Abstract
Objective
To determine whether intestinal fibroblasts in patients with Crohn’s disease (CD) have an enhanced capacity to reorganize collagen and thus cause stricture formation.
Summary Background Data
Stricture formation is a characteristic feature of CD that may distinguish it from other forms of inflammatory bowel disease.
Methods
Fibroblasts were obtained at surgery from the colon and ileum of patients with CD and ulcerative colitis (UC) and control patients. Primary fibroblast cultures were obtained by explant technique. Fibroblast contractile activity was measured using fibroblast-populated collagen lattices (FPCLs), in which the cultured fibroblasts were seeded in free-floating collagen gel matrices that they reorganize and contract. Fibroblast contractile activity was measured as the reduction of surface area (mm2) of collagen gel matrix at 24-hour intervals for 1 week.
Results
Fibroblasts from patients with CD displayed enhanced capacity to contract FPCL when compared to UC and control fibroblasts. This activity was maximal in fibroblasts recovered from strictured regions in CD. Fibroblasts from patients with UC had a contractile capacity similar to that of controls. Hydrocortisone inhibited this in vitro contractile activity in a dose-dependent manner.
Conclusions
Intestinal fibroblasts in CD possess enhanced capacity for collagen reorganization and contractile activity in vitro. This activity may be responsible for stricture formation in CD.
Stricture formation is a distinctive feature of Crohn’s disease (CD) and a frequent indication for surgical intervention. 1 The etiology of stricture formation in CD is unclear but both excess collagen deposition and smooth muscle cell accumulation may contribute. 2,3 During cutaneous wound healing, fibroblast reorganization of scar collagen leads to wound volume reduction and wound contraction. Excessive contractile activity can lead to scar contracture and consequent tissue distortion. 4 It was our hypothesis that a similar mechanism may operate in CD in response to local inflammatory mediators.
We have investigated fibroblast activity in CD by using the fibroblast-populated collagen lattice (FPCL) tissue culture technique, 4 in which explanted intestinal fibroblasts, cultured within a collagen gel lattice, bind to and reorganize collagen fibrils into a more dense and compact arrangement, resulting in lattice contraction. 4 This technique has been used extensively to study the interaction of fibroblasts and collagen in wound healing, wound fibrosis, scar contraction, and connective tissue morphogenesis.
The aim of this study was to determine whether intestinal fibroblasts obtained from patients with CD possess an enhanced ability to reorganize and compact collagen compared to fibroblasts obtained from control patients and patients with ulcerative colitis (UC).
MATERIALS AND METHODS
Primary fibroblast cultures were prepared from the serosal surface of the colon and ileum of patients undergoing surgery for CD and UC. Control fibroblast cultures were prepared from the serosa of patients undergoing intestinal resection for noninflammatory bowel conditions. Using aseptic technique, 1-cm2 serosal biopsies were obtained intraoperatively from the intestine undergoing resection. Specimens were placed in RPMI collection media (RPMI, GIBCO, Grand Island, NJ) containing 10% fetal bovine serum (FBS), supplemented with 200 U/mL penicillin, 200 U/mL streptomycin, 50 g/mL amphotericin, and 2 mmol L-glutamine.
Primary fibroblast cultures were prepared by standard explant technique 5 and grown to confluence in RPMI media containing 10% FBS, supplemented with 100 U/mL penicillin, 100 U/mL streptomycin, 25 μg/mL amphotericin, and 2 mmol L-glutamine. Cultures were maintained in 75 cm2 culture flasks (Falcon, Becton Dickinson, Franklin Lakes, NJ) at 37°C in a humidified atmosphere of 5% C02 (Forma Scientific Infrared CO2 Incubator, Margaretta, OH). Cultured fibroblasts were passaged weekly by trypsinization and were used in experiments between the first and third passages.
