Abstract
Objective
Carcinomas of the colon and rectum are the third leading cause of cancer-related deaths. Although advances in the surgical treatment of primary colorectal cancers have lead to improvements in patient survival at early tumor stages, treatment of more progressive cancers has not resulted in dramatic improvements in patient survival. However, the selection of patient subgroups based on their prognosis and other characteristics could result in improved outcomes from adjuvant therapies in patients with Dukes B and C carcinomas.
Methods
The authors reviewed the available data on the value of cell surface molecules in assessing the prognosis of colorectal carcinomas, paying specific attention to the evaluation of statistical analysis and multivariate procedures.
Results
Cell surface molecules have been identified on colorectal carcinoma cells whose expression appears to be related to malignant transformation, tumor progression, or patient prognosis. Among these cell surface molecules, various cell adhesion molecules, growth factor receptors, proteinases, and their receptors and inhibitors have been identified as potentially useful prognostic markers.
Conclusions
Although data exist on the prognostic values of certain cell surface markers, the use of multivariate analysis for the identification of valuable prognostic factors remains uncommon. Using reproducible and standardized multivariate analysis procedures, new tumor markers should be carefully examined for their biologic and prognostic relevance before being considered as potentially useful in the management of colorectal cancers.
Carcinomas of the colon and rectum will affect approximately 6% of the population (1 of 17) in the United States during their lifetime. Approximately one third of the estimated 130,000 new patients per year will die within 5 years of cancer-related problems, mostly resulting from metastatic lesions. Thus, colorectal carcinomas are the third leading cause of cancer-related deaths among women and men, 1 and they are the most important malignancies of the gut.
Colorectal carcinomas are one of the best models for the investigation of genetic alterations that lead to malignant transformation and tumor progression. Various chromosomal mutations and deletions are known to be necessary in the adenoma–carcinoma progression sequence that includes different stages of hyperplasia and malignant transformation to an invasive carcinoma. 2 Little is known, however, about the genetic alterations and cellular mechanisms responsible for the final steps in the progression sequence that lead to invasion and metastasis.
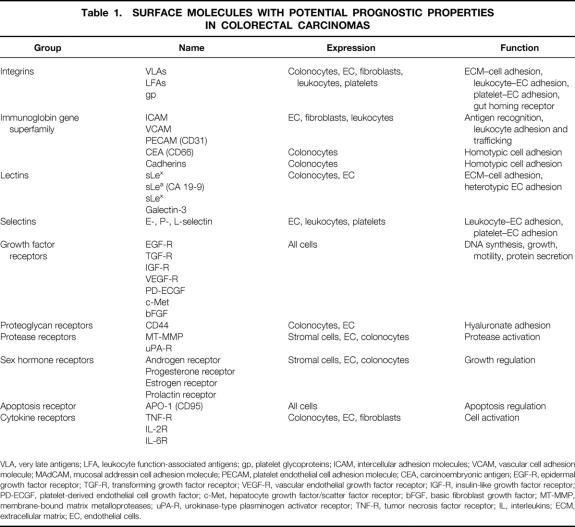
Tumor invasiveness and the development of metastases are the most important factors, besides the quality of primary surgery, in determining the prognosis of patients with colorectal carcinomas. 3,4 Patients with advanced local carcinomas or lymph node metastases can benefit from adjuvant therapy, 5 but metastatic involvement of lymph nodes or metastasis into distant organs reduces the median patient survival dramatically. 6,7 Because of the enormous differences between early and advanced stages of local tumors, the search for valuable prognostic markers remains an important subject of clinical research. Despite numerous investigations of these factors, only some of the data can be used for determining an individual patient’s risk after surgery for tumor recurrence or formation of distant metastases. For example, elevated preoperative levels of carcinoembryonic antigen (CEA) may be relevant for a patient’s prognosis, but there seems to be controversy on the usefulness of CEA determinations in individual patients. More appropriate evaluation can be achieved by following the postoperative kinetics of CEA, which more closely follows the presence of residual tumor. 8–10 Other strategies for the evaluation of patient prognosis have been developed during the last decade, such as genetic markers of progression or cellular proteins made by the tumor. This review summarizes data on surface proteins of colorectal carcinoma cells and their prognostic relevance (Table 1).
Table 1. SURFACE MOLECULES WITH POTENTIAL PROGNOSTIC PROPERTIES IN COLORECTAL CARCINOMAS
VLA, very late antigens; LFA, leukocyte function-associated antigens; gp, platelet glycoproteins; ICAM, intercellular adhesion molecules; VCAM, vascular cell adhesion molecule; MAdCAM, mucosal addressin cell adhesion molecule; PECAM, platelet endothelial cell adhesion molecule; CEA, carcinoembryonic antigen; EGF-R, epidermal growth factor receptor; TGF-R, transforming growth factor receptor; VEGF-R, vascular endothelial growth factor receptor; IGF-R, insulin-like growth factor receptor; PD-ECGF, platelet-derived endothelial cell growth factor; c-Met, hepatocyte growth factor/scatter factor receptor; bFGF, basic fibroblast growth factor; MT-MMP, membrane-bound matrix metalloproteases; uPA-R, urokinase-type plasminogen activator receptor; TNF-R, tumor necrosis factor receptor; IL, interleukins; ECM, extracellular matrix; EC, endothelial cells.
INVASIVE AND METASTATIC PROPERTIES
The prevention of death in colorectal cancer patients is dependent on understanding the mechanism of carcinoma cell spread from the primary tumor to distant organ sites. Although the pathogenesis of metastasis is the subject of numerous studies in basic and clinical research, the complex mechanisms that make a colorectal tumor cell metastatic are not well established. Colorectal cancer cells with different metastatic properties have been isolated from the same parent tumor, supporting a polyclonal character of these carcinomas. 11,12 Also, metastatic tumor cells show phenotypic instability, which leads to gradual shifts in their behavior and diversification of cells to form heterogeneous subpopulations that differ in their metastatic properties. 13 Colorectal carcinoma cells are known to undergo diversification as a result of quantitative changes in gene expression. As the tumor cells diversify, particular cell clones begin to dominate the cell population because of growth advantages and host selection. This leads to waves of clonal cell proliferation. 14
Clonal selection and diversification leading to tumor heterogeneity are major problems for the establishment of prognostic parameters for colorectal tumors. All the common techniques currently available for the characterization of carcinomas and the evaluation of their potential metastatic behavior are based on statistical analyses and the use of average values. In contrast, the development of metastases is a nonrandom process, in which the survival and distant growth of a single cell or a small number of cells can lead to death. 15 Therefore, the search continues for key events, genes, and molecules that are characteristic for the metastatic behaviors of tumor cells and that could be useful for predicting patient outcome.
The sequential model for the development of metastases involves tumor growth, neovascularization and invasion at the primary sites, followed by penetration into lymphatics and blood vessels or through the peritoneum. At the end of this process, circulating tumor cells must adhere to vessel walls of distant host organs, invade surrounding tissues, and survive and grow. 16 During these steps, interactions between tumor cells and the extracellular matrix (ECM) or surrounding cells are required. 17 Various molecules on the tumor cell surface mediate these interactions, either by direct contact, such as integrins or other adhesion molecules, or as receptors for soluble peptides or hormones, such as receptors for various growth factors. Usually the same molecules are also found on normal colonic epithelium, where they function in the maintenance of tissue structure and normal cellular regeneration.
Cell adhesion events are thought to play an important role in tumor metastasis. The detachment of tumor cells from the primary site, the homotypic interactions between tumor cells and with host cells during transport in the circulation, and the cellular interactions with the endothelium and ECM in distant organs are important for the formation of secondary tumors. The multiple surface molecules that mediate these adhesive interactions include lectins, glycosyl transferases, integrin and nonintegrin adhesion molecules, and glycolipids. The detachment of tumor cells from the primary carcinoma is characterized by the loss of cell–cell adhesion, and the cadherin–catenin systems appear to play an essential role in this process. In poorly differentiated carcinomas, loss of epithelial cell contacts is frequently observed, allowing the cells to break away from the primary tumor, invade surrounding tissue, and be released into the lymphatics and blood circulation. Adhesion of circulating tumor cells to organ endothelial cells and subendothelial ECM is mediated by several adhesion systems that may involve the same endothelial cell surface receptors used by leukocytes. For example, contacts between carcinoma cells and the microvascular endothelium seem to be related to expression of selectins, sialyl-Lewisx (sLex) and other carbohydrates, intercellular adhesion molecules (ICAM), and possibly annexins. 18 However, integrin-mediated interactions with the subendothelial ECM are among the most important determinants for organ-specific metastasis.
Penetration from the primary site into the circulation and extravasation into the host organ requires the release or activation of degradative enzymes, some classes of which appear to be metalloproteases, cathepsins, plasminogen activators, and endoglycosidases such as heparanase. Once malignant cells have arrived at and invaded secondary organ sites, they must stimulate local blood vessel growth. Neovascularization is induced and regulated by various angiogenetic molecules, which bind to specific cell surface receptors (e.g., vascular endothelial growth factor receptor, platelet-derived growth factor receptor [PDGF-R]). Finally, the neovascularized tumor must grow, and paracrine and autocrine growth factors appear to be essential in this process. Autocrine or paracrine growth factor receptors (e.g., epidermal growth factor receptor, fibroblast growth factor receptor, insulin-like growth factor) important in this process interact with intracellular proteins involved in signal transduction and can eventually modify cellular behavior.
