Abstract
Objective
To investigate the role of P-selectin and intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of lung injury associated with pancreatitis, and the relation between xanthine oxidase-derived oxidants and expression of these adhesion molecules.
Summary Background Data
In acute pancreatitis, acute respiratory distress syndrome occurs in the early stages of disease. This process is mediated by neutrophil infiltration.
Methods
Pancreatitis was induced in rats by intraductal administration of 5% sodium taurocholate. ICAM-1 and P-selectin expression was measured using radiolabeled monoclonal antibodies. Neutrophil infiltration and plasma levels of xanthine oxidase were also evaluated.
Results
Pancreatitis induces increases in P-selectin expression in lung, whereas ICAM-1 is unchanged from baseline levels. Immunoneutralization of either P-selectin or ICAM-1 prevents the infiltration of neutrophils into the lung. Xanthine and xanthine oxidase activity were increased after induction of pancreatitis. Xanthine oxidase inhibition prevents the upregulation of P-selectin in lung and neutrophil infiltration.
Conclusions
During acute pancreatitis, P-selectin is upregulated in the pulmonary endothelium and is a key determinant of leukocyte recruitment. Constitutive ICAM-1 is also involved in the process of cell infiltration into the lung. The increased expression of P-selectin appears to be triggered by a mechanism dependent on free radicals generated by xanthine oxidase released by the damaged pancreas.
Acute pancreatitis is an autodigestive process resulting in acute inflammation of the pancreas and systemic inflammation. In the most severe forms of acute pancreatitis, acute respiratory distress syndrome often occurs in the early stages of disease and can lead to early death. 1 This acute lung injury is characterized by increased pulmonary vascular permeability and noncardiogenic pulmonary edema. There is evidence indicating that this process is mediated by neutrophil infiltration. 2
The development of the inflammatory response involves sequential neutrophil–endothelial cell interactions described as rolling, activation, firm adhesion, and migration. 3 These processes are controlled by complex interactions between surface receptors on neutrophils and their corresponding endothelial cell ligands, designated as selectins, integrins, or supergene immunoglobulins. Appropriate stimulation of leukocytes or endothelial cells results in upregulation of expression of these molecules. 3 In particular, it has been reported 4 that in vitro xanthine oxidase-derived oxidants promote neutrophil–endothelial cell interactions through intercellular adhesion molecule-1 (ICAM-1) and P-selectin ligation.
A growing body of evidence indicates that pancreatitis causes a significant oxidant–antioxidant imbalance and systemic oxidative stress. 5 We have recently reported that release of xanthine and xanthine oxidase from the pancreas into the bloodstream plays an essential role in the pathogenesis of systemic complications of pancreatitis, including oxidant stress and neutrophil infiltration into the lung. 6 Conversion of xanthine dehydrogenase (XDH) to xanthine oxidase (XOD) occurs in the acinar cells during pancreatitis by the action of proteolytic enzymes activated as a consequence of cell disruption.
Accordingly, the three main objectives of this study were to investigate whether experimental pancreatitis in rats upregulates the expression of the endothelial adhesion molecules P-selectin or ICAM-1, and whether this is a unique feature of the lung or a generalized systemic response; to characterize the role of P-selectin and ICAM-1 (as molecules involved in the processes of rolling and firm adhesion) in the pathogenesis of lung injury associated with experimental pancreatitis by means of selective immunoneutralization; and to determine the possible relation between XOD-derived oxidants and induction of these adhesion molecules.
METHODS
Animal Model of Pancreatitis
Male Sprague-Dawley (Criffa, Ifa Credo, France) rats weighing 250 to 300 g were anesthetized with an intraperitoneal dose (100 mg/kg) of thiobutabarbital (Inactin, Research Biochemicals International, Natick, MA). The biliopancreatic duct was cannulated through the duodenum and the hepatic duct was closed by a small bulldog clamp. In experimental groups (n = 18; 8 for biochemical determinations, 5 for measuring the expression of ICAM-1, and 5 for measuring the expression of P-selectin), pancreatitis was induced by retrograde injection into the biliopancreatic duct of sodium taurocholate (5%; Sigma Chemical, St. Louis, MO) in a volume of 0.1 ml/100 g using a Harvard 22 infusion pump (Harvard Instruments, Edenbridge, UK). 7 Control animals (n = 18; 8 for biochemical determinations, 5 for measuring the expression of ICAM-1, and 5 for measuring the expression of P-selectin) received an intraductal infusion of saline solution (NaCl 0.9%). We have previously reported that in this model a significant neutrophil infiltration in the lung occurs 3 hours after the induction of pancreatitis. 8 Therefore, this was the time point chosen for all experiments (variations are specified).
