Abstract
Objective
To evaluate different strategies for extended resections of hilar cholangiocarcinomas on radicality and survival.
Summary Background Data
Surgical resection of hilar cholangiocarcinoma is the only potentially curative treatment. Resection of central bile duct carcinomas, however, cannot always comply with the general principles of surgical oncology to achieve wide tumor-free margins with no-touch techniques.
Methods
From 1988 to 1998, 95 patients underwent resection of hilar cholangiocarcinoma. Eighty patients had hilar and hepatic resections and 15 had liver transplantation and partial pancreatoduodenectomy (LTPP;i.e., eradication of the entire biliary tract using a no-touch technique).
Results
The 60-day death rate was 8%. The overall 1- and 5-year survival rates were 67% and 22%, respectively. Five-year survival rates after R0, R1, and R2 resections were 37%, 9%, and 0%. In a multivariate analysis, surgical radicality was the strongest determinant of survival (p < 0.001). The rate of formally curative resection (R0 resection) was significantly lower in hilar resections (29%) than in liver resections (left hemihepatectomy 59%, right hemihepatectomy 55%, right trisegmentectomy 65%; p < 0.05). The highest rate of R0 resection was observed after LTPP (93%; p < 0.05). Right trisegmentectomies achieved the highest rate of 5-year survival after R0 resection (57%). In a multivariate analysis of patient survival after R0 resection, additional portal vein resection was the only significant factor. The 5-year survival rate after formally curative liver resection with portal vein resection was 65%versus 28% without.
Conclusion
Extended resections, especially right trisegmentectomies and LTPP, resulted in the highest rate of R0 resection. Right trisegmentectomy together with portal vein resection best represents the principles of surgical oncology and may be regarded as the surgical procedure of choice. Immunosuppression limits the applicability of LTPP.
Surgical strategies in the therapy of hilar cholangiocarcinoma afford patients the best chance for significant survival. Radical resections are currently considered as optimal treatment, but <20% of patients are estimated to be amenable to a formally curative approach. 1,2 Local or hilar resections including the extrahepatic suprapancreatic biliary tract represent the least extensive resection procedures and have been shown to be safe, with a surgical death rate of <1% in selected series. 3 In principle, patients with Bismuth-Corlette type I or type II tumors can undergo hilar resections with a curative intent. In practice, failure, even after formally curative extrahepatic bile duct resection, occurs in a high percentage of patients (76%) with locoregional recurrence. 4
Hilar cholangiocarcinomas involving either the right or left hepatic duct (Bismuth-Corlette types IIIa/IIIb) are generally proposed to require resection of the respective hemiliver to achieve clear margins. Recent studies on prognostic parameters after resection identified only tumor-free margins as a common predictor of postoperative survival in multivariate analyses; histopathologic differentiation and tumor stage showed a clear correlation only in single studies. 5,6 Moreover, extended hepatic resections can be expected to increase not only the rate of curative resection but also the total number of patients amenable to surgery.
Anatomic restrictions within the biliary tree make it difficult to comply with the basic principle of surgical oncology to achieve wide tumor-free margins. In the hepatic hilum, a more distant segmental ramification of the left hepatic duct, which varies in length from 1 to 5 cm, is likely to allow more radical resections on the right than on the left. Conversely, the right hepatic duct rarely exceeds 1 cm in length. 7 In addition, the common bile duct is on the right side of the hepatoduodenal ligament, with the right hepatic artery behind its proximal portion. Therefore, left lobectomies are more likely to be prevented by an encasement of the contralateral hepatic artery than right lobectomies.
Right trisegmentectomy including the caudate lobe represents the most extended right-sided hepatic resection and can achieve the largest benefit with respect to resectability and oncologic radicality. The main obstacle to this approach is frequently the preoperative anticipation of a limited functioning hepatic remnant because of the small size of the left lateral segments in some patients or because of an insufficient postoperative compensatory enlargement from cholestasis-associated liver damage. Preoperative hypertrophy of the future remnant liver, induced by unilateral portal vein embolization, has been shown to minimize the associated risk of postoperative liver failure. 8 Decompression of the biliary system may also be indicated to improve hepatocellular function. We use a modified approach with internal stent decompression of the left side of the liver and arterial platinum-coil embolization of the right liver artery; this has been shown to be safe and to induce contralateral hypertrophy. 9
Unresectable tumors and functional restrictions, as well as the assumption that greater extirpative procedures might provide an increased chance for cure, resulted in the more radical concept of resecting the entire intrahepatic biliary tree by combining hilar resection, total hepatectomy, and liver transplantation. However, postoperative and long-term survival figures have been disappointing. In a review of 56 patients originating from 13 studies, the 5-year survival rate after total hepatectomy and liver transplantation for hilar cholangiocarcinoma was approximately 5%. 10 The unpredictable risk caused by a potentially accelerated growth of residual tumor cells during chronic immunosuppression, which has been shown after hepatectomy and liver transplantation for hepatocellular carcinoma, has been suggested as a major obstacle to longevity. 11 In addition, unrecognized microscopic or gross metastases, intraoperative tumor cell seeding, and peripancreatic tumor extension into lymphatic vessels or perineural sheaths along the distal residual bile duct served as an explanation for posttransplant failures. 12
To use a no-touch technique and to achieve a wider distal radicality, we have described the extended bile duct resection—an en bloc eradication of the entire biliary tree by combining total hepatectomy, partial pancreatoduodenectomy, extended lymphadenectomy, and liver transplantation. 13 The importance of a no-touch technique in oncologic hepatobiliary surgery is evident when considering the surgical therapy of gastrointestinal cancers. Dissection of tumor or peritumorous tissue is considered obsolete, whereas the hepatic artery and portal vein in the hepatoduodenal ligament are regularly dissected close to the tumor during preparation of the hepatic hilum.
This report is a retrospective analysis of 95 patients undergoing resection of hilar cholangiocarcinomas during the past 10 years. During this period, surgical efforts toward more radical resections have constantly increased. Therefore, our strategy, developed over 10 years, may have resulted not only in more extended resections for tumors with comparable pathologic features, but also in an increase in the total number of patients undergoing surgery.
PATIENTS AND METHODS
Patients
From October 1988 to December 1998, 95 patients (50 men, 45 women) underwent surgical resection of hilar cholangiocarcinoma. The mean age of the patients was 58.2 ± 11.1 years and the mean follow-up was 22.7 ± 24.9 months. Pathologic tumor staging was performed according to the UICC classification. Twenty patients had stage I or II tumors, 9 had stage III tumors, 55 had stage IVa tumors, and 11 had stage IVb tumors (T1, n = 4; T2, n = 26; T3, n = 65; N1, n = 51; histopathologic grading: high, n = 12; moderate, n = 55; low, n = 28). Infiltration of perineural sheaths or lymphangiosis carcinomatosa was detected in 71 (75%) and 66 (69%) patients, respectively. Longitudinal extension of the tumor was classified according to the modified Bismuth-Corlette classification as type I (n = 6), II (n = 8), IIIa (n = 27), IIIb (n = 29), and IV (n = 25). Tumors were classified as type I when there was no obstruction of the confluence, type II when the obstruction was limited to the confluence, and type IIIa or IIIb when the tumor extended into the right or left ductal ramifications. Type IV indicated bilateral involvement. The Bismuth-Corlette categories were assessed before surgery by endoscopic retrograde cholangiography or percutaneous transhepatic cholangiography. However, tumors were definitely classified by the surgeon after surgery.
