Abstract
Objective
To study the ability of bombesin (BBS) to recover gut-associated lymphoid tissue (GALT) and preserve immunity in a lethal model of Pseudomonas aeruginosa (Ps) pneumonia in mice receiving total parenteral nutrition (TPN).
Summary Background Data
TPN causes depression of mucosal immunity compared with enterally fed animals, which may explain the increased incidence of pneumonia in parenterally fed trauma patients. BBS prevents this TPN-induced GALT atrophy, depressed gastrointestinal and respiratory tract IgA levels, and impaired antiviral IgA-mediated mucosal immunity. The authors examined whether some supplement could be added to TPN to avoid this GALT atrophy and lower the incidence of infectious complications in the parenterally fed animal.
Methods
Male mice were randomized to chow or intravenous (IV) TPN. After 5 days of IV TPN, mice received 0, 1, 2, or 3 days of BBS IV three times a day and then were killed to harvest Peyer’s patch, intraepithelium, and lamina propria for cell yields. Gastrointestinal and respiratory tract IgA levels were analyzed by enzyme-linked immunosorbent assay. Next, mice underwent intranasal inoculation with liposomes alone (nonimmune) or liposome-containing Ps polysaccharide. Ps immune mice were catheterized and randomized to chow, IV TPN, or IV TPN + BBS. The liposome group received chow but no IV catheter. These mice were given an LD90 dose of intratracheal Ps, and death rates were recorded.
Results
GALT and gastrointestinal and respiratory tract IgA levels improved to those in chow-fed mice after 3 days of BBS. Immunization reduced the death rate from 92% in chow-fed liposome-only animals to 20% in immunized animals. TPN-fed animals lost their mucosal immunity, with a death rate of 86% compared with 21% in the TPN + BBS group.
Conclusion
The results demonstrate that BBS reverses TPN-induced changes in GALT and preserves mucosal immunity. Ps immunization reduces the death rate in a gram-negative pneumonia model and maintains gastrointestinal and respiratory immunity in Ps immune mice receiving IV TPN.
Nosocomial pneumonia accounts for approximately 15% of hospital-acquired infections 1 and 18 pneumonias per 1,000 ventilator days in the adult intensive care unit. 2 The overwhelming majority of bacterial isolates in these cases are aerobic gram-negative bacilli. Pseudomonas aeruginosa (Ps) was identified as the causative agent in 17% to 31%3–5 of the cases, with a subsequent death rate of 40% to 70%. 6,7 Although many etiologic factors, such as prolonged mechanical ventilation, acute pulmonary injury, and aspiration, influence the risk of pneumonia, nutrition also appears to play an important role. In clinical studies of trauma patients, those fed intravenously have a significantly higher incidence of pneumonia and intraabdominal abscess than those fed enterally. 8–10 Although the mechanism for this increased susceptibility to infection is unclear, our work suggests that enteral feeding improves the function of the major source of mucosal immunity—the gut-associated lymphoid tissue (GALT)—in both intestinal and extraintestinal sites.
The gastrointestinal tract is an important site for the regulation of postinjury hypermetabolism, 11 cytokine responses, 12 and mucosal immune defenses. 13,14 In parenterally fed animals, mucosal atrophy, 15 bacterial translocation, 16 and reduction in GALT mass and function rapidly develop. 17 The GALT contains at least 80% of the body’s plasma cells, mostly producing IgA, which is transported by the epithelial cells to coat the mucosal surfaces. 13,18 On the surfaces of the respiratory and gastrointestinal tracts, secretory IgA binds viruses and bacteria, preventing their attachment to and infection of the mucosal epithelium. 13,14,18–20 The GALT is exquisitely sensitive to route and type of nutrition as well as to specific nutrients, such as glutamine, and neuropeptides found within the enteric nervous system. 21,22
The enteric nervous system forms a vast network of neurons throughout the gastrointestinal tract. It is estimated that every cubic millimeter of intestinal tissue is innervated with 2 meters of nerve tissue. 23 Peptides synthesized within the enteric nervous system not only exert adrenergic and cholinergic actions but also regulate gut motility and secretions, mucosal growth, immune function, and mucosal defenses. 23–30 One of these neuropeptides, bombesin (BBS), is analogous to gastrin-releasing peptide in humans. 24 Administration of BBS stimulates release of most of the gut hormones and dramatically affects GALT mass and function in mice, preventing GALT atrophy associated with total parenteral nutrition (TPN). 22,25 BBS also prevents the impairment of IgA-mediated antiviral defenses induced with intravenous (IV) TPN in mice. 31 Our recent work has shown that TPN also impairs established respiratory defenses against Ps. 30 Prior immunization with Ps antigen in liposomes reduces the death rate from 90% with intratracheal administration of Ps in unimmunized mice to approximately 10%. This immunologic protection is lost, however, with IV TPN. This study investigates the ability of BBS to reverse the negative effects of TPN on immunologic responsiveness to this virulent and clinically relevant pathogen.
