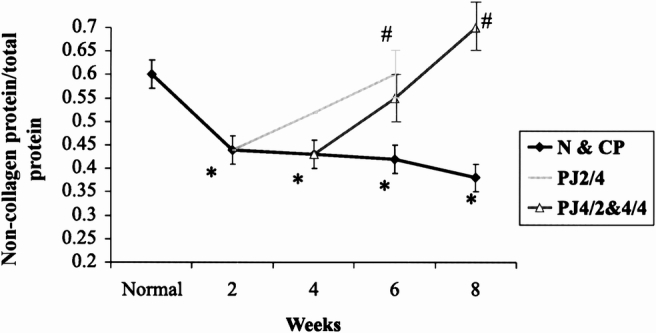
Figure 10. Effect of pancreaticojejunostomy (PJ) on noncollagen and total protein ratio (NC/TP) in chronic pancreatitis. The progressive decrease in the NC/TP after partial pancreatic duct ligation returns to normal 4 weeks after PJ. * p < 0.007 vs. normal, # p < 0.007 vs. CP.
Early Chronic Pancreatitis Studies
The surgical decompression group had PJ performed 2 weeks after partial duct ligation and then had PMBF and pHI studies performed 4 weeks later (PJ2/4). In this group, basal PMBF was restored to normal and was significantly better than the control group (CP6). The normal increase in PMBF associated with secretory stimulation, with both secretin (111 ± 15 to 148 ± 19 ml/min per 100 g, p < 0.05) and CCK (111 ± 15 to 140 ± 16 ml/min per 100 g, p < 0.05), was also restored (see Figs. 1 and 2). Surgical decompression returned basal pHI to normal (7.40 ± 0.01) and restored the normal decrease in pHI associated with secretory stimulation with secretin (7.38 ± 0.03 to 7.34 ± 0.04, p < 0.05) and CCK (7.39 ± 0.01 to 7.34 ± 0.02, p < 0.05) (see Figs. 3 and 4).
The C/TP ratio and collagen content returned to normal and were less than the control group (CP6) (see Figs. 9 and 11). The NC/TP and noncollagen protein also returned to normal and were significantly greater than the control group (CP6) (see Figs. 10 and 11).
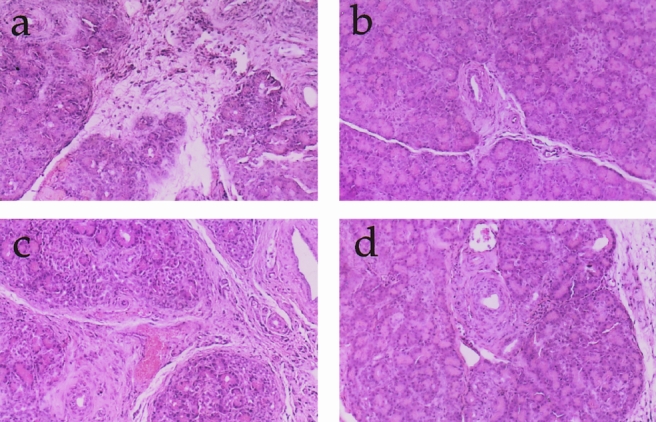
Figure 11. Histology (×50): (A) CP6, (B) PJ2/4, (C) PJ4/2, (D) PJ4/4. With the progression of chronic pancreatitis, there is marked acinar destruction fibrosis. Pancreaticojejunostomy decreases the fibrosis and preserves and may regenerate the lobular architecture.
Established Chronic Pancreatitis
The surgical decompression group had PJ performed 4 weeks after partial duct ligation and then had PMBF and pHI studies performed 2 (PJ4/2) and 4 weeks later (PJ4/4). Basal PMBF in the PJ4/2 group improved with PJ, but not significantly (52 ± 8 vs. 72 ± 6 ml/min per 100 g, p = 0.07) compared with the control group (CP6). In the PJ4/4 group (55 ± 7 vs. 82 ± 5 ml/min per 100 g, p < 0.01), PMBF was significantly higher than in the control group (CP8). The normal increase in PMBF with secretory stimulation was also restored in both groups after PJ (see Figs. 5 and 6). Surgical decompression (PJ4/2 and PJ4/4) increased the low basal pHI toward normal and was significantly better than the chronic pancreatitis controls (CP6 and CP8) (7.31 ± 0.03 vs. 7.24 ± 0.03, p < 0.01; 7.38 ± 0.02 vs. 7.24 ± 0.03, p < 0.01, respectively). Secretory stimulation with either secretin or CCK had no effect on pHI in the PJ4/2 group. In the PJ4/4 group, pHI fell from 7.35 ± 0.02 to 7.29 ± 0.02 (p < 0.05) with secretin stimulation, but CCK had no effect.
