Abstract
Objective
The survival benefit of adjuvant radiotherapy and 5-fluorouracil versus observation alone after surgery was investigated in patients with pancreatic head and periampullary cancers.
Summary Background Data
A previous study of adjuvant radiotherapy and chemotherapy in these cancers by the Gastrointestinal Tract Cancer Cooperative Group of EORTC has been followed by other studies with conflicting results.
Methods
Eligible patients with T1-2N0-1aM0 pancreatic head or T1-3N0-1aM0 periampullary cancer and histologically proven adenocarcinoma were randomized after resection.
Results
Between 1987 and 1995, 218 patients were randomized (108 patients in the observation group, 110 patients in the treatment group). Eleven patients were ineligible (five in the observation group and six in the treatment group). Baseline characteristics were comparable between the two groups. One hundred fourteen patients (55%) had pancreatic cancer (54 in the observation group and 60 in the treatment group). In the treatment arm, 21 patients (20%) received no treatment because of postoperative complications or patient refusal. In the treatment group, only minor toxicity was observed. The median duration of survival was 19.0 months for the observation group and 24.5 months in the treatment group (log-rank, p = 0.208). The 2-year survival estimates were 41% and 51%, respectively. The results when stratifying for tumor location showed a 2-year survival rate of 26% in the observation group and 34% in the treatment group (log-rank, p = 0.099) in pancreatic head cancer; in periampullary cancer, the 2-year survival rate was 63% in the observation group and 67% in the treatment group (log-rank, p = 0.737). No reduction of locoregional recurrence rates was apparent in the groups.
Conclusions
Adjuvant radiotherapy in combination with 5-fluorouracil is safe and well tolerated. However, the benefit in this study was small; routine use of adjuvant chemoradiotherapy is not warranted as standard treatment in cancer of the head of the pancreas or periampullary region.
The incidence of pancreatic cancer still seems to be increasing, and it is the fifth most common cause of cancer death in the United States. The prognosis is one of the most dismal of all cancers: approximately 95% of all patients diagnosed with pancreatic cancer will die within 1 year. 1 To date, the only potentially curative therapy is radical surgical resection. After potentially curative resection for cancer of the pancreatic head, the 1-year survival estimate is 50% to 60%, 2-year survival is 15% to 35%, and 5-year survival is 5% to 20%. In patients with periampullary carcinoma in the ampulla of Vater, distal common bile duct, or duodenum, a much better survival rate is obtained after surgical resection, with a 5-year survival rate of 40% to 60%. 2–7 After radical resection, pancreatic cancer recurs primarily within the abdominal cavity; most failures are locoregional and in the liver. Distant metastases in the lung or peritoneal cavity are seldom seen without local tumor recurrence and are characteristic of late recurrence. Therefore, a treatment approach combining local and systemic adjuvant treatment in pancreas and periampullary cancer seems interesting.
Radiation therapy with or without chemotherapy has been extensively studied in locoregional advanced disease, especially the combination of external radiotherapy combined with 5-fluorouracil (5-FU). 8–11 The possible benefit of chemoradiotherapy resulted in the use of radiotherapy combined with 5-FU as adjuvant treatment in a prospective randomized multicenter study performed by the Gastrointestinal Tumor Study Group (GITSG), in which 14 institutes participated. 12 The high incidence of pancreatic cancer and the relatively large group of participating institutions was intended to guarantee sufficient patient accrual. Radiotherapy consisted of a split course of 40 Gy—two courses of 20 Gy with an interval of 2 weeks. 5-FU was given concomitantly during the first week of radiotherapy and during 2 years thereafter. The 21 patients who received adjuvant treatment had a 2-year survival rate of 43%, significantly better than the 18% found in the control group (p < 0.03). The trial was prematurely closed because the interim analysis showed a statistically significant difference in favor of the treatment arm. Another 30 patients were treated with the same adjuvant treatment and studied by the GITSG with similar results (2-year survival rate 46%). This trial demonstrated for the first time that prolonged survival could be obtained with adjuvant treatment after surgical resection of pancreatic cancer, although this was demonstrated in only a small number of patients. 13 However, a nonrandomized study showed no significant difference in survival after adjuvant treatment as given in the GITSG trial. 14 At present, no other prospective randomized trial in pancreatic or periampullary cancers has been reported in the United States or Europe.
Recently, Yeo et al 15 described the beneficial effect of adjuvant radiotherapy and 5-FU treatment. However, in that nonrandomized study, there seemed to be a selection of patients. Patients who had a satisfactory recovery by postoperative day 60 were encouraged to accept adjuvant therapy. Patients declining radiotherapy were significantly older and had a significantly longer postoperative stay, more intensive care therapy, and a significantly greater incidence of postoperative complications.
The GITSG trial led to the use of standard adjuvant radiotherapy with 5-FU in the United States. In 1987, we initiated a prospective randomized clinical trial in collaboration with the European Organization for Research and Treatment of Cancer (EORTC) to study the effect of adjuvant radiotherapy and 5-FU.
METHODS
After resection for cancer of the head of the pancreas or periampullary region (defined as tumors in the distal common bile duct, papilla of Vater, or duodenum), patients were randomized and stratified for institution and tumor localization (pancreatic head vs. periampullary) using the minimization technique. 16 A Whipple procedure or pylorus-preserving pancreatoduodenectomy was accepted as standard resection (including total pancreatectomy). An extended lymph node resection was not performed by the surgeons participating in this study. TNM staging (according to the UICC’s 1987 guidelines) was modified for N stage. N1a-stage positive lymph nodes were located within the resection specimen, and N1b-stage positive lymph nodes were located outside the resection area—for instance, retroperitoneally along the aorta. Eligible were patients with T1-2N0-1aM0 pancreatic head cancer or T1-3N0-1aM0 periampullary cancer. Patients with stage T3 pancreatic head cancer and stage T4 periampullary cancer were excluded because of ingrowth into surrounding organs, with a limited prognosis. Twenty-nine European institutes participated in the study. Patients were randomized when the definitive pathology report was available and the patient had recovered from surgery. The treatment should start within 8 weeks after surgery; thus, patients with complications resulting in a prolonged hospital stay were not randomized. All the data were collected in a central database in the EORTC Data Center, Brussels, Belgium. Central pathology review was performed by one pathologist, who reviewed slides of the specimen and compared them with the local surgery and pathology reports.