METHODS
Animals
These studies conform to the guidelines for the care and use of laboratory animals established by the Animal Care and Use Committee of the University of Tennessee, and protocols (Fig. 1) were approved by that committee. Healthy, male ICR (Institute of Cancer Research) mice were purchased from Harlan (Indianapolis, IN) and were housed in a conventional facility accredited by the American Association for the Accreditation of Laboratory Animal Care under controlled conditions of temperature and humidity with a 12/12 light/dark cycle. Mice were fed ad libitum water and chow. During the experiments, the mice were housed in metal metabolism cages with wire-grid bottoms to eliminate coprophagia and the ingestion of bedding.
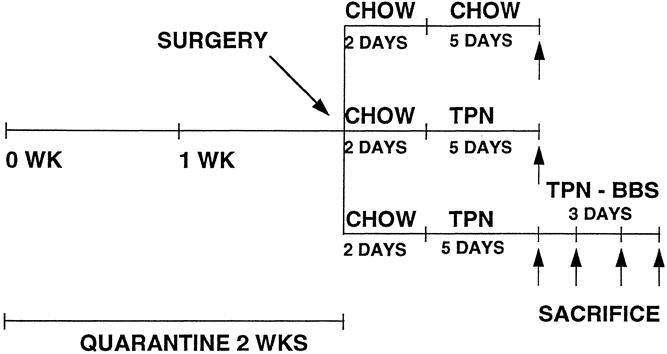
Figure 1. Protocol for gut-associated lymphoid tissue and IgA level recovery.
Experimental Design
Experimental Procedures
Six- to 8-week-old male ICR mice were anesthetized with a mixture of ketamine hydrochloride (100 mg/kg) and acepromazine maleate (10 mg/kg), and a 5-mm incision was made on the right side of the neck. A silicone catheter (0.012″ I.D. × 0.025″ O.D.; Baxter, Chicago, IL) was placed via the right jugular vein into the vena cava. The catheter was tunneled subcutaneously and exited the tail at its midpoint. The mice were partially immobilized by tail restraint to protect the catheter during infusion. This technique of infusion has proven to be an acceptable means of nutritional support and does not produce physical or biochemical evidence of stress. 32
In experiment 1, the catheterized mice were immediately connected to an infusion apparatus and a 0.9% saline solution was infused at an initial rate of 4 mL/day. During the first 2 days, the mice were allowed ad libitum access to chow and water and then were randomized to the various diets. The chow group (n = 8) received an infusion of 0.9% saline solution in addition to a standard laboratory mouse diet and water ad libitum. The TPN (n = 7) group received a standard TPN solution (prepared in the hospital pharmacy, providing 1322 kcal with a nonprotein calorie/nitrogen ratio of 168/1), which was increased over the next 24 hours to 10 mL/day. Chow and TPN animals were fed their diets for 5 days, then killed. The TPN + BBS (n = 25) group received the standard TPN solution for 5 days and then received IV injections of BBS (15 μg three times a day) for an additional 3 days and were killed on day 6 (n = 8), day 7 (n = 10), or day 8 (n = 7).
The thoracic and abdominal cavities of these animals were opened aseptically, and the animals were exsanguinated by cardiac puncture. The plasma was isolated from these blood samples for IgA analysis. Lymphocyte preparations were harvested from the entire small intestine. Once the small intestine was stripped from its mesentery, the lumen of the small intestine was lavaged with 10 mL Dulbecco’s phosphate-buffered saline (PBS), and the samples were stored for IgA analysis. The neck was aseptically opened, the distal trachea clamped, and the upper respiratory tract lavaged with 1 mL PBS to obtain nasal washings for IgA analysis. Then, the trachea was cannulated and the lower respiratory tract lavaged with 1 mL PBS.
In experiment 2, mice previously immunized against Ps were cannulated with IV catheters 10 days after immunization. All groups were given access to chow ad libitum for 2 days, then randomized to the different diets of chow (n = 15), TPN (n = 14), and TPN + BBS (n = 14). A liposome-only group (n = 12), as a nonimmune control group, was allowed ad libitum water and chow throughout the experiment but received no IV catheters. All mice were fed their respective diets for the next 5 days. The TPN + BBS group received BBS IV injections (15 μg/kg/8 hr) in addition to the standard TPN solution. After 5 days of feeding, the mice were vertically suspended and given an LD90 dose of Ps bacterial suspension (1.2 × 108 bacteria 40 μL) intratracheally using a blunt 22-gauge needle. The death rate was assessed twice each day and observed over a 48-hour period (Fig. 2).
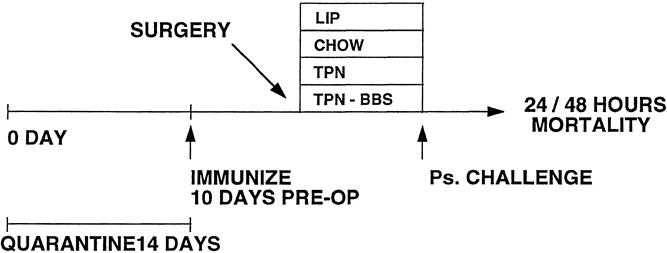
Figure 2. Protocol for bacterial pneumonia.
