Abstract
Objective
To compare transcranial motor evoked potentials (tc-MEPs) and somatosensory evoked potentials (SSEPs) as indicators of spinal cord function during thoracoabdominal aortic aneurysm repair.
Summary Background Data
Somatosensory evoked potentials reflect conduction in dorsal columns. tc-MEPs represent anterior horn motor neuron function. This is the first study to compare the techniques directly during thoracoabdominal aortic aneurysm repair.
Methods
In 38 patients, thoracoabdominal aortic aneurysm repair (type I, n = 10, type II, n = 14, type III, n = 6, type IV, n = 8) was performed using left heart bypass and segmental artery reimplantation. tc-MEP amplitudes <25% and SSEP amplitudes <50% and/or latencies >110% were considered indicators of cord ischemia. The authors compared the response of both methods to interventions and correlated the responses at the end of surgery to neurologic outcomes.
Results
Ischemic tc-MEP changes occurred in 18/38 patients and could be restored by segmental artery reperfusion (n = 12) or by increasing blood pressure (n = 6). Significant SSEP changes accompanied these tc-MEP events in only 5/18 patients, with a delay of 2 to 34 minutes. SSEPs recovered in only two patients. In another 11 patients, SSEP amplitudes fell progressively to <50% of control without parallel tc-MEP changes or association with cross-clamp events or pressure decreases. At the end of the procedure, tc-MEP amplitudes were 84 ± 46% of control. In contrast, SSEP amplitudes were <50% of control in 15 patients (39%). No paraplegia occurred.
Conclusion
In all patients, tc-MEP events could be corrected by applying protective strategies. No patient awoke paraplegic. SSEPs showed delayed ischemia detection and a high rate of false-positive results.
Despite the introduction of various strategies to protect the spinal cord during thoracoabdominal aortic aneurysm (TAAA) repair, paraplegia remains a distinct possibility. It would be advantageous if the adequacy of spinal cord blood flow could be measured continuously during these procedures. This could help to assess the efficacy of retrograde aortic perfusion; to identify segmental arteries, which are critical to the spinal cord blood supply; and to confirm successful segmental artery reimplantation. Evoked potential monitoring might offer such information. 1,2
Numerous studies have described the use of somatosensory evoked potentials (SSEPs) during TAAA repair. 3–6 Although SSEPs were claimed to be beneficial, SSEPs combined with retrograde aortic perfusion did not improve neurologic outcome in a large prospective study. 7 In addition, false-negative and false-positive results were reported. Indeed, SSEPs monitor only dorsal column function. Myogenic motor evoked potentials to transcranial stimulation (tc-MEPs) can monitor function of the ischemia-sensitive anterior horn motor neurons. In a recent study, tc-MEPs were used to guide retrograde aortic perfusion and aggressive segmental artery reimplantation in 52 patients with type I and II TAAAs, and no paraplegia occurred. 2
Until now, a direct comparison between SSEPs and tc-MEPs has never been performed. In this prospective study, SSEPs and tc-MEPs were recorded simultaneously during TAAA surgery; interventions were guided by tc-MEPs. We assessed the difference in response to perioperative interventions between both modalities.
