Abstract
Objective
To identify characteristics of the primary tumor highly associated with lymph node metastases.
Summary Background Data
Recent enthusiasm for limiting axillary lymph node dissection (ALND) in women with breast cancer may increase the likelihood that nodal metastases will be missed. Identification of characteristics of primary tumors predictive of lymph node metastases may prompt a more extensive surgical and pathologic search for metastases in patients with negative sentinel lymph nodes or limited ALND.
Methods
The authors studied 850 consecutive patients who underwent ALND for T1 breast cancer. Age, tumor size, histopathologic diagnosis, tumor differentiation, presence of lymphatic invasion, and estrogen and progesterone receptor results were studied prospectively. Stepwise logistic regression was used to identify variables independently associated with axillary lymph node metastases.
Results
Lymphatic invasion, tumor size, and age were independently associated with lymph node metastases. Fifty-one percent of the 181 patients with lymphatic invasion had axillary lymph node metastases, compared with 19% of the 669 patients without lymphatic invasion. Thirty-five percent of the 470 patients with tumors >1 cm had nodal involvement compared with 13% of the 380 patients with smaller cancers. Thirty-seven percent of the 63 women younger than age 40 had lymph node involvement compared with 25% of the 787 women older than age 40. Significant correlations were noted between lymphatic invasion and patient age and between lymphatic invasion and tumor size. The proportion of tumors with lymphatic invasion decreased progressively with increasing age and increased with increasing tumor size.
Conclusions
Axillary lymph node metastases are most significantly related to lymphatic invasion in the primary tumor, followed, in order of significance, by tumor size and patient age. Axillary nodal metastases should be suspected in the presence of lymphatic invasion of large tumors in young patients.
Axillary lymph node involvement is the most significant and durable prognostic factor for women with breast cancer. 1 Small cancers without nodal involvement have an extremely favorable prognosis. Metastasis to a single axillary node more than doubles the risk of distant disease. Consequently, nodal involvement in T1 cancers often determines whether a patient is treated with adjuvant chemotherapy. 2
The focus of recent studies has been the identification of patients unlikely to benefit from axillary lymph node dissection (ALND) because the risk of nodal metastases is extremely low. 3–13 The increasing use of sentinel lymph node biopsy may reduce the complications of ALND at the expense of increasing the risk of missed nodal metastases. In addition to identifying patients at low risk of axillary metastases, we should identify characteristics of primary tumors predictive of lymph node involvement. 4,5,7,10,14 In these patients, a limited ALND or sentinel lymph node biopsy may have a high false-negative rate. Recent enthusiasm for limiting the extent of removal of axillary lymph nodes increases the likelihood that nodal metastases will be missed. 3,9 The evolving role of sentinel lymph node biopsy must include consideration for characteristics of primary tumors that make nodal involvement likely.
To identify characteristics of primary tumors highly associated with axillary lymph node metastases, we studied consecutive patients with T1 invasive breast cancers who underwent ALND. Identifying such patients could prompt a more extensive surgical and pathologic search for metastases in a patient with negative nodes on sentinel lymph node biopsy or limited ALND.
PATIENTS AND METHODS
The pathology records of the Mount Sinai Medical Center were reviewed to identify women with invasive breast cancers measuring ≤2.0 cm who underwent definitive surgical treatment including ALND between January 1993 and July 1998. Information was prospectively collected concerning patient age, tumor size, histopathology, tumor differentiation, lymphatic invasion, estrogen and progesterone receptor levels, involvement of axillary lymph nodes, and type of surgery. In patients with multiple primary invasive tumors, the size of the largest infiltrating lesion was recorded. Histologic slides were initially read or, in a minority of cases, retrospectively reviewed by one of the authors (IJB).
Data were analyzed using the SPSS statistical program (SPSS Incorporated, Chicago, IL). Stepwise logistic regression was used to identify the most significant characteristics of the primary tumor associated with axillary lymph node involvement. The significance of differences in categorical variables was evaluated using the chi square test, and the significance of differences in continuous variables was evaluated using Student’s t test. The significance of the relation between variables was evaluated using correlation coefficients.