Bacterial Preparation
Ps was plated on a blood agar plate for fresh growth 48 hours before inoculation. The next day, a sterile cotton-tipped applicator was used to transfer the fresh Ps growth into 20 mL sterile Dulbecco’s PBS. This solution was mixed well to prepare a solution with an absorbance of 0.670 ± 0.010 at 595 nm. The bacterial number was determined by serial dilutions. After the suspension was diluted to a factor of 10−6, 0.1 mL of the solution was inoculated evenly onto three Trypticase soy agar plates. Plates were incubated overnight at 37°C. The remaining bacterial suspension was stored at 4°C overnight. On the day of bacterial inoculation, colony counts were determined using a Gallencamp colony counter. The Ps bacterial solution was centrifuged at 3500 rpm at 4°C for 30 minutes, and the supernate was discarded. The bacterial pellet was washed with 7 to 10 mL cold PBS at 3500 rpm at 4°C for 30 minutes × 3 to remove any free bacterial endotoxin. Cold PBS was added to the washed bacterial pellet to give a final concentration of 3 × 109 bacteria/mL or 1.2 × 108 bacteria/mouse.
Liposome Preparation
Bacterial polysaccharide-containing liposomes were prepared by the detergent dialysis technique, as described by Abraham et al. 33,34 Briefly, 8 μM of cholesterol, phosphatidylserine, and phosphatidylcholine (Sigma, St. Louis, MO) were combined and dried under N2, and the resulting film was lyophilized for up to 24 hours. Ten milligrams of purified alkali-labile Ps lipopolysaccharide (the gift of Dr. Gerald Pier, Channing Laboratory, Harvard University, Boston, MA) was dissolved in 500 μl HEPES buffer (150 mM NaCl, 10 mM HEPES, 1 mM EDTA, pH 7.4), added to the lipid film, and emulsified by vigorous pipetting. The emulsion was incubated for 30 minutes at 4°C, then 240 μL 1M octylglucoside (Sigma) was added and the mixture was shaken vigorously. The sample was placed in Spectra/Por dialysis tubing (molecular weight cutoff 3500; Spectrum Medical Industries, Los Angeles, CA) and dialyzed against 100 mL HEPES buffer containing 2.4 g SM-2 Bio-Beads (Bio-Rad Laboratories, Hercules, CA). After 24 hours, the liposome preparation was placed on an A5M column (Bio-Gel A5M, Bio-Rad Laboratories), and the liposome fraction (in the void volume) was collected. Polysaccharide incorporation ranged from 30% to 70%. Calculation of immunization dose was based on the original lipid concentration (15 mg) because this produced consistent immunity.
Immunization
Each mouse was immunized intranasally with liposomes containing 160 μg lipid and 30 to 70 μg polysaccharide or with liposomes (160 μg lipid) alone. Calculation of the liposome dose for immunization was based on the amount of lipid present. Mice were gently restrained by hand and 100 μL of a PBS suspension of either Ps-containing liposomes or control non-Ps–containing liposomes was placed on the nares. The mouse was allowed to inhale the inoculum. Successful inoculation was evidenced by a change in breathing pattern and brief cyanosis. Ten days later, the mice were randomized to treatment groups and underwent surgery for the placement of IV catheters.
Cell Isolation
Lymphocyte isolations from the Peyer’s patches were performed as described by Deitch et al. 35 The Peyer’s patches were excised from the serosal side of the intestine with fine-tipped scissors. The fragments were treated with type 1 collagenase (Sigma) (50 units/mL) in modified essential medium for 60 minutes at 37°C with constant rocking. After collagenase digestion, the cell suspensions were passed through nylon filters.
Lamina propria and intraepithelial lymphocytes were isolated in the following manner. After excision of the Peyer’s patches, the intestine was opened lengthwise and cut into 5-mm pieces. The pieces were incubated three times, 30 minutes each time, with prewarmed (37°C) calcium and magnesium-free Hanks balanced salt solution containing 5 mmol/L ethylenediaminetetraacetic acid (EDTA) (Sigma) in a flask on a magnetic stirrer at 20 rpm at 37°C. The supernatant containing released sloughed epithelial cells and intraepithelial lymphocytes from each incubation period were pooled and stored on ice for further purification.
To block EDTA activity, the remaining tissue pieces were incubated for 30 minutes at 37°C with RPMI 1640 (Gibco Laboratories, Gaithersburg, MD) containing 5% heat-inactivated fetal bovine serum. The RPMI and 5% fetal bovine serum mixture was decanted, and 30 mL RPMI containing 40 U collagenase per milliliter (type I, 30 U/mL; type III, 10 U/mL) and 5% inactivated fetal bovine serum was added to the flask, which was then incubated on a magnetic stirrer (100 rpm) at 37°C. The lamina propria lymphocytes were decanted from the tissue fragments. Fresh enzyme-containing medium was added, and the process was repeated twice (30 minutes each time) for a total time of 90 minutes. After the third extraction, pooled cells were gently mixed and placed on ice for 10 to 15 minutes to let larger debris settle.
The supernatants that contained lymphocytes, debris, and dead cells were centrifuged, the pellets were resuspended in 40% colloidal suspension of silica (Percoll, Pharmacia, Piscataway, NJ), and the cell suspensions were overlaid on 70% colloidal suspension of silica. After centrifugation for 20 minutes at 600 rpm at 4°C, viable lymphocytes were recovered from the 40%:70% interface and washed twice in RPMI 1640 medium. The number of cells was determined, and the viability of lymphocytes was determined by trypan blue exclusion.