PATIENTS AND METHODS
Patient Characteristics
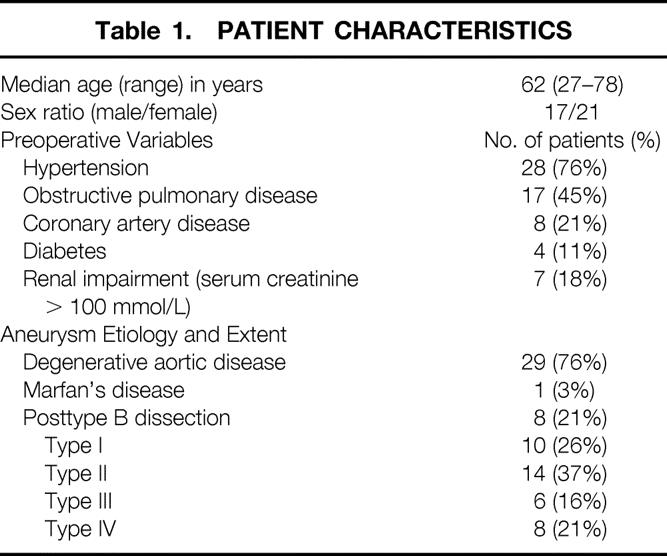
The preoperative patient characteristics are shown in Table 1. The Crawford classification was used to describe the extent of the aneurysm. 8 Forty-two consecutive patients were included in this study between February 1997 and July 1998. Two patients died during the procedure. In two patients, no reproducible SSEPs could be recorded due to a technical failure. The study population comprises the 38 patients with complete intraoperative tc-MEP and SSEP data and evaluable postoperative neurologic function. Data from 20 of these patients were analyzed in a previous report. 2
Table 1. PATIENT CHARACTERISTICS
tc-MEP Monitoring Technique
A transcranial electrical stimulator (Digitimer D 185 cortical stimulator, Welwyn, Garden City, UK) was used to evoke tc-MEPs. The stimuli were applied to the scalp with four adhesive Ag/Ag gel electrodes. The anode was placed at the vertex, and the cathode consisted of three interconnected cathodes placed behind the ears, over the mastoid bone, and on the forehead. The stimulus consisted of a train of three to five pulses, with a 2.0-ms interstimulus interval. Compound muscle action potentials were recorded from the skin over the anterior tibial muscle and forearm muscles using gel Ag/AgCl electrodes. The signals were amplified 5,000 to 20,000 times (adjusted to obtain maximal vertical resolution) and filtered between 30 and 1,500 Hz using a 3T PS-800 biologic amplifier (Twente Technology Transfer, Twente, The Netherlands). Data acquisition, processing, and analysis were performed on a computer with an AD converter and software (LabVIEW, National Instruments, Austin, TX). The computer displayed tc-MEP amplitude, proximal arterial pressure, distal arterial pressure, cerebrospinal fluid pressure, and neuromuscular blockade level on-line during the procedure, allowing visualization of all factors that might influence evoked potential interpretation. The supramaximal stimulus was assessed and tc-MEPs were recorded at a stimulus intensity of 10% above the level (typically 400 to 500 V) that produced maximal tc-MEP amplitudes.
A 25% intrapatient variation of tc-MEP amplitude was accepted as normal. Ischemic spinal cord dysfunction was considered present when tc-MEP amplitude decreased progressively below approximately 25% of baseline. This criterion is based on the assumption that an amplitude decrease <3 times the standard deviation should provide optimal detection of ischemia while limiting the false-positive rate; we previously observed a 26% within-patient variability of the tc-MEP. 1
An intervention was considered successful (i.e., to have reestablished flow to the affected spinal cord segment) when a recognizable, reproducible tc-MEP signal returned, with an amplitude progressively increasing to values >25%. tc-MEP responses of hand muscles were used to recognize potential systemic or technical causes of the tc-MEP decrease.
SSEP Monitoring Technique
The system used for tc-MEP monitoring was also used for the acquisition of SSEPs. Subdermal needle electrodes were inserted 2 cm apart at right angles to the axis of the left and right posterior tibial nerves at the ankle. For each nerve, the motor threshold was determined. The posterior tibial nerves were stimulated bilaterally with constant current wave pulses of 200 μsec duration at a frequency of 3.1 Hz (Digistim Neurotechnology, Conshohocken, PA). During surgery, the stimulus intensity was two times the motor threshold to ensure a maximal SSEP response (12 to 18 mA). Standard adhesive gel Ag/Ag electrodes were used for Fpz recordings, and Ag/Ag chloride cups were used for Cz′ (2 cm behind Cz) recordings (international 10–20 system). A ground electrode was placed behind the right ear, over the mastoid bone. The recorded signals were amplified and filtered between 5 and 250 Hz (−3 dB). The analysis time was 160 ms. The responses to 300 stimuli were averaged, and a moving average was constructed every 100 sweeps to obtain one tc-MEP and one SSEP waveform every minute. High-voltage artifacts were rejected automatically by the computer, and during diathermy SSEP acquisition was automatically halted.
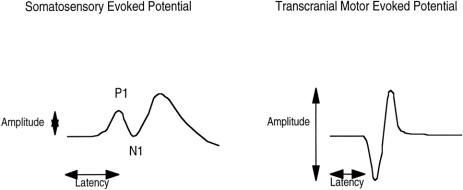
The computer displayed the SSEP waveforms in a trend plot, calculated SSEP amplitudes and latencies, and stored the waveforms on hard disk for future analysis. For each waveform, the latency of P1 peaks as well as the peak-to-peak amplitude for P1 and N1 was determined using on-screen cursors. Ischemic spinal cord dysfunction was defined as an SSEP amplitude decrease to <50% and/or a latency increase >110% of baseline values. 4 Recovery of the SSEP responses was considered complete when a recognizable, reproducible signal returned, with amplitude increasing to >50% and/or a latency decrease to <110%. An example of tc-MEP and SSEP responses is shown in Figure 1.