RESULTS
The patients ranged in age from 25 to 92 (mean 58). The invasive tumors ranged in size from microscopic, less than 1 mm, to 2 cm (mean 1.2 cm). One hundred five (12%) of the carcinomas measured up to 5 mm (median 3 mm) in greatest diameter (T1a), 275 (32%) were 5 to 10 mm (median 8 mm, T1b), and 470 (55%) were >10 mm to 20 mm (median 15 mm, T1c). Carcinomas <1 mm in diameter included 21 T1 MIC cancers (ductal carcinoma in situ with microinvasion). Seventy-three percent (n = 616) of the 850 T1 cancers were infiltrating ductal, 11% (n = 95) were infiltrating lobular, 11% (n = 92) were tubular or tubulolobular, 4% (n = 33) were colloid, and 1.5% (n = 13) were medullary. Six percent of the tumors were well differentiated, 50% were moderately differentiated, and 43% were poorly differentiated. Lymphatic invasion was identified in 21% (n = 181) of the carcinomas, and 25% (n = 216) of the 850 patients had axillary lymph node metastases. Seventy-five percent (n = 635) were estrogen receptor-positive and 63% (n = 528) were progesterone receptor-positive. Seventy-four percent (n = 628) of the patients were treated with breast conservation.
Stepwise logistic regression was used to identify variables significantly related to nodal involvement (Table 1). In step 0, age, lymphatic invasion, tumor differentiation, and tumor size were significantly related to nodal involvement. Because lymphatic invasion was the most significant of these variables, it was the first variable added to the equation predicting nodal involvement. Lymphatic invasion was significantly related to nodal involvement because the majority (51%) of patients with lymphatic invasion had axillary lymph node metastases, compared with 19% of patients without identifiable lymphatic invasion (p < 0.001, Table 2). Lymphatic invasion was not predictive of the degree of nodal involvement because the average number of involved nodes in patients with lymphatic invasion was 3.1 versus 3.2 for patients without lymphatic invasion.
Table 1. STEPWISE LOGISTIC REGRESSION WITH NODAL INVOLVEMENT AS THE DEPENDENT VARIABLE
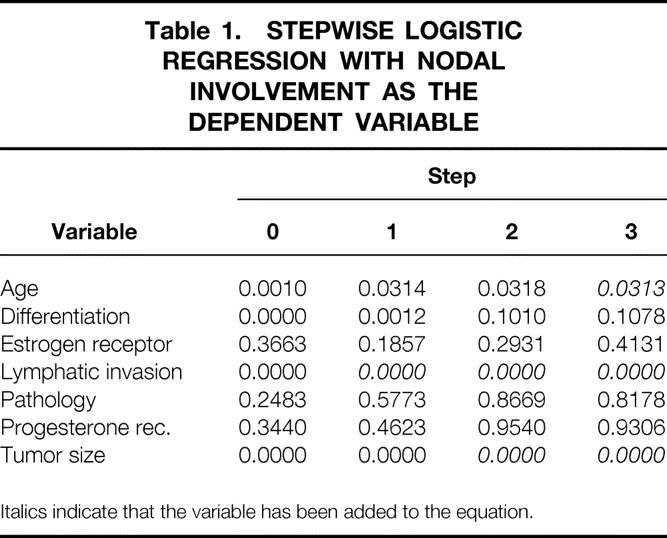
Italics indicate that the variable has been added to the equation.