Antibody Quantization
IgA was measured in intestinal washings, nasal secretions, and bronchoalveolar lavages in a sandwich enzyme-linked immunosorbent assay using a polyclonal goat anti-mouse IgA (Sigma) to coat the plate, a purified mouse IgA as standard, and a horseradish peroxidase-conjugated goat anti-mouse IgA. The intestinal washing IgA level was normalized for total recovery from the whole small intestine.
RESULTS
Body Weight
The preexperiment weights of all groups were similar. In experiment 1, the weight gain of the chow group was significantly greater than all other groups, with similar values between TPN + BBS and TPN (Table 1) . In experiment 2, the weight gain of the chow group was significantly greater than the TPN group, with no differences between the TPN and TPN + BBS groups (Table 2) .
Table 1. EXPERIMENT 1: ANIMAL BODY WEIGHT AND WEIGHT GAIN
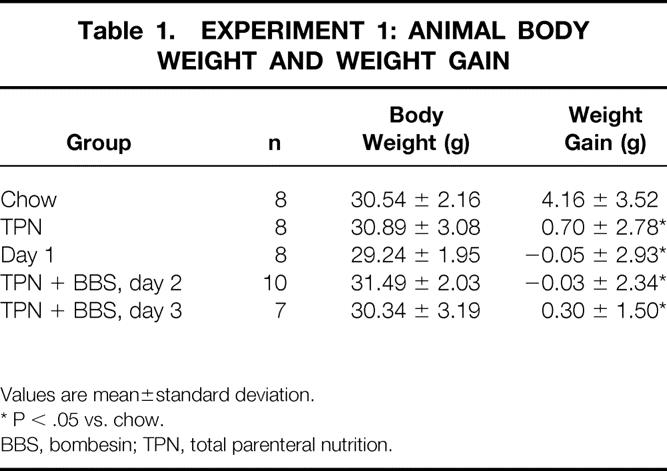
Values are mean±standard deviation.
* P < .05 vs. chow.
BBS, bombesin; TPN, total parenteral nutrition.
Table 2. EXPERIMENT 2: ANIMAL BODY WEIGHT AND WEIGHT GAIN
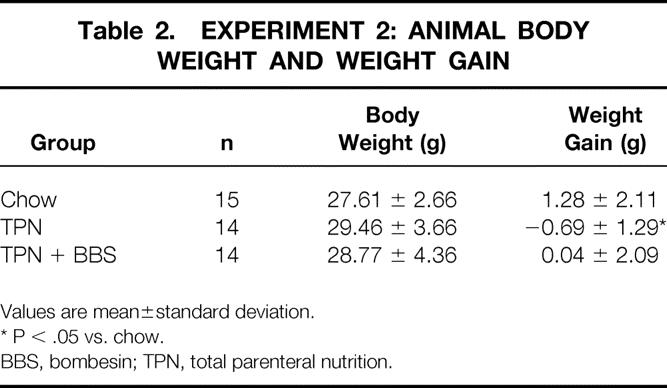
Values are mean±standard deviation.
* P < .05 vs. chow.
BBS, bombesin; TPN, total parenteral nutrition.
Experiment 1
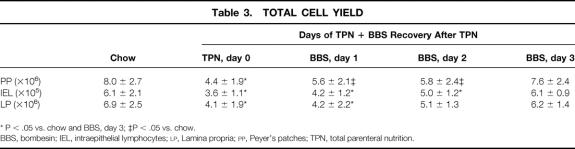
Total cell yields in the Peyer’s patches, intraepithelium, and lamina propria were significantly lower in the TPN animals than in the chow and TPN + BBS day 3 groups (P < .05) (Table 3) . All cell lines gradually returned to chow baseline after 3 days of BBS.
Table 3. TOTAL CELL YIELD
* P < .05 vs. chow and BBS, day 3; ‡P < .05 vs. chow.
BBS, bombesin; IEL, intraepithelial lymphocytes; LP, Lamina propria; PP, Peyer’s patches; TPN, total parenteral nutrition.
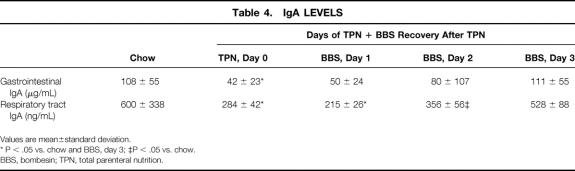
The TPN mice had significantly lower levels of IgA in the small intestine and respiratory tract than the chow and TPN + BBS day 3 groups (P < .05) (Table 4). Both respiratory and small intestine IgA levels returned to chow levels by day 3 after the addition of BBS to the TPN.
Table 4. IgA LEVELS
Values are mean±standard deviation.
* P < .05 vs. chow and BBS, day 3; ‡P < .05 vs. chow.
BBS, bombesin; TPN, total parenteral nutrition.
Experiment 2
At 24 hours, the nonimmune chow-fed mice (liposomes only) had a significantly higher death rate than the immune chow-fed (10/12 vs. 1/15, P < .05) or TPN + BBS-fed (10/12 vs. 2/14, P < .05) mice. TPN mice had a significantly greater death rate than chow-fed (11/14 vs. 1/15, P < .05) or TPN + BBS-fed (11/14 vs. 2/14, P < .05) mice (Fig. 3) . Animals receiving TPN had death rates similar to liposome-only animals.