The chemoradiotherapy regimen differed from that used in the GITSG study. 5-FU was given during the periods of radiotherapy, not thereafter, and was given as a continuous infusion instead of a bolus injection. Radiotherapy was given using a 3 or 4 field technique, starting 2 to 8 weeks after surgery. The minimal and maximal doses in the target volume were specified. Simulator films and computer planning were available for quality assurance. Doses in the liver, kidneys, and spine were not to exceed the tolerance of these normal tissues. Megavoltage photon irradiation of at least 6 MV energy was used. All fields were treated daily. The absorbed daily dose was 2 Gy, 5 fractions a week, during 2 weeks. After an interval of 2 weeks, the treatment was repeated to a total absorbed dose of 40 Gy. Chemotherapy was started on the same day before radiotherapy and consisted of a 5-FU dose of 25 mg/kg per 24 hours, with a maximal daily dose of 1500 mg. Depending on toxicity, the second course consisted of 0, 3, or 5 days of 5-FU. No 5-FU treatment during the second course of radiotherapy was given in patients with grade 3 or 4 toxicity, 3 days of 5-FU was given in patients with grade 1 or 2 toxicity, and 5 days of 5-FU was given when no toxicity occurred. Toxicity was scored according to the World Health Organization (WHO) guidelines.
Assuming an overall 2-year survival rate of 30% in the surgery-alone arm, a total of 110 deaths were necessary to detect an absolute increase of 20% (i.e., to 50% 2-year survival) on the adjuvant chemoradiotherapy arm, with a two-sided type I error of 0.05 and a power of 80%. 17 We decided to randomize 100 patients on each treatment arm and to perform the analysis when a total of 110 deaths were registered. Safety analyses were based on all eligible patients who started their treatment. Efficacy analyses (survival, progression-free survival) were performed according to the intention-to-treat principle. Survival curves were estimated using the Kaplan-Meier technique. 18 Differences in the duration of survival were compared using a two-sided log-rank test at the 0.05 level of significance. 19 The main end point of the study was the duration of survival. The two treatment arms were also compared for progression-free survival and toxicity of treatment.
RESULTS
Patient Characteristics
Between September 1987 and April 1995, 218 patients were randomized to the EORTC trial 40891, 108 patients in the observation arm (Obs) and 110 patients in the chemoradiotherapy arm (Trt). Patients were recruited from 29 centers in Europe; 3 Dutch centers and 1 French center were responsible for the randomization of 70% of all patients. Five patients in the Obs group and 6 in the Trt group were ineligible, resulting in 103 patients in the Obs group and 104 in the Trt group (Table 1). Baseline characteristics of eligible patients were comparable between the two study groups (Table 2). Central pathology review was obtained in 178 patients; in 38 patients, only local pathology data were present. From two patients, no additional data were available. After central pathology review, the diagnosis was changed in four patients in the Obs group. Pancreatic head cancer was incorrectly diagnosed in three patients, and one patient’s diagnosis was changed from unknown to periampullary cancer. In the Trt group, the diagnosis was changed to pancreatic head cancer in five patients, including one patient who had an unknown diagnosis after local pathology analysis.
Table 1. ELIGIBILITY OF RANDOMIZED PATIENTS
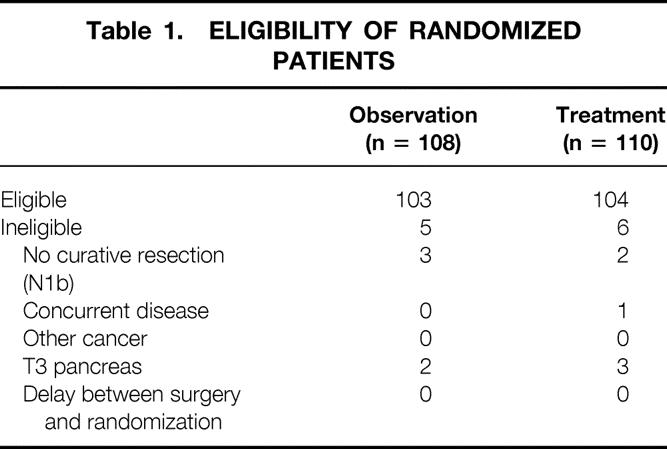
Table 2. CHARACTERISTICS OF ELIGIBLE PATIENTS
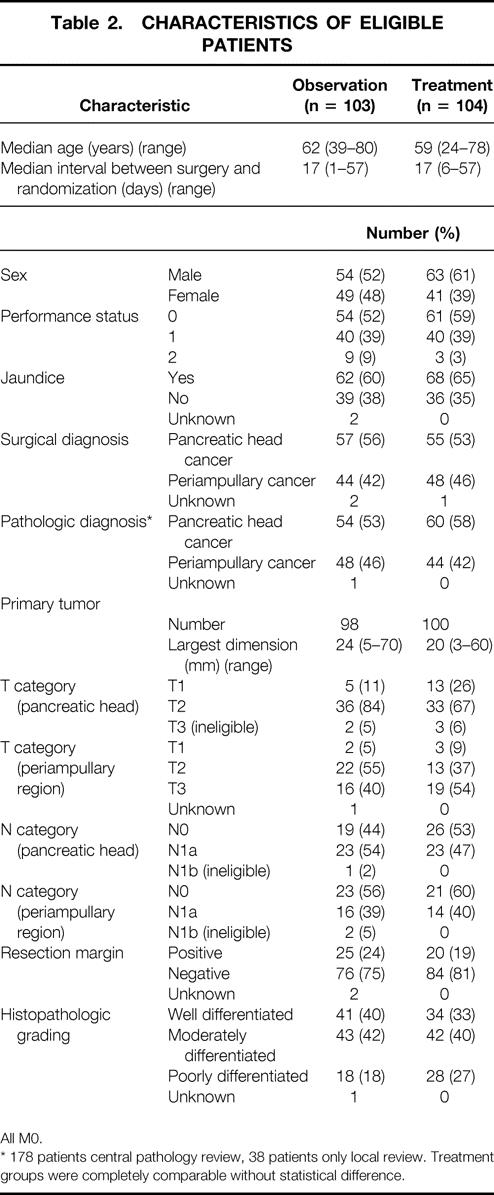
All M0.