Table 2. VARIABLES SIGNIFICANTLY RELATED TO NODAL INVOLVEMENT IN STEPWISE LOGISTIC REGRESSION (%)
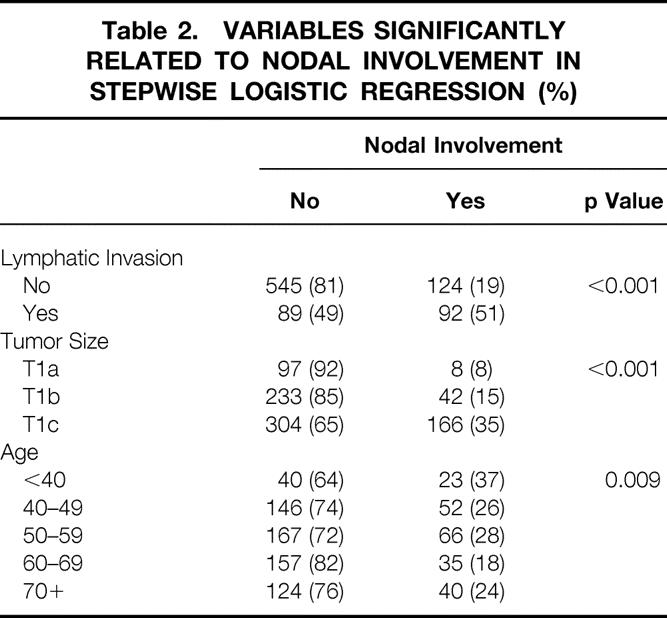
After consideration for lymphatic invasion in step 1 of the logistic regression, age, size, and differentiation continued to be significantly related to nodal involvement. Because tumor size was the most significant of these, it was added to the equation containing lymphatic invasion predicting nodal involvement. Larger tumors were more commonly associated with involved axillary lymph nodes (p < 0.001, see Table 2). Thirty-five percent (n = 166) of the 470 tumors >1 cm were associated with nodal involvement versus 13% (n = 50) of the 380 patients with smaller tumors. The average size of node-negative tumors was 1 cm versus 1.4 cm for node-positive tumors (p = 0.035).
After consideration for lymphatic invasion and tumor size in step 2 of the logistic regression, tumor differentiation was no longer significantly related to nodal involvement (see Table 1). The remaining variable significantly related to nodal involvement was patient age, and this was added to the equation containing lymphatic invasion and tumor size in step 3. Age was significantly related to nodal involvement because 37% (n = 23) of the 63 women younger than age 40 had nodal involvement compared with 25% (n = 193) of the 787 women older than age 40 (p = 0.009, see Table 2).
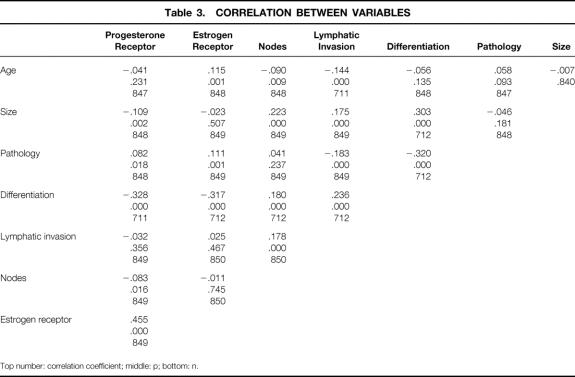
Significant correlations were noted between lymphatic invasion and patient age and between lymphatic invasion and tumor size (Table 3). The proportion of tumors with lymphatic invasion decreased progressively with increasing age: 43% of women younger than 40, 23% of women 40 to 50, 20% of women 50 to 60, 20% of women 60 to 70, and 11% of women older than 70 had tumors with lymphatic invasion (p < 0.001). The average age of patients with lymphatic invasion was 54, compared with 59 for patients without lymphatic invasion (p < 0.001). The average size of tumors with lymphatic invasion was 1.3 cm, compared with 1.1 cm for tumors without lymphatic invasion (p < 0.001), and 71% of tumors with lymphatic invasion were >1.0 cm versus 51% of carcinomas without lymphatic invasion. The proportion of tumors with lymphatic invasion increased progressively with increasing tumor size from 10% (n = 11) of the 105 T1a cancers to 15% (n = 41) of the 275 T1b and 27% (129) of the 470 T1c cancers (p < 0.001, Table 4).
Table 3. CORRELATION BETWEEN VARIABLES
Top number: correlation coefficient; middle: p; bottom: n.