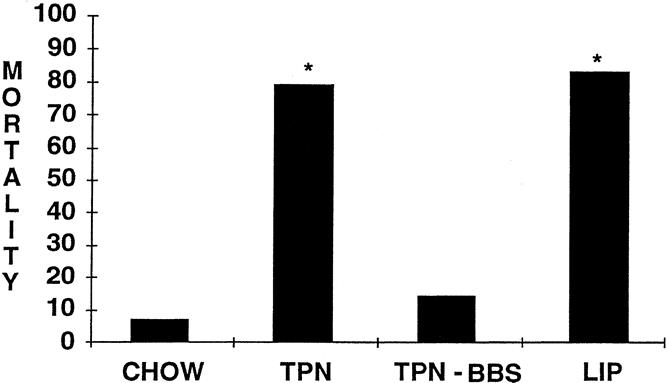
Figure 3. Twenty-four–hour death rate after bacterial pneumonia. *P < .05 vs. chow and TPN-BBS.
At 48 hours, nonimmune (liposome-only) mice had a significantly higher death rate than the chow-fed (11/12 vs. 3/15, P < .05) or TPN + BBS-fed (11/12 vs. 3/14, P < .05) mice. The death rate in TPN-fed mice was significantly higher than in chow (12/14 vs. 3/15, P < .05) or TPN + BBS-fed (12/14 vs. 3/14, P < .05) mice (Fig. 4) . The liposome-only group’s death rate was comparable to that of the TPN-fed mice (11/12 vs. 12/14).
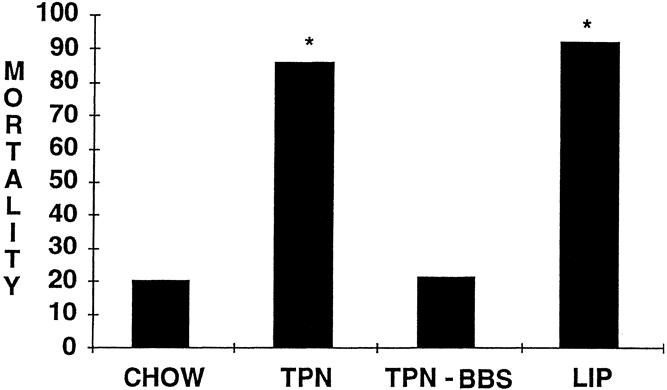
Figure 4. Forty-eight–hour death rate after bacterial pneumonia. *P < .05 vs. chow and TPN-BBS.
DISCUSSION
This work expands some of the well-established links between the nervous system and the immune system. 36 For example, during inflammation, cytokines synthesized and secreted by immune cells of the body stimulate the hypothalamic-pituitary-adrenal axis within the central nervous system. With the hypothalamic release of corticotropin-releasing hormone, ACTH stimulates the adrenal gland to release glucocorticoids to regulate the metabolic and immunologic response to injury and illness. 37,38 The enteric nervous system is known to regulate numerous gastrointestinal functions, such as motility, exocrine and endocrine secretion, microcirculatory changes, and inflammation, through mast cell interaction. 23,39,40 The enteric nervous system consists of approximately 108 neurons, with 99% of mucosal tissue no more than 13 μm from nerve tissue. 23 Nerve cell bodies are grouped into small ganglia connected by bundles of nerve processes forming two major plexuses—the myenteric and submucosal plexuses of the intestine—which are responsible for motor and secretory control of the gut, respectively. 23,39,40 The neuropeptide products of the enteric nervous system are also key elements in generalized mucosal immunity.
Neuropeptides stimulate IgA production in the gastrointestinal tract, enhance lymphocyte proliferation, and modulate phagocytic function in vitro. 41–43 BBS, a tetradecapeptide originally isolated from frog skin, is structurally related to gastrin-releasing peptide in humans. BBS mediates its effect through a neuromedin B receptor or a gastrin-releasing peptide receptor. 42,44 When activated, these receptors generate their effects through a protein kinase C pathway. 42 BBS has a host of actions in the gastrointestinal tract, including stimulation of pancreatic, gastric, and intestinal secretion and release of all gut hormones except secretin. 25 Whether it is these neuropeptides that induce the effect on mucosal immunity or the BBS itself is unclear.
From an anatomic standpoint, BBS reverses the GALT-associated atrophy associated with TPN. 22 Whereas our prior work noted the GALT cellular atrophy induced by TPN, the current study demonstrates that BBS can reverse the gut-associated changes within 3 days of its administration. One product of this cell mass, IgA, also rapidly recovers in both the respiratory and the intestinal tract in parallel with GALT cell mass. Our previous work demonstrated that GALT integrity is important to the maintenance of nasal immunity to influenza virus. 31 The current study is the first, to our knowledge, suggesting a significant function for GALT integrity in bacterial pneumonia as well. 30
We adapted the Ps pneumonia model previously described by Abraham et al. 34 These investigators immunized animals intranasally with liposomes containing Ps polysaccharide and generated a specific secretory IgA response that appeared to correlate with improved survival to a subsequent bacterial challenge. Recently, our laboratory confirmed this work, reducing the death rate from 90% in unimmunized mice to approximately 10% with immunization. Consistent with our studies of IgA-mediated antiviral immunity, immunized animals fed TPN solution IV lost this immunization protection, with death rates rising to those of unimmunized animals. In that study, chow or administration of a complex enteral diet preserved host defense against the bacterial challenge; a chemically defined enteral diet (TPN solution administered intragastrically) only partially preserved pulmonary defenses. 30 The current study convincingly shows that these pulmonary host defenses can be supported by neuropeptides without enteral feeding.