CADHERIN–CATENIN SYSTEM
Cadherins belong structurally to the immunoglobin gene superfamily, a family of transmembrane glycoproteins responsible for calcium-dependent intercellular adhesion. They are divided into >10 subclasses, which are distinct in their immunologic characteristics and tissue distributions. Cadherins are involved in subclass-specific cell–cell interactions that play a role in selective cell adhesion at various developmental tissue stages. 19 Cadherins mediate homotypic adhesion in developing tissues, and they are connected with catenins at their cytosolic domains. 20 Inactivation of cadherins causes the disruption of cell–cell adhesion, and overexpression of cadherins leads to tighter cell–cell contacts. Continued expression and functional activity of E-cadherin are required for epithelial cells to remain integrated within the epithelium. 21 Catenins belong to the group of cytoplasmic plaque proteins that connect cell surface molecules with the actin cytoskeleton. α-Catenin is required for cadherin-mediated adhesion, and it indirectly links cadherins to actin by means of a specific binding site. β-Catenin is a necessary intermediate in the linkage of α-catenin to the cytoskeleton. 21
Like other carcinomas, such as those of the breast, bladder, and lung, the expression of E-cadherin and α-catenin is downregulated in poorly differentiated colon cancer cells. 22,23 Using an immunohistochemical technique, Cowley and Smith 24 compared E-cadherin expression and tumor morphology in carcinomas that had invaded vascular spaces with extravascular carcinomas, both in primary tumors and in their lymph node metastases. In 40% of cases at the primary site, in lymph node metastases, or at both sites, E-cadherin levels were higher in the often tiny intravascular tumor compartment than in the adjacent but much larger extravascular tumor compartment. E-cadherin was poorly expressed within the extravascular compartment. In analyzing the expression of E-cadherin in colorectal carcinomas, it has been shown that this receptor may serve as an independent prognostic marker in Dukes stage B colon cancers to identify patients with a poor prognosis and designate them for adjuvant therapy after curative surgical treatment. 25 Mohri 26 wrote that the loss or heterogeneous expression of E-cadherin in colorectal cancers correlated closely with advanced clinical stage, advanced tumor penetration, undifferentiated tumor histology, widespread lymph node involvement, liver metastasis, and penetration into the lymphatic and venous channels. The loss or heterogeneous expression of E-cadherin in tumor tissues was also significantly associated with an increased incidence of tumor recurrence after apparently curative resection, reduced overall survival rates, and reduced disease-free survival rates. A multivariate analysis disclosed that the expression of E-cadherin in tumor tissue was a significant prognostic variable independent of other clinicopathologic features. However, in another study, this marker did not predict outcome in the important group of moderately differentiated Dukes B colon cancers. 27 Using in situ hybridization, low levels of expression of E-cadherin were found to be independent prognostic factors on multivariate analysis and were significantly associated with metastasis or recurrent disease in N0 colon carcinomas. 28 This has been confirmed by another group. 29 Collectively, there appears to be a statistically significant correlation between reduced expression of E-cadherin and loss of tumor differentiation. Thus, the frequency of reduced E-cadherin expression is generally greater in tumors with aggressive histopathologic characteristics, lymph node involvement, and distant metastases.
E-cadherin expression is considered to play a major role in the homotypic adhesion of cancer cells. Therefore, suppression of E-cadherin expression or its function might enhance the release of cancer cells from the primary lesion. Functionally, E-cadherin is thought to be regulated by its associated cytoplasmic proteins, including α-catenin. Normal epithelium expresses α-catenin strongly without exception. However, α- and β-catenin expression was frequently reduced (approximately 80%) in primary colorectal carcinomas. 30,31 Normal mucosa, as well as colon adenomas, showed strong membranous α-catenin expression. In the normal colon, catenin expression was observed in the crypt and surface epithelium; the cells showed reactivity both at the membrane and in the cytosol. 32 Significant downregulation of α-catenin expression in colorectal cancer has been associated with poor differentiation, higher metastatic potential, and unfavorable prognosis. 33,34 It has also been found that β-catenin forms complexes with adenomatous polyposis coli (APC) tumor suppressor protein, and β-catenin expression levels are affected by exogenously induced APC protein. The expression levels of APC protein in tumor tissues were more than three times greater than those in corresponding normal mucosa. 35,36 In coexpression experiments, approximately 80% of the colon carcinomas showed similar expression of E-cadherin and β-catenin, whereas the other tumors showed strong positive staining for E-cadherin and reduced expression of β-catenin. 37 These findings support the hypothesis that β-catenin forms a complex with E-cadherin in vivo. The downregulation of β-catenin expression is associated with malignant transformation, and colorectal cancer cells may have impaired E-cadherin–mediated cell adhesiveness because of the downregulation of catenin expression. 38
INTEGRIN SYSTEM
Integrins are heterodimeric transmembrane adhesion molecules that mediate interactions between cells and the ECM. More than 20 different integrin heterodimers are known, but β1- and β3-integrins appear to be the most important integrins expressed on tumor cells. 39 Each integrin subfamily is determined by the β-subunit. For the specificity of interactions with heterogeneous ligands, both subunits are necessary, but integrins are either monospecific or can bind to several different ECM components. Integrins are expressed on endothelial cells, epithelial cells, platelets, and leukocytes, among others, and tumor cells. 40,41 Most tissues express only a restricted number of integrins. These include those thought to function as collagen/laminin receptors. Changes in integrin receptors for fibronectin or vitronectin might have importance for the metastatic phenotypes of colorectal carcinomas.
Integrins mediate adhesion through various ECM binding sites, depending on the matrix components involved. Once cell adhesion occurs, integrin receptors generate regulatory signals in cells that allow them control over cell migration and invasion into host organs. These cell–ECM interactions are mediated through various transmembrane receptors, including integrins, which are intracellularly linked to cytoskeleton components 42 and signal transduction molecules. 43,44 Recent studies have shown that cross-talk between integrins and other cell surface receptors is involved in these signal transduction processes. 45 An example of this is the apparent cross-talk between integrin receptors and growth factor receptors. Thus, the presence of transforming growth factor-β can modulate integrin-mediated adhesive properties and differentiation states of colon carcinomas. 46
Previous studies have demonstrated a correlation between the organ preference of metastasis and the in vitro adhesion rates of malignant cells to various endothelial cells 47,48 or subendothelial ECM. 49 Using colon carcinoma cell lines with different metastatic properties, we have shown that metastatic behavior correlates with different integrin-mediated adhesive properties. 50,51 However, differences in integrin expression could not explain these differences. Therefore, integrin-mediated specific tumor cell adhesion includes complex intracellular interactions with signaling cascades and cytoskeleton components. 52,53 In addition, distinct organ site-specific basement membrane composition has been found. 17 Tumors, such as colon carcinomas, produce their own ECM components that can change peritumoral stroma composition. 54,55 This includes the release of unusual ECM components, such as oncofetal fibronectin, which is correlated with a poor prognosis in colorectal carcinomas. 56
A broad spectrum of integrin expression in certain patterns was found in normal tissues, primary tumors and metastases. 46 In normal colonocytes, α2-integrin staining was strongest in crypt cells, whereas α1, α3, and αv, and β1, β3, and β4 predominated in superficial enterocytes. In adenomas, monolayered glands showed integrin patterns that differed slightly from both crypt and superficial enterocytes. Complex glands in villous adenomas showed decreased integrin staining and basal polarization. 57 β1-Integrins were usually found to be expressed in adenomas, but the α2β1-integrin appeared to be lost in focal areas of cell contacts. 58 Colon carcinomas tend to have weaker integrin staining than adenomas or normal cells 59; however, they also show considerable heterogeneity of α2β1-integrin expression. 58 Also, α5 was frequently found to be expressed in invasive colon carcinomas, whereas the expression of this integrin subunit is usually poor or absent in normal epithelium. 60,61 In another study, however, α3 showed variable expression, with a diffuse distribution at the cell surface in peripheral areas of colorectal carcinomas, correlating with the histologic stage of malignancy, whereas the expression of α5-subunits was almost absent. 62 Various combinations of α- and β-subunits are found only in transformed cells. The α6-integrin subunit is normally paired with β1-subunits, but in colon carcinoma cells coexpression of α6- and β4-subunits was frequently found. 63 There is also evidence that the αv-subunit is involved in the regulation of apoptosis, or programmed cell death. 64 Also, colon carcinomas might induce or modify integrin expression on tumor-associated endothelial cells. The αvβ3-integrin was found to be overexpressed on tumor vasculature. 65 In contrast to the variability of integrin expression on colon carcinoma cells, analysis of adhesion molecules of the integrin family on lymphocytes immigrating into tumor tissue indicated no specific expression of individual β-integrins. 66
Correlations have been reported between integrin expression and tumor prognosis and clinical stage. The transformation from benign to malignant neoplasia was found to be associated with infiltrative growth and characterized by diminished or lost expression of α6-, β1-, and β4-integrin subunits. 67 When compared with their primary tumors, colorectal carcinoma liver metastases showed roughly similar patterns of integrin expression. In various studies, the expression of integrins in normal tissue was determined and compared with different stages of colorectal carcinomas. 62,68 Generally, a high variability of integrin expression seemed to be related to the degree of differentiation of the original tumor. 69 However, reduced α2-integrin expression was statistically associated with advanced cancer stages. A strong correlation was also observed between the expression of the α6-laminin receptor and the degree of colorectal carcinoma differentiation, invasive properties, and metastatic abilities. 25
SELECTIN SYSTEM
Selectins are adhesion molecules that use carbohydrates as receptor ligands. They are important in the interactions of cells with leukocytes/lymphocytes (L-selectin), platelets (P-selectin), and endothelial cells (E-selectin). The action or increased expression of selectins depends on cell activation of endothelial cells or leukocytes/lymphocytes by interleukins, tumor necrosis factor (TNF), or toxins and of platelets by thrombin, histamine, O2 radicals, and other procoagulatory substances. All selectins have a similar structure with a N-terminal lectin domain, epidermal growth factor-like domains, different numbers of complement binding domains, a transmembrane domain, and a short intracellular portion. Selectins mediate the recognition of carbohydrates, such as sLea, sLex, and the MECA-70 antigen. Although the members of the selectin family are structurally related, they have disparate functions. Selectins play a central role in targeting of circulating tumor cells to the endothelial cells of the host organ. In this manner, they help determine the organ preference of metastasis.