Figure 1. SSEP latency (duration in ms from stimulation to the first positive peak [P1]) and SSEP peak-to-peak amplitude (P1–N1 in μV). tc-MEP latency (duration in ms from stimulation to the first progressive negative deflection) and tc-MEP amplitude (peak-to-peak amplitude in μV).
Anesthetic Technique
Anesthesia was induced with etomidate 0.3 mg/kg and sufentanil 5 μg/kg and was maintained with sufentanil (4 μg/kg/hr) and ketamine (2 mg/kg/hr). Additional ketamine, 50 mg intravenously, was given at signs of inadequate anesthesia. Muscle relaxation was induced and maintained with vecuronium, with a closed-loop vecuronium infusion to maintain levels of neuromuscular blockade stable within a narrow range (75% to 90%). This minimized the influence of fluctuations in relaxation level on the variability of the myogenic tc-MEP signal. 9,10
Surgical Protocol
All patients underwent surgery according to a protocol previously described in detail. 2 Patients were placed in the lateral position and a catheter was introduced in the intrathecal space, maintaining cerebrospinal fluid pressure <10 mm Hg by withdrawing cerebrospinal fluid when necessary. Retrograde aortic perfusion was established by cannulation of the left atrium or pulmonary vein and the femoral artery. Heparinization was limited (0.5 mg/kg). In eight patients, cardiopulmonary bypass, with or without deep hypothermia, was used because of involvement of the aortic arch (n = 3), dissection (n = 1), or suspected prolongation of spinal cord ischemia (n = 4). Distal aortic perfusion was started before cross-clamping. The aim was to maintain distal aortic pressure at 60 mm Hg to ensure adequate perfusion of the visceral organs and legs. Dacron grafts (Sulzer Vascutek, Inchinnan, Scotland) were anastomosed using running Prolene sutures. The aorta was then sequentially clamped and intercostal arteries were reimplanted as described below.
In type II, III, and IV aneurysms, graft inclusion in the abdominal aorta was performed while the celiac, superior mesenteric, and renal arteries were selectively perfused. 11 Temperature was allowed to decrease spontaneously, reaching rectal temperatures of 31° to 34°C during the cross-clamp period.
Interventions Based on tc-MEP Changes
When aortic cross-clamping resulted in ischemic tc-MEP changes, attempts were first focused on increasing the distal aortic flow and pressure as well as the proximal arterial pressure. Significant back-bleeding from segmental arteries was managed by introducing 3F balloon occluding catheters to reduce the stealing effect from the anterior spinal artery.
When exclusion of an aortic segment resulted in ischemic tc-MEP changes, the intercostal or lumbar arteries in that segment were considered critical to the spinal cord blood supply and were immediately reattached and reperfused. If the aortic wall was not strong enough, separate Dacron grafts were selectively anastomosed to the segmental arteries, and while perfusion was established by one of the perfusion catheters (side arm of the left heart bypass), the grafts were connected to the tube graft. If tc-MEP changes indicated critical ischemia and no segmental arteries could be identified, endarterectomy of the aortic wall was performed and selective grafts were anastomosed to patent segmental arteries. If no tc-MEP changes were observed, segmental arteries were also reimplanted, but only when this was technically simple. SSEP changes with unaltered tc-MEP signals did not prompt interventions.
After surgery, leg motor function was assessed by the attending intensivist. If a neurologic deficit was suspected, an independent neurologist was consulted.
Analysis of tc-MEP and SSEP Response Patterns
Baseline tc-MEP amplitude and latency and SSEP amplitude and latency were assessed by averaging five consecutive responses before the placement of the first cross-clamp. All evoked potential variables were expressed as percentages of this baseline value. Before labeling an evoked potential event as potentially induced by spinal cord ischemia, systemic factors such as systemic hypothermia, inadvertent increases in the level of neuromuscular blockade, and peripheral ischemia of the legs were excluded.