Using this explant technique, cell lines were prepared for each of the groups: normal colon (n = 25), normal ileum (n = 25), and macroscopically normal (n = 18) and macroscopically inflamed colon (n = 26) and ileum (n = 26) in patients with CD. Cell lines were also prepared from strictured regions in the colon (n = 12) and ileum (n = 12) in patients with CD and from colon (n = 30) and ileum (n = 12) in patients with UC.
Fibroblast-Populated Collagen Lattice
Fibroblast-populated collagen lattices (FPCLs) were prepared in 35-mm sterile bacteriology-grade Petri dishes (Falcon 1008, Becton Dickinson). Washed trypsinized fibroblasts, rat tail collagen type I (Collaborative Research, Bedford, MA), and RPMI containing 10% FBS were mixed to give final concentrations of 6.25×104/mL fibroblasts and 1.25 mg/mL collagen, in a total volume of 2 mL. The mixture was allowed to set and the FPLC was then freed from the edges and bottom of the petri dish by gentle agitation and incubated at 37°C in a humidified atmosphere of 5% C02. 4
The diameter of each FPCL was measured using a grid, by a single blinded observer, at 24-hour intervals for 7 days, and was calculated as the mean of the major and minor axes; data were expressed as the surface area (mm2) of the FPLC. Matrices were fed by adding 1.0 mL of RPMI containing 10% FBS at 48 hours.
Additional FPCLs were prepared by incorporating hydrocortisone added to the medium at the time of gel formation to give a final concentration of either 0.5×10−6 mmol/L or 1.5×10−6 mmol/L per gel.
Statistical Analysis
A one-way analysis of variance (ANOVA) was applied for comparison between groups. Analysis was performed using the StatView II statistical package (Abacus Concepts, Berkeley, CA) on a Macintosh Proforma 6200 computer. All data are reported as mean±SEM. Statistical significance was achieved at P < .05.
RESULTS
Fibroblast cultures established from the colon of patients with CD displayed enhanced capacity to contract FPCL when compared to fibroblasts cultured from control patients. The reduction in gel volume was greater and the final gel surface area less at all time points, indicating an increased rate and degree of gel contraction by colonic fibroblasts in CD (Fig. 1). Fibroblast cultures established from macroscopically normal and macroscopically inflamed regions of Crohn’s colon showed no significant difference in contractile activity between the two subgroups, both of which differed from controls. Fibroblast cultures established from strictured regions of the colon in patients with CD displayed greater contractile activity than fibroblast cultures established from nonstrictured regions (Fig. 2).
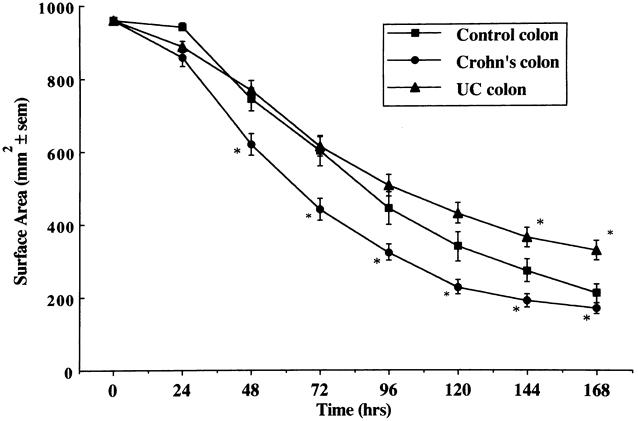
Figure 1. Comparison of the rate of contraction of fibroblast-populated collagen lattices prepared with fibroblasts cultured from control (n = 25), Crohn’s (n = 44), and ulcerative colitis colon (n = 30). Mean±SEM. *P < .05 vs. control.