Table 4. COMPARISON OF PATIENTS WITH AND WITHOUT LYMPHATIC INVASION (%)
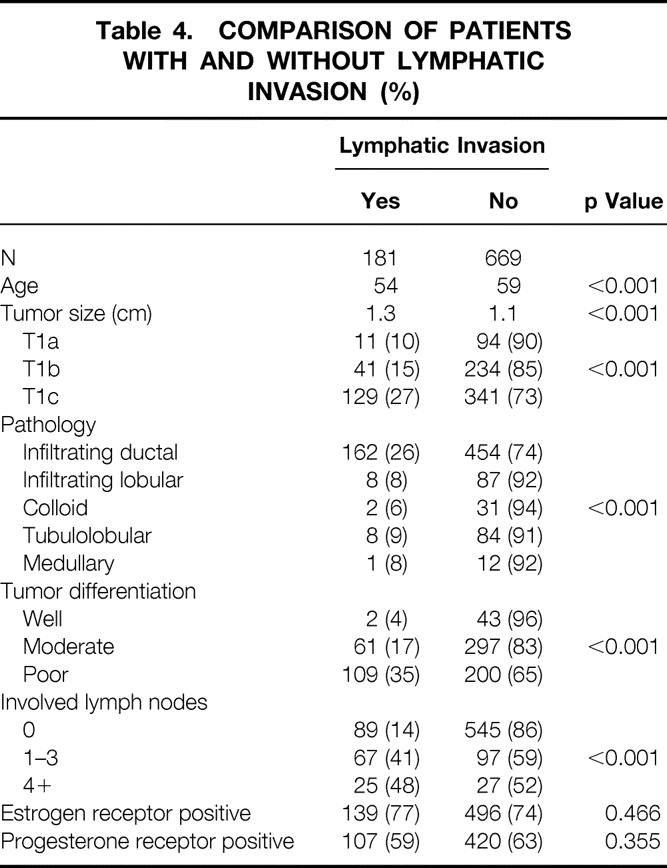
Lymphatic invasion was a strong predictor of nodal involvement in all patients except those older than 70. Lymphatic invasion increased the risk of nodal involvement >2.5 times (from 21% to 55%) in women younger than 70. In women 70 and older, the presence of lymphatic invasion increased the risk of nodal involvement 1.3 times (from 23% to 30%).
These results indicate that lymph node involvement in T1 breast cancers is most significantly related to lymphatic invasion in the primary cancer. Both increasing tumor size and young age were also significantly related to nodal involvement after consideration for lymphatic invasion. Lymphatic invasion was significantly related to tumor size and patient age. Tumor differentiation, histopathologic subtype, and estrogen and progesterone receptor status were not related to nodal involvement.
DISCUSSION
Axillary node involvement is the most significant and durable prognostic factor for women with breast cancer. 1 This is especially true for women with T1 cancers because nodal metastases double the risk of distant disease and influence therapeutic decisions. The use of systemic adjuvant chemotherapy is often determined by the presence or absence of axillary lymph node metastases. 2 Axillary lymph node involvement is found in 21% to 42% of patients with T1 lesions. 7,10,15 Variability in the incidence of nodal metastases is related to the number of lymph nodes removed and the histopathologic methods used to find metastases. The identification of characteristics of the primary tumor that are associated with nodal metastases might cause the surgeon to perform a more extensive axillary dissection and the pathologist to use methods of examining the nodes that increase the likelihood of finding metastatic disease.
The most significant characteristic of primary tumors related to nodal involvement in this study was the presence of lymphatic invasion. Lymphatic invasion has been reported in 13% to 27% of T1 cancers. 7,16,17 The presence of lymphatic invasion in cancers is associated with positive nodes in 46% to 64% of patients with T1 cancers. 6,7,10 In multivariate studies, lymphatic invasion has consistently been the most significant variable associated with nodal disease. 5–7,10 Lymphatic invasion may be regarded as the precursor of nodal involvement, and all patients with nodal involvement can be assumed to have had lymphatic invasion in the primary whether detected by the pathologist or not. However, the converse may not be true: not all patients with lymphatic invasion necessarily have nodal involvement. 16,18–20 In our study, most of the patients with lymphatic invasion had involved nodes, but almost half did not, when studied by routine histologic technique. Several studies, however, have shown that more intensive pathologic examination of axillary lymph nodes by the combined use of additional hematoxylin-and-eosin–stained levels and immunohistochemical techniques uncovers occult metastases in 10% to 24% of node-negative patients. 21–23 If it were possible to perform this level of examination routinely, it is likely that the predictive value of lymphatic invasion for nodal disease would increase even further.
