Abstract
Objective
To compare growth factor receptor expression in papilla of Vater cancer and pancreatic cancer.
Summary Background Data
Cancer of the papilla of Vater has a much better prognosis than pancreatic cancer. Earlier symptoms may result in earlier diagnosis, but different biologic growth behaviors and genetic alterations might also be explanations.
Patients and Methods
Surgical specimens from papilla of Vater cancers (24 patients) and pancreatic cancers (80 patients), normal papilla of Vater tissues (20 patients), and normal pancreatic tissues (24 patients) were frozen and fixed. The authors compared the expression of the epidermal growth factor receptor (EGFR) and c-erbB2 and c-erbB3 by Northern blot, in situ hybridization, and immunohistochemistry.
Results
In papilla of Vater cancer, Northern blots showed comparable EGFR and c-erbB2 mRNA expression but significantly lower c-erbB3 mRNA levels than in normal papilla. In pancreatic cancer, mRNA expression was enhanced compared with normal controls for EGFR (4-fold), c-erbB2 (2.5-fold), and c-erbB3 (5.2-fold). In situ hybridization confirmed this and showed mRNA expression only in cancer cells. EGFR immunohistochemical staining scores were comparable in papilla of Vater cancer (1.17 ± 0.22) and normal papilla (1.42 ± 0.25). Staining scores for c-erbB2 (2.72 ± 0.40 vs. 3.89 ± 0.37) and c-erbB3 (2.78 ± 0.35 vs. 3.89 ± 0.53) were slightly lower than controls in papilla of Vater cancer. In pancreatic cancer, immunostaining scores for EGFR, c-erbB2, and c-erbB3 were significantly higher than controls.
Conclusion
Members of the EGFR family show similar or lower expression in papilla of Vater cancer than in normal controls. In pancreatic cancer, these receptors are upregulated. This supports the hypothesis that papilla of Vater cancer and pancreatic cancer have biologic differences that may contribute to the different growth of these tumors.
The Vaterian system is composed of the segment of the distal common bile duct, the proximal main pancreatic duct, the major papilla, and the sphincteric musculature. Tumors of the papilla of Vater are rare, found in only 0.06% to 0.21% of all routine autopsy cases. 1 In clinical practice, four tumor types are described as periampullary cancers: papilla of Vater cancer, pancreatic head cancer, distal bile duct cancer, and duodenal cancer. Of these, papilla of Vater cancers are the least common, representing only 6% to 12% of all periampullary malignancies. 2 Based on these data, the population incidence of papilla of Vater cancer is estimated at 2.9 cases per million. 3 Malignancies of the papilla of Vater can arise in the epithelial cells of the ampulla region (ampullary type) and in the epithelial cells of the luminal surface (duodenal type).
Papilla of Vater cancer has a relatively good prognosis, and 5-year survival rates exceeding 40% after tumor resection have been reported by several authors. 4–8 In contrast, pancreatic cancers have the worst prognosis of the periampullary malignancies. Although the population incidence of pancreatic cancer is approximately 9 to 10 cases per 100,000, it is the fourth leading cause of cancer-related deaths in men and women in most Western countries. 9 At the time of diagnosis, most pancreatic cancers are already beyond local resectability or distant metastases are present, which limits treatment to palliative procedures. Most patients with pancreatic cancer die within 3 to 6 months of diagnosis as a result of rapid tumor progression. 10,11 In addition, most patients who undergo radical resection of pancreatic head malignancies die within the first 3 years after surgery as a result of local tumor recurrence or distant metastases, most frequently in the liver. 11 The 5-year survival rates of 0% to 25% in patients who have undergone resection of pancreatic cancer underline the aggressive growth behavior of this tumor type. 12–14
The reasons for the better prognosis of patients with papilla of Vater cancer versus those with pancreatic cancer seem obvious. The development of endoscopy has contributed to early and easy diagnosis of alterations in the papilla of Vater. In addition, it is believed that papilla of Vater cancer causes early obstructive jaundice, which brings the patients in early for medical attention and treatment. Thus, earlier diagnosis of papilla of Vater cancer is generally accepted to be a key parameter in its better prognosis.
Standard histopathologic comparison of papilla of Vater and pancreatic cancer reveals distinct differences in growth behavior between the tumor entities. Intraluminal growth is present in 40% of papilla of Vater cancers and only 2% of pancreatic cancers. The frequency of extraductal invasion is 60% in papilla of Vater cancer and 98% in pancreatic cancer. 15 Further, at comparable tumor stages, pancreatic cancer exhibits a higher frequency of lymphatic, venous, and perineural invasion than does papilla of Vater cancer. 15 The mechanisms underlying these differences are not known.
In recent years, molecular analysis in pancreatic cancer has provided new insight into the pathobiology of the disease. In pancreatic cancer, an accumulation of molecular changes is frequently present, including mutations of oncogenes and tumor suppressor genes. 16,17 These molecular alterations in combination seem to contribute to the aggressive phenotype of pancreatic cancer and to the rapid, mostly lethal, growth behavior.
In the present study, we compared the expression patterns of three closely related growth factor receptors: the epidermal growth factor receptor (EGFR), c-erbB2 (v-erb-B-related gene, a truncated version of the EGFR from the avian erythroblastosis retrovirus), and c-erbB3—in papilla of Vater cancer and pancreatic cancer. We found significant differences between papilla of Vater cancer and pancreatic cancer in the expression patterns of these three factors, suggesting that differences in the molecular profile of the tumors account, at least in part, for differences in their prognosis.