Monoclonal Antibodies
The monoclonal antibodies (mAbs) used for the in vivo assessment of ICAM-1 and P-selectin expression, and for immunoneutralization, were 1A29, a mouse IgG1 against ICAM-1, and RMP-1, a murine IgG2a against rat and mouse P-selectin. P-23, a nonbinding murine IgG1 directed against human but not rat P-selectin, and UPC-10, a murine IgG2a, were used as control isotype-matched nonbinding immunoglobulins for ICAM-1 and P-selectin determination, respectively. 9 1A29, RMP-1, and P23 were scaled up and purified by protein A/G chromatography at Upjohn Laboratories (Kalamazoo, MI). UPC-10 was purchased from Sigma Chemical Co. (St. Louis, MI).
Treatment Groups
In one group, circulating XOD was inhibited by an infusion of oxypurinol dissolved in bicarbonate-buffered saline into the portal vein (10 mM; 0.066 ml/min) during 30 minutes, after pancreatitis induction (n = 18; 8 for biochemical determinations, 5 for measuring the expression of ICAM-1, and 5 for measuring the expression of P-selectin). In two other groups, a mAb directed against ICAM-1 (1A29; 2 mg/kg; n = 8) or P-selectin (RMP-1; 60 μg/kg; n = 8) was intravenously administered immediately before induction of pancreatitis. The dose of 1A29 has been reported to block leukocyte adhesion in a variety of conditions. 10 The dose of RMP-1 has been shown to abrogate leukocyte rolling in intestinal vessels of colitic rats. 11 Three hours after induction, samples of plasma, pancreas, and lung were obtained, immediately frozen, and maintained at −80°C until assayed.
Measurement of ICAM-1 and P-Selectin Expression
Radioiodination of mAbs
Binding mAbs directed against ICAM-1 (1A29) and P-selectin (RMP-1) were labeled with 125I (Du Pont NEN, Boston, MA), whereas the isotype-matched nonbinding mAbs (P23, UPC-10) were labeled with 131I (Du Pont NEN). Radioiodination of mAbs was performed by the iodogen method. 12 Labeled mAbs were stored at 4°C and used within 3 weeks after the labeling procedure. The specific activity of labeled mAbs was 0.5 mCi/mg.
Experimental Protocols
To determine the expression of ICAM-1 and P-selectin, the right carotid artery and right jugular vein were cannulated. Rats were randomly distributed in two groups (n = 5): control rats (biliopancreatic tract saline infusion) and pancreatitis rats (taurocholate infusion). P-selectin was also measured in an additional group of pancreatitic rats receiving treatment with oxypurinol.
Three hours after induction of pancreatitis, a mixture of 3 μg 125I-anti-ICAM-1 mAb (1A29), 247 μg unlabeled 1A29, and 5 μg 131I-nonbinding mAb (P23) was administered through the jugular vein catheter. For P-selectin measurements, 5 μg 125I-anti-P-selectin mAb (RMP-1), 10 μg unlabeled RMP-1, and 5μg 131I-nonbinding mAb (UPC-10) were administered. A blood sample was obtained through the carotid artery catheter 5 minutes after injection of the mAbs mixture. The animals were then heparinized (1 mg/kg sodium heparin given intravenously) and rapidly exsanguinated by vascular perfusion of sodium bicarbonate buffer via the jugular vein with simultaneous blood withdrawal via the carotid artery, followed by perfusion of sodium bicarbonate buffer via the carotid artery after the inferior cava vein was severed at the thoracic level. Entire organs were then harvested and weighed.
Calculation
125I (binding mAb) and 131I (nonbinding mAb) activities in different organs and in 100-μl aliquots of cell-free plasma were counted in a Cobra II gamma counter (Packard, Meridian, Australia) with automatic correction for background activity and spillover. The injected activity in each experiment was calculated by counting a 5-μl sample of the mixture containing the radiolabeled mAbs. The accumulated activity of each mAb in an organ was expressed as ng of binding mAb/g of tissue. The formula used to calculate expression of an adhesion molecule was as follows:
Endothelial expression = ([cpm125I organ × g−1 × cpm125I injected−1] − [cpm131I organ × g−1 × cpm131I injected−1] × [cpm125I in plasma/cpm131I in plasma]) × ng injected binding mAb.
Biochemical Assays
Lipase
Plasma lipase was determined by using reagent kits from Boehringer-Mannheim (Mannheim, Germany) according to the supplier’s specifications.