* 178 patients central pathology review, 38 patients only local review. Treatment groups were completely comparable without statistical difference.
Treatment Data
Ten patients randomized in the Trt group (n = 104) refused to start treatment after randomization. In another 11 patients, problems developed after randomization that contraindicated adjuvant treatment. In one patient, long-lasting septic shock developed as a result of leakage of the pancreaticojejunostomy; in four patients, rapid progression occurred; one patient had only one functional kidney, located within the radiation field; and another five patients had other reasons not to start treatment, varying from the wrong treatment arm to late lung embolism after discharge. In two patients, no data at all could be obtained. As a result, a total of 81 patients could be evaluated for treatment toxicity (50 patients with pancreatic head cancer and 31 patients with periampullary cancer), and 207 patients for survival analysis.
Chemoradiotherapy
Ninety-three percent of the treated patients received 40 Gy radiation therapy. Only one patient received one course of radiation therapy (20 Gy) as a result of a persistent duodenal ulcer; it was treated with antacids and histamine blockers but did not heal after 6 weeks. As a result, the second course was canceled. The total absorbed dose varied between 30 and 43 Gy in another five patients because of local practices in the radiation department where the patient was treated.
The total dose of 5-FU was 6800 to 16,000 mg (median 12,000 mg). When corrected for body weight, the total dose was 99 to 275 mg/kg (median 197 mg/kg). We considered the maximal theoretical dose of 5-FU in the protocol to be 25 mg × body weight (kg) × 9 days, taking into consideration that a total daily dose should not exceed 1500 mg. The maximal theoretical dose was 13,500 mg. The total dose of 5-FU given, expressed as a percentage of the maximal theoretical dose, was 50% to 122% (median 90%).
Toxicity
Thirty-five patients (44%) received only 3 days of 5-FU infusion during the second course of radiotherapy because of grade 1 or 2 acute toxicity. No leukopenia or thrombopenia worse than WHO grade 1 was observed, and the daily dose of 5-FU was never reduced. Minor toxicity was observed in seven patients, with a maximal WHO grade 3 toxicity, especially nausea and vomiting. These toxicities were always treated by conservative methods. Only one instance of major toxicity was observed in the 81 patients available for analysis; this patient was described above.
Progression-Free Survival
Of the 207 eligible patients, 68 (66%) in the Obs group and 67 (65%) in the Trt group had progressive disease, independent from the localization of the tumor. The site of first progression and the site of distant progression are shown in Table 3. In the 37 patients classified as “other,” 13 had peritoneal carcinomatosis, 5 had distant lymph node metastases, and 19 had metastases varying from bone to brain metastases, equally divided between the Obs and Trt groups. There was no advantage of adjuvant treatment in progression-free survival in the total group of eligible patients, nor in the patients with pancreatic head cancer, nor in the patients with periampullary cancer, particularly not in the locoregional recurrence rate. Median progression-free survival was 16 months in the 103 patients in the Obs group and 17.4 months in the 104 patients in the Trt group. The 2-year progression-free survival rates were 38% (95% CI, 28% to 48%) and 37% (95% CI, 27% to 47%), respectively (p = 0.643, relative risk 0.9 [95% CI, 0.7 to 1.3]).
Table 3. PROGRESSION STATUS (ALL ELIGIBLE PATIENTS)
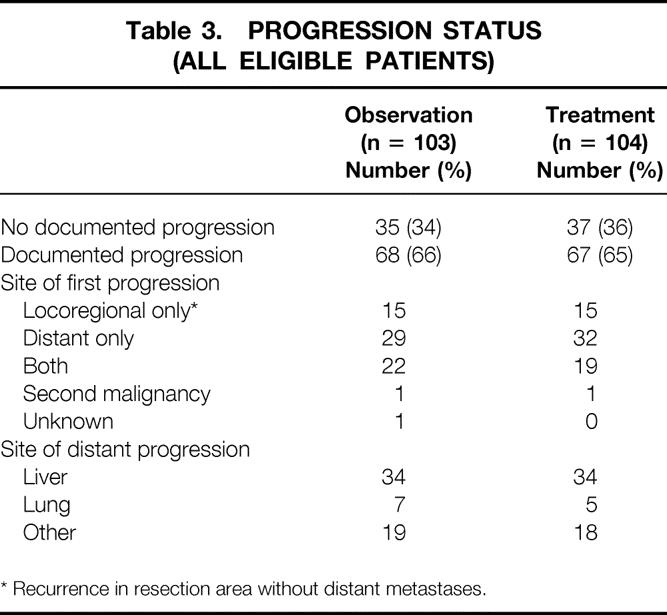
* Recurrence in resection area without distant metastases.
Duration of Survival
In all randomized patients, the cause of death was malignant disease in 125 patients (68 Obs, 57 Trt), in-hospital death in 3 patients (0 Obs, 3 Trt), nonmalignant/nontoxic death in 8 patients (3 Obs, 5 Trt), and unknown in 8 patients (5 Obs, 3 Trt). Survival was not influenced by treatment in randomized patients or in eligible patients. Survival of all eligible patients is shown in Figure 1. Median survival was 19 months in the Obs group and 24.5 months in the Trt group; the 2-year survival rates were 41% (95% CI, 31% to 51%) in the Obs group and 51% (95% CI, 41% to 61%) in the Trt group (p = 0.208). The relative risk was 0.8 (95% CI, 0.6 to 1.1). Taking into account the location of the tumor, similar results were found. The survival data and survival curves of the eligible patients in these two groups are shown in Table 4 and in Figures 2 and 3. In the patients with cancer of the head of the pancreas, a larger difference was found, but again it was not significant. The median duration of survival was 12.6 months in the Obs group and 17.1 months in the Trt group. Follow-up and number of patients seem to be insufficient to draw conclusions, but a trend in favor for adjuvant treatment was shown (p = 0.099).