PATIENTS AND METHODS
Patients and Tissue Collection
The studies were approved by the Human Subject Committee of the University of Bern. Twenty normal papilla of Vater tissue samples were obtained from 9 female and 11 male previously healthy organ donors, median age 41 years (range 7 to 60) through an organ donor program in which the whole pancreas and the duodenum were removed. The papilla of Vater was completely cut out after longitudinal opening of the duodenum.
Papilla of Vater cancer tissues were obtained from 14 female and 10 male patients, median age 73 years (range 50 to 87), who underwent a partial duodenopancreatectomy (Whipple resection) (n = 20), a biliary bypass operation (n = 3), or an exploratory laparotomy (n = 1). In patients without tumor resection, the tumor tissue was collected during endoscopy and extensive biopsy material was taken for analysis. The diagnosis of papilla of Vater cancer was confirmed by the preoperative clinical investigation, endoscopic tumor biopsy, and histopathologic examination of the resected tumor specimens. According to the TNM classification of the UICC, 18 there were 5 stage I, 5 stage II, 10 stage III, and 4 stage IV tumors. Tumor grading showed 6 well-differentiated tumors, 13 moderately differentiated tumors, and 5 poorly differentiated tumors.
Normal human pancreatic tissue samples were obtained from 14 women and 10 men, free of pancreatic disease, through an organ donor program in which no candidates for pancreatic transplantation were available. The median age of the organ donors was 42 years (range 7 to 57). All normal tissue samples were obtained from the head of the pancreas.
Pancreatic cancer tissues were obtained from 80 patients (36 women and 44 men) undergoing a partial duodenopancreatectomy (Whipple resection). The median age of the patients was 64 years (range 31 to 84). According to the TNM classification, there were 23 stage I, 11 stage II, 42 stage III, and 4 stage IV tumors. Tumor grading showed 7 well-differentiated tumors, 41 moderately differentiated tumors, and 32 poorly differentiated tumors.
Freshly removed tissue samples were immediately fixed in Bouin’s or paraformaldehyde solution and paraffin-embedded for immunohistochemistry and in situ hybridization. Concomitantly, tissues for RNA extraction were snap-frozen in liquid nitrogen in the operating or endoscopy room on surgical removal and maintained at −80°C until use.
Northern Blot Analysis
After the extraction of total RNA and gel electrophoresis, the RNA was electrotransferred onto nylon membranes (Gene Screen, Du Pont, Boston, MA) and cross-linked by ultraviolet irradiation. 19,20 The filters were then prehybridized, hybridized, and washed under highly stringent conditions. The blots were prehybridized overnight at 65°C, then hybridized for 18 hours at 65°C in the presence of 1 × 106 cpm/ml of the 32P-labeled antisense EGFR, c-erbB2, and c-erbB3 cRNA probes, respectively, washed twice at 65°C in 1× sodium saline citrate (SSC) and 0.5% sodium dodecyl sulfate (SDS), and rinsed twice at 65°C in 0.1× SSC and 0.5% SDS.
To assess equivalent RNA loading, all blots were rehybridized with a mouse 32P-labeled 7S cDNA probe, which cross-hybridizes with human 7S RNA. 19,20
All blots were exposed at −80°C to Fuji x-ray film with Kodak intensifying screens. The intensity of the radiographic bands was quantified by a computerized video system and Image-pro-plus 3.0 software (Media Cybernetics, Silver Spring, MD). The ratios of the optical densities of the RNA levels (EGFR/7S, c-erbB2/7S, and c-erbB3/7S) were calculated for each sample.
In Situ Hybridization
In situ hybridization was performed as previously reported. 19–21 Briefly, pancreatic tissue samples were fixed in paraformaldehyde and paraffin-embedded. The tissue sections (2 to 4 μm) were deparaffinized, dehydrated, and incubated in 0.2M HCl for 20 minutes. The sections were treated with proteinase K (50 μg/ml; Boehringer Mannheim, Mannheim, Germany), prehybridized at 50°C and hybridized overnight at 50°C. The final concentration of the digoxigenin-labeled probes was approximately 0.5 ng/μl. After hybridization, the sections were washed and treated with RNase (Boehringer Mannheim). The samples were then incubated with an antidigoxigenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim) (dilution 1:500). For color reaction, 5-bromo-4-chloro-3-indolyl phosphate and nitro-blue tetrazolium (Sigma, Buchs, Switzerland) were used. For control experiments, the slides were incubated with RNase or with the corresponding sense probes. Pretreatment of the slides with RNase abolished the hybridization signal produced by the antisense probe. Further, incubation with the sense probe failed to produce in situ hybridization signals.
Probe Synthesis
A 560 bp EcoRI fragment of the human EGF receptor HER pA21, 17 a 400 bp BamHI/EcoRI fragment of human pHER2-436-1 cDNA 22 (American Type Culture Collection, Rockville, MD), and a 454 bp c-erbB3 cDNA 23 generated by reverse transcription–polymerase chain reaction were used.
The 7S probe consisted of a 190 bp BamHI/BamHI fragment of mouse 7S cDNA, which cross-hybridizes with human 7S.
For Northern blot analysis, EGFR, c-erbB2, and c-erbB3 antisense cRNA probes were radiolabeled with [alpha-32P] CTP and the 7S cDNA probe was labeled with [alpha-32P] dCTP (both Du Pont).