Surgical Procedures
The surgical procedures comprised hilar resections (n = 14), left hepatic resections (segments 1 to 4; n = 29), right-sided hemihepatectomies (segments 1 and 5 through 8; n = 11), right trisegmentectomies (segments 1 and 4 through 8; n = 26), and liver transplantation and partial pancreatoduodenectomy (LTPP; n = 15). A change in protocol occurred over this 10-year period. Hilar resections with curative intent were performed only in the early years of the program. In general, surgical radicality increased. This is partly reflected by the introduction of LTPP in 1992, and preoperative unilateral biliary decompression and arterial embolization before right trisegmentectomy in 1995. In neither group did patients undergo adjuvant radiotherapy or chemotherapy.
Resections were considered formally curative (R0 resection) when there was no evidence of gross residual disease (R2 resection) or microscopic infiltration of the resection and dissection margins (R1 resection). This classification was used despite the reservation that doubts may exist as to whether lymph node-positive tumors are surgically curable.
Hilar resections included the extrahepatic suprapancreatic bile duct and were performed with a curative intent in Bismuth-Corlette type I and II carcinomas or as a palliative procedure in patients not suitable for liver resection or those with advanced tumors. Few patients with Bismuth-Corlette type II carcinomas underwent right or left hepatic resections in an intent to achieve wider tumor-free margins. Generally, right or left hepatic resections were performed for Bismuth-Corlette type IIIa or IIIb tumors, respectively. In the group of left-sided hepatectomies, the extent of the procedure varied only with respect to the resected liver parenchyma, whereas the resection of the right hepatic duct was limited by its first segmental ramification, which regularly occurred within 1 cm from the hepatic hilum. Therefore, all left hemihepatectomies are analyzed as a common category.
In contrast, right-sided hepatic resections are differentiated as anatomic hemihepatectomies and trisegmentectomies—that is, procedures without or with resection of segment 4 and the total left hepatic duct, respectively. This distinction is required because of the more distant ramification of the left hepatic duct into segmental ducts 2 and 3, which may extend 5 cm from the hepatic hilum, allowing more radical resections than on the right side. 7 In all patients who underwent right trisegmentectomy, resection of segments 1 and 4 through 8 was performed.
To minimize the risk of postoperative liver failure associated with right trisegmentectomies, left hepatic hypertrophy was induced in 11 patients undergoing right trisegmentectomy by left hepatic biliary decompression and embolization of the right hepatic artery. The aim of this combined treatment was to achieve maximal hypertrophy of segments 2 and 3. Since 1995, all patients considered suitable for right trisegmentectomy were eligible for this procedure. In brief, endoscopic retrograde insertion of inner drainage for decompression of the left segments was combined with placement of 4 to 15 embolization platinum coils in the right hepatic artery. 9 Resection was performed 27 to 75 days after embolization, when serum bilirubin levels had dropped to <5 mg/dl and volumetric helical CT measurements disclosed an increase in volume of the left hemiliver of 11% to 68% (median 35%). No patient had severe complications from the embolization procedure.
The combined LTPP procedure has been performed since 1992 and has been described in detail. 13 It offers the advantage of wide tumor-free margins, and as a no-touch technique, it dispenses with the need to dissect the hepatoduodenal ligament. An attempt was made to select patients with less advanced tumors because it is known from other cancers that there is a limited chance of cure at an advanced stage, even with excellent surgical technique. Immunosuppression consisted of cyclosporine A, prednisolone, azathioprine, and antithymocyte globulin. Concomitant treatment was performed as described previously. 14 After 7 of 15 LTPP procedures, a 7-day course of postoperative octreotide was included in the protocol.
In the groups of partial hepatic resections, 23 patients underwent additional resection of the portal vein (6 left hepatic resections, 3 right hemihepatectomies, 14 right trisegmentectomies). The indication for resection of the portal vein bifurcation was tight adherence of the portal bifurcation to the resection specimen, which at surgery could not be judged with respect to pathology. Carcinomatous invasion was always suspected in these patients, although it was macroscopically indistinguishable from adherence and perivascular fibrosis. 15
Statistical Analysis
Statistical analysis of patient survival was performed according to the Kaplan-Meier method, calculated from the time of surgery until January 4, 1999. Comparison of patient survival in different groups was performing using the log-rank test. To assess the outcome of a surgical treatment in terms of oncologic criteria, the survival analyses with respect to the various procedures and to different tumor characteristics were restricted to R0 resections, excluding postoperative deaths. A multivariate analysis of parameters influencing survival was performed for this group of patients, as well as for all patients in the study. The variables surgical radicality, tumor stage, Bismuth-Corlette category, lymph node infiltration, histopathologic differentiation, lymphangiosis carcinomatosa, perineural sheath infiltration, and type of surgical procedure were analyzed applying the Cox multiple stepwise regression model. Categorical and continuous variables were compared using the chi square test and the Mann-Whitney test, respectively.
RESULTS
Death and Complications
The 30- and 60-day death rates were 6% (n = 6) and 8% (n = 8), respectively. Among these eight patients, two had undergone a formally curative liver resection and two had undergone LTPP. The other four patients died during the postoperative course after palliative procedures. The 60-day death rates according to the surgical procedures were 10% (n = 3) after left hemihepatectomy, 9% (n = 1) after right hemihepatectomy, 8% (n = 2) after right trisegmentectomy, and 13% (n = 2) after LTPP. In the group of hilar resections, no patient died of postoperative complications within 60 days after surgery. The main cause of death after partial liver resections was hepatic failure. In the LTPP group, two patients with pancreatic fistulas died, one of hemorrhage of the arterial anastomosis at the celiac axis and another of multiple organ failures after severe pancreatitis. Chronologically, these were patients number 4 and 6 in a group of 15 undergoing LTPP for hilar cholangiocarcinoma.
The postoperative complication rate was 59% (n = 56). It increased with the extent of resection and was lowest after hilar resections (43.%; n = 6). The most common complications were cholangitis (n = 17), hepatic insufficiency (n = 12), and bile leakage from the hepatic resection line (n = 11). Pleural effusions requiring percutaneous drainage (n = 5) occurred after right trisegmentectomies exclusively. The highest complication rate was observed after LTPP (80%; n = 12). Specific problems in this group were pancreatic fistulas (n = 4) and cellular graft rejections (n = 3) resulting from insufficient enteral cyclosporine uptake in the early phase of the study. Rejections were successfully treated by conversion to oral tacrolimus. 16
Surgical Radicality
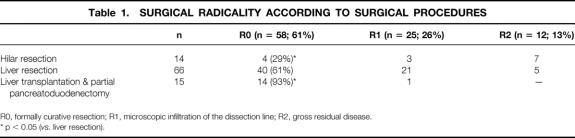
Formally curative (R0) resections were achieved in 58 patients (61%). In 25 patients (26%), microscopic infiltration of the dissection margin (R1 resection) was disclosed histologically, and 12 patients (13%) had gross residual disease (R2 resection). The rates of R0 resection for the different surgical procedures are given in Table 1. Among the groups who underwent partial liver resection (R0 resections: n = 40; 61%), the rates of R0 resection did not differ significantly. Hilar resections resulted in significantly fewer R0 resections than partial liver resections (29%vs. 61%; p < 0.05). Incomplete resections in the group of hilar resections were the result of invasion of the proximal or distal dissection margin and lateral infiltration of vascular hilar structures.