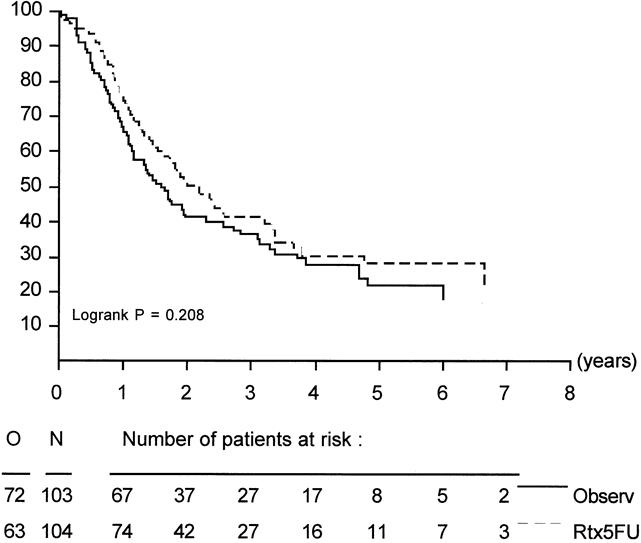
Figure 1. Duration of survival in all eligible patients.
Table 4. SURVIVAL DATA BY TUMOR AND TREATMENT ARM
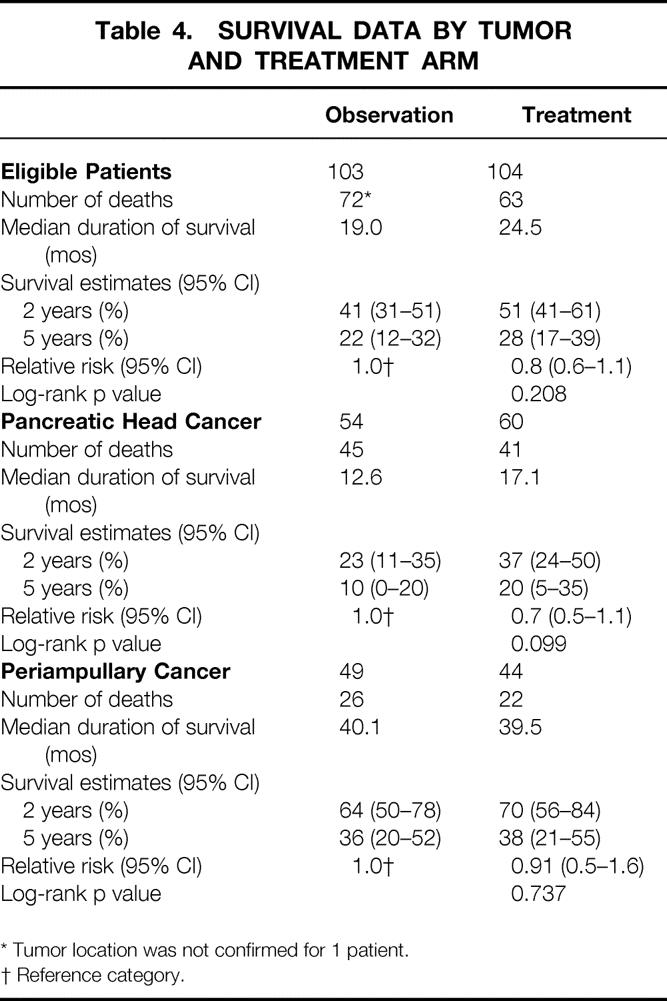
* Tumor location was not confirmed for 1 patient.
† Reference category.
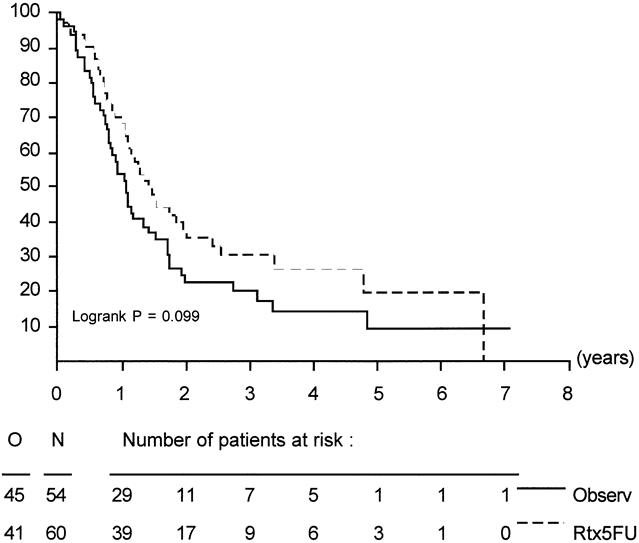
Figure 2. Duration of survival in eligible patients with pancreatic head cancer.
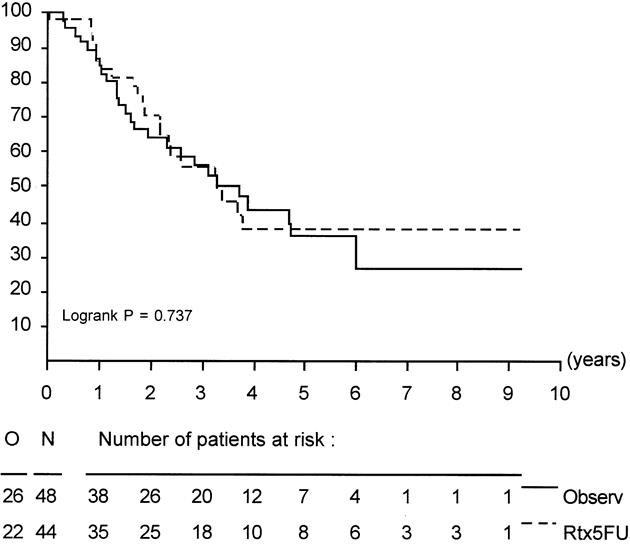
Figure 3. Duration of survival in eligible patients with periampullary cancer.