The temporal relation between particular perioperative events, such as placement of aortic clamps or sudden pressure decreases, and tc-MEP and SSEP data was analyzed. SSEP or tc-MEP changes were linked to these events only when they occurred within 50 minutes of the onset of the event. 7 Finally, the responses of both techniques at the end of the procedure were evaluated for their accuracy in predicting postoperative leg motor function.
Presentation of Data
All data are expressed as mean ± standard error of the mean, except for data that demonstrated skewed distributions, which are shown as medians and range.
RESULTS
Of the 38 analyzed patients, the in-hospital death rate was 8%. The cause of death in three patients was a systemic inflammatory response syndrome, caused by pulmonary complications. Major postoperative complications included pulmonary insufficiency in 20 patients (53%), myocardial infarction in 1 (3%), dysrhythmias in 12 (32%), and minor stroke in 2 (5%). Two patients had renal failure requiring temporary dialysis. Early or late paraplegia did not occur in any patient.
Baseline tc-MEP amplitudes and latencies were 1447 μV (216 to 3684) and 32 ms (21 to 39), respectively. Baseline SSEP amplitudes and latencies were 1.9 μV (0.4 to 11.2) and 46 ms (38 to 58), respectively.
Eighteen segmental arteries were reimplanted in 6 type I TAAAs, 89 in 14 type II TAAAs, 19 in 6 type III TAAAs, and 13 in 6 type IV TAAAs. In the remaining six patients (four type I and two type IV aneurysms), no segmental arteries were reimplanted and no tc-MEP changes occurred. tc-MEP amplitudes were 96 ± 28% of baseline at the end of the procedure in these six patients.
tc-MEP and Accompanying SSEP Events
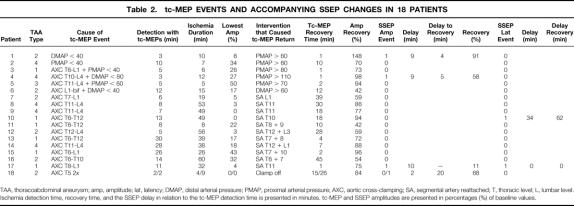
Nineteen tc-MEP amplitude decreases occurred in 18 patients (Table 2). Two tc-MEP events were caused by isolated proximal or distal arterial pressure decreases <40 mm Hg (patients 1 and 2). After restoring the arterial pressure to >60 mm Hg, tc-MEPs recovered rapidly in both patients. In only one patient was the tc-MEP event accompanied by an SSEP decrease and recovery, but with a delay of 9 minutes. Thereafter, SSEP amplitudes progressively decreased to <50% for the rest of the surgical procedure. Another four tc-MEP events (patients 3 through 6) occurred when the arterial pressure decreased during the exclusion of an aortic segment. In these patients, an arterial pressure correction was sufficient to restore the tc-MEP responses. The SSEP amplitudes decreased to <50% in only one of these patients, but with a delay of 9 minutes. The SSEP responses recovered 5 minutes after the tc-MEP recovery, but again decreased to <50% later in the procedure. Segmental arteries were reimplanted in all four patients.
Table 2. tc-MEP EVENTS AND ACCOMPANYING SSEP CHANGES IN 18 PATIENTS
TAA, thoracoabdominal aneurysm; amp, amplitude; lat, latency; DMAP, distal arterial pressure; PMAP, proximal arterial pressure; AXC, aortic cross-clamping; SA, segmental artery reattached; T, thoracic level; L, lumbar level. Ischemia detection time, recovery time, and the SSEP delay in relation to the tc-MEP detection time is presented in minutes. tc-MEP and SSEP amplitudes are presented in percentages (%) of baseline values.
Thirteen tc-MEP events (patients 7 through 18) were the result of aortic cross-clamping causing exclusion of an aortic segment. In 11 of these 13 events, tc-MEP recovery followed reperfusion of the reattached segmental arteries. The two remaining events occurred in one patient (patient 18). The aorta was cross-clamped twice to revise the proximal anastomosis. Distal aortic perfusion was no longer present, and both brief clamping episodes resulted in immediate tc-MEP loss. The tc-MEP changes were accompanied by SSEP amplitude decreases to <50% in only two patients (patients 17 and 18). In one, SSEP amplitudes decreased to <50%, 10 minutes after tc-MEPs had decreased to <25%, but the SSEP responses did not recover after reperfusion. In the other patient, in whom the proximal anastomosis was revised, SSEP amplitudes decreased to <50% only after the second clamping episode, and 2 minutes later than the tc-MEP decrease. Recovery of the SSEP responses occurred 20 minutes later than tc-MEP recovery. In patient 10, only SSEP latency changes accompanied the tc-MEP decrease, but with a delay of 34 minutes. Recovery of the tc-MEP responses was followed by the return to normal of SSEP latency, with a delay of 62 minutes.