For in situ hybridization, EGFR, c-erbB2, and c-erbB3 antisense and sense cRNA probes were labeled with digoxigenin. 19–21
Immunohistochemistry
Consecutive 3- to 5-μm paraffin-embedded tissue sections were subjected to immunostaining using the streptavidin-peroxidase technique (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD), as previously reported. 17,19,21 After deparaffinization and rehydration, the tissue sections were submerged in Tris buffered saline (TBS) solution (10 mM Tris-HCl, 0.85% NaCl, pH 7.4) containing 0.1% (v/v) Triton X-100, and then washed for 5 minutes in TBS buffer. Endogenous peroxidase activity was blocked by incubating the slides in methanol and in methanol/0.6% hydrogen peroxide. After treatment with hyaluronidase, the sections were incubated with normal goat serum and incubated with the primary antibodies as follows: monoclonal anti-EGFR antibody (Sigma Immuno Chemicals, St. Louis, MO) (1:150 dilution), polyclonal anti-c-erbB2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (1:100 dilution), and anti-c-erbB3 antibody (Santa Cruz Biotechnology) (1:320 dilution). The antibodies have no cross-reactivity with each other. Bound antibody was detected with a biotinylated goat antimouse or antirabbit IgG secondary antibody and a streptavidin-peroxidase complex (Kirkegaard & Perry), followed by incubation with diaminobenzidine tetrahydrochloride (0.05%) as the substrate and counterstaining with Mayer’s hematoxylin. To ensure specificity of the primary antibodies, consecutive sections were incubated either in the absence of the primary antibody or with a nonimmunized rabbit IgG antibody. In these cases, no immunostaining was detected.
Histopathologic analysis was performed by two independent observers blinded to patient status, followed by resolution of any differences by joint review and consultation with a third observer. The immunohistochemical results of EGFR, c-erbB2, and c-erbB3 analysis were scored in a semiquantitative fashion. Both the intensity of immunostaining and the percentage of immunoreactive cells were analyzed as previously described. 24 In brief, the evaluators recorded the percentage of positively staining cells in four categories: 0 = no staining in tumor cells, 1 = 0% to 33% of the cells exhibit immunoreactivity, 2 = 33% to 66% of the cells exhibit immunoreactivity, 3 = 66% or more of the cells exhibit immunoreactivity. In addition, the staining intensity was recorded in four intensity categories: 0 = no immunostaining, 1 = weak immunostaining, 2 = moderate immunostaining, and 3 = intense immunostaining. For each tissue sample, the immunohistochemical staining score was calculated by multiplying the score of the percentages of immunopositive cancer cells by the intensity score.
Statistical Analysis
The data are given as median and range or as mean ± SEM. For statistical analysis, Student’s t test was used. In all cases, significance was defined as p < 0.05.
RESULTS
Northern Blot Analysis
All normal papilla of Vater tissue samples exhibited moderate EGFR mRNA expression levels. c-erbB2 mRNA expression levels in these samples were lower than those of EGFR, whereas strong mRNA expression was found for c-erbB3 (Fig. 1). In papilla of Vater cancer samples, EGFR mRNA expression was similar to that found in the normal tissues. Compared with normal papilla of Vater tissues, the cancer samples exhibited similar c-erbB2 mRNA levels. In contrast, significantly reduced c-erbB3 mRNA expression was found in the papilla of Vater cancer samples compared with normal controls. By densitometry, c-erbB3 mRNA levels were 2.4-fold lower (p < 0.05) in the papilla of Vater cancer samples than in the normal papilla of Vater samples.
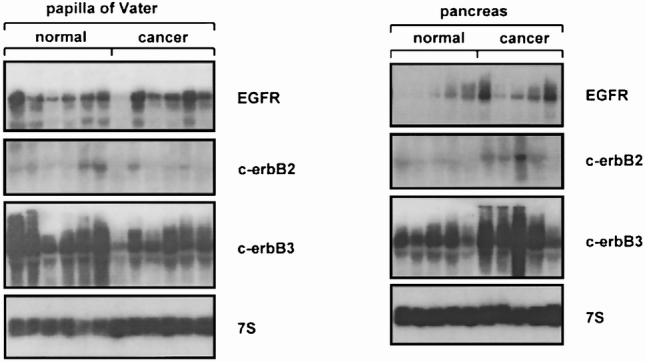
Figure 1. Northern blot analysis of papilla of Vater and pancreatic tissue samples for EGFR, c-erbB2, and c-erbB3. mRNA expression of EGFR was similar, c-erbB2 mRNA levels were slightly lower, and c-erbB3 mRNA levels were significantly reduced in papilla of Vater cancer samples compared with normal tissues. In contrast, mRNA expression of EGFR, c-erbB2, and c-erbB3 was significantly increased in pancreatic cancer samples compared with normal tissues. 7S hybridization was used to verify equivalent RNA loading.
In contrast to papilla of Vater cancer, pancreatic cancer samples exhibited enhanced EGFR, c-erbB2, and c-erbB3 mRNA expression compared with the normal pancreas. Densitometric analysis revealed a 4-fold, a 2.5-fold, and a 5.2-fold increase in EGFR (p < 0.05), c-erbB2 (p < 0.05), and c-erbB3 (p < 0.05) mRNA levels in pancreatic cancer samples versus the normal control pancreas.
In Situ Hybridization
In situ hybridization was performed to determine the exact sites of expression of EGFR, c-erbB2, and c-erbB3 mRNA in normal and cancerous papilla of Vater tissues, and in the normal and cancerous pancreas.
In the normal papilla of Vater, moderate EGFR mRNA staining was mainly present in the epithelial cells (Fig. 2). c-erbB2 mRNA staining was found in the same cells, but the intensity of the c-erbB2 in situ hybridization signals was weaker than that of EGFR. Further, moderate c-erbB3 mRNA staining was visible in the epithelial cells of the normal papilla of Vater.