DISCUSSION
The prognosis of pancreatic cancer is dismal. Even if the tumor is resectable, the incidence of locoregional recurrence is high, up to 80%, and in most cases is combined with distant metastases. Patients with periampullary carcinoma have a more favorable outcome, with a 5-year survival rate of 50% to 70%. 2,4,6,7,20–22 To improve survival, several therapeutic modalities have been used (intraoperative radiotherapy, chemotherapy, and combinations of both) in patients with irresectable and resectable disease, with varying success. 8,10,11,14,23–29 The first study showing an effect of adjuvant treatment in pancreatic cancer was the GITSG trial. 12 The 2-year survival rate after adjuvant treatment in 21 patients was 43%, significantly better than after surgery alone (18%, p < 0.03). Another 30 patients were treated with the same treatment regimen with comparable results. 13
Because of the long accrual time and the small number of patients entered in the GITSG study, we decided to assess the possible value of radiotherapy and 5-FU as an adjuvant to surgery in a larger group of patients. A prospective randomized trial was initiated in 1987 in collaboration with the EORTC. The only treatment modification compared with the GITSG trial was a reduction in 5-FU therapy: the 5-FU was limited to the first week of each radiation course. A total of 218 patients with pancreatic head and periampullary cancer were randomized in the study after resection. Radiotherapy and 5-FU treatment did not induce major toxicity (the worst WHO scale toxicity was 3); all patients but the one with severe toxicity completed the treatment. However, despite the success of this treatment in the only other randomized study, 12 no advantage in progression-free survival or duration of overall survival was shown in this large group of patients for either cancer. Nevertheless, the difference in survival in patients with pancreatic head cancer seemed to be larger than in those with periampullary cancer. The median duration of survival for patients with pancreatic head cancer was 12.6 months in the Obs group and 17.1 months in the Trt group (p = 0.099). However, this was an exploratory analysis: the sample size was not large enough to draw any separate conclusions. Also, it should be noted that N1a-stage patients were included in the trial. It is questionable whether this variance from the GITSG study makes any difference in outcome.
It is not likely that the difference in results from the GITSG trial is due to the modification of the treatment schedule. In pancreatic cancer, 5-FU alone is known to have a limited effect, although this hypothesis cannot be proven because of the different adjuvant treatment regimen used in this study. 30 Further, an advantage in survival would be expected by the reduction of local recurrence, which was not apparent in the study groups. The radiation dose of 40 Gy may not have been high enough to prevent local recurrence. This suggests that radiotherapy with 40 Gy does not prevent local recurrence and hence is not effective as adjuvant treatment in pancreatic or periampullary cancer. Because of the central review of pathology results, in almost 75% of cases there was no misdiagnosis in location or grade of the tumor.
The question remains as to what kind of adjuvant therapy should be proposed, given that surgery alone is inadequate. Radiotherapy with chemotherapy has been applied as neoadjuvant treatment with some success, but the value of this treatment is not yet proven. 23,30–34 New chemotherapeutic agents such as docetaxel, gemcitabine, and topotecan have been studied, with response rates of 10% to 25%. 35 Link et al described a technique of intraarterial infusion chemotherapy using the celiac axis after resection; they found promising results with respect to median survival, but only in 18 patients after curative resection. 36–38
In summary, at present there seems to be no effective adjuvant treatment available for patients with cancer of the pancreas or periampullary region. Our results indicate that there is no indication for radiotherapy and 5-FU as standard adjuvant treatment after curative resection, although it can be used safely and is well tolerated.
Acknowledgments
The following physicians participated in the trial: Nordlinger B, Hospital St. Antoine, Paris, France; Gay F, Hospital Erasme, Brussels, Belgium; Gignoux M, CHU, Caen, France; Boerma EJ, Kennemer Gasthuis, Haarlem, The Netherlands; Greve JWM, University Hospital, Maastricht, The Netherlands; Rauschecker H, University Hospital, Göttingen, Germany; Brassier D, CHG Ballanger, Aulnay-sur-Bois, France; Buset M, Hospital Brugmann, Brussels, Belgium; Veeze M, Rotterdam Cancer Center, Rotterdam, The Netherlands; Oberlin P, CH Villeneuve, St. Georges, France; Descottes B, CHRU, Limoges, France; Guimares Dos Santos J, IPO, Porto, Portugal; Lenroit JP, CHG, Longjumeau, France; Wals J, Gregorius Hospital, Brunssum, The Netherlands; De Greve J, AZVUB, Brussels, Belgium; Rougier P, IGR, Villejuif, France; Millat P, CHU, Montpellier, France; Ollier JC, CMCO, Schiltingheim, France; Pezet D, Hospital Clermont Ferrand, Clermont Ferrand, France; Nitti D, University di Padova, Padova, Italy; Silva Ferreira J, Hospital San Joao, Porto, Portugal; Hay J, Hospital Louis Mourier, Colombes, France; Estourgie RJA, St. Laurentius Hospital, Roermond, The Netherlands.
Discussion
DR. J NEOPTOLEMOS (Liverpool, United Kingdom): This phase III study of adjuvant chemoradiotherapy comprising 40 Gy of postoperative external-beam radiotherapy with combined 5-FU is the largest phase III adjuvant trial in pancreatic cancer and the most important to date. The most widely quoted adjuvant trial was undertaken by the Gastrointestinal Tumor Study Group, first published in 1985 and subsequently published with additional follow-up data along with an additional registration group in 1987.