Table 1. SURGICAL RADICALITY ACCORDING TO SURGICAL PROCEDURES
R0, formally curative resection; R1, microscopic infiltration of the dissection line; R2, gross residual disease.
* p < 0.05 (vs. liver resection).
The highest rate of R0 resection was observed after LTPP (n = 14; 93%); this differed significantly from the groups of hilar resections (p < 0.01) and partial liver resections (p < 0.05). Only one noncurative (R1) LTPP was performed in a female patient, who had waited 3 months after evaluation for a suitable graft. Distal paraaortic lymph nodes were positive and the right ovary was infiltrated by a metastasis. These findings were not evident until the surgical procedure was already advanced. In the resection specimen of another patient undergoing a formally curative LTPP, a positive lymph node was detected at the right renal vein and the tumor was classified as UICC stage IVb.
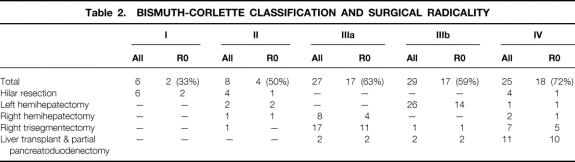
In the various Bismuth-Corlette categories, R0 resections were achieved in 43% of type I/II, 63% of type IIIa, 59% of type IIIb, and 72% of type IV tumors (Table 2). Except for four patients, all patients with Bismuth-Corlette type I or II carcinomas (n = 14) were treated by hilar resection. All noncurative resections in type I/II tumors were hilar resections, with the exception of one trisegmentectomy (R1 resection) in which distal radicality along perineural sheaths and lymphatics had not been achieved. Type IIIa tumors were mainly resected by right hemihepatectomies or trisegmentectomies, resulting in 50% R0 resections (4 of 8 patients) for hemihepatectomies and 65% R0 resections (11 of 17 patients) for trisegmentectomies. Left hemihepatectomies were performed in most patients with type IIIb tumors and were classified as formally curative in 14 of 26 resections (54%). One type IIIb carcinoma was resected by a right trisegmentectomy (R0 resection). A formally curative resection could be achieved because the tumor infiltrated the segment 4 duct but not the left lateral segments. In type IV tumors, R0 resections were achieved in 18 of 25 patients (72%; right trisegmentectomies, 5/7 patients; LTPP, 10/11 patients).
Table 2. BISMUTH-CORLETTE CLASSIFICATION AND SURGICAL RADICALITY
Survival Rates
Overall 1- and 5-year survival rates, including the postoperative deaths, were 67% and 22%, respectively. Figure 1 shows the survival rate with respect to surgical radicality. One-year survival rates after R0, R1, and R2 resections, excluding the postoperative deaths, were 86%, 58%, and 50%, respectively (p < 0.0001); the 5-year survival rate was 37% after R0 resections and 9% after R1 resections, with only one patient after R1 resection (trisegmentectomy) being alive for >5 years. No patient survived beyond 22 months after a R2 resection. Median survival was 36 months, 15 months, and 13 months after R0, R1, and R2 resections, respectively. In a multivariate analysis, surgical radicality was the most relevant determinant of patient survival.
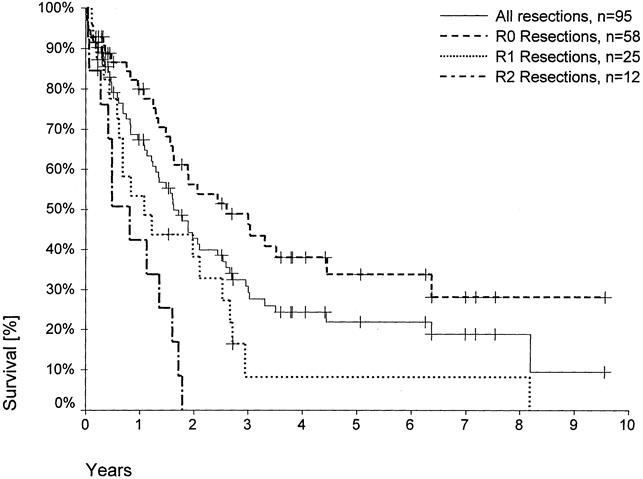
Figure 1. Actuarial patient survival according to surgical radicality (R0 resections, n = 58; R1 resections, n = 25; R2 resections, n = 12; p < 0.001). Individual patients still alive during follow-up are indicated by marks on the curves.
The survival analyses according to the different surgical procedures as well as to histopathologic parameters included only R0 resections without the postoperative deaths—that is, 4 patients after hilar resections, 16 patients after left hemihepatectomies, 6 patients after right hemihepatectomies, 16 patients after right trisegmentectomies, and 11 patients after LTPP (one patient excluded despite an R0 resection because of a UICC stage IVb tumor).
The 5-year survival rate was 28% after left hemihepatectomies, 50% after right hemihepatectomies, 57% after right trisegmentectomies, and 38% after LTPP (Fig. 2). In the group of hilar resections, no patient survived for 5 years. Accordingly, there was no 5-year survival in the Bismuth-Corlette type I/II group (Fig. 3). Five-year survival rates in the Bismuth-Corlette type IIIa, IIIb, and IV groups were 48%, 40%, and 34%, respectively.
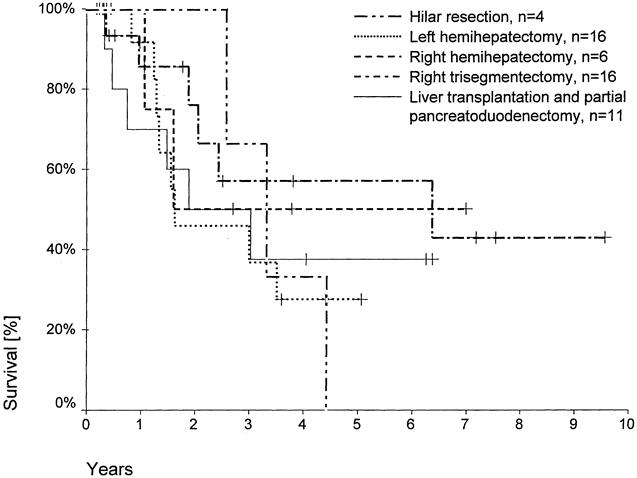
Figure 2. Actuarial patient survival according to surgical procedure (R0 resections, 60-day deaths excluded). Individual patients still alive during follow-up are indicated by marks on the curves.
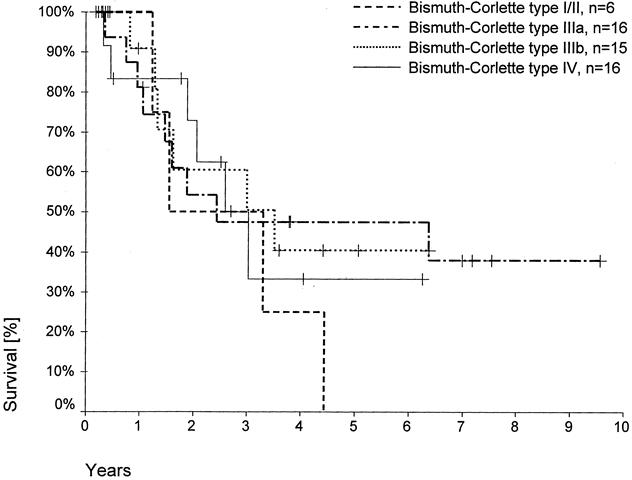
Figure 3. Actuarial patient survival according to Bismuth-Corlette category (R0 resections, 60-day deaths excluded). Individual patients still alive during follow-up are indicated by marks on the curves.