Myeloperoxidase
Tissue myeloperoxidase was measured photometrically, employing 3,3′,5,5′- as a substrate. 13 Samples were macerated with 0.5% hexadecyl-trimethylammonium bromide in 50 mM phosphate buffer, pH 6.0. Homogenates were then disrupted for 30 seconds using a Labsonic (B. Braun, Melsongen, Germany) sonicator at 20% power and were subsequently snap-frozen in dry ice and thawed on three consecutive occasions before a final 30-second sonication. Samples were incubated at 60°C for 2 hours and then spun down at 4000 g for 12 minutes. Supernatants were collected for myeloperoxidase assay. Enzyme activity was assessed photometrically at 630 nm. The assay mixture consisted of 20 ml supernatant, 10 ml tetramethylbenzidine (final concentration 1.6 mM) dissolved in DMSO, and 70 ml H2O2 (final concentration 3.0 mM) diluted in 80 mM phosphate buffer, pH 5.4. An enzyme unit is defined as the amount of enzyme that produces an increase of 1 absorbance unit per minute.
XDH and XOD Activity
Activity of XDH and XOD in plasma was assayed according to the method described by Stirpe and Della Corte. 14 A total of 500 μl plasma supernatant was passed through a Sephadex G-25 small column. The eluted fractions were incubated for 30 minutes in the presence of dithiothreitol (1 mM) to prevent the artifactual conversion to XOD. Activities were measured spectrophotometrically on the basis of uric acid formation at 292 nm in the presence or absence of 0.60 nM NAD+, respectively. Xanthine (60 mM) was used as substrate. The kinetics of the reaction was recorded for 10 minutes at 20°C.
Xanthine
For the analysis of xanthine concentration, 500 μl blood obtained from the portal vein was placed in 1.0 ml 3.6% perchloric acid at 0.5°C for 30 minutes. After centrifugation, the supernatants were neutralized with a potassium carbonate–potassium hydroxide solution to pH 6.5 to 7.0. The assay mixture consisted of Na2CO3(0.05 M, pH 10.2), EDTA (10−4 M); nitroblue tetrazolium (1.5 × 10−4 M), and XOD (100 U/l). The reduction of nitroblue tetrazolium to formazan was recorded at 560 nm.
Protein Measurement
Total protein concentration in homogenates was determined using a commercial kit from BioRad (Munich, Germany).
Statistical Analysis
Data are expressed as mean ± SEM. Means of different groups were compared using a one-way analysis of variance. The Mann-Whitney test was performed to evaluate significant differences between groups. Differences were assumed to be significant when p < 0.05.
RESULTS
ICAM-1 and P-Selectin Expression
Table 1 summarizes the baseline expression of ICAM-1 and P-selectin in all the organs studied and the effects of pancreatitis on the expression of these adhesion molecules. ICAM-1 expression was not altered in any of the organs studied as a consequence of pancreatitis. Because maximal upregulation of ICAM-1 in response to other stimuli such as endotoxin occurs 5 to 9 hours after stimulation, additional experiments were performed 6 hours (instead of 3) after induction of pancreatitis; these confirmed that ICAM-1 expression remained unmodified. In contrast to ICAM-1, P-selectin expression markedly increased in the pancreas and lung after pancreatitis induction, whereas it remained unchanged in the other splanchnic and systemic organs studied.
Table 1. LEVELS OF P-SELECTIN AND ICAM-1 EXPRESSION
Values are expressed as mean ± SEM (n = 5).
* p < 0.05 vs. control.
Effects of Immunoneutralization of ICAM-1 or P-Selectin on Pancreatitis-Induced Lung Damage
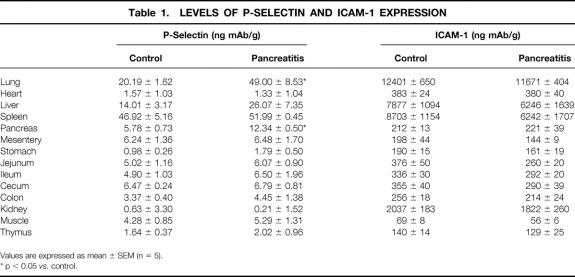
The level of neutrophil infiltration in the pancreas and lung is shown in Figure 1. As expected, pancreatitis induced a significant infiltration of neutrophils into the pancreas and lung, measured as myeloperoxidase activity. In the lung, the increase in myeloperoxidase activity was abrogated by treatment with mAbs against either ICAM-1 or P-selectin. In the pancreas, P-selectin blockage prevented the increase in MPO activity, but anti-ICAM-1 treatment had no effect on neutrophil infiltration.