On the basis of this study, along with questionable retrospective nonrandomized and biased studies, a number of units, particularly from North America, hold the firm opinion that adjuvant radiochemotherapy should be regarded as standard therapy following resection for pancreatic ductal adenocarcinoma. The Gastrointestinal Tumor Study Group (GITSG) included a total of only 43 patients in their randomized controlled study. The regimen comprised 40 Gy of postoperative external-beam radiochemotherapy, with 5-FU given on the first 3 days of each 2-week 20-Gy fraction. This was then followed by intravenous bolus 5-FU weekly for 2 years, or until relapse. The 5-year survival rate in the treatment group was 18%versus 8% for the control group. The major problem with this trial was that far too few patients were entered into the study. Moreover, it took more than 8 years to complete through many institutions around the United States. The average recruitment per institution was less than 1 patient per year. The confidence intervals of the study were so large that they would easily overlap with many other studies in terms of 5-year survival rate in which no adjuvant treatment had been given. Because of serious doubts as to the statistical validity of this study, a further 30 patients were treated according to the study protocol, achieving a similar result. Unfortunately, this additional study could not retrieve the deficiencies of the original GITSG study.
There is a second randomized controlled trial from Norway which is not often referred to. In this study, a total of 43 patients with ductal adenocarcinoma were randomized to adjuvant treatment or to observation. Treatment comprised combination chemotherapy of 5-FU, doxorubicin, and mitomycin C. The treatment group survived significantly longer (23 months) versus the control group (11 months). Unfortunately, there was no improvement in 5-year survival, being 4% in the treatment arm and 8% in the control group. The toxicity of the combination chemotherapy treatment used in the study was so substantial that it would be difficult to advise its use on a routine basis, particularly given the lack of any longer-term survival.
Thus, the study organized by the EORTC is of enormous importance. Most of the patients were recruited from Holland, but other participating units were drawn from France, Belgium, Germany, Portugal, and Italy. Analysis was by intention-to-treat. Overall, the study did not reveal any statistically significant differences in long-term survival. Taken at face value, this study supports the notion that adjuvant treatment following resection for pancreatic ductal adenocarcinoma is of no value.
There are two important points to be made about this study. Firstly, unlike the GITSG trial, radiochemotherapy was given without continuing 5-FU on a weekly basis—in other words, 5-FU was only given during the course of radiotherapy. Thus, the benefit that was observed in the GITSG study, apart from there being a statistical type 2 error, could have been due to the follow-up chemotherapy comprising weekly 5-FU bolus injections. Secondly, it is possible that the EORTC study is also underpowered. In the observation arm, of 108 patients randomized, only 103 were eligible. Of these, 54 had pancreatic ductal adenocarcinoma, 48 had ampullary cancer, and 1 was of unknown status. The problem arises in the comparative treatment arm. Of 110 patients randomized to the treatment arm, 104 were eligible. Unfortunately, 10 patients refused and 11 did not start the treatment, and on 2 patients there were no data. This leaves only 81 patients. The paper, however, identifies 60 patients with pancreatic ductal adenocarcinoma and 44 patients with ampullary cancer that were eligible for treatment initially without the exclusions. Unfortunately, we are given no information as to the distribution of these patients to the two arms. If we assume that the 23 patients who did not receive treatment were equally distributed between the two histological types, then less than 50 patients with pancreatic ductal adenocarcinoma actually received the treatment.
Power calculations are as follows. To detect 20% differences from a standard survival of 30% would require a minimum of 55 patients in each treatment group using 5% significance at 80% power. In fact, 55 patients in each treatment group will allow a detection of 20% differences from a standard survival ranging from 20% to 40%, but always at 80% power. At 90% power, a minimum of 73 patients in each group would be required. Although there were 60 patients with pancreatic ductal adenocarcinoma, because there was a large dropout rate, the sample size is likely to be too small. The results seem inconclusive and may mirror an inadequate sample size. The confidence interval of the response rates spans from 0.5% to 1.1%, and yet this small sample achieves borderline significance (log-rank = 0.099). This suggests that there is evidence of a treatment difference which may have reached statistical significance with an increased number of patients.
At the present time, we are also seeing the final recruitment phase for the European Study Group for Pancreatic Cancer Trial 1 (ESPAC-1). This study randomizes patients with pancreatic ductal adenocarcinoma into four groups, as follows: a treatment arm comprising radiochemotherapy followed by weekly 5-FU and folinic acid for 6 months; the second group comprises radiochemotherapy as undertaken in the EORTC study; the third group comprises chemotherapy only, comprising 5-FU and folinic acid given weekly for 6 months; the final and fourth group is an observation arm.
The trial, which is the largest trial in pancreatic cancer to date, has recruited nearly 550 patients from specialist units around Europe. This trial should answer several important questions. Firstly, whether adjuvant treatment using conventional approaches is better than observation. Secondly, it will answer the question as to which approach is superior compared to others. For example, radiochemotherapy without follow-on chemotherapy is a relatively short course of treatment and would obviously be attractive. Chemotherapy alone for 6 months has the attraction that radiotherapy, with its own set of toxicities, could be avoided. Finally, it may be that the only effective adjuvant treatment is one which combines radiochemotherapy and follow-on chemotherapy. As a precursor to the ESPAC trial, the UK Pancreatic Cancer Group (UKPACA) organized a study along the lines of the GITSG as a phase II study and confirmed that toxicity was acceptable.
Dr. J. Klinkenbijl and colleagues from the EORTC are to be congratulated for taking forward the clinical science of the treatment of pancreatic cancer. The results of the ESPAC trial, with recruitment due to close at the end of 1999, could be combined with those of EORTC to provide a metaanalysis as additional support for the results of the ESPAC trial one way or the other.
Further progress in the management of pancreatic cancer requires many more trials similar to that organized by the EORTC, which are randomized and undertaken to the high qualities set by this study.
DR. J. KLINKENBIJL (Arnhem, The Netherlands): Thank you very much for your comment. Of course we could have continued, but we made very strict end points for the study beforehand, and there were 50% of deaths in the treated group of patients when we stopped the study. This was our cutoff point. Another fact was that in the treatment group, especially in the pancreatic head cancer treatment group, there were no survivors anymore. This would not change by continuing, so the difference would not be greater at 2 years, 4 years, or 5 years. If we were to wait in the observation group, the difference would only be smaller after a longer follow-up period.