Various selectins are expressed in different tissues and on different cell types. E-selectin is expressed on endothelium but not on colonocytes. 70 Approximately 30% of the intraepithelial lymphocytes in the colonic epithelium express L-selectin. 71 E-selectin was shown to be present on the endothelial cells of small vessels adjacent to cancer cell nests both in primary and in metastatic lesions. In these tissues, E-selectin was observed on the endothelial cells lining the lumen of small vessels. The degree of expression of E-selectin was inversely correlated with the distance of the blood vessels from the cancer cell nests: endothelial cells adjacent to the metastatic lesion expressed E-selectin more extensively than those adjacent to the primary tumor foci. 72 Serum E-selectin levels were also significantly elevated in the patients with metastasis versus those without. 73 There were also weak but significant correlations between serum E-selectin levels and CEA or CA 19-9 levels. 73 However, in one study E-selectin immunostaining did not correlate with cell infiltration. 74 Studies on the prognostic value of selectins for estimating patient survival have not been published, so it is difficult to assess the potential value of using selectin expression for patient prognosis.
LECTINS AND GLYCOCONJUGATES
Normal colonic epithelial cells undergo maturation as they traverse the crypt to the luminal surface, and changes occur during this process in the expression of specific cell surface oligosaccharides. The binding of lectins to goblet cell mucins and other glycoconjugates changes as the cells migrate from the crypt and undergo differentiation. These sialylated carbohydrate structures on mucins play a role in colorectal cell adhesive interactions involving both basement membrane ECM and endothelial cell-associated ligands.
The affinities and antigenic structures of the sialylated carbohydrates are regulated by the activities of glycosyl-transferases and other membrane-bound enzymes, some of which are upregulated in colon carcinomas. 75 Additional stepwise modifications in glycoconjugate expression occur in premalignant and malignant neoplasms. 76 Various glycoconjugates differ in their affinities for different cellular components, local distribution within crypts, and regional distribution between right (ascending colon) and left (rectum) segments of the large bowel. 77 CA 19-9 and sLex are tumor-associated antigens that have been found expressed in the whole colorectum, whereas other sialylated carbohydrates, such as sLeb and sLey, were found only in the distal colon. 78
Colorectal carcinomas with increased metastatic potential and with a poor prognosis are characterized by a high content of certain carbohydrate antigens. The levels of these carbohydrate antigens apparently increase during colorectal carcinoma progression from nonmetastatic to metastatic tumors. 79 For example, the levels of tumor-associated sLex antigens were inversely correlated with the postsurgical survival of patients with colon carcinoma, as revealed by retrospective studies. 80 Disease-free survival rates of patients with sLex-positive tumors were significantly poorer than those with sLex-negative tumors. A multivariate analysis revealed that the sLex status was an independent predictive factor for colorectal disease recurrence, depth of invasion, and histologic type, whereas sLea status, age, gender, tumor location, nodal status, and vessel invasion were not. 81 Increased sialylation of mucin-associated carbohydrates, such as sLex, is generally characteristic of colon cancer cells that are likely to metastasize. Metastases have been found to express decreases in mucin core structures, reciprocal increases in sialylated mucins, and increases in peripheral sLex compared with the primary tumors from which they arose. 82 The levels of this carbohydrate antigen apparently increase during colorectal carcinoma progression from nonmetastatic to metastatic tumors, and they inversely correlate with postoperative survival. 83
Previously, certain antigens related to blood serum antigens were correlated with tumor progression and prognosis. For example, the CA 19-9 antigen was studied for years before it was identified as a monosialosyl Lea blood group antigen. Levels in adenoma and carcinoma specimens were significantly higher than in the normal mucosa. 84,85 Higher tumor stages correlated with higher tissue marker values of CA 19-9. 86 Other lectins, such as lactose-binding lectins or galectin-3, also showed significant correlations to the Dukes stages and appear to be related to neoplastic transformation and metastatic progression. 87,88 These results were related to other known prognostic factors such as CEA. 89
IMMUNOGLOBIN SUPERGENE FAMILY
Cell adhesion molecules with an immunoglobin-like (Ig-like) structure in their extracellular portions are thought to have wide-ranging functions and to participate in a variety of homophylic and heterophylic interactions. 90 Members of this family, such as ICAM-1, ICAM-2, and vascular cell adhesion molecule (VCAM-1), are known to participate in heterotypic cell–cell adhesion. Receptors for certain growth factors (e.g., PDGF, colony-stimulating factor-1), T-cell receptors (CD4, CD8), tumor cell antigens (CEA), and a group of molecules that mediate cell–cell interactions between platelets and endothelial cells (CD31, ICAM-1, VCAM-1) belong to various Ig-like subgroups. This latter group takes part in cell–cell interactions by binding to other adhesion molecules, such as integrins or selectins, that are important in tumor cell interactions and metastasis.
Immunohistochemical localization and in situ hybridization have revealed a lack or low expression of ICAM-1 on normal colonic epithelium. 70 In colonic tissues, ICAM-1 immunostaining was restricted to the ECM and vascular endothelium. The vast majority of normal tissue samples revealed only faint ICAM-1 immunoreactivity; however, moderate to strong immunostaining was found in >80% of cancerous tissues. ICAM-1 was more intensely expressed in well-differentiated carcinomas as well as in the adenomatous parts and transition zones of cancers. In normal tissues, VCAM was seen only in isolated lymphoid aggregates. 70 Similar to ICAM-1, colon cancers exhibited markedly enhanced VCAM-1 immunostaining in the endothelial cells of small blood vessels. The intense vascular immunostaining of ICAM-1 and VCAM-1 was associated with the presence of CD3-positive T lymphocytes. 74
CEA is a highly glycosylated cell surface protein and a member of the Ig-like superfamily. It is produced in large amounts in essentially all colon and several other adenocarcinomas; therefore, it has been widely used as a clinical tumor marker. Endothelial cells express CEA on their cell surfaces. Therefore, CEA-expressing adenocarcinomas may adhere to endothelial cells, in part by CEA–CEA interactions. Thus, CEA interactions may facilitate tumor cell extravasation and hematogenous metastasis formation. 91 CEA is expressed intracellularly as well as extracellularly. The intracellular expression of CEA appears to be associated with the degree of atypia in histologic sections. 92 The concentrations of CEA in tumor specimens showed a high degree of correlation with the risk of relapse. 93,94 Conversely, there was no correlation between tissue CEA content and tumor differentiation. 95 Immunohistochemical expression confirmed the predictive value of CEA contents in colorectal tumor specimens. 96 Serum CEA levels and the CEA tissue contents determined by immunohistochemical staining correlated with patient survival, and they appear to have similar prognostic values. 95
Ig-like receptors have also been useful in assessing angiogenesis, a crucial step in tumor growth and progression. Its quantitation by microvessel counting has prognostic value in several types of malignancies. The expression of endothelial cell-specific CD31 has been used to evaluate the onset of angiogenesis in colorectal tumors, and microvessel quantitation has been used to assess its prognostic significance. The density of microvessels in the tumor can be determined using endothelial cell-specific antigens and is a reliable marker for the angiogenesis that is an early, critical step in colorectal tumorigenesis. High expression of CD31 was not associated with metastasis formation, disease stage, or patient survival 97; however, using von Willebrand factor for endothelium-specific immunostaining, high microvascular counts were a prognostic predictor for a longer survival time independent of Dukes stage. 98 The presence of p53 protein overexpression was also found to be associated with a high microvascular density. 99
CD44, ITS ISOFORMS AND RECEPTORS
CD44 is a cell surface adhesion molecule family with different splice variants that is expressed on endothelial cells and various tumor cells. 100 Multiple functions have been attributed to the CD44 family of molecules. CD44 plays a role in the production and catabolism of hyaluronate, which is primarily located in the liver and lymph nodes. It mediates cell–cell contacts with glycosaminoglycans, such as hyaluronate, on fibroblasts, on endothelial and hematopoietic cells, and in the ECM. Hyaluronate and CD44 have been proposed to be important in tumor invasiveness, cell migration, and angiogenesis. 100 CD44 splice variants are frequently but not always expressed in advanced states of tumor progression. In colorectal carcinogenesis, expression of exon v5 is an early tumor marker because it can be detected on small dysplastic polyps but not on normal colon epithelium. 101 The loss of expression of the CD44-v6 isoform seems to be associated with a poor prognosis in colorectal cancer because of the development of tumor metastases. 102 For example, CD44-v6 immunoreactivity was detected in 100% of adenomas and in >90% of colorectal carcinomas, but expression was mostly weak in only approximately one third of liver metastases. Normal mucosa shows weak subnuclear localization of CD44-v6 after immunostaining. Overall correlations were not found with tumor type, stage, or patient survival by Coppola et al. 103 Another study reported a significant correlation between expression of CD44-v6, Dukes stage, metastasis, and patient survival. 104 Expression of CD44H, CD44-v9, and CD44-v6 was decreased compared with corresponding primary colorectal tumors, 105 and this group also showed that increasing CD44-v6 expression correlated with progressive tumor stage and differentiation. 106 These correlations were confirmed at the mRNA level using reverse transcriptase–polymerase chain reaction. 107 Using a multivariate analysis, the expression of another CD44 exon, CD44v8-10, has emerged as an independent prognostic indicator for lymph node and hematogenous metastasis and overall survival. 108 In addition to CD44, one of its commonly found receptors, hyaluronate, has also been correlated with colorectal cancer survival and recurrence. The intensity of hyaluronate immunostaining in tumor epithelium independently predicted survival and recurrence-free survival. 109
GROWTH FACTOR RECEPTORS
Growth factor receptors mediate a wide diversity of signals from the cell surface into the cell. Specific receptor occupation with growth factors can induce or inhibit cell growth, motility, and protein expression or secretion. Their autocrine or paracrine activity seems to be coregulated by other cell signaling systems, such as integrins (discussed above). Growth factor receptors are found on all cells, but their pattern of expression is highly heterogeneous and dynamic. Growth factor receptors are transmembrane molecules that often have enzymatic activity, mostly kinase activities, at their cytosolic domains. Their signaling pathways to the nucleus are often associated with oncogene products, such as the central protooncogene Ras, which is found in all eukaryotic cells.