The C/TP ratio and collagen content increased, whereas NC/TP and noncollagen protein content decreased, in the CP glands at 4, 6, and 8 weeks after ductal ligation (see Figs. 9 through 11). Surgical decompression restored the collagen content, C/TP, noncollagen protein content, and NC/TP to normal after 4 weeks of pancreatic ductal decompression (see Figs. 10 and 11).
DISCUSSION
Chronic pancreatitis is characterized by severe, intractable abdominal pain, which is the main indication for surgery. Ductal obstruction as an etiologic factor accounts for 5% of patients with CP in North America and Europe; alcohol is the major cause (>70%). There is an element of ductal hypertension in the latter group as a result of strictures, protein plugs, intraductal calculi, and inelastic capsule. 2,3 The elevated pancreatic interstitial pressure in CP decreases with surgical decompression. The absence of a decrease in interstitial pressure is associated with failure of the PJ to relieve pain. 13 Recently, a combination of endoscopic pancreatic sphincterotomy, extracorporeal shockwave lithotripsy, and stenting has been used to clear the pancreatic duct and decrease intraductal pressure. 14 It has been suggested that if there is no relief of pain with pancreatic stenting, then surgical decompression is unlikely to be effective. 15 However, experimental data from a feline model of obstructive CP, using an isolated perfused pancreas preparation, has shown that surgical decompression in CP is more effective than stenting in decreasing interstitial pressure and pancreatic vascular resistance. 16 Also, Dumonceau et al performed endoscopic pancreatic sphincterotomy and attempted to clear the duct of intraductal stones in 70 patients 14; they were successful in 35 (50%). In this subgroup of patients, pain relief was on the order of 90% immediately and 54% at 2 years, rates comparable with surgery. There was a close relation between duct clearance and the disappearance of pain.
The feline model of partial duct obstruction is an ideal model to study the pathophysiology of CP. In CP, basal interstitial pressure was shown to be higher and PMBF and pHI lower than in the normal gland. 2,9 Basal pHI is a good index of ischemia, especially because basal pancreatic secretion is negligible. Secretin stimulation results in a further increase in the interstitial pressure and a decrease in PMBF, 2,9 thus suggesting that a compartment syndrome may exist with underlying ischemia in CP. This is similar to the clinical situation in painful CP regardless of etiology, where interstitial and ductal pressures are elevated and PMBF and pHI are low. 9,15 Interestingly, pain is often precipitated by alcohol and secretory stimulation, both of which cause a further rise in the increased interstitial pressure and cause the low PMBF to decrease further. 17,18
Pancreaticojejunostomy has been shown to decrease interstitial pressure in CP, but the effects on PMBF and pHI have not previously been studied. In the early CP group, PJ was found to improve the low basal PMBF and pHI to normal levels and to restore the normal increase in blood flow with secretion. Similarly, in the established CP group, although basal PMBF and pHI improved significantly, they did not reach normal levels. Increasing the time interval of pancreatic duct decompression from 2 to 4 weeks produced no significant difference in the improved PMBF and pHI. The effect of a longer period of ductal decompression was not studied in this established CP group. PJ not only improves basal PMBF and pHI but also restores the normal hyperemic response to secretion.