DR. NEOPTOLEMOS: Why did you not continue to recruit patients?
DR. KLINKENBIJL: The reason was that we had a cutoff point at the death of half of the patients in the follow-up group. At the time when we did the interim analysis, this point was achieved, so we stopped the intake of patients. Afterwards, we can suggest that maybe another 50 or 60 patients might have made a significant difference, but I do not think so. All the treated patients had died, but not the observed patients, so this is only speculation. I do not know. Maybe your results are going to be better.
PROF. E. FARTHMANN (Freiburg, Germany): I too want to congratulate the authors on the important study and the nice presentation, and I thank you for giving me the manuscript beforehand to go through it. I have prepared a slide as well to put this study into the context of the other studies that have been mentioned already. This is the second prospective randomized study that has been performed. The GITSG study has been mentioned already. As we have heard, they did find a significant difference in a small number of patients. Besides the Johns Hopkins study, which is retrospective, there is another retrospective one, not randomized, from Milwaukee. So we have to go by the first two studies, and we are eagerly awaiting the results of the still-running ESPAC-1 trial.
So what have we learned? We know from your study that this treatment is well tolerated. It can be applied to a large population. My point of criticism has been mentioned already. I think it is very difficult to evaluate this form of treatment if we do not separate pancreatic head carcinoma and periampullary tumors. We have heard why this is necessary, and I think this should be done in further studies. Nevertheless, you have demonstrated that locoregional recurrences were not different between the observation and the treatment group, and since the lymph node stage and the rate of positive resection margins were even slightly higher in the observation group, I think these data are quite hard, although unexpected. However, from your manuscript, I concluded that with respect to grading, the treatment group was worse. There were 27% poorly differentiated versus 18%. This would be my first question. Might this have influenced the results at all? Then you showed a small benefit towards survival, and I think your intent-to-treat analysis is perfectly correct. However, if this survival analysis were based on all eligible patients who really started their treatment, could you speculate that this difference might have reached statistical significance? One more point: 80% of your patients with pancreatic cancer had tumor extending beyond the pancreas. These were formerly T2 tumors, and now they are T3 tumors, right? However, only 50% were classified as node-positive. This is a little bit surprising because we would expect a rate of somewhere between 70% or 90% of lymph node-positive patients if they have an involved resection margin. Therefore, my question: was the extent of lymphadenectomy controlled in any way?
DR. KLINKENBIJL: No, it was not.
POF. FARTHMANN: And finally, to look to the future and to a future trial, if you were to start a new one, would you suggest changing the medication, or would you consider switching to neoadjuvant treatment, which is what we are doing right now?
DR. KLINKENBIJL: I completely agree that after doing the trial, it would have been nicer, and I think better, to include only pancreatic head cancer, but as you could see on the slides, a number of patients were switched from a diagnosis of periampullary to pancreatic head, and so on. Because there is a slight bias in this patient group, I think you have to include the periampullary group. The differentiation grade in the treatment group of the pancreatic head cancer was worse in the periampullary than the observation group. Maybe that is the reason why the results are not different, but we did not look separately for that. When we looked at all the patients with the intention-to-treat, and we did perform that analysis, there was no difference in survival. It does not matter if it was a p value of 0.1; it was not significant at all.
Standardized lymph node dissection was not performed. It was only a normal Whipple or normal PPPD procedure, without an extended lymph node dissection. If I get the opportunity to participate in a new study, I would suggest performing neoadjuvant chemoradiotherapy. I think that is the best way to try to get better results.
PROF. H. BEGER (Ulm, Germany): This is surely a very important step forward in our knowledge in terms of the treatment of ductal pancreatic cancer. I have two questions. In terms of recurrence, we have to discriminate between liver metastases, local lymph node, peritoneal, and the pancreatic bed recurrence. Did you discriminate in your follow-up for the type of recurrence? Because the patient had a local lymph node dissection, not an extended lymph node dissection, it might be important for the interpretation of your results to have knowledge of the type of local and/or peritoneal recurrence. The tendency is quite impressive in terms of the difference in the outcome of the patients in the treatment group.
Secondly, if you add the micrometastases, almost 100% of the patients have a metastatic state in ductal pancreatic cancer. My question is directed to the doses you have applied. What about the doses of the local radiation? Most of the studies, for example the GITS group, used 56 Gy applied locally, and this maybe opened an opportunity to improve the results by reduction or prevention of local recurrence.
DR. KLINKENBIJL (Closing Discussion): The local recurrences were only diagnosed with CT scanning, ultrasound, etc. There was no histological proof, but the local recurrences are reliably seen on the CT scan on the place of the pancreatic head. Everything outside of that little block of tissue was mentioned as distant metastases. Also, lymph nodes along the aorta, in the hepatoduodenal ligament, and secondaries in the liver are staged as distant metastases.
It is possible that the 56 Gy in the earlier studies could enhance a little the survival, but would have more effect on local recurrence. However, the toxicity of that dose is greater and more severe than the 40 Gy. Maybe intraoperative radiotherapy is an option. A lot of studies have been undertaken, but it is very difficult to perform, and not all centers have this opportunity. I think the neoadjuvant regimen is more and more going to be the solution for this.
Footnotes
Reprint requests: Johannes Jeekel, MD, PhD, University Hospital Rotterdam-Dijkzigt, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
Presented at the Sixth Annual Meeting of the European Surgical Association, at the Royal College of Surgeons of England, London, United Kingdom, April 23–24, 1999.
The Parthenon Trust provided the EORTC Data Center with support for this research.
Accepted for publication July 1999.
