Abstract
Objective
To analyze the outcome of 200 patients with gastrointestinal stromal tumor (GIST) who were treated at a single institution and followed up prospectively.
Summary Background Data
A GIST is a visceral sarcoma that arises from the gastrointestinal tract. Surgical resection is the mainstay of treatment because adjuvant therapy is unproven.
Methods
Two hundred patients with malignant GIST were admitted and treated at Memorial Hospital during the past 16 years. Patient, tumor, and treatment variables were analyzed to identify patterns of tumor recurrence and factors that predict survival.
Results
Of the 200 patients, 46% had primary disease without metastasis, 47% had metastasis, and 7% had isolated local recurrence. In patients with primary disease who underwent complete resection of gross disease (n = 80), the 5-year actuarial survival rate was 54%, and survival was predicted by tumor size but not microscopic margins of resection. Recurrence of disease after resection was predominantly intraabdominal and involved the original tumor site, peritoneum, and liver.
Conclusions
GISTs are uncommon sarcomas. Tumor size predicts disease-specific survival in patients with primary disease who undergo complete gross resection. Tumor recurrence tends to be intraabdominal. Investigational protocols are indicated to reduce the rate of recurrence after resection and to improve the outcome for patients with GIST.
Gastrointestinal stromal tumor (GIST) is an uncommon visceral sarcoma that arises predominantly in the gastrointestinal tract. During the past three decades, there has been considerable debate regarding its nomenclature, cellular origin, diagnosis, and prognosis. 1 Due to their similar appearance by light microscopy, GISTs were previously thought to be smooth muscle neoplasms, and most were classified as leiomyosarcoma. With the advent of immunohistochemistry and electron microscopy, 2 it became apparent that GISTs may have myogenic features (smooth muscle GIST), 3 neural attributes (gastrointestinal autonomic nerve tumor), 4–6 or characteristics of both muscle and nerve (mixed GIST), or may lack differentiation (GIST not otherwise specified).
The precise cellular origin of GIST recently has been proposed to be the interstitial cell of Cajal, an intestinal pacemaker cell. 7 This postulate is supported by the finding that GISTs have cell markers similar to those of the normal Cajal cell. They stain for the myeloid stem cell antigen CD34 in 52% to 72% of cases 7,8 and are frequently marked by the presence of the c-kit protooncogene. 7,9 On ultrastructural examination, the Cajal cell has characteristics of both smooth muscle and neural differentiation. Thus, neoplastic Cajal cells could preferentially express one, both, or neither of these features, accounting for the variants of GIST.
Most of the published reports of GISTs contain few patients, span long periods, include benign mesenchymal tumors, 10,11 and do not distinguish between primary and recurrent disease. 12–14 Previously, we reported our initial experience with 38 patients with GIST. 15 In this study, we analyzed our experience of 200 patients with GIST to identify the variables predictive of survival and the patterns of disease recurrence.
METHODS
Clinicopathologic Variables
From July 1982 to February 1998, 200 patients age 16 years or older with the diagnosis of GIST were evaluated and treated at Memorial Sloan-Kettering Cancer Center. Patients were followed up prospectively and entered into the Sarcoma Database of the Department of Surgery. Patient, tumor, and treatment variables were recorded.
Patient data included age, sex, race, and presentation status. Presentation status reflects the extent of disease and the history of prior treatment when the patient was first seen at Memorial Hospital; it was categorized as primary, metastatic, or locally recurrent. Patients with primary disease had a recently discovered intraabdominal tumor without metastasis. All patients with any metastatic disease were considered to have a metastatic presentation, regardless of whether they had received prior therapy or also had local recurrence.
The histologic diagnosis of all tumors was confirmed by members of the pathology department at Memorial Hospital. All tumors were regarded as histologically malignant, and patients with GIST of uncertain malignant potential (four cases) were excluded from this study. Tumor size was recorded as the largest diameter in any dimension of the primary tumor and was stratified as ≤5 cm, 5 to 10 cm, or >10 cm. Margins of resected specimens were analyzed for the presence of microscopic disease.