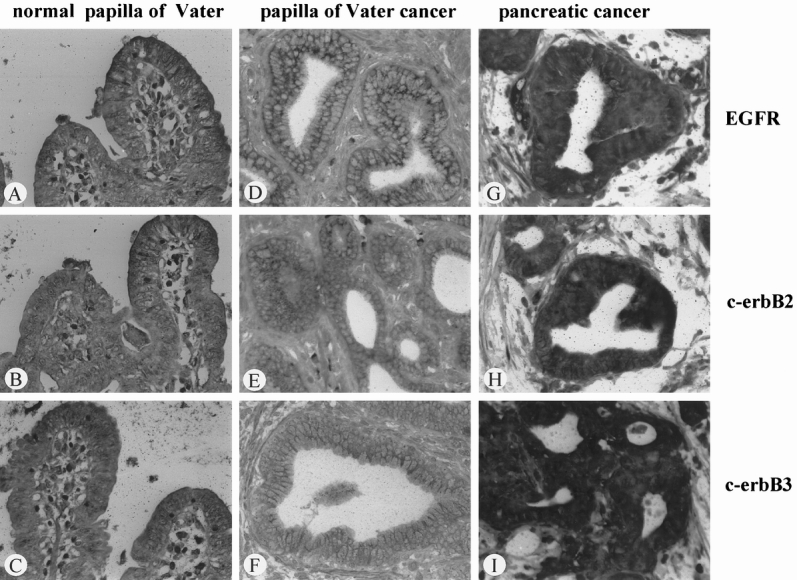
Figure 2. In situ hybridization of EGFR (A, D, G), c-erbB2 (B, E, H), and c-erbB3 mRNA (C, F, I) in normal papilla of Vater tissue samples (A through C), papilla of Vater cancer (D through F), and pancreatic cancer (G through I). Whereas in pancreatic cancer the in situ hybridization signals were markedly increased in comparison with normal tissues, in papilla of Vater cancer samples the mRNA signals were in general weaker than in normal tissues. (Original magnification ×300)
In the papilla of Vater cancer tissues, cytoplasmic EGFR, c-erbB2, and c-erbB3 mRNA staining was present in the cancer cells. Comparison of tissue sections from normal and papilla of Vater cancer tissues, processed simultaneously under the same incubation conditions, revealed that signal intensity for EGFR was similar in cancerous and normal sections, whereas the signals for c-erbB2 and c-erbB3 were generally weaker in cancerous tissues than in normal tissues. This difference in staining was most obvious for c-erbB3, where only faint mRNA signals were detectable in the cancer cells.
In comparison with the normal pancreas, pancreatic cancer samples showed a marked increase in staining intensity for EGFR, c-erbB2, and c-erbB3 mRNA in the cytoplasm of the cancer cells.
Immunohistochemical Analysis
EGFR, c-erbB2 (p185), and c-erbB3 were localized by immunohistochemical staining. Immunostaining showed results similar to those of in situ hybridization. In the normal papilla of Vater tissue samples, moderate EGFR, c-erbB2, and c-erbB3 immunoreactivity was present in the epithelial cells (Fig. 3). In papilla of Vater cancer compared with normal tissues, cancer cells exhibited similar EGFR immunoreactivity, and for c-erbB2 and c-erbB3 less immunoreactivity was found.
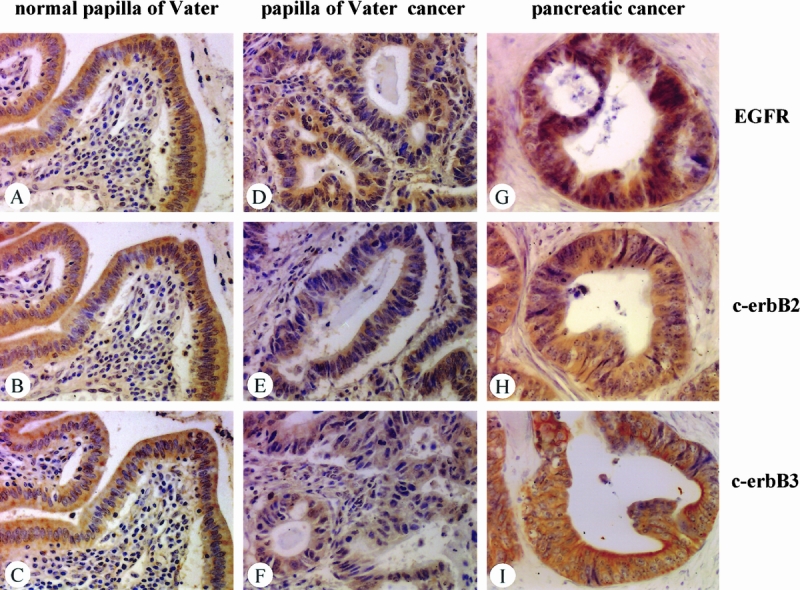
Figure 3. Immunohistochemical analysis of EGFR (A, D, G), c-erbB2 (B, E, H), and c-erbB3 (C, F, I) in normal papilla of Vater tissue samples (A through C), papilla of Vater cancer (D through F), and pancreatic cancer (G through I). Whereas in pancreatic cancer the intensity of the immunohistochemical signals was increased in comparison with normal tissues, in papilla of Vater cancer samples immunoreactivity was similar or slightly decreased compared with normal tissues. (Original magnification ×300)
Opposite findings occurred in pancreatic cancer sections. Whereas in the normal pancreas weak EGFR immunostaining was present in ductal and some acinar cells, most pancreatic cancer samples exhibited strong EGFR immunoreactivity. Similar findings were made for c-erbB2 and c-erbB3, with weak immunoreactivity in the normal pancreas and moderate to strong immunostaining in most pancreatic cancer samples in the cancer cells.