tc-MEPs decreased to <25% a median of 8 minutes (range 2 to 30) after the placement of the aortic clamps. Reperfusion of the segmental arteries occurred within 38 minutes (range 4 to 60), after which tc-MEPs recovered to >25% of control within 18 minutes (range 1 to 45). The duration of tc-MEP recovery showed a trend to inverse correlation with the time to detect ischemia—that is, when an amplitude decrease rapidly followed aortic clamping, recovery was prolonged. In contrast, when the decrease was gradual, recovery followed immediately (r = −0.5, CI 0.8 to 0.05, p = 0.07).
As shown in Table 2, interventions aimed at improving spinal cord perfusion were performed in six patients in whom the tc-MEP decrease did not reach the 25% criterion (patients 2, 3, 4, 5, 15, and 16). In four of these patients, perioperative interpretation of the raw tc-MEP amplitudes suggested a decrease of approximately 25%, but analysis of the tc-MEP amplitudes as a percentage of control revealed that amplitudes decreased only to 26% to 34%. In the two other patients, in whom the decreases reached 50% and 43%, the progressive decrease was followed by successful corrections of the spinal cord perfusion before the 25% level was reached. In these six patients, the recovery time was calculated as the time in which, after reperfusion, reproducible and progressively increasing responses increased to >50% of control. At the end of the procedure, tc-MEP amplitudes were >25% of control in all patients, with amplitudes of 84 ± 46%. In the patients in whom a tc-MEP event occurred, amplitudes recovered to 91 ± 72%.
Significant tc-MEP latency changes occurred as rapidly as tc-MEP amplitude changes (p = 0.4). tc-MEP latencies were 97 ± 5% at the end of the procedure for the entire group and 97 ± 6% for the group with tc-MEP events. In the four patients in whom SSEP amplitudes decreased to <50% (patients 1, 4, 17, and 18), a simultaneous SSEP latency increase to >110% occurred in only one patient. After reperfusion, SSEP latency recovered within 1 minute in this patient, but SSEP amplitudes did not exceed 11% for the remaining time.
SSEP Events Not Associated With tc-MEP Events
In 13 patients, 13 SSEP events occurred that were not associated with ischemic tc-MEP changes. Two of these events occurred in patients who also had tc-MEP events, but during different episodes of the procedure. In one patient, a sudden distal arterial pressure decrease accounted for the SSEP amplitude decreases, with a detection delay of 5 minutes. Pressure correction did not result in recovery of the responses, and SSEP amplitude was 31% at the end of surgery. Another patient showed SSEP changes 13 minutes after correction of a pressure decrease to <40 mm Hg lasting 7 minutes. SSEPs recovered completely in this patient. The remaining 11 patients showed SSEP changes that could not be associated with cross-clamp or pressure events, but a progressive SSEP amplitude decrease to <50% was observed during the procedure. SSEP latency changes did not accompany these amplitude changes. At the end of the procedure, 15 patients demonstrated SSEP amplitudes <50% of control, with an average value of 28 ± 10%.
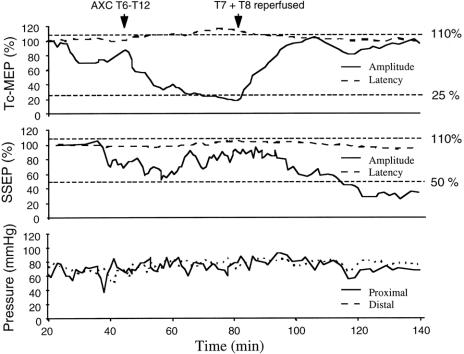
Figure 2 shows tc-MEP and SSEP changes during the procedure in one patient who underwent repair of a type I TAAA. The significant tc-MEP changes after aortic cross-clamping were not accompanied by significant SSEP changes, but a gradual and progressive SSEP amplitude decrease to <50% was observed after the cross-clamping episode, without a temporal relation with aortic cross-clamping or pressure events.