The respiratory tract is constantly exposed to the external environment and a multitude of toxins, viruses, and bacteria. Although most of the cleansing occurs in the upper respiratory tract, defense systems of the lower respiratory tract are also developed to fight infectious challenges. In addition to mucociliary clearance, the respiratory tree has a rich complement of substances with antibacterial activities, including secretory IgA, lysozymes, lactoferrin, and defensins. 45–47 In addition, the lower respiratory tree can recruit numerous inflammatory cells, including neutrophils, lymphocytes, and macrophages, which, in times of infection, can participate in the response. Not surprisingly, the respiratory tract is richly innervated with afferent and efferent nerve endings containing neuropeptides such as substance P, gastrin-releasing peptide/BBS, and vasoactive intestinal peptide. 48–50 Although the lower respiratory effects have not been studied, administration of BBS to the nasal mucosa in humans stimulates submucosal glands to produce secretions rich in hexose-containing glycoconjugates, total protein, and lysozyme. 51
It is unclear how TPN impairs pulmonary defenses and thus how BBS preserves respiratory defenses, but the GALT appears to play an important role. In studies of establishing respiratory immunity to Ps, Cripps et al tested the efficacy of mucosal and systemic immunization with killed Ps. 52 The most effective site for immunizing animals against the subsequent bacterial challenge was intra-Peyer’s patch immunization, followed in decreasing degrees of effectiveness by a combination oral and intratracheal, intratracheal, subcutaneous, and oral immunization. T lymphocytes harvested from the thoracic duct of rats primed by intra-Peyer’s patch inoculation of nontypeable Haemophilus influenzae and intravenously transfused into unimmunized recipient rats were capable of conferring specific immunologic protection in the recipient rats. 53 We have noted a decrease in the number of GALT lymphocytes and in the normal CD4/CD8 ratio in the lamina propria of TPN-fed animals; this, together with a decrease in the size of Peyer’s patches, may contribute to the susceptibility of TPN-fed immune animals to infection. 17
We suspect the important mechanism for protection is the preservation of IgA levels, which remain normal with BBS therapy but drop with IV TPN. Although our results and those of Abraham 34 show merely an association, increasing levels of respiratory IgA do correlate with survival to a bacterial challenge. IgA has traditionally been viewed as a noninflammatory immunoglobulin, but recent studies have challenged this concept while providing an explanation for other proposed mechanisms. In addition to preventing attachment of bacteria to mucosal surfaces and thus avoiding the initial step in invasive infection, IgA may play a role in neutrophil-mediated bacterial clearance because it can opsonize bacteria, inducing phagocytosis and the respiratory burst in neutrophils. 54 This may explain the findings of Cripps et al, 52 which suggested that pulmonary neutrophils were the most important element in the pulmonary response after immunizations with Ps. In those studies, after intra-Peyer’s patch immunization with killed Ps, survival after bacterial challenge correlated best with enhanced recruitment of neutrophils and neutrophil activity in bronchoalveolar lavage specimens obtained after the bacterial challenge. Serum and bronchoalveolar antibody levels did not appear to correlate with improved survival after the bacterial challenge. It is unclear, however, whether the antibodies measured in those lavage specimens accurately reflected the true antibody levels. Specimens were obtained after the bacterial challenge and centrifuged, and antibody levels were measured in the supernatant. These levels could have been falsely interpreted as low because antibody bound to the bacteria themselves and lost during the separation of the bacteria and cellular elements from the supernatant would not be measured. In addition, permeability changes in the epithelium induced by the bacterial challenge almost certainly contaminated the specimens with serum and serum antibodies, thus masking the actual airway concentrations of IgA. We speculate that high levels of IgA bound the intratracheal Ps, reducing the infective load and thus reducing the lethality of the bacterial dose. Studies are underway to substantiate that hypothesis.
Another cell type, the pulmonary macrophage, has been speculated to be important in protection against bacterial pneumonia. When pulmonary macrophages were eliminated in mice by the administration of dichlormethylene diphosphonate-encapsulated liposomes, an otherwise nonlethal dose of Klebsiella administered intratracheally became uniformly fatal. 55 Increased recruitment of neutrophils with apparent normal phagocytic function into the lungs was associated with decreased bacterial clearance and an increased death rate compared with control animals. These results were at odds with those of Cripps et al, 52 which implicated neutrophils as the primary predictor of survival. Unfortunately, respiratory tract IgA levels could not be measured in these experiments, and because macrophages transformed into dendritic cells could also have been affected by the administration of this drug, death could be related to changes in cytokine signals, reduced airway levels of IgA, or decreased neutrophil function. Defects in pulmonary macrophages and disturbances in their cytokine levels have been noted in TPN-fed animals. 56
Our results reveal a strong association between preservation of GALT and IgA levels and improved survival to bacterial challenge. The neuropeptide BBS appears to provide the signal to the mucosal immune system that reverses TPN-associated host defense defects. Although our understanding of the mechanisms behind the interaction of the enteric nervous system and the immunologic system is limited, this experiment continues to add support to the common mucosal immune system hypothesis. These results, as well as those from our previous work, consistently demonstrate that support of the GALT results in functional preservation of respiratory defenses to both viruses and bacteria. Although the preferred route of nutrition is via the gut, supplemental neuropeptide may be a way to improve host defenses in patients restricted to parenteral feeding.