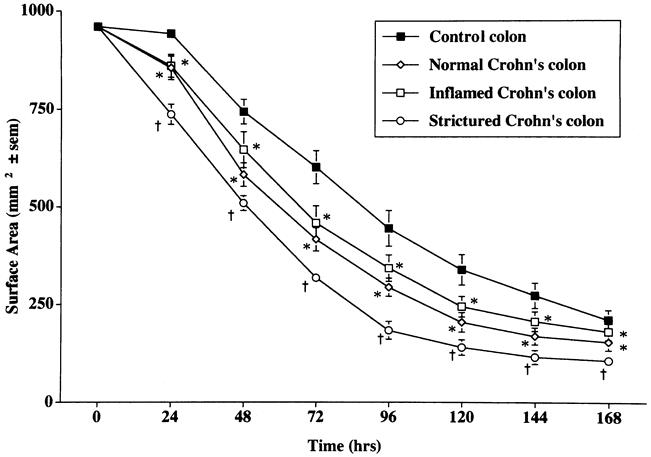
Figure 2. Comparison of the rate of contraction of fibroblast-populated collagen lattices prepared with fibroblasts cultured from control colon (n = 25), macroscopically normal Crohn’s colon (n = 18), macroscopically inflamed Crohn’s colon (n = 26), and strictured regions in Crohn’s colon (n = 12). Mean±SEM. *P < .05 vs. control colon; †P < .05 vs. control, macroscopically normal, and macroscopically inflamed colon.
A similar pattern of enhanced capacity to contract FPCLs was seen with fibroblast cultures established from the ileum of patients with CD when compared with control patients (Fig. 3). Fibroblast cultures established from macroscopically normal and macroscopically inflamed ileum in CD showed no significant difference in contractile activity between the two subgroups; however, both were different from controls (Fig. 4). Fibroblast cultures established from macroscopically strictured regions of the ileum in patients with CD displayed greater contractile activity than fibroblast cultures established from nonstrictured regions (Fig. 4).
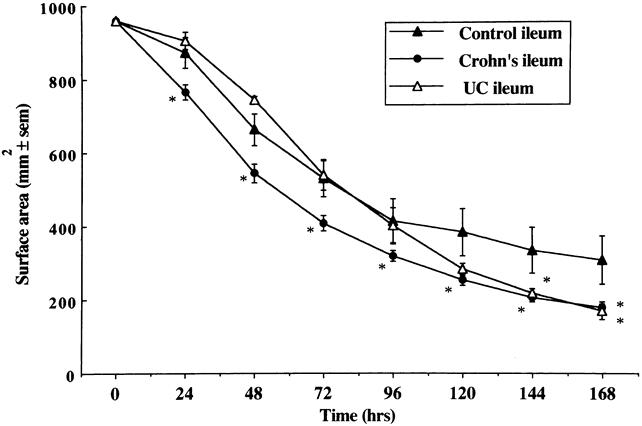
Figure 3. Comparison of the rate of contraction of fibroblast-populated collagen lattices prepared with fibroblasts cultured from control (n = 25), Crohn’s (n = 44), and ulcerative colitis ileum (n = 12). Mean±SEM. *P < .05 vs. control ileum.
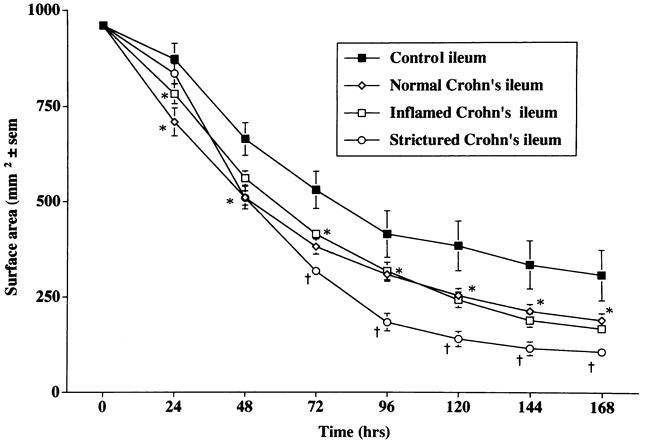
Figure 4. Comparison of the rate of contraction of fibroblast-populated collagen lattices prepared with fibroblasts cultured from control ileum (n = 25), macroscopically normal Crohn’s ileum (n = 18), macroscopically inflamed Crohn’s ileum (n = 26), and strictured regions in Crohn’s ileum (n = 12). Mean ± SEM. *P < .05 vs. control ileum; †P < .05 vs. control, macroscopically normal, and macroscopically inflamed ileum.