Figure 2. The significant tc-MEP amplitude and latency changes after thoracic cross-clamping were not accompanied by significant SSEP changes. Thereafter, SSEP amplitude decreased gradually to values <50%, whereas tc-MEPs recovered to values within the normal range after reperfusion. Arterial pressures were stable during the procedure, and the patient awoke with normal neurologic function.
Evoked Potential Events Not Related to Spinal Cord Ischemia
In 14 patients, 18 transient events occurred in which a significant evoked potential change could be credited to peripheral ischemia, increases in the level of neuromuscular blockade, or systemic hypothermia. In the five patients in whom peripheral ischemia occurred, a unilateral tc-MEP amplitude decrease was observed, accompanied by a latency increase to >110%. This resulted in a 25% tc-MEP amplitude decrease within 38 ± 5 minutes and a unilateral disappearance within 62 ± 9 minutes after the initiation of extracorporeal circulation. When unilateral tc-MEPs disappeared as a result of peripheral ischemia, the combined SSEP amplitude showed an average reduction to 45 ± 6%.
Temporary increases in the level of neuromuscular blockade occurred in two patients despite the closed-loop system. In both patients, intravenous magnesium had been administered to decrease cardiac hyperexcitation. The levels of neuromuscular blockade increased from 75% to 98% and from 75% to 97%, respectively. Bilateral tc-MEP amplitudes decreased to 13% and 11% within 25 and 22 minutes, respectively. tc-MEP latencies did not change during these events.
Temporary systemic hypothermia caused significant evoked potential changes in nine patients. In three patients, SSEP latencies increased to >110%. In four patients, both tc-MEP and SSEP latencies significantly increased, with systemic temperatures varying from 26° to 32.6°C. In two patients, transient tc-MEP and SSEP disappearance occurred as a result of transient deep systemic hypothermia (19.5° and 22°C).
Prediction of Postoperative Neurologic Function
None of the 38 patients had paraplegia after the procedure. In all patients, tc-MEPs exceeded the 25% criterion and predicted leg motor function correctly. SSEPs, however, demonstrated ischemic values at the end of the procedure in 15 patients (39%).
DISCUSSION
In this clinical study, tc-MEPs were used to guide the surgical repair of TAAAs, and no neurologic deficit occurred. Intact tc-MEPs were present at the end of the procedure in all patients. SSEPs, however, showed false-positive results in 39% of the patients. In addition, significant tc-MEP changes, caused by either aortic cross-clamping or blood pressure decreases, were accompanied by SSEP changes in only 22% of the patients.
This is the first clinical study comparing tc-MEPs with SSEPs for spinal cord function monitoring during TAAA repair. Because both were recorded concurrently, we had the opportunity to compare evoked potential changes in response to intraoperative events, such as sequential cross-clamping and blood pressure changes. The majority of events that produced spinal cord ischemia, as evidenced by significant tc-MEP amplitude decreases, could be detected and successfully corrected before any SSEP abnormality occurred. This suggests that SSEPs offer little additional benefit to tc-MEP monitoring.