Semiquantitative Analysis of Immunohistochemical Results
The immunohistochemical staining score for EGFR was 1.44 ± 0.25 in the normal papilla of Vater samples and 1.17 ± 0.22 for papilla of Vater cancer samples. This difference was not statistically significant (p = 0.57). For c-erbB2, the immunohistochemical staining score was 3.89 ± 0.37 in the normal papilla of Vater and 2.72 ± 0.40 in papilla of Vater cancer (p = 0.058). For c-erbB3 immunoreactivity as well, there was a tendency toward lower staining in the papilla of Vater cancer samples (immunohistochemical staining score 2.78 ± 0.35) compared with the normal papilla (3.89 ± 0.53) (p = 0.057).
In contrast to the papilla of Vater samples, increased immunoreactivity of EGFR, c-erbB2, and c-erbB3 compared with normal controls was present in 60.5%, 49.4%, and 56.8%, respectively, of the pancreatic cancer samples. The immunohistochemical staining scores in the pancreatic cancer samples were 6.04 ± 0.32, 5.16 ± 0.29, and 5.76 ± 0.31 for EGFR, c-erbB2, and c-erbB3, respectively. Statistical analysis revealed that the differences in immunoreactivity between normal and pancreatic cancer samples were highly significant (p < 0.01).
DISCUSSION
The EGFR family comprises four structurally homologous transmembrane proteins with intracellular tyrosine kinase activity. The EGFR, also known as human EGFR 1 (HER-1), is probably the best-known and most-studied growth factor receptor of this family. 25 Other members of this family are c-erbB2 (also named HER-2), 26 c-erbB3 (also named HER-3), 27 and c-erbB4 28 (also named HER-4). They consist of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain. 25 The intracellular domain has tyrosine kinase activity. Receptor activation by the binding of specific ligands to the extracellular receptor domain leads to the formation of receptor oligomers. 25 However, the formation of heterodimers between members of the EGFR family has been recognized as an important mechanism in signal transduction. 29 Activation of the receptors leads to various methods of intracellular stimulation, including increased DNA synthesis, and changes in cell motility and cell metabolism. 30 Enhanced expression of EGFR leads to malignant transformation, and receptor activation is associated with cell proliferation and tumor growth in cancer cells. Similar characteristics for cell growth and proliferation have been reported for the other members of the EGFR family.
The term “periampullary cancer” includes four different tumor entities in the pancreatic head region. Although the surgical treatment concept in all four malignancies consists of a pylorus-preserving or a classical pancreaticoduodenectomy (Whipple resection), the long-term outcome is different. After Whipple resection of pancreatic, duodenal, distal bile duct, and papilla of Vater cancer, 5-year survival rates of 0% to 25%, 20% to 30%, 18% to 54%, and 15% to 56%, respectively, have been reported. 12–14,31–37
The reasons for the different prognoses are not clearly understood. In papilla of Vater cancer, it is postulated that visible jaundice resulting from obstruction of the common bile duct brings the patient to the doctor earlier, leading to earlier diagnosis. The establishment of the diagnosis at an early stage of disease is believed to be the predominant reason for the better prognosis.
In the present study, the expression and localization of EGFR, c-erbB2, and c-erbB3 were analyzed in normal papilla of Vater and papilla of Vater cancer samples, and the findings were compared with those in pancreatic cancer. Northern blot analysis revealed comparable expression of EGFR and c-erbB2 mRNA in normal and cancerous papilla of Vater samples, whereas c-erbB3 mRNA expression was significantly lower in the papilla of Vater cancer samples than in normal samples. Immunohistochemical analysis showed no significant difference in EGFR, c-erbB2, and c-erbB3 immunostaining in the papilla of Vater cancer samples. In contrast, in pancreatic cancer there was significantly enhanced EGFR, c-erbB2, and c-erbB3 mRNA expression and higher immunostaining in the cancer samples than in normal tissues.
These findings clearly show that there are differences in the molecular alterations between cancers of the papilla of Vater and of the pancreas. These findings further indicate that members of the EGFR family seem to play a minor role in the pathogenesis of papilla of Vater cancer, and that they should not be taken for prognostic discrimination in this malignancy.
For a long time, it was not known why pancreatic cancer grows so aggressively and metastasizes early into distant organs. Recent molecular research in pancreatic cancer has revealed that several growth factor receptors and their activating ligands are markedly overexpressed in the cancer cells. Further, growth-inhibiting pathways are frequently inactivated in pancreatic cancer, either by the lack of a sufficient number of signal transmitting receptors on the cell surface or by mutation or deletion of intracellular signal transmitters. 24,38–40 In our present study, we could confirm that EGFR, c-erbB2, and c-erbB3 are increased in pancreatic cancer compared with the normal pancreas. The upregulation of EGFR and c-erbB3 in pancreatic cancer cells enhances cell proliferation in vitro and in vivo, resulting in a more aggressive tumor phenotype. 23,41 Interestingly, papilla of Vater cancer cells seem to behave differently with regard to regulation of these growth-promoting factors, with equal or lower mRNA expression and lower protein staining of the three members of the EGFR family, than normal papilla of Vater samples. This indicates that on the molecular level, clear differences exist between papilla of Vater cancer and pancreatic cancer.
The lack of upregulation of these growth factor receptors in papilla of Vater cancer cells might account at least in part for the fact that this tumor type does not grow as aggressively as pancreatic cancer. It also indicates that from a molecular point of view, papilla of Vater cancer is a distinctly separate tumor entity, with a lower malignancy potential than pancreatic cancer. Further investigation is needed to clarify whether the molecular differences between pancreatic cancer and papilla of Vater cancer are limited to local growth-promoting factors (growth factors and their receptors), or whether parameters promoting tumor invasion and metastasis are also differently regulated in these malignancies.