References
- 1.Parker SL, Tony T, Bolden S, Wingo PA. Cancer Statistics, 1997. CA Cancer J Clin 1997; 47: 5–27. [DOI] [PubMed] [Google Scholar]
- 2.Trede M. Survival after pancreatoduodenectomy; 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trede M. The surgical treatment of pancreatic carcinoma. Surgery 1985; 97: 28–35. [PubMed] [Google Scholar]
- 4.Klinkenbijl JHG, Jeekel J, Schmitz PIM, et al. Carcinoma of the pancreas and periampullary region: palliation versus cure. Br J Surg 1993; 80: 1575–1578. [DOI] [PubMed] [Google Scholar]
- 5.Wanebo HJ, Vezeridis MP. Pancreatic carcinoma in perspective. A continuing challenge. Cancer 1996; 78 (3 Suppl): 580–591. [DOI] [PubMed] [Google Scholar]
- 6.Wade TP, Coplin MA, Virgo KS, Johnson FE. Periampullary cancer treatment in U.S. Department of Veterans Affairs hospitals: 1987–1991. Surgery 1994; 116: 819–825. [PubMed] [Google Scholar]
- 7.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg 1995; 221: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high-dose (6000 Rads) radiation alone, moderate-dose radiation (4000 Rads + 5-fluorouracil), and high-dose radiation + 5-fluorouracil. Cancer 1981; 48: 1705–1710. [DOI] [PubMed] [Google Scholar]
- 9.GITSG. A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. Ann Surg 1979; 189: 205–208. [PMC free article] [PubMed] [Google Scholar]
- 10.GITSG. Radiation therapy combined with Adriamycin or 5-fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Cancer 1985; 56: 2563–2568. [DOI] [PubMed] [Google Scholar]
- 11.Treurniet-Donker AD, Mierlo van MJM, Putten van LJ. Localized unresectable pancreatic cancer. Int J Radiation Oncology Biol Phys 1990; 18: 59–62. [DOI] [PubMed] [Google Scholar]
- 12.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985; 120: 899–903. [DOI] [PubMed] [Google Scholar]
- 13.GITSG. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 1987; 59: 2006–2010. [DOI] [PubMed] [Google Scholar]
- 14.Neoptolemos JP, Baker P, Johnson CD, Cunningham D, Spooner D, for the UK Pancreatic Group. Adjuvant radiotherapy and follow-on chemotherapy in patients with pancreatic cancer. Results of the UK Pancreatic Cancer Group study (UKPACA-1). GI Cancer 1998; 2: 235–245. [Google Scholar]
- 15.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic carcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997; 225: 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31: 103–115. [PubMed] [Google Scholar]
- 17.Freedman LS. Tables of the number of patients required in clinical trials using the log-rank test. Statistics in Medicine 1982; 1: 121–129. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Ass 1958; 53: 457–481. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170. [PubMed] [Google Scholar]
- 20.Nitecki SS, Sarr MG, Colby TV, Heerden van JA. Long-term survival after resection for ductal carcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillemoe KD. Current management of pancreatic carcinoma. Ann Surg 1995; 221: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg 1994; 81: 102–107. [DOI] [PubMed] [Google Scholar]
- 23.Jeekel J, Treurniet-Donker AD. Treatment perspectives in locally advanced unresectable pancreatic cancer. Br J Surg 1991; 78: 1332–1334. [DOI] [PubMed] [Google Scholar]
- 24.Whittington R, Bryer MP, Haller DG, Solin LJ, Rosato EF. Adjuvant therapy of resected adenocarcinoma of the pancreas. Int J Radiation Oncology Biol Phys 1991; 21: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 25.Foo ML, Gunderson LL, Nagorney DM, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation ± 5-fluorouracil. Int J Radiation Oncol Biol Phys 1993; 26: 483–489. [DOI] [PubMed] [Google Scholar]
- 26.Taylor OM, Benson EA, McMahon MJ. Clinical trial of tamoxifen in patients with irresectable pancreatic adenocarcinoma. Br J Surg 1993; 80: 384–386. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone PA, Sindelar WF. Patterns of disease recurrence following definitive therapy of adenocarcinoma of the pancreas using surgery and adjuvant radiotherapy: correlations of a clinical trial. Int J Radiation Oncol Biol Phys 1993; 27: 831–834. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg L, Barkun AN, Denis MH, Pollak M. Low-dose octreotide and tamoxifen in the treatment of adenocarcinoma of the pancreas. Cancer 1995; 75: 23–28. [DOI] [PubMed] [Google Scholar]
- 29.Bakkevold KE, Arnesjo B, Dahl O, Kambestad B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater—results of a controlled, prospective, randomised multicenter study. Eur J Cancer 1993; 29A: 698–703. [DOI] [PubMed] [Google Scholar]
- 30.Robertson JM, Shewach DS, Lawrence TS. Preclinical studies of chemotherapy and radiation therapy for pancreatic carcinoma. Cancer 1996; 78: 674–679. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman JP, Weese JL, Solin LJ, et al. A pilot study of preoperative chemoradiation for patients with localized adenocarcinoma of the pancreas. Am J Surg 1995; 169: 71–77. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa O, Ohigashi H, Imaoka S, et al. Is the long-term survival rate improved by preoperative irradiation prior to Whipple’s procedure for adenocarcinoma of the pancreatic head. Arch Surg 1994; 129: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 33.Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. Cancer 1993; 72: 2124–2133. [DOI] [PubMed] [Google Scholar]
- 34.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992; 127: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman JP, O’Dwyer P, Agarwal P, Salazar H, Ahmad N. Preoperative chemoradiotherapy for localized pancreatic carcinoma. Cancer 1996; 78: 592–597. [DOI] [PubMed] [Google Scholar]
- 36.Link KH, Gansauge F, Pillasch J, Beger HG. Multimodal therapies in ductal pancreatic cancer. The future. Int J Pancreatol 1997; 21: 71–83. [DOI] [PubMed] [Google Scholar]
- 37.Link KH, Gansauge F, Rilinger N, Beger HG. Celiac artery adjuvant chemotherapy. Results of a prospective trial. Int J Pancreatol 1997; 21: 65–69. [DOI] [PubMed] [Google Scholar]
- 38.Link KH, Gansauge F, Gorich J, Leder GH, Rilinger N, Beger HG. Palliative and adjuvant regional chemotherapy in pancreatic cancer. Eur J Surg Oncol 1997; 23: 409–414. [DOI] [PubMed] [Google Scholar]