Recurrent tumors arose mainly at the former resection line, as peritoneal carcinomatosis or as distant metastases. In the group of patients who underwent LTPP, recurrent malignancy was observed in 8 of 15, including 4 patients with peritoneal carcinomatosis. The other four patients had tumors at the jejunojejunal anastomosis, at the terminal ileum, or within the abdominal wall (n = 2), where postoperative wound drainage and preoperative percutaneous transhepatic drainage had been in place, respectively.
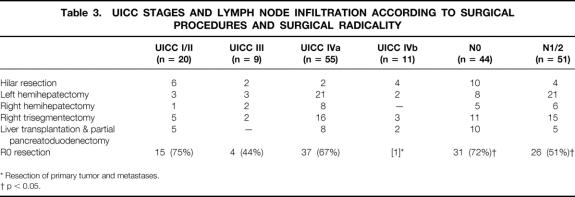
Lymph node-negative hilar cholangiocarcinomas were detected in 44 patients (46%), whereas infiltration of lymph nodes occurred in 51 patients (54%). Lymph node-negative tumors outnumbered lymph node-positive tumors only in the hilar resection and LTPP groups (Table 3). With respect to surgical radicality, significantly more R0 resections were performed in lymph node-negative than in lymph node-positive patients (72%vs. 51%; p < 0.05). Five-year survival rates after R0 resection of lymph node-negative and -positive carcinomas were 45% and 28%, respectively.
Table 3. UICC STAGES AND LYMPH NODE INFILTRATION ACCORDING TO SURGICAL PROCEDURES AND SURGICAL RADICALITY
* Resection of primary tumor and metastases.
† p < 0.05.
Positive lymph nodes frequently coincided with tumors infiltrating the surrounding structures (stage T3). Therefore, only 9 patients had UICC stage III tumors, but 55 had UICC stage IVa tumors (see Table 3). UICC stage I/II tumors (n = 20) predominated only in the hilar resection group, whereas the other procedures were predominantly performed in patients with UICC IVa carcinomas. The rate of R0 resection did not differ between UICC stages I/II, III, and IVa tumors (see Table 3). Patient survival rates were comparable after resection of UICC stage I/II (42% 5-year survival) and stage IVa tumors (39% 5-year survival;Fig. 4). ). No patient with a UICC stage III carcinoma survived beyond 40 months.
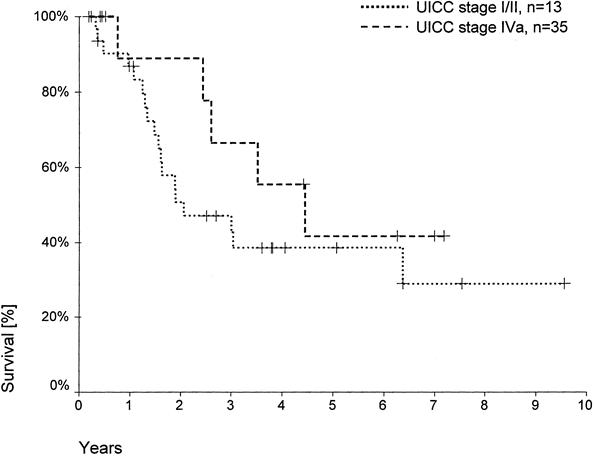
Figure 4. Actuarial patient survival according to UICC stage (R0 resections, 60-day deaths excluded). Individual patients still alive during follow-up are indicated by marks on the curves
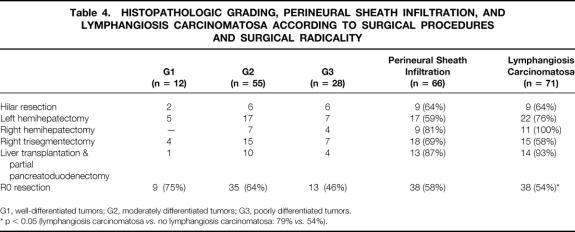
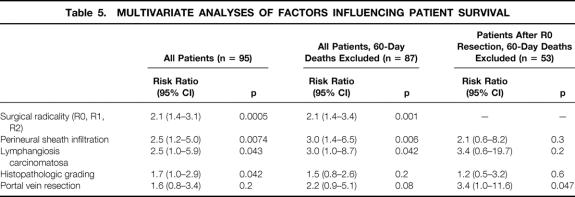
The different grades of histopathologic differentiation were distributed evenly in the groups of surgical procedures (Table 4). The postoperative death rate increased significantly in patients with poorly differentiated tumors, where 5 of 28 patients (18%) died within 60 days after surgery. In contrast, in patients with well or moderately differentiated carcinomas, 3 of 67 patients (4%) died within 60 days after surgery (p < 0.05). In the multivariate analysis, the histopathologic grading was a significant determinant of patient survival in the total series, but not when the postoperative deaths were excluded (Table 5). The rates of R0 resection of well-, moderately, or poorly differentiated hilar cholangiocarcinomas were 75%, 63%, and 48%, respectively. The difference did not reach statistical significance. The 5-year survival rates after R0 resection, excluding the postoperative deaths, in the groups of well-, moderately, or poorly differentiated tumors were 44%, 37%, and 34%, respectively.
Table 4. HISTOPATHOLOGIC GRADING, PERINEURAL SHEATH INFILTRATION, AND LYMPHANGIOSIS CARCINOMATOSA ACCORDING TO SURGICAL PROCEDURES AND SURGICAL RADICALITY
G1, well-differentiated tumors; G2, moderately differentiated tumors; G3, poorly differentiated tumors.
* p < 0.05 (lymphangiosis carcinomatosa vs. no lymphangiosis carcinomatosa: 79%vs. 54%).
Table 5. MULTIVARIATE ANALYSES OF FACTORS INFLUENCING PATIENT SURVIVAL
Perineural Sheath Infiltration and Lymphangiosis Carcinomatosa
Infiltration of perineural sheaths was detected in 66 specimens (69%). The rate of R0 resection in these patients was 58% (n = 38), compared with 66% (n = 19) in patients with hilar cholangiocarcinoma without perineural sheath infiltration (see Table 4). The 1- and 5-year survival rates after R0 resection of tumors with perineural sheath infiltration were 100% and 37%, respectively, compared with 82% and 37% in the other patients (not significant). According to surgical procedure, the highest rates of perineural sheath infiltration were observed in the groups of right hemihepatectomies (81%, n = 9) and LTPP (87%, n = 13), although the increase in frequency did not reach statistical significance.