The most important growth factors for determination of the growth properties of epithelial malignancies are epidermal growth factor (EGF) and transforming growth factor (TGF). No consensus about the involvement of the EGF receptor (EGF-R) in colorectal carcinomas has been attained, although it is assumed to play a role in the invasion and metastasis to lymph nodes and in recurrence at regional and distant sites. EGF-R has been detected both in adenomas and carcinomas. 110 Significantly increased levels of EGF and EGF-R were found in some neoplastic samples compared with surrounding mucosa, 111 but increased expression of EGF-R seems to be uncommon in colonic adenocarcinomas. 112 Some studies found significant correlations between EGF-R protein expression (or its mRNA) with Dukes classification, differentiation, and survival, whereas others could not confirm these results. 113–116 The expression of other growth factor receptors, such as TGF-R and amphiregulin (AR), have been correlated with Dukes stage and differentiation. 113 Often EGF-R and TGFs and their receptors are coexpressed in colorectal tissues. EGF-R expression and its mRNA levels appear to be related to TGFα staining in normal and adenomatous tissue. 117 TGFβ type I and II receptors were found to be overexpressed in tumors compared with normal samples, and there appeared to be a relation between the abundance of type II receptors and the degree of differentiation of the colorectal tumors, but not the Dukes staging or the locations of the carcinomas. 118
HER-2/neu oncogene encodes a transmembrane tyrosine kinase receptor with homology to EGF-R and is often amplified or overexpressed in adenocarcinomas. Normal mucosa does not usually express HER-2/neu protein, but a significant number of benign lesions and adenocarcinomas were found to overexpress this protein. Carcinomas were significantly more positive than benign lesions. A significant correlation was found with differentiation, Dukes classification, and relapse-free and postoperative survival. 119
AR, a protein structurally related to EGF and TGFα, is also functionally related to this family of growth regulatory molecules and can bind and activate EGF-R. Immunostaining and in situ hybridization detected AR protein and its mRNA in primary and metastatic colorectal tumors in liver but not in normal colon or uninvolved liver. 120
Vascular endothelial growth factor (VEGF) is a well-known tumor and normal cell growth factor that induces angiogenesis. Expression of VEGF was found to be significantly reduced in metastatic colorectal liver tumors compared with primary lesions. However, the levels of VEGF in primary colorectal tumors did not predict risk of liver metastasis or survival duration in one study. 121 In another study, tumors with high VEGF expression and detection of the high-affinity VEGF receptor (KDR) on tumor endothelium were associated with metastasis formation. 122 Further studies indicated that patients with high VEGF expression in their primary colorectal cancers had a high likelihood of recurrence. 123 Various isoforms of VEGF have been identified in colorectal cancers. The detection of mRNA isoforms correlated with metastasis and a poor prognosis. 124
The insulin-like growth factor (IGF) is known to induce or modify growth properties in various tissues. Although its receptor is expressed on colorectal carcinoma cells, none of the clinicopathologic parameters showed any association with IGF-1R status. Differences were not observed in the overall survival period between patients with IGF-1R–positive tumors and those with IGF-1R–negative tumors. 125 Other growth factor receptors, such as those for platelet-derived endothelial cell growth factor, c-Met (receptor for hepatocyte growth factor/scatter factor), or fibroblast growth factor, were investigated in animal models, where they demonstrated various correlations with colorectal tumorigenesis, invasion, or tumor vessel count. 126 However, data on human colon cancer specimens did not show significant correlations between the expression of these receptors and patient prognosis. 127
PROTEASE ACTIVATORS AND PROTEASES
Although proteases are mostly nonintegral membrane or secreted molecules, they can be found in membrane receptor-bound forms or closely related to specific receptors that are involved in the release and activation of degradative enzymes. Therefore, proteases and their receptors should be considered as cell surface or surface-related molecules. Various kinds of degradative enzymes are involved in the dissolution of tumor-surrounding ECM as a prerequisite for tumor invasion at the primary site. Degradation of the ECM is also required for extravasation of tumor cells into the distant host organs. Various classes of degradative enzymes can be released by malignant cells and surrounding stromal cells, including the plasminogen activators, cathepsins, metalloproteinases, and endoglycosidases. Cells can also produce inhibitors of degradative enzymes. Therefore, maintenance or disturbance of the degradative enzyme/inhibitor balance plays an essential role in invasion and metastasis formation.
Receptor-bound urokinase-type plasminogen activator (uPA) and its receptor (uPA-R) and its inhibitor (PAI-1) seem to play an important role in the dissolution of the surrounding ECM and the formation of tumor stroma. Secreted uPA binds with high affinity to its specific receptor uPA-R on the cell surface. These processes appear to be prerequisites for invasion and metastasis. The binding of uPA to uPA-R has at least two important consequences: it enhances the rate of plasminogen activation on the cell surface, and it focuses the uPA proteolytic activity at the leading front of migrating cells. 128 Several recent findings suggest that surface-bound uPA is essential for the invasive ability of tumor cells, although the emerging data suggest concerted action of uPA and uPA-R with other secreted and cell-bound proteases, such as metalloproteinases and cathepsin B. Increased uPA, uPA-R, or PAI-1/2 correlated with tumor progression and shortened disease-free or overall survival. 129 Interestingly, the numbers of uPA-R–positive cells along the invasive margins of tumors were significantly less in patients with liver metastases than in patients without liver metastasis, and the uPA-R–positive cells were also less in cases with an infiltrating margin than in cases with an expanding margin. 130 This suggests that simple relations between one degradative enzyme and its membrane receptor, such as uPA–uPA-R, with tumor progression may be an oversimplification. Disturbances in plasmin formation take place in distinct stromal compartments, but not on epithelial cells. These imbalances appear to be maximized in invasive neoplasias. 131 Thus, it may be that changes in uPA–uPA-R may be more important than the absolute levels of these markers. Low tissue plasminogen activator (tPA), high levels of uPA-related antigen, and a high uPA:tPA antigen ratio as well as PAI-2 antigen were associated with poor overall survival. 132,133
Expression of cathepsin D has been suggested to affect the invasiveness of carcinoma cells. Secretion of cathepsins appears to be mostly by stromal cells, such as fibroblasts. In colorectal carcinomas, cathepsin D was also found to be expressed by malignant cells. Because colorectal carcinomas showed a high variance of immunostaining for cathepsin D, the prognostic value of its expression remains uncertain. Although overexpression of cathepsin D was found in some studies, 134–136 its independent prognostic value was described for patient survival and Dukes stage in only one report. 135 Colorectal carcinomas express higher levels of cathepsin L than normal colonic tissues. 137 However, studies on the prognostic value of cathepsin L are not available.
Matrix metalloproteases (MMP) are a family of metal-dependent endopeptidases with proteolytic activities for various components of the ECM. Their activity is regulated by specific tissue inhibitors of metalloproteinases (TIMP) and by activation through membrane-bound MMP (MT-MMP). 138 Depending on their substrate specificity, MMPs are grouped into collagenases (or gelatinases), stromelysins, matrilysin, and MT-MMP. The most important collagenases are MMP-2 and -9, which can hydrolyze ECM collagens. Using quantitative zymography for detection of proteolytic activity, higher amounts of MMP-2 and -9 were found in carcinomas, correlating with Dukes stage but not differentiation or survival. 138–140 Further, in specimens from metastases originating from primary tumors of the colon, significantly enhanced type IV collagen degrading enzyme activity was observed relative to the primary tumor. 141 Immunologic staining has also been seen in tumor-infiltrating neutrophils and macrophages located adjacent to invasive tumor glands where cancer cells were not stained. In normal colon tissue, staining for MMPs was seen only in scattered neutrophils in vessels and in macrophages in Peyer’s patches. 142 The degree of tissue expression of MMP-9 by host cells in colorectal cancers appeared to be inversely associated with liver metastasis and an infiltrating growth pattern. 130 Both increased levels of proenzyme and active enzyme forms of gelatinase A (MMP-2) and increased cathepsin B activity were localized in regions of tumor invasion compared with the levels found in a matched number of normal epithelial cells. In this study, the levels of progelatinase B (MMP-9) were also increased in the tumors. 143
Other MMPs have been found to be expressed by colorectal cancer cells or stromal cells. The presence of MMP-1 (fibroblast-type collagenase) in colorectal cancer was found to be associated with a poor prognosis and had prognostic value independent of Dukes stage. 144 MMP-11 (stromelysin-3) expression was characteristic for tumors of epithelial origin and was overexpressed in colon carcinomas, including in situ lesions. 145 These results were confirmed by detection of mRNA for all stromelysins, which were expressed in the majority of colon carcinomas examined. 146 The overexpression of MMP-11 was localized in stromal fibroblasts and correlated with tumor invasion and progression. 147 Matrilysin (MMP-7) mRNA was also detected in cancerous tissue but not in adjacent normal colon tissue. 148 This upregulation of secretion and the activation of matrilysin apparently occur during malignant conversion of colonic epithelium. 149 In an interesting study, the expression of this MMP-7 mRNA was used to detect occult lymph node metastases with a high sensitivity. 150
Inhibitors of MMPs have also been examined for their expression in colorectal carcinomas and normal epithelium. For example, TIMP-1 and TIMP-2 were immunolocalized in scattered stromal cells, whereas epithelial cells of normal mucosa and hyperplastic polyps were weakly stained. Immunolocalization of TIMPs demonstrated gradual increases from tubular adenomas to villous adenomas, and in situ carcinomas showed a definite positive immunolocalization. 151 The distribution of TIMP-1 mRNA and protein showed similar increases in expression during malignant transformation. 152
The expression of several proteinases and their inhibitors in a given tumor may provide information independent of clinical stage and may identify crucial variations in tumor behavior. On occasion these data have been combined, and it was shown in one study that cathepsins and MMPs could be combined into proteinase profiles. 153 The combination of MMP-9 and cathepsin B and L showed significant correlation with tumor stage. Moreover, a combined role for MMP-9, uPA, and uPA-R expression has been assumed in colon cancer tissue as important cancer progression/promoting factors, but they might also be related to host defense mechanisms when they are expressed by infiltrating host cells.