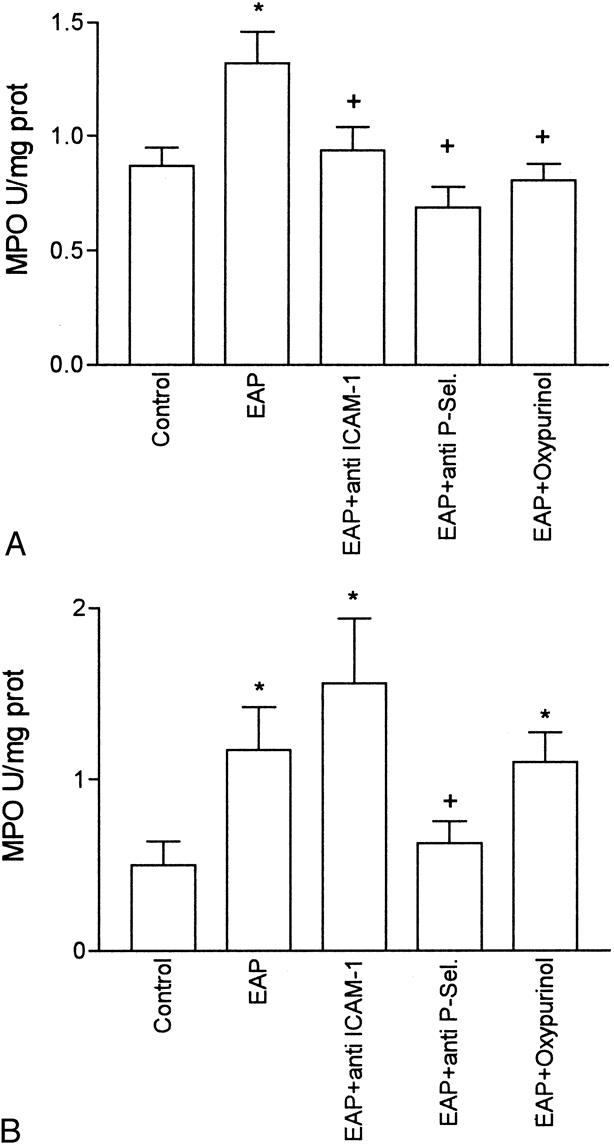
Figure 1. Myeloperoxidase activity in the lung (A) and pancreas (B), expressed as U/mg prot. *, p < 0.05 vs. control; +, p < 0.05 vs. pancreatitis (n = 8).
XOD and XDH in Plasma
Total XDH + XOD activity in plasma significantly increased after induction of pancreatitis (Fig. 2). In addition, increased conversion of XDH to XOD occurred as a consequence of pancreatitis. In control animals, XOD represented only 15% of the total enzymatic activity. Ater induction of pancreatitis, this proportion increased to 43%. Administration of oxypurinol inhibited the total activity of XDH + XOD without modifying the conversion of XDH to XOD.
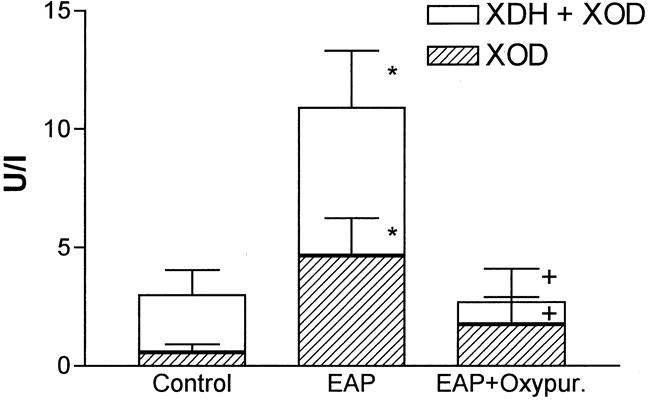
Figure 2. Xanthine oxidase (XOD) and dehydrogenase (XDH) activities in plasma. *, p > 0.05 vs. control; +, p < 0.05 vs. pancreatitis (n = 8).
Plasma Xanthine Levels
Figure 3 shows the amount of xanthine in plasma at 3 hours after induction of pancreatitis. There were no significant differences between the control and pancreatitis groups. However, inhibition of XDH and XOD with oxypurinol resulted in significant increases in xanthine levels, probably because of the lack of metabolism of xanthine to uric acid.
Figure 3. Xanthine levels in plasma expressed as μmol/l. +, p < 0.05 vs. pancreatitis (n = 8).
Effect of XOD Inhibition on P-Selectin Expression and Neutrophil Recruitment
Oxypurinol treatment completely prevented the increase in P-selectin expression in the lung associated with acute pancreatitis (Fig. 4). This treatment had no effect on the expression of P-selectin in the pancreas. In addition, administration of oxypurinol did not modify the degree of cell infiltration in the pancreas, but it abolished the increase in myeloperoxidase activity in the lung, indicating the absence of neutrophil infiltration (see Fig. 1).