Fibroblast cultures established from both colon (Fig. 1) and ileum (Fig. 3) of patients with UC displayed a pattern of FPCL contraction that was not significantly different from fibroblast cultures established from normal colon and ileum.
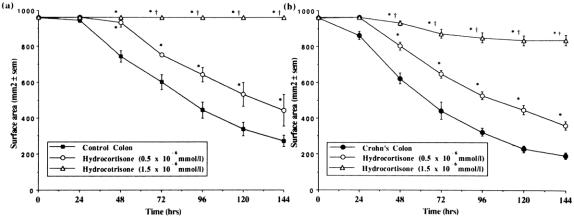
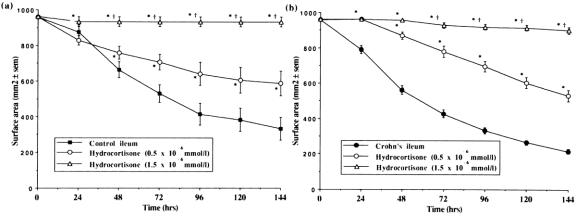
The addition of hydrocortisone to FPCLs prepared with control colonic fibroblasts led to a reduction in the rate and extent of gel contraction. This effect was dose-dependent with 1.5 × 10−6 mmol/L hydrocortisone causing total inhibition of fibroblast-mediated contraction in these gels (Fig. 5A). An 84% inhibition of gel contraction was observed after the addition of hydrocortisone to gels prepared with Crohn’s colonic fibroblasts (Fig. 5B). A similar pattern was seen in FPCLs prepared with ileal fibroblasts, with 1.5 × 10−6 mmol/L hydrocortisone causing almost total inhibition of gel contraction by both control (Fig. 6A) and Crohn’s ileal fibroblasts (Fig. 6B).
Figure 5. Comparison of the rate of contraction of fibroblast-populated collagen lattices prepared with fibroblasts cultured from (A) control colon (n = 25), and (B) Crohn’s colon (n = 44) after addition of hydrocortisone at either 0.5×10−6 mmol/L (n = 8, n = 24) or 1.5×10−6 mmol/L (n = 8, n = 20), respectively. Mean±SEM. *P < .05 vs. no hydrocortisone; †P < .05 vs. hydrocortisone 0.5×10−6 mmol/L.
Figure 6. Comparison of the rate of contraction of fibroblast-populated collagen lattices prepared with fibroblasts cultured from (A) control ileum (n = 16), and (B) Crohn’s ileum (n = 44) after addition of hydrocortisone at either 0.5×10−6 mmol/L (n = 8, n = 19) or 1.5×10−6 mmol/L (n = 8, n = 19), respectively. Mean±SEM. *P < .05 vs. no hydrocortisone; †P < .05 vs. hydrocortisone 0.5×10−6 mmol/L.
DISCUSSION
This study has shown that intestinal fibroblasts recovered from both the colon and ileum of patients with CD display enhanced capacity for collagen reorganization and matrix contraction when compared to intestinal fibroblasts from both control and UC patients. This enhanced contractile activity was found in fibroblasts recovered from both macroscopically normal and macroscopically inflamed regions and was inhibited by hydrocortisone in a dose-dependent manner.