However, the design of this clinical study has several limitations. Because we did not have an independent measure of spinal cord blood flow and surgical decisions were based on tc-MEPs, a possibility exists that several tc-MEP events might have been false positives. This issue can be resolved only in a controlled experimental setup in which a spinal cord segment is made ischemic. Nonetheless, we suspect that few episodes were indeed false positives, because tc-MEPs recovered promptly in all patients after either blood pressure increases or segmental artery reimplantation. Further, this rapid response is in agreement with other experimental reports, in which tc-MEPs disappeared rapidly after the onset of spinal cord ischemia. 12–14
In some patients, the tc-MEP amplitude decrease was more gradual. In these patients, restoration of spinal cord blood flow resulted in immediate recovery of the signals. In contrast, in patients in whom tc-MEPs disappeared rapidly, tc-MEP recovery was delayed. One possible explanation for these observations is that during TAAA repair, some manipulations produce a state of borderline spinal cord perfusion, sufficient to decrease transmission in the motor pathways but compatible with neuronal survival. Conversely, the rapid loss of tc-MEP signals might reflect complete interruption of blood flow, representing a higher risk for neuronal injury. This hypothesis is further supported by the observation that tc-MEP recovery seemed delayed when the signal was lost abruptly. We conclude that the tc-MEP amplitude changes apparently reflected the adequacy of spinal cord blood flow accurately. This is in accordance with an experimental study in which the reduction of spinal cord blood flow correlated with motor evoked potential amplitudes. 13
In this study, we could not find a consistent relation between SSEPs and intraoperative interventions. When SSEP changes accompanied tc-MEP changes, they always occurred with a delay. The difference in response time between tc-MEPs and SSEPs to spinal cord ischemia can be explained physiologically. Axonal conduction of SSEP responses in the dorsal columns is thought to be accomplished in a nonsynaptic fashion and requires little energy expenditure. Consequently, this relative resistance to ischemia could explain the delayed SSEP response. 15–17 In addition, aortic clamping results in an interruption of flow in the anterior spinal artery, which could result in selective anterior horn damage. In a large prospective study, Crawford et al observed ischemia detection times of up to 54 minutes with SSEP and adequate distal aortic perfusion, which precluded timely localization of critical segmental arteries. 7 In our study, the majority of tc-MEP events had recovered as a result of corrective interventions before SSEP abnormalities were detected. Although the average duration of aortic cross-clamping or blood pressure decreases was 38 minutes, in 78% of the patients SSEPs were still normal at the end of the ischemic episode. These observations suggest a high false-negative rate of SSEP monitoring during TAAA surgery.
False-positive SSEPs were present in 39% of the patients at the end of the procedure. In many of these patients, a gradual decrease in SSEP amplitude occurred during the surgical procedure, without a temporal relation to pressure or cross-clamping events. This “fade-out phenomenon” was previously described by Cunningham et al. 4 They attributed this slow but progressive decrease to inadequate distal aortic perfusion, but this was not the case in our patients. There is no physiologic explanation for this observation, although it might be the result of physiologic adaptation to the repetitive 3.1-Hz stimulation for a prolonged period.
In conclusion, tc-MEPs accurately guided the management of spinal cord protective strategies during TAAA surgery, and no paraplegia occurred. Concurrent SSEP monitoring did not offer additional benefit due to the delayed responses to ischemia and a high incidence of false-positive results.
Discussion
PROF. J. COLLIN (Oxford, United Kingdom): The differences between tc-MEP and SSEP are very interesting. I wonder if you can explain why SSEP shows so many false positives at the end of the operative procedure, while apparently being insensitive as an indicator of problems preoperatively. I am also interested to know how long it took to reattach the segmental arteries in the 10 patients with ischemic tc-MEP changes, whether the changes persisted while the arteries were being reattached, and how long it took for the MEP changes to correct after reestablishment of spinal cord blood flow.
PROF. MICHAEL J. JACOBS (Amsterdam, The Netherlands): Somatosensory evoked potentials evaluate conduction through the dorsal horn, which in fact is less sensitive to ischemia than the anterior horn. It therefore can occur that the anterior horn (motor function) is severely ischemic and the dorsal horn still sufficiently perfused. In general, it takes around 10 minutes to perform an end-to-end anastomosis to reattach one or two intercostal arteries. As soon as the segmental arteries are reattached, perfusion is reestablished. There is a reversed correlation with the time for MEPs to disappear and to restore after reperfusion; gradually disappearing MEPs will restore fairly quickly, whereas rapidly decreased MEPs will take much more reperfusion time to restore spinal cord integrity.
DR. J. VANVROONHOFEN (Utrecht, The Netherlands): Professor Jacobs and his coworkers are to be congratulated for their continuing efforts to lower the morbidity associated with the surgical treatment in this very difficult group of patients. Having had the opportunity to study the manuscript, I would first of all like to ask Professor Jacobs if he could give us some information on how these patients were selected, if it was a consecutive series of operations, in the context of the fact that some of the data seem to be reported in a recent publication in the Journal of Vascular Surgery. Having had no neurological deficits at all, a commendable feat, it might seem to me that tc-MEP monitoring could be too sensitive. In other words, could you please give us some more arguments why you think that tc-MEP monitoring is indeed indispensable?
I enjoyed your paper.
PROF. JACOBS: This is indeed a series of consecutive patients. MEPs are extremely sensitive; intraoperative changes correspond with clinical outcome. For example, some patients showed decreased MEPs in one leg which correspond with weakness in that particular leg. If MEPs disappear, the patient will awake with paraplegia. Therefore, any significant change will be attached with a surgical solution to restore MEPs.