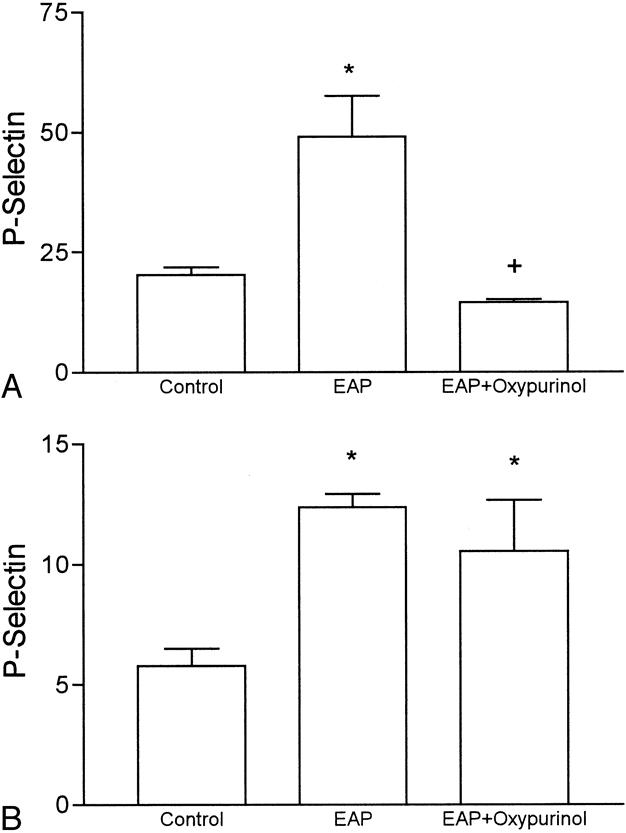
Figure 4. P-selectin expression in the lung (A) and pancreas (B) 3 hours after induction of pancreatitis. Results are expressed as μg mAb/g tissue. *, p < 0.05 vs. control; +, p < 0.05 vs. pancreatitis (n = 5).
Severity of Pancreatitic Damage
Figure 5 shows the levels of plasma lipase 3 hours after induction. Pancreatitis was associated with a significant increase in lipase levels, reflecting the degree of pancreatic damage in this experimental model. Administration of oxypurinol or mAb against ICAM-1 or P-selectin did not modify the severity of pancreatic damage.
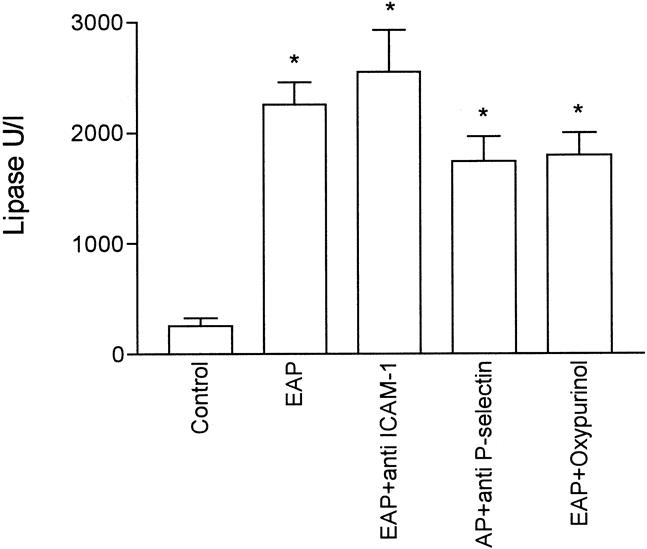
Figure 5. Lipase levels in plasma of all experimental groups. Values are expressed as U/l. *, p < 0.05 vs. control (n = 8).
DISCUSSION
Acute respiratory distress syndrome is a common early complication of acute pancreatitis that leads to significant complications or death in approximately one third of patients with severe forms. Various experimental approaches have provided evidence that this is a neutrophil-mediated event, but the mechanisms responsible for neutrophil accumulation remain unknown. 2,15,16 Neutrophil recruitment and extravasation at sites of inflammation depend in part on the expression of P-selectin and ICAM-1 on the endothelium. Immunoneutralization of these adhesion molecules has been shown to afford protection in a variety of experimental inflammatory conditions. 17
In the present study, acute pancreatitis was induced by intraductal administration of 5% sodium taurocholate. In this model, the inflammatory process in the lung can be observed 3 hours after induction. 8 This fact was evidenced by the increase in myeloperoxidase activity in the lung and pancreas, reflecting the existence of neutrophil accumulation (see Fig. 1).
When measuring P-selectin and ICAM-1 expression after induction of pancreatitis, we found that P-selectin levels significantly increased in the pancreas and in the lung, whereas P-selectin remained at baseline levels in all other organs evaluated (see Table 1). This result is consistent with the fact that the lung and pancreas are the early targets for the inflammatory process associated with acute pancreatitis. 1 In contrast, ICAM-1 expression did not change with the induction of pancreatitis in any of the organs evaluated. However, baseline expression of ICAM-1 is already high, particularly in the lung. 10 In the current study, these values turned out to be 17-fold higher than those obtained for P-selectin after induction of pancreatitis.