OTHER SURFACE MARKERS
Various other cell surface markers have been examined for their usefulness as prognostic factors. Histocompatibility antigen-A, -B, -C, and -DR expression in colorectal carcinoma seems to be irrelevant in vivo, and they are not related to the survival and growth of residual tumor cells after putatively curative colorectal tumor resection. 154,155 The expression of the motility-related protein-1 or CD9 showed a significant correlation with higher frequency of venous vessel invasion and liver metastasis. 156
Although overexpression has been described for sex hormone receptors, such as androgen, progesterone, prolactin, and estrogen receptors, correlations with histologic findings, clinical stage, or prognosis have not been established. 157–159
Some cell surface receptors regulate cell survival, and these are of interest in malignant tumors with high growth fractions or low death rates. APO-1 is a cell membrane protein identical to the Fas antigen (now designated CD95). It is a member of the NGF/TNF receptor superfamily, which is strongly involved in the regulation of apoptosis. Using immunohistochemistry, APO-1 was found to be expressed routinely at the basolateral membrane surface of normal colon epithelia. In a minor fraction of colon adenomas and in approximately 40% of the carcinomas, APO-1 expression was diminished. APO-1 expression was completely abrogated in approximately 50% of carcinomas, predominantly in the nonmucinous type, and the level of APO-1 expression in carcinomas was correlated with the mucinous type. 160
Various cytokines and interleukins (IL) can take part in the activation and regulation of cellular functions, such as endothelial cell activation and leukocyte trafficking. Therefore, the expression of cytokines and their receptors is thought to be an important determinant for tumor cell behavior. When colorectal tumor cells were examined for IL-6, a large subset of colon cancer specimens were strongly immunostained. The expression of IL-6 was less conspicuous and less frequent in the epithelial cells of normal colonic mucosa compared with colon carcinomas. IL-6 receptor mRNA was also detected at twice the levels in colonic carcinomas than in normal colon tissues. 161 One study investigated soluble IL receptors in the peripheral blood from patients with colon carcinoma and found significantly higher levels of sIL-2-R that correlated with clinical stage. 162 However, the relation of IL levels in the blood might be related to immune response in these patients. 163 Data on the prognostic value of cytokines and their receptors are not available.
CONCLUSIONS
The establishment of useful prognostic markers for colorectal cancer appears to have utility in determining prognosis as well as adjuvant therapies that might be clinically applied. Thus, selecting patients based on their prognosis may lead to more appropriate subgrouping of candidates for particular adjuvant therapies. Numerous cell surface or surface-related molecules have been identified that are functionally involved in neoplastic transformation, tumor progression, and the development of metastases. Therefore, it is not surprising that many data exist on the prognostic values of these markers. However, few studies have used multivariate analyses to identify valuable prognostic factors. Future examinations of potentially useful markers will have to take this into consideration. Also, the more immediate search for prognostic indicators and metastatic site preferences in tumors expressing particular molecular profiles has been partly hampered by the small numbers of patients studied by most investigators. Thus, marker trends in tumor subsets barely achieve significance in most studies.
Currently, adhesion molecules, such as β1-, β4-, and α6-integrins, E-cadherin and its intracellular partner proteins α-, β-catenin, CD44-v6 and other splice variants, sLex, CEA, as well as the angiogenesis-related molecules von Willebrand factor, VEGF, MMP-9, uPA and its receptor (uPA-R) and inhibitor (PAI), appear to be the most significant prognostic markers for patients with colorectal cancer. Some studies suggest that EGF-R and cathepsin D also have prognostic significance. Taken together, several surface molecules were found to be useful for the evaluation of prognosis, but in some studies contradicting results, possibly because of small numbers of patients, different sources (genetics) of patients, or use of univariate analyses or other methodologic considerations, will require further investigation on the usefulness of these markers in assessing colorectal cancer outcome.
Perhaps the next phase should be to evaluate promising markers in defined combinations in larger studies to establish the relative importance of these markers in tumor prognosis, survival, and response to treatment. The usefulness of surface markers in routinely processed archival material from human tumor specimens must also be carefully examined in multivariate analyses. Future developments in therapy, such as definition of subgroups for particular adjuvant therapies, may rely on the knowledge that may emerge from such work. 164 New markers for evaluation of an individual tumor’s progression and prognosis should also be undertaken. This can be achieved only with a better understanding of the cell surface biochemistry and molecular biology of colorectal carcinomas. All potential prognostic factors will have to demonstrate clear biologic relevance using reproducible and standardized procedures to be useful in the management of colorectal cancers.
Footnotes
Correspondence: Garth L. Nicolson, PhD, The Institute for Molecular Medicine, 15162 Triton Lane, Huntington Beach, CA 92649-10941.
Accepted for publication March 1, 1999.
References
- 1.American Cancer Society, Surveillance Research, 1998. Vital Statistics of the United States, 1997.
- 2.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal tumor development. N Engl J Med 1988; 319:525–532. [DOI] [PubMed] [Google Scholar]
- 3.Haier J. Indication and strategy of surgical treatment in metastatic colorectal cancer. In: Buhr HJ, Runkel N, eds. Operationskurs: Kolorektales Karzinom. Leipzig: Ambrosius Barth Verlag; 1997: 138–145.
- 4.Hermanek P Jr, Wiebelt H, Riedl S, et al. Long-term results of surgical therapy of colon cancer. Results of the Colorectal Cancer Study Group. Chirurgie 1994; 65:287–297. [PubMed] [Google Scholar]
- 5.Köhne CH. Advantages in the development of chemotherapy for colorectal carcinomas. Med Klinik 1996; 91:33–37. [PubMed] [Google Scholar]
- 6.Steinberg SM, Barkin JS, Kaplan RS, et al. Prognostic indicators of colon tumors: the Gastrointestinal Tumor Study Group experience. Cancer 1986; 57:1866–1870. [DOI] [PubMed] [Google Scholar]
- 7.Gall FP, Hermanek P. Change and current status of surgical treatment of colorectal cancer. Report of experiences of the Erlangen Surgical University Clinic. Chirurgie 1992; 63:227–236. [PubMed] [Google Scholar]
- 8.Hohenberger P, Schlag PM, Gernet T, Herfarth C. Pre- and postoperative carcinoembryonic antigen determinations in hepatic resection for colorectal metastases. Predictive value and implications for adjuvant treatment based on multivariate analysis. Ann Surg 1994; 219:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu DZ, Erickson CA, Russell MP, et al. Prognostic significance of carcinoembryonic antigen in colorectal carcinoma. Serum levels before and after resection and before recurrence. Arch Surg 1991; 126:314–316. [DOI] [PubMed] [Google Scholar]
- 10.Fortner JG, Silva JS, Golbey R, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg 1984; 199:3096–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman M, Eisenbach L. What makes a tumor metastatic? Sci Am 1988;40–47. [DOI] [PubMed] [Google Scholar]
- 12.De Both NJ, Vermey M, Groen N, et al. Clonal growth of colorectal-carcinoma cell lines transplanted to nude mice. Int J Cancer 1997; 72:1137–1141. [DOI] [PubMed] [Google Scholar]
- 13.Nicolson GL. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochem Biophys Acta 1988; 948:175–224. [DOI] [PubMed] [Google Scholar]
- 14.Moustafa A, Nicolson GL. Breast cancer metastasis-associated genes: prognostic significance and therapeutic implications. Oncol Res 1997; 7:505–525. [PubMed] [Google Scholar]
- 15.Fidler IJ. Critical factors in the biology of human cancer metastasis. Cancer Res 1990; 50:6130–6138. [PubMed] [Google Scholar]
- 16.Nicolson GL. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metast Rev 1988; 7:143–188. [DOI] [PubMed] [Google Scholar]
- 17.Nicolson GL. Tumor cell interaction with the vascular endothelium and their role in cancer metastasis. In: Goldberg ID, Rosen EM, eds. Epithelial–Mesenchymal Interactions in Cancer. Basel: Birkhäuser Verlag; 1995: 123–156. [DOI] [PubMed]
- 18.Menger MD, Vollmer B. Adhesion molecules as determinants of disease: from molecular biology to surgical research. Br J Surg 1996; 83:588–601. [DOI] [PubMed] [Google Scholar]
- 19.Behrens J. Cell contacts, differentiation, and invasiveness of epithelial cells. Invas Metast 1994; 14:61–70. [PubMed] [Google Scholar]
- 20.Shiozaki H, Oka H, Inoue M, et al. E-cadherin mediated adhesion systems in cancer cells. Cancer 1996; 77(suppl):1605–1613. [DOI] [PubMed] [Google Scholar]
- 21.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996; 84:345–357. [DOI] [PubMed] [Google Scholar]