Fibroblast-mediated contraction of FPCLs occurs as a result of fibroblast locomotion within the collagen gel matrix. 4,6 Fibroblasts migrate within the gel and, in the process, compact the originally randomly distributed collagen fibers into organized bundles with consequent reduction in volume. Similar activity takes place within the healing wound, leading to scar contraction. 4 Fibroblasts interact with the extracellular matrix through cell surface receptors belonging to the integrin family. Mg++-enhanced fibroblast adherence to collagen type I is mediated by the α2β1 integrin, 7 while fibroblast adherence to fibronectin is mediated by the α4β1 integrin. 8
Clinical and experimental investigations have shown that during normal wound healing, fibroblasts the acquire morphologic and biochemical features of smooth muscle cells, including expression of alpha smooth muscle actin with the appearance fibrillar cytosolic elements. 9 Collagen gel contraction is mediated by these myofibroblasts that express alpha smooth muscle actin, which in turn serves as a functional marker for a fibroblast subtype that rapidly remodels the extracellular matrix. 10 Persistence of myofibroblasts is characteristic of hypertrophic scar formation, in which they are involved in continuing extracellular matrix accumulation and organization. 11 In contrast, during normal wound healing, myofibroblasts disappear as scar maturity occurs, presumably by a process of apoptosis.
We have shown that intestinal fibroblast-mediated collagen contraction is inhibited by hydrocortisone in a dose-dependent manner. Although the degree of inhibition with hydrocortisone was similar in both CD and controls, the effect has potentially greater significance in CD, because stricturing will only take place in the presence of excessive new collagen deposition, as occurs in an area of Crohn’s inflammation. The mechanism by which steroids inhibit collagen reorganization remains to be elucidated, but interference with the binding of fibroblasts with collagen has been postulated. 12
Type I collagen is the major collagen subtype present in normal intestine, accounting for approximately 70% of total collagen; type III and type V collagen account for approximately 20% and 12%, respectively. 2 In areas of stricture formation in CD, both the collagen content and the relative amounts of type III 3 and type V 2 collagen are increased. Fibroblasts isolated from such strictures have been found to produce more collagen than fibroblasts from normal intestine, particularly collagen of the type III phenotype. 3 In situ hybridization studies have shown increased type III procollagen messenger RNA steady-state levels in intestinal subepithelial lamina propria fibroblasts present in strictured areas in CD, when compared to fibroblasts in UC or normal intestine. 13 These differences allow useful comparison with the changes in collagen deposition that occur during cutaneous wound healing. 14 Healing granulation tissue contains a high concentration of type III collagen, 15 and deposition of Type V collagen is also increased. 16 Hypertrophic scars have proportionately more type III and V collagen and less type I collagen. 4,14 Fibroblast-populated collagen lattices made using type III collagen contract faster and more completely than FPCLs made with type I collagen. 4 Stricture formation thus may occur as a consequence of enhanced fibroblast contractile activity occurring in an extracellular matrix, the composition of which favors increased compaction and stricturing.
The increased contractile activity in fibroblasts, which we have shown in all the sampled regions in CD, suggests an inherently increased contractile capacity of intestinal fibroblasts in these patients. Sustained in vitro alterations in fibroblast contractile activity have been previously documented. 17,18 It remains to be determined whether these changes are due to prolonged inflammatory stimulation, selection of fibroblast subpopulations, or an intrinsic property of fibroblasts in CD. It is likely that inflammatory cytokines, in particular TGF-β and γ-IFN, are responsible for the observed alterations in fibroblast phenotypic response. 19,20 Whether this effect in CD is due to universal modulation of all intestinal fibroblasts or to proliferation of a fibroblast clonal subtype that possesses enhanced contractile activity remains to be determined.
Footnotes
Correspondence: P. Ronan O’Connell, MD, FRCSI, Surgical Professorial Unit, Mater Misericordiae Hospital, 47 Eccles St., Dublin 7, Ireland. E-mail: rocon@indigo.ie
This work was supported by a grant from the Mater College for Postgraduate Education and Research.
Accepted for publication July 26, 1999.
References
- 1.Kirsner J, Shorter R. Inflammatory bowel disease. Philadelphia: Lea & Febiger; 1980: 510.