To evaluate the role of P-selectin and ICAM-1 in the neutrophil recruitment in this model of inflammation in the pancreas and lung, we administered mAbs at blocking doses simultaneously with the induction of pancreatitis. Under these conditions, the observed increase in myeloperoxidase activity in the lung was abrogated by anti-ICAM-1 mAb (see Fig. 1). This result could not be attributed to changes in the severity of pancreatitis, because mAb treatment did not modify the plasma levels of lipase activity (see Fig. 5), indicating a similar intensity of acinar cell damage. These data indicate that the constitutive, relatively high, level of expression of ICAM-1 contributes to neutrophil recruitment into the lung, even in the absence of molecular upregulation. This result is in accordance with a recent report of reduced lung damage associated with acute pancreatitis in ICAM-1–deficient mice. 18 In contrast, ICAM-1 blockade had no effect in neutrophil infiltration into the pancreas.
An additional endothelial ligand for pancreatitis-induced neutrophil adherence appears to be P-selectin. This molecule is expressed on the surface of endothelial cells and plays an essential role in the initial tethering of leukocytes to the endothelium, known as rolling. 19 P-selectin is also expressed on platelets, but because in our experimental design the animals were exsanguinated, the interference of this expression may be minimal. In recent years, studies have shown the involvement of P-selectin in the pathogenesis of a variety of pathologic conditions. 20,21 In the present work, we also found that P-selectin expression, both in the lung and the pancreas, markedly increased after induction of pancreatic damage (see Table 1), and immunoneutralization of this adhesion molecule resulted in prevention of the increase in myeloperoxidase activity in the lung and pancreas (see Fig. 1). It appears that induction of P-selectin expression is one of the events that trigger neutrophil infiltration into the lung during acute pancreatitis. This provides a possible therapeutic target for the treatment of systemic disorders associated with severe forms of pancreatitis. P-selectin blockade by means of mAbs or soluble oligosaccharides containing sialyl Lewisx could be effective in preventing neutrophil infiltration into the lung in patients with acute pancreatitis. Our results also indicate that immunoneutralization of ICAM-1 may be of therapeutic value to prevent lung dysfunction resulting from acute pancreatitis. However, because ICAM-1 has been proposed to mediate normal leukocyte recirculation, the latter strategy might alter physiologic processes that would otherwise be preserved by selective blockade of P-selectin.
According to these results, mediators released by the pancreas into the blood during pancreatitis induce the expression of P-selectin in lung endothelial cells. It has been reported that reactive oxygen species generated by XOD could act as inducers for the expression of P-selectin in vitro. 4 Although P-selectin expression in response to stimuli such as thrombin or histamine is usually transient (minutes), activation of endothelial cells by free radicals results in the prolonged (hours) surface expression of P-selectin. 22 Consequently, it may be hypothesized that XOD-derived oxidants may act as mediators of lung inflammation associated with pancreatitis by triggering the expression of P-selectin. We have recently shown that xanthine and XOD are released into the bloodstream as a consequence of pancreatitis, and XOD-derived oxidants act as mediators of the inflammatory response in the lung. 6 Acinar cell disruption during pancreatitis induces the activation of proteolytic enzymes and the conversion of XDH to its oxidase form. This fact was evidenced by increased plasma levels of both the dehydrogenase and oxidase forms, but the proportion of oxidase was greatly increased in pancreatitis with respect to controls (see Fig. 2). In addition, xanthine released by the damaged pancreas (see Fig. 3) can act as a substrate for the enzyme, generating in turn superoxide radical. The fact that this reaction effectively occurs is evidenced by the increase in xanthine levels detected under XOD inhibition.
Oxypurinol treatment prevented the increases in myeloperoxidase activity in the lung without modifying the levels in the pancreas (see Fig. 1). When measuring the expression of P-selectin under XOD inhibition, we found that the increase observed in the lung after induction of pancreatitis was completely abrogated by oxypurinol administration (see Fig. 4). As it occurs with myeloperoxidase activity, XOD inhibition had no effect on the levels of P-selectin expressed in the pancreas. Collectively, these observations indicate that free radicals released by XOD act as mediators inducing the expression of P-selectin in lung endothelial cells.
The role of oxygen free radicals has been extensively investigated in different models of pancreatitis. 23,24 There is evidence indicating that XOD is the main source of these mediators in pancreatic tissue. 25 However, neutrophils are recognized as effectors of lung injury when activated to generate superoxide radical by the action of the enzyme NADPH oxidase. 15 Our results indicate that circulating xanthine and XOD released by the pancreas during the course of pancreatitis link both systems of cell damage. Of course, other mediators could participate in this process. Increased levels of circulating tumor necrosis factor-α, interleukin-1, phospholipase A2, and activated complement have been reported in pancreatitis. These proinflammatory agents could also play a role in the process of P-selectin induction, but the superoxide radical generated by XOD seems to be a key mediator in this mechanism. Nevertheless, caution is needed when extrapolating these results to other animal species. Considerable interspecies differences regarding the roles of xanthine and XOD as well as the antioxidant capacity have been reported. The reason why these changes are restricted to the lung without modifying the expression of P-selectin in other organs remains to be elucidated.