Discussion
PROF. A. EGGERMONT (Rotterdam, The Netherlands): I would like to congratulate you on a very nice study. What your group has been doing gives credence to an already-known notion that tumors of the papilla of Vater and pancreatic cancers are two distinct entities. These are actually two very different diseases with a remarkable difference in prognosis. We already know from previous histopathology studies that the level of extraductal tumor invasion is close to 100% in pancreatic cancer, whereas it is about 60% in tumors of the papilla of Vater. With your methodology to detect three biologic determinants that can further the understanding of why these diseases are different, and from the fact that your three methodologies—in situ hybridization, immunohistochemistry, and Northern blot analysis—are coherent, you see a downregulation of this receptor family in papilla of Vater tumors, whereas you see a significantly enhanced expression in cancer of the head of the pancreas. What I would like to know is whether at the level of the individual patient it is truly a prognostic factor. I would like to see scatter diagrams which identify each patient, and where you can correlate the level of the receptor expression, tumor stage, or length of survival. Then you would actually be able to address and answer the next question, which I think is more relevant, and that is: is there a cut-off level of expression of these receptors above which patients have rapidly progressive disease? The prognosis is so dismal that you would have the impetus to further work on in this field because you may indeed have identified an independent prognostic factor. That would change the field rather remarkably because, at this point in time, we can only retrospectively investigate the material and determine prognostic factors after having performed a major and perhaps nonbeneficial surgical intervention. Could this type of study lead, and there I would like you to speculate, to methodologies where, at the preoperative stage, you may be able to identify which patient has such a dismal prognosis? By finding these types of level through fine-needle aspiration and PCR work of the material, then you could know which patients not to operate on and which patients should be operated on. That is one of the major tasks we should have in this field of surgical oncology, to know which patients we should not be operating on anymore. I would like to have your comments on that.
DR. H. FRIESS (Bern, Switzerland): We have discussed all the points you mentioned concerning the data analysis when we were looking at our results. We came to the conclusion that there are two reasons not to do it by the scatter analysis that you suggest. Firstly, our analysis includes only a low number of papilla of Vater cancer patients. Only 20 of the 24 patients underwent a resection, so if we make a survival analysis in these patients, for example, we would have only 20 evaluable patients. If you split these 20 patients into further subgroups, such as higher versus lower expression levels, you can do a statistical analysis, of course, but the significance of the answer would be very limited due to the low patient numbers. In the pancreatic cancer patients, we analyzed the data as you suggest. We divided the patients into subgroups according to the expression levels of growth factor receptors, and a variety of other markers, and could identify patients who had a poor prognosis and patients who had better prognosis. If we were to do such an analysis of papilla of Vater cancer, we would find that this kind of analysis makes no sense because the studied receptors are downregulated in all samples. Thus, these receptors seem not to play a major role in the tumor pathogenesis of papilla of Vater cancers. In pancreatic cancer, overexpression of these receptors is present in approximately half of the patients. If you find an overexpression of, for example, the EGF receptor in conjunction with its stimulatory ligands, these patients have a poorer prognosis in comparison to patients who have overexpression of only the receptor or only the ligands. In papilla of Vater cancers we see the opposite. In all of the samples from these cancers, the levels of the studied receptors are lower than in normal samples. So if I would look for a prognostic marker, I would not choose members of the EGF receptor family as candidates. In papilla of Vater cancer, there might exist other molecular alterations which are finally responsible for converting the normal cells of the papilla of Vater into malignant cells. I think that we have first to identify these factors which are activated in papilla of Vater cancer, and then do the kind of analysis with these factors which you suggested.
PROF. D. JAECK (Strasbourg, France): In a previous study analyzing EGF receptors in chronic pancreatitis, you also reported modifications in the EGF receptors in case of enlargement of the head of the pancreas. You did not mention these results today, and I would be interested to have your comment on this point. Do you believe that simultaneous analysis of suppressor genes and proliferation index could be helpful in determining the prognosis of this kind of tumor? In the literature I could find some results which seem discordant with yours: in a study made by Lee et al, published in 1995, periampullary tumors which were benign were compared to those which were malignant. They showed that, in malignant tumors, there was a high level of EGF receptors. Could you also comment on these findings? Did you observe good correlation between your biological results and the patient follow-up? In your study both groups are not homogeneous, and it would be of interest to determine a prognostic factor for each patient indicating whether this patient is likely to develop metastases, recurrence, or not. Finally, do you intend to go into the DNA not only to make a study of the receptors but also of the genes to see if you can find more reliable prognostic factors in DNA modifications?
DR. FRIESS: First I would like to comment on the paper that we have published about c-erbB2 in chronic pancreatitis and the correlation with the pancreatic head enlargement. We have analyzed several growth factors and growth factor receptors in pancreatic cancer and also in chronic pancreatitis. In chronic pancreatitis we found a subgroup of patients which always showed marked overexpression of various growth factors and growth factor receptors. However, only c-erbB2 was positively correlated with the enlargement of the pancreatic head. When the levels of overexpression of these factors are compared in chronic pancreatitis and pancreatic cancer, the levels are lower in chronic pancreatitis. If we localize these factors, they are strongly expressed in the metaplastic cells, which seem to be the cells where the remodeling of the pancreatic gland takes place. However, with regard to pancreatic cancer, we have also noticed that the overexpression of only one growth factor receptor in a specimen seems not to be enough to make a normal cell malignant. Enhanced expression of one factor might result in more cell proliferation, but this is not sufficient to transform a normal pancreatic cell into a malignant one. We believe that pancreatic cancer, for example, results from an accumulation of a variety of molecular alterations. You need overexpression of growth factor receptors and downregulation of suppressive pathways like p53 mutations or mutations of other tumor suppressor genes in combination. We also know that in pancreatic cancer, alterations in physiologically existing inhibitory pathways like the TGF-beta pathways occur, and we have recently identified several alterations in the TGF-beta pathway which contribute to the disturbance of the inhibitory action of TGF-beta. These combinations of molecular alterations make the difference between a benign disease, where you also have overexpression of some growth factor receptors, and a malignant disease.