The risk of involved nodes also increases with increasing tumor size. 1,3,5–10,13 Three percent to 11% of T1a, 13% to 17% of T1b, and 26% to 35% of T1c cancers have lymph node metastases, irrespective of the presence or absence of lymphatic invasion. As tumors increase in size, they are more likely to exhibit both lymphatic invasion and positive lymph nodes. 3,7 One might speculate that tumor size predicts nodal disease simply because it also predicts lymphatic invasion, which remains occult because of pathologic sampling issues. Assessment of lymphatic invasion in breast cancer is most productive with the histologic examination of greater amounts of tissue and is most accurately assessed at the periphery of the lesion. As invasive tumors increase in size, a smaller percentage of the tumor area is histologically examined. Further, as the size increases, the peripheral areas of the tumor increase and the most relevant sites for assessment of lymphatic invasion may receive relatively less pathologic attention. Thus, larger tumors may predict node positivity because of occult histologic lymphatic invasion.
Sampling error cannot explain the independent association of patient age with nodal involvement, which has been reported in studies both with and without multivariate analysis. 5–7 In our study, 43% of women younger than 40 had lymphatic invasion and 37% had involved nodes. Nodal involvement in women with or without lymphatic invasion was more frequent in women younger than 40. Young age is also an independent prognostic factor for women with breast cancer, independent of nodal involvement and tumor size. 24 Several studies have attributed the independent association of young age with prognosis to association with other prognostic factors, such as tumor grade or proliferative measures. 25,26 However, in large studies, young age has prognostic resilience comparable to nodal involvement. 4,27 The prognostic value of age remains unexplained.
This study of T1 invasive breast cancers found that lymphatic invasion, tumor size, and age are independently associated with axillary lymph node metastasis. Lymphatic invasion, whether histologically evident or not, is a prerequisite to lymph node metastases. Tumor size may be independently associated with nodal metastases because inherent limitations to pathologic sampling cause lymphatic invasion to be missed in larger tumors. The independent association of age with lymph node metastases and, in other studies, with poor prognosis has not been explained by the association of age with other prognostic variables. These results suggest that surgeons and pathologists should be diligent and thorough in searching for lymph node metastases in young patients with large tumors or lymphatic invasion. Examining additional levels and immunohistochemical studies on all axillary lymph nodes in such cases is not feasible. A reasonable compromise would be to perform sentinel lymph node biopsy in such patients so that the lymph nodes most likely to harbor metastasis are identified and examined with additional methods. Sentinel lymphadenectomy not only serves to avoid full node dissection but also may increase the precision of pathologic staging of the axilla, especially in patients at high risk for lymph node metastasis.
Footnotes
Correspondence: Paul Ian Tartter, MD, Box 1259, Dept. of Surgery, Mount Sinai Medical Center, New York, NY 10029.
Accepted for publication April 5, 1999.