- 2.Graham M, Diegelmann R, Elson C, et al. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterol 1988; 94:257–265. [DOI] [PubMed] [Google Scholar]
- 3.Stallmach A, Detlef S, Riese H, et al. Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn’s disease. Gastroenterol 1992; 102:1920–1929. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich HP. The role of connective tissue matrix in wound healing. In: Barbul A, Pines E, Caldwell M, Hunt TK, eds. Growth factors and other aspects of wound healing: biological and clinical implications. New York: Alan R Liss; 1988: 243–258. [PubMed]
- 5.Freshney RI. Disaggregation of the tissue and primary culture. In: Freshney RI, ed. Culture of animal cells: a manual of basic tissue culture techniques. New York: Alan R Liss; 1987: 113–115.
- 6.Andujar M, Melin M, Gurret S, Grimaud J. Cell migration influences collagen gel contraction. J Submicrosc Cytol Pathol 1992; 24:145–154. [PubMed] [Google Scholar]
- 7.Lange T, Bielinskey A, Kirchberg K, et al. Mg2+ and Ca2+ differentially regulate beta 1 integrin-mediated adhesion of dermal fibroblasts and keratinocytes to various extracellular matrix proteins. Exp Cell Res 1994; 214:381–388. [DOI] [PubMed] [Google Scholar]
- 8.Gailit J, Pierschbacher M, Clarke R. Expression of functional alpha 4 beta 1 integrin by human dermal fibroblasts. J Invest Dermatol 1993; 100:323–328. [DOI] [PubMed] [Google Scholar]
- 9.Desmouliere A, Rubbia Brandt L, Abdiu A, et al. Alpha smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma interferon. Exp Cell Res 1992; 201:64–73. [DOI] [PubMed] [Google Scholar]
- 10.Acora P, Mc Colloch C. Dependence of collagen remodeling on alpha smooth muscle actin expression by fibroblasts. J Cell Physiol 1994; 159:161–175. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt-Graff A, Desmouliere A, Gabbiani G. Heterogenicity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch 1994; 425:3–24. [DOI] [PubMed] [Google Scholar]
- 12.Coulomb B, Dubertret L, Bell E, Touraine R. The contractility of fibroblasts in a collagen lattice is reduced by corticosteroids. J Invest Dermatol 1984; 82:341–344 [DOI] [PubMed] [Google Scholar]
- 13.Matthes H, Herbst H, Schuppan D, et al. Cellular localization of procollagen gene transcripts in inflammatory bowel disease. Gastroenterology 1992; 102:431–442. [DOI] [PubMed] [Google Scholar]
- 14.Bailey AJ, Bazin S, Simms TJ, et al. Characterization of the collagen of hypertrophic and normal scars. Acta Biochem Biophys 1975; 405:412–421. [DOI] [PubMed] [Google Scholar]
- 15.Barnes MJ, Morton LF, Bennet RC, Bailey AJ. Studies in collagen synthesis in the mature dermal scar in the guinea pig. Biochem Soc 1975; 3:917–920. [Google Scholar]
- 16.Ehrlich H, White B. The identification of a & b collagen chains in hypertrophic scar. Exp Mol Pathol 1981; 24:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Finesmith TH, Broadley KN, Davidson JM. Fibroblasts from wounds of different stages of repair vary in their ability to contract a collagen gel in response to growth factors. J Cell Physiol 1990; 144:99–107. [DOI] [PubMed] [Google Scholar]
- 18.Regan MC, Kirk SJ, Wasserkrug HL, Barbul A. The wound environment as a regulator of fibroblast phenotype. J Surg Res 1991; 50:442–448. [DOI] [PubMed] [Google Scholar]
- 19.Zang HY, Gharace Kermani M, Zhang K, Karmial S, Phan SH. Lung fibroblast alpha smooth muscle actin expression and contractile phenotype in bleomycin induced pulmonary fibrosis. Am J Pathol 1996; 148:572–537. [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce GF, Vande Berg J, Rudolph R, et al. Platelet-derived growth factor-BB and tissue growth factor B1 selectively modulate glycosaminoglycans, collagen and myofibroblasts in excisional wounds. Am J Pathol 1991; 138:629–646. [PMC free article] [PubMed] [Google Scholar]