As mentioned before, in papilla of Vater cancer, alterations in the gene levels of members of the epidermal growth factor receptor family do not seem to contribute to the malignant phenotype of these tumors, because we found downregulation of the receptors in the cancer cells in comparison to the normal cells. We were surprised by these findings because we expected that growth factor receptors of the EGF receptor family would be upregulated in papilla of Vater cancer because these tumors are quite aggressive. However, the papilla of Vater cancer cells have less growth factor receptor expression than the normal cells. Therefore, I do not want to go further with this analysis because I think it will not be fruitful. In the papilla of Vater cancer samples, we have to identify the mechanisms and factors which are important in the conversion of a normal cell into a malignant cell, and in this context members of the epidermal growth factor receptor family seem to be less important in papilla of Vater cancer compared to pancreatic cancer. If we identify factors which are overexpressed in the papilla of Vater cancer cells, we have to do further research with these genes.
PROF. H. BEGER (Ulm, Germany): I have two points. You have nicely shown the difference between pancreatic cancer and papillary cancer in terms of the growth factors. However, you investigated in the major proportion of your examinations the localized cancer stage and stage III. Localized cancers usually do not involve the lymph nodes. They are in the level of the duodenal mucosa and submucosa. Have you compared advanced papillary cancer with infiltration of the pancreas, which worsens the prognosis? The second point is: You included in your DNA analysis DNA from normal or dysplastic cells, which might cause a final result that is not representative for molecular changes of papillary cancer.
DR. FRIESS (Closing Discussion): The advantage of doing studies in pancreatic cancer is that data are much stronger than those in papilla of Vater cancer, because the patient numbers are much higher. In pancreatic cancer we found that in advanced tumor stages, the analyzed growth factor receptors are more strongly expressed than in early tumor stages. Therefore, we believe that these growth factor receptors are involved in tumor progression, rather than in tumor initiation. In papilla of Vater cancer, there was not a big difference in the expression of the analyzed growth factor receptors between the different tumor stages, so the receptors are just downregulated, or they disappear, independently of the tumor stage. As I have already mentioned, our study included only a small number of papilla of Vater cancers in early and in advanced tumor stages, and therefore the data are not sufficient to do a strong statistical analysis in subgroups. We agree completely with your statement in pancreatic cancer. In advanced tumor stages, there is a higher expression of the receptors, and you can do correlation analysis with these factors and clinical patient data. We did not do microdissection. For Northern blot analysis, we always collected tumor material from the center of the tumor, because we believe that the highest density of tumor cells is there. However, by histological analysis we could nicely differentiate between dysplasia and malignant cells, and we could confirm by immunostaining our Northern blot results. The receptors are downregulated in the papilla of Vater cancer cells.
Footnotes
Correspondence: Markus W. Büchler, MD, Dept. of Visceral and Transplantation Surgery, University of Bern, Inselspital, CH-3010 Bern, Switzerland.
Presented at the Sixth Annual Meeting of the European Surgical Association, at the Royal College of Surgeons of England, London, United Kingdom, April 23–24, 1999.
Supported by Swiss National Fund grant SNF 32-49494.
Accepted for publication July 1999.
References
- 1.Frierson HF Jr. The gross anatomy and histology of the gallbladder, extrahepatic bile ducts, Vaterian system, and minor papilla. Am J Surg Pathol 1989; 13: 146–162. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Talbot IC, Carr-Locke DL, et al. Treatment and outcome in 52 consecutive cases of ampullary carcinoma. Br J Surg 1987; 74: 957–961. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JB, Cooper MJ, Williamson RC. Adenocarcinoma of the extrahepatic biliary tree. Ann R Coll Surg Engl 1985; 67: 139–143. [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao A, Harada A, Nonami T, et al. Prognosis of cancer of the duodenal papilla of Vater in relation to clinicopathological tumor extension. Hepatogastroenterology 1994; 41: 73–78. [PubMed] [Google Scholar]
- 5.Ihse I, Axelson J, Al-Sharaf K, Andren-Sandberg A. Surgery of periampullary cancer. Dig Surg 1994; 11: 402–407. [Google Scholar]
- 6.Nakao A, Harada A, Nonami T, et al. The results and problems of surgical treatment of cancer of the duodenal papilla of Vater. Nippon Geka Gakkai Zasshi 1992; 93: 805–810. [PubMed] [Google Scholar]
- 7.Willett CG, Warshaw AL, Convery K, Compton CC. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet 1993; 176: 33–38. [PubMed] [Google Scholar]
- 8.Beger HG, Treitschke F, Poch B. Adenocarcinoma of the ampulla of Vater—operative treatment and results. In: Beger HG, Warshaw AL, Büchler MW, et al. The pancreas. Oxford: Blackwell Science, 1998: 1328–1331.