Footnotes
Correspondence: Cor J. Kalkman, MD, PhD, Dept. of Anesthesiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Presented at the Sixth Annual Meeting of the European Surgical Association, at the Royal College of Surgeons of England, London, United Kingdom, April 23–24, 1999.
Accepted for publication July 1999.
References
- 1.Haan de P, Kalkman CJ, de Mol BA, et al. Efficacy of transcranial motor-evoked myogenic potentials to detect spinal cord ischemia during operations for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 1997; 113: 87–100. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs MJ, Meylaerts SA, de Haan P, et al. Strategies to prevent neurologic deficit based on motor-evoked potentials in type I and II thoracoabdominal aortic aneurysm repair. J Vasc Surg 1999; 29: 48–59. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham JN Jr, Laschinger JC, Merkin HA, et al. Measurement of spinal cord ischemia during operations upon the thoracic aorta: initial clinical experience. Ann Surg 1982; 196: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham JN Jr, Laschinger JC, Spencer FC. Monitoring of somatosensory evoked potentials during surgical procedures on the thoracoabdominal aorta. IV. Clinical observations and results. J Thorac Cardiovasc Surg 1987; 94: 275–285. [PubMed] [Google Scholar]
- 5.Grabitz K, Sandmann W, Stuhmeier K, et al. The risk of ischemic spinal cord injury in patients undergoing graft replacement for thoracoabdominal aortic aneurysms. J Vasc Surg 1996; 23: 230–240. [DOI] [PubMed] [Google Scholar]
- 6.Griepp RB, Ergin MA, Galla JD, et al. Looking for the artery of Adamkiewicz: a quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg 1996; 112: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 7.Crawford ES, Mizrahi EM, Hess KR, et al. The impact of distal aortic perfusion and somatosensory evoked potential monitoring on prevention of paraplegia after aortic aneurysm. J Thorac Cardiovasc Surg 1988; 95: 357–367. [PubMed] [Google Scholar]
- 8.Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986; 3: 389–404. [DOI] [PubMed] [Google Scholar]
- 9.Kalkman CJ, Drummond JC, Kennelly NA, et al. Intraoperative monitoring of tibialis anterior muscle motor evoked responses to transcranial electrical stimulation during partial neuromuscular blockade. Anesth Analg 1992; 75: 584–589. [DOI] [PubMed] [Google Scholar]
- 10.Adams DC, Emerson RG, Heyer EJ, et al. Monitoring of intraoperative motor-evoked potentials under conditions of controlled neuromuscular blockade. Anesth Analg 1993; 77: 913–918. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs MJ, de Mol BA, Legemate DA, et al. Retrograde aortic and selective organ perfusion during thoracoabdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 1997; 14: 360–366. [DOI] [PubMed] [Google Scholar]
- 12.Haan de P, Kalkman CJ, Ubags LH, et al. A comparison of the sensitivity of epidural and myogenic transcranial motor-evoked responses in the detection of acute spinal cord ischemia in the rabbit. Anesth Analg 1996; 83: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 13.Reuter DG, Tacker WA Jr, Badylak SF, et al. Correlation of motor-evoked potential response to ischemic spinal cord damage. J Thorac Cardiovasc Surg 1992; 104: 262–272. [PubMed] [Google Scholar]
- 14.Kraus KH, Pope ER, O’Brien D, Hay BL. The effects of aortic occlusion on transcranially induced evoked potentials in the dog. Vet Surg 1990; 19: 341–347. [DOI] [PubMed] [Google Scholar]
- 15.Kobrine AI, Evans DE, Rizzoli HV. The effects of ischemia on long-tract neural conduction in the spinal cord. J Neurosurg 1979; 50: 639–644. [DOI] [PubMed] [Google Scholar]
- 16.Machida M, Yamada T, Ross M, et al. Effect of spinal cord ischemia on compound muscle action potentials and spinal evoked potentials following spinal cord stimulation in the dog. J Spinal Disord 1990; 3: 345–352. [PubMed] [Google Scholar]
- 17.Machida M, Weinstein SL, Yamada T, et al. Dissociation of muscle action potentials and spinal somatosensory evoked potentials after ischemic damage of spinal cord. Spine 1988; 13: 1119–1124. [DOI] [PubMed] [Google Scholar]