In conclusion, this work indicates that during experimental acute pancreatitis induced by sodium taurocholate, P-selectin is upregulated in the pulmonary endothelium, promoting the recruitment of neutrophils into the lung. Constitutively expressed ICAM-1 is also involved in the process of cell infiltration into the lung. The increased expression of P-selectin appears to be triggered in this model of pancreatitis by a mechanism dependent on free radicals generated by circulating xanthine and XOD released into the bloodstream by the damaged pancreas. Therefore, both adhesion molecules and XOD are potential therapeutic targets for the prevention of lung dysfunction in acute severe pancreatitis.
Discussion
PROF. O. TERPSTRA (Leiden, The Netherlands): I enjoyed your presentation and the beautiful slides. I would like to congratulate you and your group on this important contribution in elucidating the pathogenesis of the ARDS in acute pancreatitis. I think it is important because ARDS causes a lot of morbidity and mortality in this group of patients. However, I wonder how you explain the phenomenon that inhibition of P-selectin prevents the infiltration of neutrophils in the lung and the pancreas, while immunoneutralization of the ICAM-1 occurs only in the lung, but not in the pancreas. You demonstrated that inhibition of that adhesion molecule does have an effect on the lipase concentration. What is your explanation for that?
The third question is: How can we extrapolate these findings to the human situation? Should we treat our patients with acute pancreatitis with radical scavengers or with a monoclonal antibody in this stage?
DR. D. CLOSA (Barcelona, Spain): In fact, lack of effect of ICAM-1 treatment in the pancreas was not highly surprising, since we have found very low levels of ICAM-1 expression in the pancreas. We conclude that we found an effect of treatment with monoclonal antibodies only in the organs that constitutively expressed ICAM-1, such as lung, or where the molecule has been upregulated, such as P-selectin in pancreas and lung. ICAM-1 is present in very low levels in pancreas and is not upregulated during acute pancreatitis; thus, the lack of effect of anti-ICAM-1 treatment in the pancreas is not really surprising.
With respect to the case of humans, the involvement of xanthine oxidase has been largely discussed, but it appears that the inhibition of xanthine oxidase only shows protection in experimental models when inhibitors are administered prior to the induction of pancreatitis. Probably, administration of antioxidants after the induction of pancreatitis works too late to prevent the expression of P-selectin. In this sense, the use of monoclonal antibodies or antisense oligonucleotides to block P-selectin could be more useful for the treatment of the systemic disorders in the case of pancreatitis.
PROF. H. BEGER (Ulm, Germany): Two recent papers confirmed your hypothesis that adhesion molecules are important in the generation of multisystem organ failure in experimental acute pancreatitis because the application of anti-ICAM produced a reduction in pulmonary insufficiency and the degree of local inflammation. I have a question in terms of your results. You have shown that ICAM was not very much expressed in the local area of acute pancreatitis, in contrast to the pulmonary tissue and in contrast to the liver. How do you explain this? These papers using anti-ICAM as treatment in experimental pancreatitis have shown convincingly that the local inflammation process was reduced by application of an antiadhesion molecule treatment.
DR. CLOSA (Closing Discussion): The problem with ICAM-1 is that it shows great differences as a function of the animal model of pancreatitis. The high levels of ICAM-1 found in control animals are surprising, since recent work using a mouse model of pancreatitis reported low basal levels of ICAM-1 in lung and an increase in the expression of this molecule after the induction of pancreatitis. Probably rats do not need to increase the expression of ICAM-1, since in basal conditions this molecule is present in sufficient levels. In the case of the pancreas, the problem could be the model of pancreatitis. Taurocholate-induced pancreatitis is a good model to study the systemic effect of the disease, but it is probably too severe, and we suspect that the results shown in pancreas cannot be physiologically interpreted, since the local destruction due to the taurocholate could affect the expression of ICAM-1.
Footnotes
Correspondence: Daniel Closa, PhD, Department of Medical Bioanalysis, IIBB-CSIC; IDIBAPS, C/Roselló, 173, 7° 08036 Barcelona, Spain.
Presented at the Sixth Annual Meeting of the European Surgical Association, at the Royal College of Surgeons of England, London, United Kingdom, April 23–24, 1999.
Supported by SAF 97/0040, FIS 99/0107, FIS 96/1815-SAT; E. Folch was supported by a grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer.
Accepted for publication July 1999.