- 22.Breen E, Steele G Jr, Mercurio AM. Role of the E-cadherin/alpha-catenin complex in modulating cell–cell and cell–matrix adhesive properties of invasive colon carcinoma cells. Ann Surg Oncol 1995; 2:378–385. [DOI] [PubMed] [Google Scholar]
- 23.Breen E, Clarke A, Steele G Jr, Mercurio AM. Poorly differentiated colon carcinoma cell lines deficient in alpha-catenin expression express high levels of surface E-cadherin but lack Ca(2+)-dependent cell-cell adhesion. Cell Adhes Commun 1993; 1:239–250. [DOI] [PubMed] [Google Scholar]
- 24.Cowley GP, Smith ME. Modulation of E-cadherin expression and morphological phenotype in the intravascular component of adenocarcinomas. Int J Cancer 1995; 60:325–329. [DOI] [PubMed] [Google Scholar]
- 25.Streit M, Schmidt R, Hilgenfeld RU, et al. Adhesion receptors in malignant transformation and dissemination of gastrointestinal tumors. Recent Results Cancer Res 1996; 142:19–50. [DOI] [PubMed] [Google Scholar]
- 26.Mohri Y. Prognostic significance of E-cadherin expression in human colorectal cancer tissue. Surg Today 1997; 27:606–612. [DOI] [PubMed] [Google Scholar]
- 27.Ilyas M, Novelli M, Wilkinson K, et al. Tumour recurrence is associated with Jass grouping but not with differences in E-cadherin expression in moderately differentiated Dukes’ B colorectal cancers. J Clin Pathol 1997; 50:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitadai Y, Ellis LM, Tucker SL, et al. Multiparametric in situ mRNA hybridization analysis to predict disease recurrence in patients with colon carcinoma. Am J Pathol 1996; 149:1541–1551. [PMC free article] [PubMed] [Google Scholar]
- 29.Dorudi S, Hanby AM, Poulsom R, et al. Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer 1995; 71:614–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka H, Kadowaki T, Matsui S, et al. Immunohistochemical detection of alpha-catenin expression in human cancers. Am J Pathol 1994; 144:667–674. [PMC free article] [PubMed] [Google Scholar]
- 31.Takayama T, Shiozaki H, Shibamoto S, et al. Beta-catenin expression in human cancers. Am J Pathol 1996; 148:39–46. [PMC free article] [PubMed] [Google Scholar]
- 32.Skoudy A, Gomez S, Fabre M, Garcia de Herreros A. p120-catenin expression in human colorectal cancer. Int J Cancer 1996; 68:14–20. [DOI] [PubMed] [Google Scholar]
- 33.Shiozaki H, Gofuku J, Inoue M, Tamura S. Correlation between the intercellular adhesion molecule E-cadherin and its associated protein (alpha-catenin) expression and metastasis in human digestive cancers. Nippon Rinsho 1995; 53:1602–1606. [PubMed] [Google Scholar]
- 34.Raftopoulos I, Davaris P, Karatzas G, et al. Level of alpha-catenin expression in colorectal cancer correlates with invasiveness, metastatic potential, and survival. J Surg Oncol 1998; 68:92–99. [DOI] [PubMed] [Google Scholar]
- 35.Senda T, Miyashiro I, Matsumine A, et al. The tumor suppressor protein APC colocalizes with beta-catenin in the colon epithelial cells. Biochem Biophys Res Commun 1996; 223:329–334. [DOI] [PubMed] [Google Scholar]
- 36.Inomata M, Ochiai A, Akimoto S, et al. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res 1996; 56:2213–2217. [PubMed] [Google Scholar]
- 37.Monden M. Beta-catenin expression in human cancers. Am J Pathol 1996; 148:39–46. [PMC free article] [PubMed] [Google Scholar]
- 38.Shiozaki H, Iihara K, Oka H, et al. Immunohistochemical detection of alpha-catenin expression in human cancers. Am J Pathol 1994; 144:667–674. [PMC free article] [PubMed] [Google Scholar]
- 39.Hynes RO. Integrins: a family of cell surface receptors. Cell 1987; 48:549–554. [DOI] [PubMed] [Google Scholar]
- 40.Feldman LP, Shin KE, Natale RB, et al. β1 integrin expression on human small cell lung cancer cells. Cancer Res 1991; 51:1065–1070. [PubMed] [Google Scholar]
- 41.Gehlsen KR, Davis GE, Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis 1992; 10:111–120. [DOI] [PubMed] [Google Scholar]
- 42.Chen YP, O’Toole TE, Shipley T, et al. “Inside-out” signal transduction inhibited by isolated integrin cytoplasmic domains. J Biol Chem 1994; 269:18307–18310. [PubMed] [Google Scholar]
- 43.Miyamoto S, Teramoto H, Coso OA, et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 1995; 131:791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995; 11:549–599. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Ze’ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Op Cell Biol 1997; 9:99–108. [DOI] [PubMed] [Google Scholar]
- 46.Agrez MV, Bates RC. Colorectal cancer and the integrin family of cell adhesion receptors: current status and future directions. Eur J Cancer 1994; 30A:2166–2170. [DOI] [PubMed] [Google Scholar]
- 47.Tressler RJ, Belloni PN, Nicolson GL. Correlation of inhibition of adhesion of large cell lymphoma and hepatic sinusoidal endothelial cells by RGD-containing peptide polymers with metastatic potential: role of integrin-dependent and -independent adhesion mechanisms. Cancer Comm 1989; 1:55–63. [DOI] [PubMed] [Google Scholar]
- 48.Sawada H, Wakabayashi H, Nawa A, et al. Differential motility stimulation but not growth stimulation or adhesion of metastatic human colorectal carcinoma cells by target organ-derived liver sinusoidal endothelial cells. Clin Exp Metastasis 1996; 14:308–314. [DOI] [PubMed] [Google Scholar]
- 49.Lichtner RB, Belloni PN, Nicolson GL. Differential adhesion of metastatic rat mammary carcinoma cells to organ-derived microvessel endothelial cells and subendothelial matrix. Exp Cell Biol 1989; 57:146–152. [DOI] [PubMed] [Google Scholar]
- 50.Haier J, Nasralla M, Nicolson GL. Different adhesion properties of highly and poorly metastatic HT29 colon carcinoma cells with extracellular matrix components: role of integrin expression and cytoskeletal components. Br J Cancer 1999; 80:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haier J, Nasralla M, Buhr HJ, Nicolson GL. Different integrin-mediated adhesion of poorly and highly metastatic colon carcinoma cell lines on extracellular matrix. Langenbecks Arch Surg 1998; 309(suppl):307–313. [PubMed] [Google Scholar]
- 52.Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Op Cell Biol 1995; 7:681–689. [DOI] [PubMed] [Google Scholar]
- 53.Haier J, Nasralla M, Nicolson GL. Influence of phosphotyrosine kinase inhibitors on adhesive properties of highly and poorly metastatic HT29 colon carcinoma cells to collagen. Int J Colorect Dis 1999; 14:119–127. [DOI] [PubMed] [Google Scholar]
- 54.Burtin P, Chavanel G, Foidart JM. Immunofluorescence study of the antigens of the basement membrane and the peritumoral stroma in human colonic adenocarcinomas. Ann NY Acad Sci 1983; 420:229–236. [DOI] [PubMed] [Google Scholar]
- 55.Grigioni WF, Biagini G, Errico AD, et al. Behaviour of basement membrane antigens in gastric and colorectal cancer: immunohistochemical study. Acta Pathol Jpn 1986; 36:173–184. [DOI] [PubMed] [Google Scholar]
- 56.Inufusa H, Nakamura M, Adachi T, et al. Localization of oncofetal and normal fibronectin in colorectal cancer. Cancer 1995; 75:2802–2808. [DOI] [PubMed] [Google Scholar]
- 57.Koukoulis GK, Virtanen I, Moll R, et al. Immunolocalization of integrins in the normal and neoplastic colonic epithelium. Virchows Arch B Cell Pathol Incl Mol Pathol 1993; 63:373–383. [DOI] [PubMed] [Google Scholar]
- 58.Koretz K, Schlag P, Boumsell L, Möller P. Expression of VLA-alpha 2, VLA-alpha 6, and VLA-beta 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol 1991; 138:741–750. [PMC free article] [PubMed] [Google Scholar]
- 59.Bosman FT, de Bruïne A, Flohil C, et al. Epithelial-stromal interactions in colon cancer. Int J Dev Biol 1993; 37:203–211. [PubMed] [Google Scholar]
- 60.Koretz K, Brüderlein S, Henne C, et al. Comparative evaluation of integrin alpha- and beta-chain expression in colorectal carcinoma cell lines and in their tumours of origin. Virchows Arch 1994; 425:229–236. [DOI] [PubMed] [Google Scholar]
- 61.Gong J, Wang D, Sun L, et al. Role of alpha5beta1 integrin in determining malignant properties of colon carcinoma cells. Cell Growth Differ 1997; 8:83–90. [PubMed] [Google Scholar]
- 62.Takazawa H. Association between expression of integrin (VLA-3, VLA-5) and malignancy in human colon-cancer. Nippon Rinsho 1995; 53:1672–1677. [PubMed] [Google Scholar]
- 63.Hemler ME, Crouse C, Sonnenberg A. Association of the VLA alpha 6 subunit with a novel protein. A possible alternative to the common VLA beta 1 subunit on certain cell lines. J Biol Chem 1989; 264:6529–6535. [PubMed] [Google Scholar]
- 64.Williams GT. Programmed cell death: apoptosis and oncogenesis. Cell 1991; 65:1097–1098. [DOI] [PubMed] [Google Scholar]
- 65.Max R, Gerritsen RR, Nooijen PT, et al. Immunohistochemical analysis of integrin αvβ3 expression on tumor-associated vessels of human carcinomas. Int J Cancer 1997; 71:320–324. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Barcini M, Bidaurrazaga I, Neaud V. Variations in the expression of cell-adhesion molecules on liver-associated lymphocytes and peripheral-blood lymphocytes in patients with and without liver metastasis. Int J Cancer 1995; 61:475–479. [DOI] [PubMed] [Google Scholar]
- 67.Stallmach A, von Lampe B, Matthes H, et al. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malignant transformation. Gut 1992; 33:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindmark G, Gerdin B, Pahlman L, et al. Interconnection of integrins alpha2 and alpha3 and structure of the basal membrane in colorectal cancer: relation to survival. Eur J Surg Oncol 1993; 19:50–60. [PubMed] [Google Scholar]