Lymphangiosis carcinomatosa occurred in 71 patients (75%). A significantly increased rate of R0 resection (79%, n = 19) was observed for tumors negative for lymphangiosis carcinomatosa when compared with positive tumors (54%, n = 38; p < 0.05). After R0 resection, 1- and 5-year survival rates were 100% and 48%, respectively, in patients without lymphangiosis carcinomatosa versus 79% and 30% in the other patients, respectively (not significant). The fact that almost all tumors in patients undergoing right hemihepatectomy (100%, n = 11) or LTPP (93%, n = 14) showed lymphangiosis carcinomatosa did not result in a significant accumulation (see Table 4). Apart from the surgical radicality, perineural sheath infiltration and lymphangiosis carcinomatosa were independent variables determining patient survival in a multivariate analysis of the total series, irrespective of an exclusion of the postoperative deaths. In the selected subset of patients undergoing R0 resection, a significant influence on survival no longer prevailed (see Table 5).
Portal Vein Resection
Resection of the portal vein was performed in 23 patients undergoing partial liver resections, which were formally curative in 14 patients (61%;Fig. 5, Table 6). No postoperative deaths occurred among these 14 patients. The overall postoperative death rate after portal vein resection was 17% (n = 4); deaths occurred only after noncurative resections. A direct relation between portal vein resection and postoperative death was not evident. In these 23 patients, the proportion of R0 resections was highest in those undergoing right hemihepatectomies (2 of 3 patients) or trisegmentectomies (10 of 14 patients) and lowest in patients undergoing left hemihepatectomies (2 of 6 patients). However, this difference did not reach statistical significance. Histologically confirmed tumor infiltration into a resected portal vein was detected in five specimens (22%) and perivascular tumor growth in three specimens (13%). Comparing the share of tumorous portal vein infiltrations with respect to the various surgical procedures of liver resection, half of the patients undergoing a left hemihepatectomy with portal vein resection, 2 of the 14 patients undergoing right trisegmentectomy with portal vein resection, and 0 patients undergoing right hemihepatectomy had vascular tumor infiltration. In this subset of patients, a significantly increased number of portal vein infiltrations occurred after left-sided versus right-sided liver resections (50%vs. 12%; p < 0.05). R0 resections were achieved in 14 patients (61%) in whom partial hepatic resections and additional portal vein resections had been performed (2 of 6 patients with left hemihepatectomies, 2 of 3 patients with right hemihepatectomies, and 10 of 14 patients with right trisegmentectomies). One- and 5-year survival rates after formally curative partial liver resections with additional portal vein resection were 100% and 65%, respectively, versus 85% after 1 year and 0% after 5 years when there was no additional portal vein resection (p = 0.036). Only portal vein resections were identified as an independent variable in a multivariate analysis of patients after R0 resection (see Table 5). After additional portal vein resection, 13 patients survived >2 years, including only one patient after a left hemihepatectomy (right-sided vs. left-sided resections, p < 0.05 for postoperative survival > 2 years).
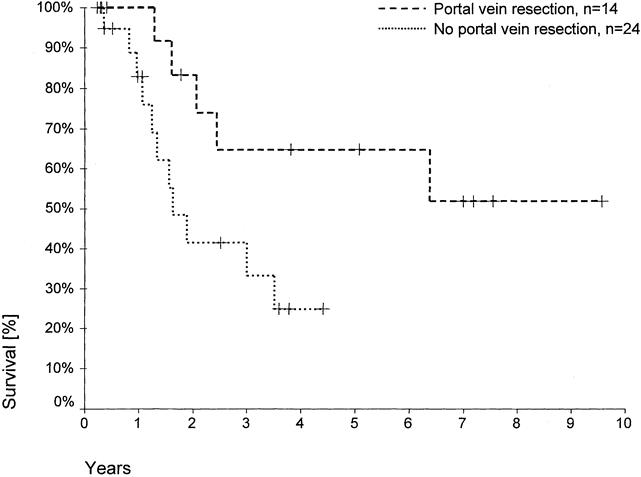
Figure 5. Actuarial patient survival according to additional portal vein resections after liver resection (R0 resections, 60-day deaths excluded; p = 0.036). Individual patients still alive during follow-up are indicated by marks on the curves.
Table 6. LIVER RESECTIONS WITH PORTAL VEIN RESECTIONS
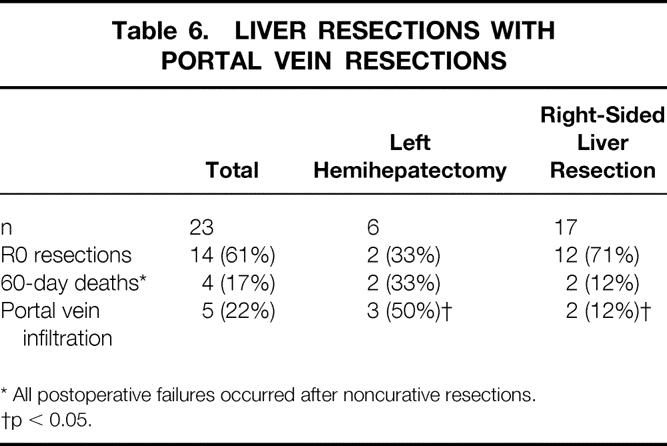
* All postoperative failures occurred after noncurative resections.
†p < 0.05.
Preoperative Biliary Decompression
Preoperative biliary decompression had been performed in 40 patients undergoing partial hepatic resections (61%; inner drainage, n = 35; percutaneous transhepatic drainage, n = 3; nasobiliary drainage, n = 2) and resulted in a significant decrease in the number of patients with elevated preoperative serum bilirubin levels (75%vs. 96%; p < 0.001). Among the patients with elevated serum bilirubin levels, mean levels were significantly lower in the decompression group (8.1 ± 6.8 mg/dl vs. 15.2 ± 8.6 mg/dl; p < 0.05). Three patients died in each group (i.e., 8% of the patients with and 12% of the patients without biliary decompression before liver resection); the respective rates were 20%versus 35% for postoperative hepatic insufficiency, 25%versus 15% for postoperative cholangitis, and 20%versus 15% for postoperative bile leakage. Neither difference reached statistical significance. One- and 5-year survival rates in the decompression group after formally curative liver resections, excluding the postoperative deaths, were 89% and 41%, respectively, compared with 91% and 45% in patients in whom decompression had not been performed. Comparing patient survival irrespective of preoperative decompression for those undergoing hepatic resection with serum bilirubin levels less than or more than 10 mg/dl, the respective rates were 92%versus 78% after 1 year and 41%versus 31% after 5 years (not significant).
DISCUSSION
Surgical Radicality
Long-term survival is the most conclusive parameter in the assessment of therapeutic strategies for malignant tumors. In our series of 95 patients undergoing resection of hilar cholangiocarcinoma, 5-year survival was observed almost exclusively in patients in whom a formally curative resection (R0) had been performed. Multivariate analysis revealed surgical radicality, perineural sheath infiltration, lymphangiosis carcinomatosa, and histopathologic grading as variables significantly influencing patient survival. Surgical radicality had been shown in multivariate analyses of previous studies to be the only parameter with a significant impact on survival identified in all reports. 1,5,6 The question remains of how to achieve an R0 resection in patients with hilar cholangiocarcinoma.