References
- 1.Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin 1997; 47:28–51. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Buzdar AU. Current status of adjuvant systemic therapy for primary breast cancer: progress and controversy. CA Cancer J Clin 1995; 45:199–226. [DOI] [PubMed] [Google Scholar]
- 3.Port ER, Tan LK, Borgen PI, Van Zee KJ. Incidence of axillary lymph node metastases in T1a and T1b breast carcinoma. Ann Surg Oncol 1998; 5:23–27. [DOI] [PubMed] [Google Scholar]
- 4.Ravdin PM, De Laurentiis M, Vendely T, Clark GM. Prediction of axillary lymph node status in breast cancer patients by use of prognostic indicators. J Natl Cancer Inst 1994; 86:1771–1775. [DOI] [PubMed] [Google Scholar]
- 5.Olivotto IA, Jackson JSH, Mates D, et al. Prediction of axillary lymph node involvement of women with invasive breast carcinoma. Cancer 1998; 83:948–955. [PubMed] [Google Scholar]
- 6.Fein DA, Fowble BL, Hanlon AL, et al. Identification of women with T1–T2 breast cancer at low risk of positive axillary nodes. J Surg Oncol 1997; 65:34–39. [DOI] [PubMed] [Google Scholar]
- 7.Chadha M, Chabon AB, Friedmann P, Vikram B. Predictors of axillary lymph node metastases in patients with T1 breast cancer. Cancer 1994; 73:350–353. [DOI] [PubMed] [Google Scholar]
- 8.Mustafa IA, Cole B, Wanebo HJ, et al. The impact of histopathology in nodal metastases in minimal breast cancer. Arch Surg 1997; 132:384–391. [DOI] [PubMed] [Google Scholar]
- 9.Velanovich V, Szymanski W. Lymph node metastasis in breast cancer: common prognostic markers lack predictive value. Ann Surg Oncol 1998; 5:613–619. [DOI] [PubMed] [Google Scholar]
- 10.Barth A, Craig PH, Silverstein MJ. Predictors of axillary lymph node metastases in patients with T1 breast carcinoma. Cancer 1997; 79:1918–1922. [PubMed] [Google Scholar]
- 11.Cady B. Use of primary breast carcinoma characteristics to predict lymph node metastases. Cancer 1997; 79:1856–1861. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein MJ, Barth A. Use of primary breast carcinoma characteristics to predict lymph node metastases: reply. Cancer 1997; 79:1862–1864. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein MJ, Gierson ED, Waisman JR, et al. Axillary lymph node dissection for T1a breast carcinoma. Cancer 1994; 73:664–667. [DOI] [PubMed] [Google Scholar]
- 14.Choong PL, deSilva JSC, Dawkins HJS, et al. Predicting axillary lymph node metastases in breast carcinoma patients. Breast Cancer Res Treat 1996; 37:135–149. [DOI] [PubMed] [Google Scholar]
- 15.Toma S, Bonassi S, Puntoni R, Guido N. Primary tumor site, size, patient’s age and axillary lymph nodes in breast cancers. Tumori 1986; 72:259–265. [DOI] [PubMed] [Google Scholar]
- 16.Clemente CG, Boracchi P, Andreola S, et al. Peritumoral lymphatic invasion in patients with node-negative mammary duct carcinoma. Cancer 1992; 69:1396–1403. [DOI] [PubMed] [Google Scholar]
- 17.Lauria R, Perrone F, Carlomagno C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995; 76:1772–1778. [DOI] [PubMed] [Google Scholar]
- 18.Nime FA, Rosen PP, Thaler HT, et al. Prognostic significance of tumor emboli in intramammary lymphatics in patients with mammary carcinoma. Am J Surg Path 1977; 1:25–30. [DOI] [PubMed] [Google Scholar]
- 19.Bettelheim R, Penman HG, Thornton-Jones H, Neville AM. Prognostic significance of peritumoral vascular invasion in breast cancer. Br J Cancer 1984; 50:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen PP. Invasive duct carcinoma. In: Rosen PP, ed. Rosen’s breast pathology. Philadelphia: Lippincott-Raven; 1997: 275–293.
- 21.Clare SE, Sener SF, Wilkens W, et al. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol 1997; 4:447–451. [DOI] [PubMed] [Google Scholar]
- 22.Fischer ER, Swamidoss S, Lee CH, et al. Detection and significance of occult axillary node metastases in patients with invasive breast cancer. Cancer 1978; 42:2025–2031. [DOI] [PubMed] [Google Scholar]
- 23.International (Ludwig) Breast Cancer Study Group. Prognostic importance of occult axillary lymph node micrometastases from breast cancer. Lancet 1990; 335:1565–1568. [PubMed] [Google Scholar]
- 24.De La Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet 1993; 341:1039–1043. [DOI] [PubMed] [Google Scholar]
- 25.Kollias J, Elston CW, Ellis IO, et al. Early-onset breast cancer—histopathological and prognostic considerations. Br J Cancer 1997; 75:1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillet D, Kennedy C, Carmalt H. Breast cancer in young women. Aust NZ J Surg 1997; 67:761–764. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Simkovich-Heerdt A, Tran KN, et al. Women 35 years of age or younger have higher locoregional relapse rates after undergoing breast conservation therapy. J Am Coll Surg 1998; 187:1–8. [DOI] [PubMed] [Google Scholar]