- 69.Daneker GW, Piazza AJ, Stelle GD, Mercurio AM. Relationship between extracellular matrix interactions and degree of differentiation in human colon carcinoma cell lines. Cancer Res 1989; 49:681–686. [PubMed] [Google Scholar]
- 70.Bloom S, Simmons D, Jewell DP. Adhesion molecules intercellular adhesion molecule-1 (ICAM-1), ICAM-3 and B7 are not expressed by epithelium in normal or inflamed colon. Clin Exp Immunol 1995; 101:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seibold Schmid B, Cong Y, Shu FY, et al. Regional differences in L-selectin expression in murine intestinal lymphocytes. Gastroenterology 1998; 114:965–974. [DOI] [PubMed] [Google Scholar]
- 72.Kannagi R, Ito K, Watanabe T, Kondo K, et al. Expression of E-selectin on endothelial cells of small veins in human colorectal cancer. Int J Cancer 1995; 61:455–460. [DOI] [PubMed] [Google Scholar]
- 73.Mitsuoka C, Kannagi R. Clinical significance of circulating soluble E-selectin (ELAM-1) in patients with cancers. Nippon Rinsho 1995; 53:1770–1775. [PubMed] [Google Scholar]
- 74.Maurer CA, Friess H, Kretschmann B, et al. Over-expression of ICAM-1, VCAM-1 and ELAM-1 might influence tumor progression in colorectal cancer. Int J Cancer 1998; 79:76–81. [DOI] [PubMed] [Google Scholar]
- 75.Kudo T, Ikehara Y, Togayachi A, et al. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab Invest 1998; 78:797–811. [PubMed] [Google Scholar]
- 76.Kolar Z, Altavilla G. Lectin histochemistry of colonic adenomas. Appl Pathol 1989; 7:42–53. [PubMed] [Google Scholar]
- 77.Lee YS. Lectin reactivity in human large bowel. Pathology 1987; 19:397–401. [DOI] [PubMed] [Google Scholar]
- 78.Asai S, Watanabe T, Sakamoto J, et al. Expression and prognostic indicators of type 1 and type 2 Lewis blood group antigens in colorectal cancers. Nippon Geka Gakkai Zasshi 1994; 95:753–762. [PubMed] [Google Scholar]
- 79.Boland CR. Mucin histochemistry in colonic polyps and cancer. Semin Surg Oncol 1987; 3:183–189. [DOI] [PubMed] [Google Scholar]
- 80.Irimura T, Nakamori S, Matsushita Y, et al. Colorectal cancer metastasis determined by carbohydrate-mediated cell adhesion: role of sialyl-Lex antigens. Semin Cancer Biol 1993; 4:319–324. [PubMed] [Google Scholar]
- 81.Nakamori S, Kameyama M, Imaoka S, et al. Involvement of carbohydrate antigen sialyl Lewis(x) in colorectal cancer metastasis. Dis Colon Rectum 1997; 40:420–431. [DOI] [PubMed] [Google Scholar]
- 82.Bresalier RS, Ho SB, Schoeppner HL, et al. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology 1996; 110:1354–1367. [DOI] [PubMed] [Google Scholar]
- 83.Izumi Y, Kawamura YJ, Irimura T. Carbohydrate antigens in carcinoma invasion and metastasis. Nippon Geka Gakkai Zasshi 1996; 97:140–144. [PubMed] [Google Scholar]
- 84.Itzkowitz SH, Yuan M, Fukushi Y, et al. Immunohistochemical comparison of Lea, monosialosyl Lea CA 19-9, and disialosyl Lea antigens in human colorectal and pancreatic tissues. Cancer Res 1988; 48:3834–3842. [PubMed] [Google Scholar]
- 85.Fischbach W, Mössner J. Tissue concentrations of CEA and CA 19-9 in the carcinogenesis of colorectal carcinoma exemplified by the adenoma-carcinoma sequence. Res Exp Med (Berl) 1988; 188:101–114. [DOI] [PubMed] [Google Scholar]
- 86.Quentmeier A, Möller P, Schwarz V, et al. Carcinoembryonic antigen, CA 19-9, and CA 125 in normal and carcinomatous human colorectal tissue. Cancer 1987; 60:2261–2266. [DOI] [PubMed] [Google Scholar]
- 87.Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer 1995; 75:2818–2826. [DOI] [PubMed] [Google Scholar]
- 88.Lotan R, Matsushita Y, Ohannesian D, et al. Lactose-binding lectin expression in human colorectal carcinomas. Relation to tumor progression. Carbohydr Res 1991; 213:47–57. [DOI] [PubMed] [Google Scholar]
- 89.Irimura T, Matsushita Y, Sutton RC, et al. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages. Cancer Res 1991; 51:387–93. [PubMed] [Google Scholar]
- 90.Benton LD, Khan M, Greco RS. Integrins, adhesion molecules and surgical research. Surg Gynecol Obstet 1993; 177:311–327. [PubMed] [Google Scholar]
- 91.Majuri ML, Hakkarainen M, Paavonen T, Renkonen R. Carcinoembryonic antigen is expressed on endothelial cells. A putative mediator of tumor cell extravasation and metastasis. APMIS 1994; 102:432–438. [PubMed] [Google Scholar]
- 92.Battistelli S, Marcheggiani F, Stella F, et al. Histochemical demonstration of CEA in pathology of the colorectum. Boll Soc Ital Biol Sper 1990; 66:985–992. [PubMed] [Google Scholar]
- 93.Gebauer G, Müller Ruchholtz W. Tumor marker concentrations in normal and malignant tissues of colorectal cancer patients and their prognostic relevance. Anticancer Res 1997; 17:2939–2942. [PubMed] [Google Scholar]
- 94.Cosimelli M, De Peppo F, Castelli M, et al. Multivariate analysis of a tissue CEA, TPA, and CA 19-9 quantitative study in colorectal cancer patients. A preliminary finding. Dis Colon Rectum 1989; 32:389–397. [DOI] [PubMed] [Google Scholar]
- 95.Cunningham L, Stocking B, Halter SA, Kalemeris G. Immunoperoxidase staining of carcinoembryonic antigen as a prognostic indicator in colorectal carcinoma. Dis Colon Rectum 1986; 29:111–116. [DOI] [PubMed] [Google Scholar]
- 96.Brown RW, Campagna LB, Dunn JK, Cagle PT. Immunohistochemical identification of tumor markers in metastatic adenocarcinoma. A diagnostic adjunct in the determination of primary site. Am J Clin Pathol 1997; 107:12–19. [DOI] [PubMed] [Google Scholar]
- 97.Bossi P, Viale G, Lee AK, et al. Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res 1995; 55:5049–5053. [PubMed] [Google Scholar]
- 98.Lindmark G, Gerdin B, Sundberg C, et al. Prognostic significance of the microvascular count in colorectal cancer. J Clin Oncol 1996; 14:461–466. [DOI] [PubMed] [Google Scholar]
- 99.Vermeulen PB, Roland L, Mertens V, et al. Correlation of intratumoral microvessel density and p53 protein overexpression in human colorectal adenocarcinoma. Microvasc Res 1996; 51:164–174. [DOI] [PubMed] [Google Scholar]
- 100.Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells 1991; 3:347–350. [PubMed] [Google Scholar]
- 101.Herrlich P, Pals S, Ponta H. CD44 in colon cancer. Eur J Cancer 1995; 31A:1110–1112. [DOI] [PubMed] [Google Scholar]
- 102.Streit M, Schmidt R, Hilgenfeld RU, et al. Adhesion receptors in malignant transformation and dissemination of gastrointestinal tumors. J Mol Med 1996; 74:253–268. [DOI] [PubMed] [Google Scholar]
- 103.Coppola D, Hyacinthe M, Fu L, et al. CD44v6 expression in human colorectal carcinoma. Hum Pathol 1998; 29:627–635. [DOI] [PubMed] [Google Scholar]
- 104.Nihei Z, Ichikawa W, Kojima K, et al. The positive relationship between the expression of CD44 variant 6 and prognosis in colorectal cancer. Surg Today 1996; 26:760–761. [DOI] [PubMed] [Google Scholar]
- 105.Finke LH, Zörb C, Haensch W, et al. Expression of CD44 isoforms—a new histopathologic parameter in colorectal carcinoma? Zentralbl Chir 1996; 121:450–454. [PubMed] [Google Scholar]
- 106.Günthert U, Stauder R, Mayer B, et al. Are CD44 variant isoforms involved in human tumour progression? Cancer Surv 1995; 24:19–42. [PubMed] [Google Scholar]
- 107.Finn L, Dougherty G, Finley G, et al. Alternative splicing of CD44 pre-mRNA in human colorectal tumors. Biochem Biophys Res Commun 1994; 200:1015–1022. [DOI] [PubMed] [Google Scholar]
- 108.Yamaguchi A, Urano T, Goi T, et al. Expression of a CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer patients. J Clin Oncol 1996; 14:1122–1127. [DOI] [PubMed] [Google Scholar]
- 109.Ropponen K, Tammi M, Parkkinen J, et al. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998; 58:342–347. [PubMed] [Google Scholar]
- 110.Hayashi Y, Widjono YW, Ohta K, et al. Expression of EGF, EGF-receptor, p53, v-erb B and ras p21 in colorectal neoplasms by immunostaining paraffin-embedded tissues. Pathol Int 1994; 44:124–130. [DOI] [PubMed] [Google Scholar]
- 111.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol 1998; 37:285–289. [DOI] [PubMed] [Google Scholar]
- 112.Rothbauer E, Mann K, Wiebecke B, et al. Epidermal growth factor receptors and epidermal growth factor-like activity in colorectal mucosa, adenomas and carcinomas. Klin Wochenschr 1989; 67:518–523. [DOI] [PubMed] [Google Scholar]
- 113.Langlois N, Walker LG, Smith I, et al. Expression of growth factor receptors is not a prognostic indicator in young patients with colorectal cancer. J R Coll Surg Edinb 1997; 42:98–101. [PubMed] [Google Scholar]
- 114.Saeki T, Salomon DS, Johnson GR, et al. Association of epidermal growth factor-related peptides and type I receptor tyrosine kinase receptors with prognosis of human colorectal carcinomas. Jpn J Clin Oncol 1995; 25:240–249. [PubMed] [Google Scholar]
- 115.Koretz K, Schlag P, Möller P. Expression of epidermal growth factor receptor in normal colorectal mucosa, adenoma, and carcinoma. Virchows Arch A Pathol Anat Histopathol 1990; 416:343–349. [DOI] [PubMed] [Google Scholar]
- 116.Komuta K, Koji T, Izumi S, et al. Expression of epidermal growth factor receptor messenger RNA in human colorectal carcinomas assessed by non-radioactive in-situ hybridization. Eur J Surg Oncol 1995; 21:269–275. [DOI] [PubMed] [Google Scholar]