The tumor’s location in the hepatic hilum and also the type of growth create a situation that impairs the chances for cure. The nodular type with a well-demarcated tumor is rarely found in cholangiocarcinoma, and a fibrous capsule, which can frequently be detected in hepatocellular carcinoma, is almost always absent. Conversely, the periductal type, with infiltration and proliferation along thickened bile duct walls with minimal mass formation, is exclusively observed in the hilar type of cholangiocarcinoma. 17,18 Apart from local spread by lymphatic vessels, microscopic tumor extension can independently be observed through perineural spaces, predominantly in the hepatofugal direction because of the intrahepatic narrowing of the perineurium. 19,20 Therefore, the already difficult diagnosis of hilar cholangiocarcinoma, including its differentiation from benign fibrosing diseases at the hepatic confluence (especially primary sclerosing cholangitis, primary biliary cirrhosis, and Caroli disease), is complicated by a tumor extension that even during surgery can hardly be defined and that may easily reach beyond its palpable confines.
In the group of patients undergoing hilar resection, the rate of R0 resection was 30%, in accord with previously reported data. 2,15,21 In our series, half of the 14 hilar resections were palliative procedures in patients with locally extensive disease or distant metastases. However, even in those undergoing R0 hilar resections, no 5-year survival was observed, emphasizing the relativity of the terms “formally curative” or “R0 resection.” Moreover, the apparent paradox that in our study patients with more extensive tumors had a better survival rate is most likely the result of the increased radicality in the group of wider resections. Tumor-free margins of <10 mm, which are not uncommon in oncologic hepatobiliary surgery, are considered insufficient for all gastrointestinal carcinomas. In a review of the pattern of recurrence after formally curative hilar resections, 76% of these patients had locoregional recurrence, an unacceptable figure for other tumors (e.g., rectal cancer). 4,22 Distant metastases developed in most patients exhibiting a locoregional recurrence but were the site of first failure in only 24%. 4
The most promising strategy to yield additional tumor-free distance is an extension of the resection line toward the left-lateral segments until the ramification of the left hepatic duct by performing a right trisegmentectomy including the caudate lobe. In our series, the rates of R0 resection (65%) and of 5-year survival (57%) after right trisegmentectomy exceeded those of all other resection groups (excluding LTPP). Moreover, these results were achieved despite the highest share of Bismuth-Corlette type IV carcinomas (27%) when compared with the other resection groups (13%). Indeed, five of the seven right trisegmentectomies performed for Bismuth-Corlette type IV tumors were formally curative.
The worst outcome in terms of 5-year survival (28%) after formally curative partial hepatic resections was observed in patients undergoing left hemihepatectomy. The R0 resection rate was 59%, and most patients had Bismuth-Corlette type IIIb carcinomas. However, the longitudinal radicality along the right hepatic duct is unlikely to be comparable to the contralateral clearance because of the early right segmental ramification into anterior and posterior ducts. Not only right trisegmentectomies but also right hemihepatectomies (R0 resection rate, 55%) compared favorably with left hemihepatectomies, with a 5-year survival rate of 50%. Right hemihepatectomies were predominantly performed for Bismuth-Corlette type IIIa tumors. A lower proportion of lymph node-positive carcinomas in the right hemihepatectomy group is in contradistinction to a greater number of histologically high-grade tumors in the left hemihepatectomy group, although this difference did not reach statistical significance. Moreover, the highest rates of lymphangiosis carcinomatosa and perineural space infiltration occurred in the right hemihepatectomy group.
Portal Vein Resection
The superior survival rate after right hemihepatectomies may raise questions with respect to the lateral rather than the longitudinal radicality in left hemihepatectomies. The left hepatic artery runs through a portion of the hepatoduodenal ligament that is frequently not directly infiltrated by a hilar cholangiocarcinoma. In contrast, the right hepatic artery, which must be preserved during left hemihepatectomy, crosses directly behind the choledochal bifurcation. Tumorous tissue, which may macroscopically be indistinguishable from adherence or perivascular fibrosis, must regularly be stripped off the artery. Histologic data about involvement of the artery are usually not available (nor were they in our study). Therefore, it was all the more interesting to find that infiltration or perivascular involvement after 23 additional portal vein resections could be disclosed in only 22% and 13% of the specimens, respectively. Although fibrosis had previously been reported to be more common than histologic invasion, 15 it is an unprecedented finding that direct portal vein infiltration occurred significantly more often after left hemihepatectomies (50%) than after right-sided liver resections (12%). This is another clue for an insufficient lateral radicality, especially in left-sided resections performed for hilar cholangiocarcinoma.
In a multivariate analysis after R0 resection, additional resection of the portal vein was the only variable with a significant influence on patient survival. The 5-year survival rate was 65% after combined hepatic and portal vein resection, compared with no 5-year survivors after liver resection without an additional portal vein resection. A putative increase in lateral radicality by resecting the portal vein may be one explanation. In addition, a significantly increased share of 2-year survivors in the group of right hepatic resections with portal vein resection (12 patients after right-sided resections compared with only 1 patient after left hemihepatectomy) suggests a side-specific problem as well. The above-mentioned differing relation of the right and left vascular branches with respect to the bifurcation of the hepatocholedochal duct also result in different surgical approaches. Whereas dissection of the hilar region is required in left-sided lobectomies, it can frequently be left untouched during right-sided hepatectomies. No-touch techniques are generally used in oncologic surgery to prevent dissemination of tumor cells. Right-sided liver resections follow this principle more closely than left lobectomies, all the more so if the resection line is extended to the left portion of the hepatoduodenal ligament by portal vein resection. Thus, it is unnecessary to strip the right portal vein branch of the hepatic duct bifurcation, and the portal bifurcation can be left undisturbed as well, which further decreases the required extent of hepatoduodenal dissection. These arguments may support the concept of resection of the portal vein bifurcation. However, all portal vein resections in the present study were directed by the assumed need to achieve local tumor clearance.
LTPP
Intraabdominal tumor seeding confronts the surgeon with a problem that is probably as important as lateral and longitudinal radicality. It is still common practice in some centers to establish the diagnosis of cholangiocarcinoma by open biopsies, although dissection of tumor or peritumorous tissue is considered obsolete for other carcinomas. In rectal cancer, for example, not only perforation of the tumor but even dissection of the mesorectum, which is considered a “holy plane,” has been identified as a main reason for local recurrence. 22,23 The “holy plane” in oncologic surgery of the extrahepatic biliary tract may be the hepatoduodenal ligament, which is regularly dissected during preparation of the hepatic artery and portal vein for liver resections or transplantation.
To comply with the basic rules of oncologic surgery—wide tumor-free margins and no dissection of tumor-bearing areas—we have described the extended bile duct resection (LTPP) as an en bloc eradication of the entire biliary tree by a no-touch technique combining total hepatectomy, partial pancreatoduodenectomy, extended lymphadenectomy, and liver transplantation. 13 Despite an attempted selection of patients with early tumor stages, the postoperative classification revealed UICC stage IV tumors in 10 of 15 and Bismuth-Corlette type IV tumors in 11 patients. Difficulties in the assessment of patients with tumors arising at the hepatic confluence include not only staging but also the differentiation from benign fibrosing disease, because the current state of diagnostic imaging fails to discriminate reliably between these lesions. 24,25 In contrast, the staging systems themselves suffer from inaccuracies. For example, the UICC classification combines lymph node-positive and -negative tumors in a common stage (IVa). Important pathologic variables such as perineural sheath infiltration and lymphangiosis carcinomatosa are not included at all. The Bismuth-Corlette diagnosis, which relies on preoperative cholangiography, frequently must be revised after surgery. 26 Tumor extension into the right and left ducts can easily remain invisible during cholangiography, as long as the lumen is not obstructed. Therefore, a preoperative classification is likely to be reliable only with respect to obstruction or nonobstruction of the primary confluence.