Footnotes
Correspondence: Kenneth A. Kudsk, MD, Dept. of Surgery, University of Tennessee, 956 Court Ave., Suite E228, Memphis, TN 38163.
Supported by NIH Grant 5 RO1 GM53439 (KAK) and NIH Grant AI-01359 (KRB).
Accepted for publication July 13, 1999.
References
- 1.Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Sem Respir Infect 1996; 11:32–53. [PubMed] [Google Scholar]
- 2.Jarvis WR, Edwards JE, Culver DH, et al. Nosocomial infection rates in adult and pediatric intensive care units in the United States. Am J Med 1991; 91:185S–191S. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Nosocomial infection surveillance. Surveillance Summaries 1986; 35:1755. [Google Scholar]
- 4.Shaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med 1991; 3B(suppl):72S–75S. [DOI] [PubMed] [Google Scholar]
- 5.Fagon JY, Chastre J, Domart Y, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation: prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis 1989; 139:877–884. [DOI] [PubMed] [Google Scholar]
- 6.Brewer SC, Wunderink RG, Jones CB, Leeper KY Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 1996; 104:1019–1029. [DOI] [PubMed] [Google Scholar]
- 7.Fagon JY, Chastre J, Hance AJ, et al. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 1993; 94:281–288. [DOI] [PubMed] [Google Scholar]
- 8.Kudsk KA, Croce MA, Fabian TC, et al. Enteral vs. parenteral feeding: effects on septic morbidity following blunt and penetrating trauma. Ann Surg 1992; 215:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore FA, Moore EE, Jones TN, et al. TEN vs. TPN following major abdominal trauma: reduced septic morbidity. J Trauma 1989; 29:916–923. [DOI] [PubMed] [Google Scholar]
- 10.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma: a prospective, randomized study. J Trauma 1986; 26:874–881. [DOI] [PubMed] [Google Scholar]
- 11.Wilmore DW, Smith RJ, O’Dwyer ST, et al. The gut: a central organ after surgical stress. Surgery 1988; 104:917–923. [PubMed] [Google Scholar]
- 12.Ogle CK, Guo X, Hasselgren PO, et al. The gut as a source of inflammatory cytokines after stimulation with endotoxin. Eur J Surg 1997; 163:45–51. [PubMed] [Google Scholar]
- 13.Svanborg C. Bacterial adherence and mucosal immunity. In: Ogra PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, eds. Handbook of Mucosal Immunology. San Diego: Academic Press; 1994: 71–78.
- 14.Winner L III, Mack J, Weltzin R, et al. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun 1991; 59:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LR, Copeland EM, Dudrick SJ, et al. Structural and hormonal alterations in the gastrointestinal tract of parenteral fed rats. Gastroenterology 1975; 68:1177–1183. [PubMed] [Google Scholar]
- 16.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1888; 104:185–190. [PubMed] [Google Scholar]
- 17.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 1995; 39:44–52. [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P. Distribution and characteristics of mucosal immunoglobulin producing cells. In: Ogra PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, eds. Handbook of Mucosal Immunology. San Diego: Academic Press; 1994: 251–262.
- 19.Renegar KB, Small PA Jr. IgA mediation of murine nasal anti-influenza immunity. J Virol 1991; 65:2146–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilian M, Russell MW. Function of mucosal immunoglobulins. In: Ogra PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, eds. Handbook of Mucosal Immunology. San Diego: Academic Press; 1994: 127–137.
- 21.Li J, Kudsk KA, Janu P, Reneger KB. Effect of glutamine-enriched total parenteral nutrition on small intestinal gut-associated lymphoid tissue and upper respiratory tract immunity. Surgery 1996; 121:542–549. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Kudsk KA, Hamidian M, Gocinski BL. Bombesin affects mucosal immunity and gut-associated lymphoid tissue in intravenously fed mice. Arch Surg 1995; 130:1164–1170. [DOI] [PubMed] [Google Scholar]
- 23.Ottaway CA. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am 1991; 20:511–529. [PubMed] [Google Scholar]
- 24.Hildebrand P, Werth B, Beglinger C, et al. Human gastrin-releasing peptide: biological potency in humans. Reg Peptides 1991; 36:423–433. [DOI] [PubMed] [Google Scholar]
- 25.Greely GH Jr, Newman J. Enteric bombesin-like peptides. In: Thompson JC, Greely GH Jr, Rayford PL, Townsend CM Jr, eds. Gastrointestinal Endocrinology. New York: McGraw-Hill; 1987: 322–329.