- 9.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin 1997; 47: 5–27. [DOI] [PubMed] [Google Scholar]
- 10.Friess H, Uhl W, Beger HG, Büchler MW. Surgical treatment of pancreatic cancer. Dig Surg 1994; 11: 378–386. [Google Scholar]
- 11.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–465. [DOI] [PubMed] [Google Scholar]
- 12.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihse I, Anderson H, Andren-Sandberg A. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg 1996; 20: 288–293. [DOI] [PubMed] [Google Scholar]
- 14.van Wagensveld BA, Coene PP, van Gulik TM, et al. Outcome of palliative biliary and gastric bypass surgery for pancreatic head carcinoma in 126 patients. Br J Surg 1997; 84: 1402–1406. [PubMed] [Google Scholar]
- 15.Yamaguchi K, Enjoji M, Tsuneyoshi M. Pancreatoduodenal carcinoma: a clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19–9. J Surg Oncol 1991; 47; 148–154. [DOI] [PubMed] [Google Scholar]
- 16.Friess H, Berberat P, Schilling M, et al. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med 1996; 74: 35–42. [DOI] [PubMed] [Google Scholar]
- 17.Korc M, Chandrasekar B, Yamanaka Y, et al. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest 1992; 90: 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittekind C, Wagner G. TNM-Klassifikation maligner Tumoren. Berlin: Springer, 1997.
- 19.Friess H, Cantero D, Graber H, et al. Enhanced urokinase plasminogen activation in chronic pancreatitis suggests a role in its pathogenesis. Gastroenterology 1997; 113: 904–913. [DOI] [PubMed] [Google Scholar]
- 20.Guo XZ, Friess H, Maurer C, et al. KAI1 is unchanged in metastatic and non-metastatic esophageal and gastric cancers. Cancer Res 1998; 58: 753–758. [PubMed] [Google Scholar]
- 21.Guo XZ, Friess H, Di Mola FF, et al. KAI1, a new metastasis suppressor gene, is reduced in metastatic hepatocellular carcinoma. Hepatology 1998; 28: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 22.Friess H, Yamanaka Y, Büchler M, et al. A subgroup of patients with chronic pancreatitis overexpress the c-erbB-2 protooncogene. Ann Surg 1994; 220: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friess H, Yamanaka Y, Kobrin MS, et al. Enhanced erbB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1995; 1: 1413–1420. [PubMed] [Google Scholar]
- 24.Lu Z, Friess H, Graber HU, et al. The presence of two signaling TGF-β receptors in human pancreatic cancer correlates with advanced tumor stage. Dig Dis Sci 1997; 42: 2054–2063. [DOI] [PubMed] [Google Scholar]
- 25.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990; 61: 203–212. [DOI] [PubMed] [Google Scholar]
- 26.Coussens L, Yank-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 1985; 230: 1132–1139. [DOI] [PubMed] [Google Scholar]
- 27.Kraus MH, Issing W, Miki T, et al. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci USA 1989; 86: 9193–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plowman GD, Culouscou JM, Whitney GS, et al. Ligand-specific activation of HER-4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci USA 1995; 90: 1746–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzahar E, Levkowitz G, Karunagaran D, et al. ErbB-3 and ErbB-4 function as the respective low- and high-affinity receptors of all Neu differentiation factor/heregulin isoforms. J Biol Chem 1994; 269: 25226–25233. [PubMed] [Google Scholar]
- 30.Petch LA, Harris J, Raymond VW, et al. A truncated, secreted form of the epidermal growth factor receptor is encoded by an alternatively spliced transcript in normal rat tissue. Mol Cell Biol 1990; 10: 2973–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SM, Kim SH, Choi SY, Kim YC. Surgical treatment of periampullary cancer: review of 766 surgical experiences of 8 hospitals. J Korean Med Sci 1992; 7: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakkevold KE, Kambestad B. Long-term survival following radical and palliative treatment of patients with carcinoma of the pancreas and papilla of Vater: the prognostic factors influencing the long-term results. A prospective multicentre study. Eur J Surg Oncol 1993; 19: 147–161. [PubMed] [Google Scholar]
- 33.Böttger T, Zech J, Weber W, et al. Prognostically relevant factors in cancer of Vater’s ampulla (Prognostisch relevante Faktoren beim Carcinom der Papilla Vateri). Langenbecks Arch Chir 1989; 374: 358–362. [DOI] [PubMed] [Google Scholar]
- 34.Kayahara M, Nagakawa T, Ohta T, et al. Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery 1997; 121: 611–617. [DOI] [PubMed] [Google Scholar]
- 35.Nagakawa T, Konishi I, Higashino Y, et al. The spread and prognosis of carcinoma in the region of the pancreatic head. Jpn J Surg 1989; 19: 510–518. [DOI] [PubMed] [Google Scholar]
- 36.Wade TP, Prasad CN, Virgo KS, Johnson FE. Experience with distal bile duct cancers in U.S. Veterans Affairs hospitals: 1987–1991. J Surg Oncol 1997; 64: 242–245. [DOI] [PubMed] [Google Scholar]
- 37.Fong Y, Blumgart LH, Lin E, et al. Outcome of treatment for distal bile duct cancer. Br J Surg 1996; 83: 1712–1715. [DOI] [PubMed] [Google Scholar]
- 38.Friess H, Yamanaka Y, Büchler M, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 1993; 105: 1846–1856. [DOI] [PubMed] [Google Scholar]
- 39.Wagner M, Kleeff J, Lopez ME, et al. Transfection of the type I TGF-beta receptor restores TGF-beta responsiveness in pancreatic cancer. Int J Cancer 1998; 78: 255–260. [DOI] [PubMed] [Google Scholar]
- 40.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996; 271, 5247:350–353. [DOI] [PubMed]
- 41.Yamanaka Y, Friess H, Kobrin MS, et al. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res 1993; 13: 565–570. [PubMed] [Google Scholar]