In the normal pancreas, PMBF increases with secretion and pHI decreases transiently. This transient decrease in pHI reflects increased hydrogen ion production during both protein and bicarbonate secretion and occurs with both secretin and CCK stimulation. With the evolution of CP, secretory output decreases and the transient decrease in pHI is lost, and basal pH and PMBF also decrease. 9 In the early CP group, PJ was performed 2 weeks after partial pancreatic duct ligation. At this stage, the transient decrease in pHI with secretion was present and PJ prevented the loss of this response, suggesting that PJ prevents further loss of exocrine function in early CP. In established CP, 2 weeks after PJ there was no change in the low pHI with secretory stimulation. Four weeks after PJ, a transient decrease in pHI was seen with both CCK and secretin stimulation, but this was significant only in the latter group, suggesting that there was some restoration of bicarbonate secretion.
During the evolution of CP in the partial ductal obstruction model, pancreatic secretion diminishes and there is a progressive increase in fibrosis with acinar and ductal cell destruction. In these experiments, the effects of PJ on fibrosis were studied. Quantitative assessment of the degree of fibrosis in human CP has been performed previously, using colorimetric and morphometric techniques. Valderrama et al, using the selective capacity of Sirius red to stain collagen protein and fast green FCF to stain noncollagen protein, showed a good correlation (r = 0.85) between colorimetric and morphometric assessment of the fibrosis in CP. 12 We used a computer-assisted, morphometric assessment of the C/TP ratio to assess the degree of fibrosis, using Sirius red and fast green FCF. In the feline model of CP, fibrosis increased markedly after 2 weeks of ductal ligation, followed by a slower gradual increase. Basal PMBF and pHI also markedly decreased after 2 weeks of ductal ligation, followed by a gradual decrease. This suggests that there is a progressive increase in the degree of ischemia. Transforming growth factor β1 is thought to be a mediator of fibrosis in many disease processes, including cirrhosis of the liver and idiopathic pulmonary fibrosis. 19 Van Laethem et al have shown that transforming growth factor β1 plays an important role in the development of fibrosis in CP. 20 Interestingly, hypoxia has been shown to release transforming growth factor β. 21 PJ restores the degree of fibrosis, both in the early and established CP groups, to normal levels.
Tiscornia and Dreiling 22 showed that acute ductal obstruction results in a decrease in exocrine function and a marked increase in fibrosis in dogs, assessed subjectively. Subsequent excision of the stricture and reanastomosis of the duct resulted in recovery of secretory function over a long period of time and a decrease in fibrosis, suggesting that recovery from ductal obstruction is possible both in terms of morphologic and secretory function. This is in agreement with the Marseilles 1984 classification, which defined obstructive CP as a reversible condition in terms of both morphology and secretory function. 23 Because an element of pancreatic ductal obstruction exists both in alcoholic CP and obstructive CP, it is not surprising that Nealon and Thompson found that surgical decompression in the form of PJ results in a delay in the deterioration in exocrine function in humans with CP. 7
We propose that regardless of the etiology of CP, a compartment syndrome exists and is associated with underlying ischemia. PJ is effective in painful CP by reducing ductal hypertension, improving PMBF, and reducing tissue acidosis. The evidence presented here suggests that PJ may even improve exocrine secretion and decrease fibrosis in CP.
Footnotes
Correspondence: Ameet Patel, FRCS, Dept. of Surgery, King’s College Hospital, Denmark Hill, London SE5 9RS, United Kingdom.
Supported by Veterans Administration Merit Review Awards (HAR, SWA).
Accepted for publication May 24, 1999.