- 26.Ghatei MA, Jung RT, Stevenson JC, et al. Bombesin: action on gut hormones and calcium in man. J Clin Endocrin Metab 1982; 54:980–985. [DOI] [PubMed] [Google Scholar]
- 27.Miyata M, Rayford PL, Thompson JC. Hormonal (gastrin, secretin, cholecystokinin) and secretory effects of bombesin and duodenal acidification in dogs. Surgery 1980; 87:209–215. [PubMed] [Google Scholar]
- 28.Bienenstock J, Perdue M, Stanisz A, Stead R. Neurohormonal regulation of gastrointestinal immunity. Gastroenterology 1987; 93:1431–1434. [DOI] [PubMed] [Google Scholar]
- 29.Jin GF, Guo YS, Houston CW. Bombesin: an activator of specific aeromonas antibody secretion in rat intestine. Dig Dis Sci 1989; 34:1708–1712. [DOI] [PubMed] [Google Scholar]
- 30.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg 1999; 229:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janu PG, Kudsk KA, Li J, Renegar KB. Effects of bombesin on impairment of upper respiratory tract immunity induced by total parenteral nutrition. Arch Surg 1997; 132:89–93. [DOI] [PubMed] [Google Scholar]
- 32.Sitren HS, Heller PA, Bailey LB, Cedra JJ. Total parenteral nutrition in the mouse: development of a technique. J Parenteral Enteral Nutr 1983; 7:582–586. [DOI] [PubMed] [Google Scholar]
- 33.Philippot J, Mutaftschiev S, Liautard JP. A very mild method allowing the encapsulation of very high amounts of macromolecules into very large (1000 nm) unilamellar liposomes. Biochem Biophys Acta 1983; 734:137–143. [Google Scholar]
- 34.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine 1992; 10:461–468. [DOI] [PubMed] [Google Scholar]
- 35.Deitch EA, Xu D, Qi L. Different lymphocyte compartments respond differently to mitogenic stimulation after thermal injury. Ann Surg 1990; 211:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sternberg EM, Chrousos GP, Wilder RI, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med 1992; 117:854–866. [DOI] [PubMed] [Google Scholar]
- 37.Bernton EW, Beach JE, Holaday JW, et al. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science 1987; 238:519–521. [DOI] [PubMed] [Google Scholar]
- 38.Besedovsky HO, Rey DA, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986; 233:652–654. [DOI] [PubMed] [Google Scholar]
- 39.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med 1996; 33:1106–1115. [DOI] [PubMed] [Google Scholar]
- 40.Debas HT, Mulvihill SJ. Neuroendocrine design of the gut. Am J Surg 1991; 161:243–249. [DOI] [PubMed] [Google Scholar]
- 41.Jin GF, Guo YS, Houston C. Bombesin: an activator of specific aeromonas antibody secretion in rat intestine. Dig Dis Sci 1989; 34:1708–1712. [DOI] [PubMed] [Google Scholar]
- 42.Rio MD, Hernanz A, Fuente M. Bombesin, gastrin-releasing peptide, and neuromedin C modulate murine lymphocyte proliferation through adherent accessory cells and activate protein kinase C. Peptides 1994; 15:15–22. [DOI] [PubMed] [Google Scholar]
- 43.Jin GF, Guo YS, Smith R, Houston CW. The effect of bombesin-related peptides on the phagocytic function of mouse phagocyte in vitro. Peptides 1990; 11:393–396. [DOI] [PubMed] [Google Scholar]
- 44.Woodruff GN, Hall MD, Reynolds T, Pinnock RD. Bombesin receptors in the brain. Ann NY Acad Sci 1996; 780:223–243. [DOI] [PubMed] [Google Scholar]
- 45.Rutland J, Iongh RU. Random ciliary orientation. N Engl J Med 1990; 323:1681–1684. [DOI] [PubMed] [Google Scholar]
- 46.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 1996; 369:319–322. [DOI] [PubMed] [Google Scholar]
- 47.Romberger D, Floreani AA, Robbins RA, Thompson AB. Respiratory tract defense mechanisms. In: Baum GL, Wolinsky E, eds. Textbook of Pulmonary Diseases. Boston: Little, Brown; 1994: 23–46.
- 48.Barnes PJ. The third nervous system in the lung: physiology and clinical perspectives. Thorax 1984; 34:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobonya RE. Normal anatomy and development of the lung. In: Baum GL, Wolinsky E, eds. Textbook of Pulmonary Diseases. Boston: Little, Brown; 1994: 3–21.
- 50.Barnes PJ, Baraniuk JN, Belvisi MG. Neuropeptides in the respiratory tract (part II). Am Rev Respir Dis 1991; 144:1391–1399. [DOI] [PubMed] [Google Scholar]
- 51.Baraniuk JN, Silver PB, Lundgren JD, et al. Bombesin stimulates human nasal mucous and serous cell secretion in vivo. Am J Physiol 1992; 262:L48–L52. [DOI] [PubMed] [Google Scholar]
- 52.Cripps AW, Dunkley ML, Clancy RL. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infections in rats. Infect Immun 1994; 62:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace FJ, Cripps AW, Clancy RL, et al. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunology 1991; 74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 54.Gorter PS, Hiemstra PC, Leijh ME, et al. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology 1987; 61:303–309. [PMC free article] [PubMed] [Google Scholar]
- 55.Broug-Holub EB, Toews GB, Iwaarden JF, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increase neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 1997; 65:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shou J, Lappin J, Daly JM. Impairment of pulmonary macrophage function with total parenteral nutrition. Ann Surg 1994; 219:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]