References
- 1.Klöppel G, Maillet B. Pathology of acute and chronic pancreatitis. Pancreas 1993; 8: 659–670. [DOI] [PubMed] [Google Scholar]
- 2.Guice KS, Oldham KT, Caty MG, et al. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg 1989; 210: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panés J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 1998; 114: 1066–1090. [DOI] [PubMed] [Google Scholar]
- 4.Terada LS, Hybertson BM, Connelly KG, et al. XO increases neutrophil adherence to endothelial cells by a dual ICAM-1 and P-selectin-mediated mechanism. J Appl Physiol 1997; 82: 866–873. [DOI] [PubMed] [Google Scholar]
- 5.Braganza JM, Scott P, Bilton D, et al. Evidence for early oxidative stress in acute pancreatitis. Int J Pancreatol 1995; 17: 69–81. [DOI] [PubMed] [Google Scholar]
- 6.Folch E, Gelpí E, Roselló-Catafau J, Closa D. Free radicals generated by xanthine oxidase mediate pancreatitis associated organ failure. Dig Dis Sci 1998; 43: 2405–2410. [DOI] [PubMed] [Google Scholar]
- 7.Aho HJ, Suonpää K, Ahola RA, Nevalainen TJ. Experimental pancreatitis in the rat: ductal factors in sodium taurocholate-induced acute pancreatitis. Exp Path 1984; 25: 73–79. [DOI] [PubMed] [Google Scholar]
- 8.Folch E, Closa D, Prats N, et al. Leukotriene generation and neutrophil infiltration after experimental acute pancreatitis. Inflammation 1998; 22: 83–93. [DOI] [PubMed] [Google Scholar]
- 9.Panés J, Perry MA, Anderson DC, et al. Portal hypertension enhances endotoxin-induced intercellular adhesion molecule 1 up-regulation in the rat. Gastroenterology 1996; 110: 866–874. [DOI] [PubMed] [Google Scholar]
- 10.Panés J, Perry MA, Anderson DC, et al. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol 1995; 269: A1955–1964. [DOI] [PubMed] [Google Scholar]
- 11.Sans M, Panés J, Ardite E, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in TNBS-induced colitis. Gastroenterology 1999; 116: 874–883. [DOI] [PubMed] [Google Scholar]
- 12.Fraker PJ, Speck JC. Protein and cell membrane iodinations with a sparingly soluble chloroamine 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem Biophys Res Comm 1978; 80: 849–855. [DOI] [PubMed] [Google Scholar]
- 13.Trush MA, Egner PA, Kensler TW. Myeloperoxidase as a biomarker of skin irritation and inflammation. Fd Chem Toxic 1994; 32: 143–147. [DOI] [PubMed] [Google Scholar]
- 14.Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase. J Biol Chem 1969; 244: 3855–3863. [PubMed] [Google Scholar]
- 15.Murakami H, Nakao A, Kishimoto W, et al. Detection of O2 generation and neutrophil accumulation in rat lungs after acute necrotizing pancreatitis. Surgery 1995; 118: 547–554. [DOI] [PubMed] [Google Scholar]
- 16.Inoue S, Nakao A, Kishimoto W, et al. Antineutrophil antibody attenuates the severity of acute lung injury in rats with experimental acute pancreatitis. Arch Surg 1995; 130: 93–98. [DOI] [PubMed] [Google Scholar]
- 17.Panés J, Perry M, Granger DN. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br J Pharmacol 1999; 126: 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frossard JL, Saluja A, Bhagat L, et al. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis-associated lung injury. Gastroenterology 1999; 116: 694–701. [DOI] [PubMed] [Google Scholar]
- 19.Lasky LA. A “roll” in acute inflammation. Curr Biol 1993; 3: 680–682. [DOI] [PubMed] [Google Scholar]
- 20.Carden DL, Young JA, Granger DN. Pulmonary microvascular injury after intestinal ischemia-reperfusion: role of P-selectin. J Appl Physiol 1993; 75: 2529–2534. [DOI] [PubMed] [Google Scholar]
- 21.Albert RK. Mechanisms of the adult respiratory distress syndrome: selectins. Lancet 1994; 344: 215–219. [DOI] [PubMed] [Google Scholar]
- 22.Doré M, Sirois J. Regulation of P-selectin expression by inflammatory mediators in canine jugular endothelial cells. Vet Pathol 1996; 33: 662–671. [DOI] [PubMed] [Google Scholar]
- 23.Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg 1984; 200: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Closa D, Hotter G, Roselló-Catafau J, et al. Prostanoids and oxygen free radicals in early stages of experimental acute pancreatitis. Dig Dis Sci 1994; 39: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 25.Closa D, Bulbena O, Hotter G, et al. Xanthine oxidase activation in cerulein- and taurocholate-induced acute pancreatitis in rats. Arch Int Physiol Biol 1994; 102: 167–170. [DOI] [PubMed] [Google Scholar]