Formally curative liver transplantations and partial pancreatoduodenectomies were achieved in all but one patient, in whom a metastasis at the right ovary was not apparent until the procedure had already reached an irreversible stage. The 5-year survival rate was a disappointing 38%. However, the total number of more advanced tumors and the rate of R0 resection (i.e., the number of patients for survival analysis) were increased in comparison to the other groups of surgical procedures. An analysis of the late failures disclosed implantation metastases in about half of the patients with tumor recurrence. The pathway is not fully apparent, although exfoliation of tumor cells is likely to be the cause. 27
Even more disappointing results are reported after abdominal organ cluster transplantation, performed by Starzl et al in Pittsburgh. 28,29 This procedure was designed to resect the whole complexity of visceral organs deriving during embryonal life from the portion of the foregut that later in life differentiates into the duodenum, liver, and pancreas. In the subset of patients with cholangiocarcinoma, postoperative death and 5-year survival rates were 20% and 14%, respectively. Most of these patients underwent additional transplantation of the pancreas, and all postoperative deaths could be related to the pancreatic graft. Transplantation of the liver alone was advocated for some patients, despite the creation of diabetes mellitus, to avoid the considerable rates of death and complications associated with the pancreas graft.
Death Rates
After LTPP, two patients (13%) died of complications associated with the pancreatic tail. After the protocol was changed to include the routine administration of octreotide, we did not observe any more severe complications. Although a postoperative death rate of 13% represents a considerable share of the patients, it is still on the low end of death rates after extensive procedures for hilar cholangiocarcinoma. 29,30 The same holds true for the entire series, in which a 60-day death rate of 8% demonstrated that an aggressive approach resulting in an increase in surgical radicality does not increase the death rate. 5,6,31
Preoperative biliary decompression has been discussed as a means of decreasing postoperative death and complications. 32 Indeed, a serum bilirubin level > 10 mg/dl had been identified as an unfavorable indicator for postoperative survival. 6 In our patients, preoperative biliary decompression was conceptualized only in the unilateral approach before right trisegmentectomy; in the other patients, preoperative drainage of the biliary tract had almost exclusively been performed previously by the referring gastroenterologist. Former randomized trials did not demonstrate an advantage associated with preoperative external biliary drainage. 33 However, older studies mainly involved bypass surgery and included a low proportion of hepatectomies (i.e., the group most likely to have insufficient postoperative compensatory enlargement of the parenchyma as a result of cholestasis-associated liver damage). In our series, the rates of postoperative death and hepatic insufficiency increased by ≥50% when patients underwent liver resection without prior decompression. However, these differences did not reach statistical significance, and neither preoperative stenting nor a serum bilirubin level <10 mg/dl had a significant influence on survival. Theoretically, the group with biliary decompression may also have represented a favorable selection—that is, a group in whom intubation of the tumor was still possible.
Interestingly, the histopathologic grade was a determinant of 60-day death. We have no conclusive explanation for this finding. Presumably, more aggressive tumors resulted in a more aggressive surgical approach, or patients may have been in a more compromised general condition before surgery.
CONCLUSION
Multivariate analysis disclosed surgical radicality as the most relevant determinant for patient survival. More extended resections, especially right trisegmentectomies and LTPP, not only resulted in an increased rate of R0 resection but also allowed the inclusion of patients deemed previously to have unresectable tumors. Because of the tumor biology of hilar cholangiocarcinoma and anatomic considerations, hilar resections cannot be considered potentially curative. In contrast, right trisegmentectomy and portal vein resection should be regarded as the surgical procedure of choice, because longitudinal and lateral radicality as well as the use of no-touch techniques within the hepatic hilum are best combined in this approach. LTPP may offer additional advantages. However, the risk of tumor recurrence from posttransplant immunosuppression is probably reflected by a high rate of implantation metastases, which at the present time limit the applicability of this method.
Discussion
PROF. H. BISMUTH (Villejuif, France): I congratulate you, Peter, for a very nice paper on your experience with this tumor and its resection. I have two questions and one comment.
Firstly, how did you know before surgery that the patients were resectable? If you did know, how did you choose the type of resection to perform? Nimura, for instance, describes the use of percutaneous cholangiography of each lobe, and even of each segment, in order to see precisely which part of the intrahepatic biliary tree and the corresponding segment he has to resect. How do you decide before surgery, for instance between right hepatectomy, extended right hepatectomy, or transplantation?
The second question concerns the induced hypertrophy in the remnant liver. This approach is of great importance for increasing the resectability of some patients in whom a too-small future remnant is a contraindication to resection. We perform preoperative portal vein embolization, usually by a percutaneous approach. Why do you use arterial embolization? My experience is that it is less efficient at inducing hypertrophy than portal embolization.
The comment applies to some surgeons, but mainly to the other specialists—the radiologists and endoscopists—who most often stent these patients before knowing if they are candidates for radical resection or not. After stenting they are most often not. Your paper stresses that the first objective in the treatment of hilar cholangiocarcinoma, as with all cancers, is radical excision. These specialists, and sometimes gastroenterologists too, say that we surgeons are too aggressive, but what is aggressivity? To use adequate tools against malignant disease is not aggressivity, but logical strategy.
PROF. P. NEUHAUS (Berlin, Germany): Thank you, Henri, for your comment. Concerning the preoperative decision-making, it is important to note that many patients are referred to our institution after they had already undergone ERC and stent insertion by gastroenterologists. Obviously, gastroenterologists consider treatment of hilar cholangiocarcinomas mainly in terms of palliation. Currently, we use a different approach by combining MRCP, then a selective ERC, with stenting of the segments to be preserved. As described in our paper, the remnant liver will mostly be comprised of segments 2 and 3. Therefore, we attempt to confirm the possibility of a segment 1 and 4 to 8 resection. Only if this procedure is not possible, more extensive evaluation regarding other types of liver resection are performed. We consider PTCD as an important additional tool, but we also have reservations about the risk of needle track metastases, especially in the subset of patients undergoing transplantation with subsequent immunosuppression.
The concept of liver transplantation and partial pancreatoduodenectomy is not part of our general approach. These patients were selected on the basis of a good general condition, an age below 60 years, and a less advanced tumor stage, though this did not hold true after the histopathological workup of the specimens.
The last point is about arterial versus portal embolization. Our arguments in favor of a unilateral arterial embolization were the arterial supply of the biliary tree and the large experience of our radiologists with this technique. To our knowledge, other groups have not reported about the effects of arterial embolization in livers with cholestasis due to hilar cholangiocarcinomas. We are, of course, aware of the excellent data of Makuuchi’s and Nimura’s groups, and think it would be interesting to compare the different techniques in this special setting.
PROF. D. CHERQUI (Creteil, France): First of all, I want to say that the numbers and the results were very impressive. I have three questions.
Usually in these types of disease, the treatment of choice is percutaneous biliary drainage. Personally, I do not use that because, like you, I am afraid of implantation of tumor cells within the tract,which I also have seen in some of my patients. So our policy is to operate without drainage in these patients with jaundice. Recently a study from Hong Kong showed a very high incidence of cholangitis when a retrograde cholangiogram and stenting were performed. You said earlier that your endoscopists were very good, but the point is that when you use that, by definition, you infect the bile. You cannot always avoid to have contrast injection above the stricture. If it is not drained, then it becomes infected. So I would like to know if you have infectious complications.