References
- 1.Hakaim AG, Broughan TA, Vogt DP, Hermann RE. Long-term results of the surgical management of chronic pancreatitis. Am J Surg 1994; 60:306–308. [PubMed] [Google Scholar]
- 2.Reber HA, Karanjia ND, Alvarez C, et al. Pancreatic blood flow in cats with chronic pancreatitis. Gastroenterology 1992; 103:652–659. [PubMed] [Google Scholar]
- 3.Ebbehøj N, Borly L, Madsen P, Matzen P. Pancreatic tissue fluid pressure during drainage operations for chronic pancreatitis. Scand J Gastroenterol 1990; 25:1041–1045. [DOI] [PubMed] [Google Scholar]
- 4.Gress TM, Muller-Pillasch F, Lerch MM, et al. Balance of expression of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in chronic pancreatitis. Z Gastroenterol 1994; 32:221–225. [PubMed] [Google Scholar]
- 5.Bedossa P, Lemaigre G, Bacci J, Martin E. Quantitative estimation of the collagen content in normal and pathologic pancreas tissue. Digestion 1989; 44:7–13. [DOI] [PubMed] [Google Scholar]
- 6.Nagata A, Homma T, Oguchi H, et al. Study of chronic alcoholic pancreatitis by means of serial pancreozymin-secretin tests. Digestion 1986; 33:135–145. [DOI] [PubMed] [Google Scholar]
- 7.Nealon WH, Thompson JC. Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression. A longitudinal prospective analysis of the modified Puestow procedure. Ann Surg 1993; 217:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley SW, Schwarz M, Alvarez C, Nguyen TN, Dovenko A, Reber HA. Pancreatic interstitial pH regulation: effects of secretory stimulation. Surgery 1994; 115:503–509. [PubMed] [Google Scholar]
- 9.Patel AG, Toyama MT, Alverez C, Nguyen T, Ashley SW, Reber HA. Pancreatic interstitial pH in human and feline chronic pancreatitis. Gastroenterology 1995; 109:1639–1645. [DOI] [PubMed] [Google Scholar]
- 10.Ashley SW, Cheung LY. Measurement of gastric mucosal blood flow by hydrogen gas clearance. Am J Physiol 1984; 247:G339–G345. [DOI] [PubMed] [Google Scholar]
- 11.Livingston EH, Reedy T, Leung FW, Guth PH. Computerized curve fitting in the analysis of hydrogen gas clearance curves. Am J Physiology 1989; 257:G668–G675. [DOI] [PubMed] [Google Scholar]
- 12.Valderrama R, Navarro S, Campo E, et al. Quantitative measurement of fibrosis in pancreatic tissue. Evaluation of a colorimetric method. Intl J Pancreatol 1991; 10:23–29. [DOI] [PubMed] [Google Scholar]
- 13.Ebbehøj N, Christensen E, Madsen P. Prediction of the outcome of pancreaticogastrostomy for pain in chronic pancreatitis. Scand J Gastroenterol 1987; 22:337–342. [DOI] [PubMed] [Google Scholar]
- 14.Dumonceau JM, Deviere J, Le Moine O, et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc 1996; 43 (6):547–555. [DOI] [PubMed] [Google Scholar]
- 15.Cremer M. Endoscopy, treatment of the future in chronic pancreatitis? Gastroenterol Clin Biol 1993; 17:787–791. [PubMed] [Google Scholar]
- 16.Reber PU, Patel AG, Lewis MT, Ashley SW, Reber HA. Stenting does not decompress interstitial pressure as effectively as surgery in experimental chronic pancreatitis. Surgery 1998; 124:561–567. [PubMed] [Google Scholar]
- 17.Lewis MPN, Lo SK, Reber P, et al. Endoscopic measurement of pancreatic blood flow: alterations in patients with chronic pancreatitis. Gastroenterology 1996; 110:A4111. [Google Scholar]
- 18.Toyama MT, Patel AG, Nguyen T, Ashley SW, Reber HA. Effect of ethanol on pancreatic interstitial pH and blood flow in cats with chronic pancreatitis. Ann Surg 1997; 225:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med 1994; 331:1286–1291. [DOI] [PubMed] [Google Scholar]
- 20.Van Laethem JL, Deviere J, Resibois A, et al. Localization of transforming growth factor beta-1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 1995; 108:1873–1881. [DOI] [PubMed] [Google Scholar]
- 21.Falanga V, Qian SW, Danielpour D, Katz MH, Roberts AB, Sporn MB. Hypoxia upregulates the synthesis of TGF-beta-1 by human dermal fibroblasts. J Invest Dermatol 1991; 97:634–637. [DOI] [PubMed] [Google Scholar]
- 22.Tiscornia OM, Dreiling DA. Recovery of pancreatic exocrine secretory capacity following prolonged ductal obstruction: bicarbonate and amylase response to hormonal stimulation. Ann Surg 1966; 164:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyr KE, Singer MV, Sarles H, eds. Revised classification of pancreatitis. In: Pancreatitis: concepts and classification. Amsterdam: Elsevier Science Publishers BV; 1984:XXIII–XXV.