- 117.Markowitz SD, Molkentin K, Gerbic C, et al. Growth stimulation by coexpression of transforming growth factor-alpha and epidermal growth factor-receptor in normal and adenomatous human colon epithelium. J Clin Invest 1990; 86:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eskinazi R, Resibois A, Svoboda M, et al. Expression of transforming growth factor beta receptors in normal human colon and sporadic adenocarcinomas. Gastroenterology 1998; 114:1211–1120. [DOI] [PubMed] [Google Scholar]
- 119.Kapitanovic S, Radosevic S, Kapitanovic M, et al. The expression of p185 (HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology 1997; 112:1103–1113. [DOI] [PubMed] [Google Scholar]
- 120.Ciardiello F, Kim N, Saeki T, et al. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci USA 1991; 88:7792–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berney CR, Yang JL, Fisher RJ, et al. Vascular endothelial growth factor expression is reduced in liver metastasis from colorectal cancer and correlates with urokinase-type plasminogen activator. Anticancer Res 1998; 18:973–977. [PubMed] [Google Scholar]
- 122.Takahashi Y, Kitadai Y, Bucana CD, et al. Expression of vascular endothelial factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55:3964–3968. [PubMed] [Google Scholar]
- 123.Takahashi Y, Tucker SL, Kitadai Y, et al. Vessel counts and VEGF expression as prognostic factors in node-negative colon cancer. Arch Surg 1997; 132:541–546. [DOI] [PubMed] [Google Scholar]
- 124.Tokunaga T, Oshika Y, Abe Y, et al. Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer 1998; 77:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bhatavdekar JM, Patel DD, Shah NG, et al. Prognostic value of insulin-like growth factor-1 receptors in patients with colon/rectal cancer: correlation with plasma prolactin. Eur J Surg Oncol 1995; 21:23–26. [DOI] [PubMed] [Google Scholar]
- 126.Radinsky R, Risin S, Fan D, et al. Level and function of EGF-R predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res 1995; 1:19–31. [PubMed] [Google Scholar]
- 127.Takahashi Y, Bucana CD, Liu W, et al. Platelet-derived endothelial cell growth factor in human colon cancer angiogenesis: role of infiltrating cells. J Natl Cancer Inst 1996; 88:1146–1151. [DOI] [PubMed] [Google Scholar]
- 128.Conese M, Blasi F. The urokinase/urokinase-receptor system and cancer invasion. Baillieres Clin Haematol 1995; 8:365–389. [DOI] [PubMed] [Google Scholar]
- 129.Schmitt M, Harbeck N, Thomssen C, et al. Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost 1997; 78:285–296. [PubMed] [Google Scholar]
- 130.Takeha S, Fujiyama Y, Bamba T, et al. Stromal expression of MMP-9 and urokinase receptor is inversely associated with liver metastasis and with infiltrating growth in human colorectal cancer: a novel approach from immune/inflammatory aspect. Jpn J Cancer Res 1997; 88:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Delbaldo C, Cunningham M, Vassalli JD, Sappino AP. Plasmin-catalyzed proteolysis in colorectal neoplasia. Cancer Res 1995; 55:4688–4695. [PubMed] [Google Scholar]
- 132.Ganesh S, Sier CFM, Griffioen G, et al. Prognostic relevance of plasminogen activators and their inhibitors in colorectal cancer. Cancer Res 1994; 54:4065–4071. [PubMed] [Google Scholar]
- 133.Gandolfo GM, Conti L, Vercillo M. Fibrinolysis components as prognostic markers in breast cancer and colorectal carcinoma. Anticancer Res 1996; 16:2155–2159. [PubMed] [Google Scholar]
- 134.Mayer A, Fritz E, Fortelny R, et al. Immunohistochemical evaluation of cathepsin D expression in colorectal cancer. Cancer Invest 1997; 15:106–110. [DOI] [PubMed] [Google Scholar]
- 135.Theodoropoulos GE, Panoussopoulos D, Lazaris AC, Golematis BC. Evaluation of cathepsin D immunostaining in colorectal adenocarcinoma. J Surg Oncol 1997; 65:242–248. [DOI] [PubMed] [Google Scholar]
- 136.Valentini AM, Pirrelli M, Armentano R, Caruso ML. The immunohistochemical expression of cathepsin D in colorectal cancer. Anticancer Res 1996; 16:77–80. [PubMed] [Google Scholar]
- 137.Chauhan SS, Goldstein LJ, Gottesman MM. Expression of cathepsin L in human tumors. Cancer Res 1991; 51:1478–1481. [PubMed] [Google Scholar]
- 138.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res 1991; 51:5054s–5059s. [PubMed] [Google Scholar]
- 139.Liabakk NB, Talbot I, Smith RA, et al. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenase in colorectal cancer. Cancer Res 1996; 56:190–196. [PubMed] [Google Scholar]
- 140.Karakiulakis G, Papanikolaou C, Jankovic SM, et al. Increased type IV collagen-degrading activity in metastases originating from primary tumors of the human colon. Invas Metast 1997; 17:158–68. [PubMed] [Google Scholar]
- 141.Abdalla SA, Jeziorska M, Schofield P, et al. Gelatinase B (MMP-9) expression and survival in colorectal cancer patients. Int J Colorect Dis 1997; 12:342–343. [DOI] [PubMed] [Google Scholar]
- 142.Nielsen BS, Timshel S, Kjeldsen L, et al. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer 1996; 65:57–62. [DOI] [PubMed] [Google Scholar]
- 143.Emmert Buck MR, Roth MJ, Zhuang Z, et al. Increased gelatinase A (MMP-2) and cathepsin B activity in invasive tumor regions of human colon cancer samples. Am J Pathol 1994; 145:1285–1290. [PMC free article] [PubMed] [Google Scholar]
- 144.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med 1996; 2:461–462. [DOI] [PubMed] [Google Scholar]
- 145.Kossakowska AE, Huchcroft SA, Urbanski SJ, Edwards DR. Comparative analysis of the expression patterns of metalloproteinases and their inhibitors in breast neoplasia, sporadic colorectal neoplasia, pulmonary carcinomas and malignant non-Hodgkin’s lymphomas in humans. Br J Cancer 1996; 73:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McDonnell S, Navre M, Coffey RJ Jr, Matrisian LM. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog 1991; 4:527–533. [DOI] [PubMed] [Google Scholar]
- 147.Porte H, Chastre E, Prevot S, et al. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer 1995; 64:70–75. [DOI] [PubMed] [Google Scholar]
- 148.Yoshimoto M, Itoh F, Yamamoto H, et al. Expression of MMP-7 (PUMP-1) mRNA in human colorectal cancers. Int J Cancer 1993; 54:614–618. [DOI] [PubMed] [Google Scholar]
- 149.Itoh F, Yamamoto H, Hinoda Y, Imai K. Enhanced secretion and activation of matrilysin during malignant conversion of human colorectal epithelium and its relationship with invasive potential of colon cancer cells. Cancer 1996; 77:1717–1721. [DOI] [PubMed] [Google Scholar]
- 150.Ichikawa Y, Ishikawa T, Momiyama N, et al. Detection of regional lymph node metastases in colon cancer by using RT-PCR for matrix metalloproteinase 7, matrilysin. Clin Exp Metastasis 1998; 16:3–8. [DOI] [PubMed] [Google Scholar]
- 151.Tomita T, Iwata K. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in colonic adenomas-adenocarcinomas. Dis Colon Rectum 1996; 39:1255–1264. [DOI] [PubMed] [Google Scholar]
- 152.Zeng ZS, Guillem JG. Distinct pattern of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 mRNA expression in human colorectal cancer and liver metastases. Br J Cancer 1995; 72:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Murnane MJ, Shuja S, Del Re E, et al. Characterizing human colorectal carcinomas by proteolytic profile. In Vivo 1997; 11:209–216. [PubMed] [Google Scholar]
- 154.Möller P, Momburg F, Koretz K, et al. Influence of major histocompatibility complex class I and II antigens on survival in colorectal carcinoma. Cancer Res 1991; 51:729–736. [PubMed] [Google Scholar]
- 155.Mori M, Mimori K, Shiraishi T, et al. Motility-related protein 1 (MRP1/CD9) expression in colon cancer. Clin Cancer Res 1998; 4:1507–1510. [PubMed] [Google Scholar]
- 156.Stebbings WS, Farthing MJ, Vinson GP, et al. Androgen receptors in rectal and colonic cancer. Dis Colon Rectum 1986; 29:95–98. [DOI] [PubMed] [Google Scholar]
- 157.Bhatavdekar J, Patel D, Ghosh N, et al. Interrelationship of prolactin and its receptor in carcinoma of colon and rectum: a preliminary report. J Surg Oncol 1994; 55:246—249. [DOI] [PubMed] [Google Scholar]
- 158.d’Istria M, Fasano S, Catuogno F, et al. Androgen and progesterone receptors in colonic and rectal cancers. Dis Colon Rectum 1986; 29:263–265. [DOI] [PubMed] [Google Scholar]
- 159.Norazmi M, Hohmann AW, Skinner JM, Bradley J. Expression of MHC class I and class II antigens in colonic carcinomas. Pathology 1989; 21:248–253. [DOI] [PubMed] [Google Scholar]
- 160.Möller P, Koretz K, Leithäuser F, et al. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer 1994; 57:371–377. [DOI] [PubMed] [Google Scholar]
- 161.Shirota K, LeDuy L, Yuan SY, Jothy S. Interleukin-6 and its receptor are expressed in human intestinal epithelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol 1990; 58:303–308. [DOI] [PubMed] [Google Scholar]
- 162.Berghella AM, Pellegrini P, Piancatelli D, et al. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunol Immunother 1994; 38:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berghella AM, Pellegrini P, Del Beato T, et al. The significance of an increase in soluble interleukin-2 receptor level in colorectal cancer and its biological regulating role in the physiological switching of the immune response cytokine network from TA1 to TH2 and back. Cancer Immunol Immunother 1998; 45:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pignatelli M, Stamp G. Integrins in tumour development and spread. Cancer Surv 1995; 24:113–127. [PubMed] [Google Scholar]