My second question is about segment 1. You did not mention if you were removing segment 1 systematically as part of the resection.
My third question concerns the resection. I agree that it would be better always to do right trisegmentectomies or extended right hepatectomies, but some of the tumors extend in the left lobe and some of them are sometimes associated with atrophy of the left lobe, so you cannot always choose to do that. Sometimes you have to do what the tumor tells you to do. So how often can you actually do this right trisegmentectomy?
PROF. NEUHAUS (Closing Discussion): First we compared the groups of patients undergoing liver resection with preoperative biliary decompression to those in whom a stent or a PTCD had not been inserted, and could not observe any significant differences. The rates of postoperative mortality (8%vs. 12%) and hepatic insufficiency (20%vs. 35%) decreased slightly in the decompression group, whereas cholangitis (25%vs. 15%) occurred more frequently after decompression. However, these figures represent a historical situation rather than the present status. Today, almost all patients are stented, and it is our current experience that the old advice not to do so because of the fear of infection is largely unfounded. Moreover, our endoscopists have analyzed the data of patients who did not undergo surgery and in whom they have drained only one major segment, in comparison to two to four stents, which had been used formerly. They conclude from their data that the infection rate will not increase if only one major segment is drained, unless the contrast media had been forcefully injected into the biliary system.
Secondly, segment 1 should be routinely resected, as it generally drains directly into the hepatic bifurcation or within a distance of 1 cm.
Thirdly, the usual condition is that in the bifurcation, you have a tumor which is small and extends to both sides. As we do not really know whether it is a type IIIA or type IIIB tumor, we decided to resect within the umbilical fissure in order to use the length of the left hepatic duct. In this resection line, we can almost always achieve clear margins.\
Footnotes
Correspondence: Peter Neuhaus, MD, Dept. of Surgery, Charité-Virchow Klinikum, Humboldt University Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany.
Presented at the Sixth Annual Meeting of the European Surgical Association, at the Royal College of Surgeons of England, London, United Kingdom, April 23–24, 1999.
Accepted for publication July 1999.
References
- 1.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992; 215: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumgart LH, Benjamin IS. Liver resection for bile duct cancer. Surg Clin North Am 1989; 69: 323–337. [DOI] [PubMed] [Google Scholar]
- 3.Bengmark S, Blumgart LH, Launois B. Liver resection in high bile duct tumors. In: Bengmark S, Blumgart LH, eds. Liver surgery. Edinburgh: Churchill Livingstone; 1986: 81–87.
- 4.Mittal B, Deutsch M, Iwatsuki S. Primary cancers of the extrahepatic biliary passages. Int J Radiat Oncol Biol Phys 1985; 11: 849–855. [DOI] [PubMed] [Google Scholar]
- 5.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg 1996; 224: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg 1996; 223: 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 8.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990; 107: 521–527. [PubMed] [Google Scholar]
- 9.Vogl TJ, Balzer JO, Dette K, et al. Initially unresectable hilar cholangiocarcinoma: hepatic regeneration after transarterial embolization. Radiology 1998; 208: 217–222. [DOI] [PubMed] [Google Scholar]
- 10.Curley SA, Levin B, Rich TA. Management of specific malignancies: liver and bile ducts. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, eds. Clinical oncology. New York: Churchill Livingstone; 1995: 1305–1372.
- 11.Yokoyama I, Carr B, Saitsu H, et al. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer 1991; 68: 2095–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckurts KTE, Hölscher AH, Bauer TH, Siewert JR. Malignant tumors of the hepatic bifurcation: results of surgical therapy and prognostic factors. Chirurg 1997; 68: 378–384. [DOI] [PubMed] [Google Scholar]
- 13.Neuhaus P, Blumhardt G. Extended bile duct resection: a new oncological approach to the treatment of central bile duct carcinomas? Description of method and early results. Langenbecks Arch Chir 1994; 379: 123–128. [DOI] [PubMed] [Google Scholar]
- 14.Jonas S, Kling N, Bechstein WO, et al. Rejection episodes after liver transplantation during primary immunosuppression with FK506 or a cyclosporine-based regimen: a controlled prospective randomized trial. Clin Transpl 1995; 9: 406–414. [PubMed] [Google Scholar]
- 15.Beazley RM, Hadjis N, Benjamin IS. Clinicopathological subjects of high bile duct cancer: experience with resection and bypass surgical treatments. Ann Surg 1984; 199: 623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas S, Bechstein WO, Lemmens HP, et al. Conversion to tacrolimus after liver transplantation. Transpl Int 1996; 9: 23–31. [DOI] [PubMed] [Google Scholar]
- 17.Colombari R, Tsui WMS. Biliary tumors of the liver. Semin Liv Dis 1995; 15: 402–413. [DOI] [PubMed] [Google Scholar]
- 18.Sugihara S, Kojiro M. Pathology of cholangiocarcinoma. In: Okuda K, Ishak KG, eds. Neoplasms of the liver. Tokyo: Springer Verlag; 1987: 236–301.
- 19.Bhuiya MR, Nimura Y, Kamiya J, et al. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg 1992; 215: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol 1983; 139: 217–238. [DOI] [PubMed] [Google Scholar]
- 21.Mizumoto R, Kawarada Y, Suzuki H. Surgical treatment of hilar carcinoma of the bile duct. Surg Gynecol Obstet 1986; 162: 153–158. [PubMed] [Google Scholar]
- 22.Heald RJ, Ryall R. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 23.Heald RJ. The “holy plane” of rectal surgery. J R Soc Med 1988; 81: 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbeek PCM, van Leeuwen DJ, de Wit LT, et al. Benign fibrosing disease at the hepatic confluence mimicking Klatskin tumors. Surgery 1992; 112: 866–871. [PubMed] [Google Scholar]
- 25.Wetter LA, Ring EJ, Pellegrini CA, Way LW. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg 1991; 161: 57–63. [DOI] [PubMed] [Google Scholar]
- 26.Gerhards MF, van Gulik TM, Bosma A, et al. Long-term survival after resection of proximal bile duct carcinoma (Klatskin tumors). World J Surg 1999; 23: 91–96. [DOI] [PubMed] [Google Scholar]
- 27.Verbeek PCM, van der Heyde MN, Ramsoekh T, Bosma A. Clinical significance of implantation metastases after surgical treatment of cholangiocarcinoma. Semin Liv Dis 1990; 10: 142–144. [DOI] [PubMed] [Google Scholar]
- 28.Alessiani M, Tzakis A, Todo S, et al. Assessment of five-year experience with abdominal cluster transplantation. J Am Coll Surg 1995; 180: 1–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg 1989; 210: 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepatogastroenterology 1991; 38: 170–175. [PubMed] [Google Scholar]
- 31.Reding R, Buard JL, Lebeau G, Launois B. Surgical management of 552 carcinomas of the extrahepatic bile ducts (gallbladder and periampullary tumors excluded). Results of the French Association survey. Ann Surg 1991; 219: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada T, Yasuda H. Value of preoperative biliary drainage prior to bile duct cancer surgery: results of a retrospective review. Asian J Surg 1996; 19: 84–87. [Google Scholar]
- 33.Hatfield ARW, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice: a prospective controlled clinical trial. Lancet 1982; 2: 896–899. [DOI] [PubMed] [Google Scholar]