The surgeons at our institution share a treatment philosophy with regard to GIST that emphasizes complete gross removal of the tumor. Resections are classified as incomplete when the tumor is unresectable at exploration or when gross residual disease is present after resection. Complete resection is considered to be the excision of all gross disease, regardless of microscopic margins. Resection of metastases is performed in selected patients in whom the primary tumor is controlled. Systemic chemotherapy and radiation therapy were excluded from the analyses in this report because they were used in sporadic fashion.
Survival Analysis and Statistics
Patient, tumor, and treatment variables were analyzed for their relation to outcome. Survival and recurrence were the end points in this study. Only deaths confirmed to be the result of disease were counted as events; other deaths were censored at the time of death. Local recurrence was defined as the reappearance of tumor at the initial site of the primary tumor. Metastasis was recorded when the tumor spread to the liver, lymph nodes, or extraabdominal sites. All times were calculated from the first admission date to Memorial Hospital to the last day of follow-up or death. Actuarial survival was determined by Kaplan–Meier analysis. The relations of patient, tumor, and treatment characteristics to outcome were tested by univariate analysis using log-rank. P < .05 was considered statistically significant. Multivariate analysis was performed with the Cox proportional hazards model, and only variables that were deemed statistically significant were included in the final Cox model.
RESULTS
Entire Population
Patient Characteristics
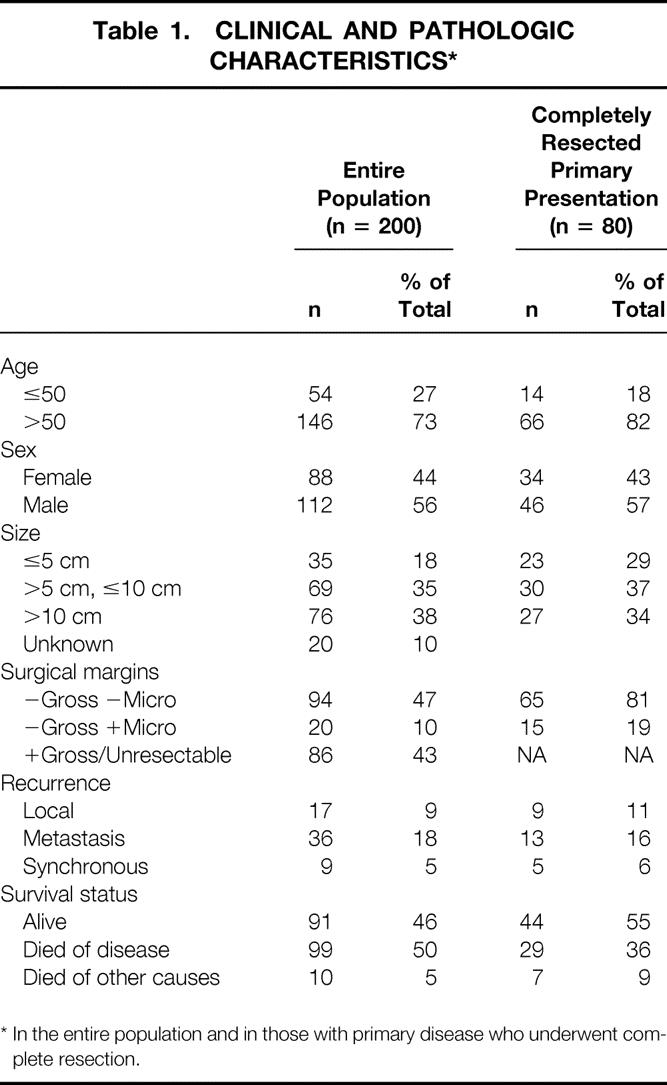
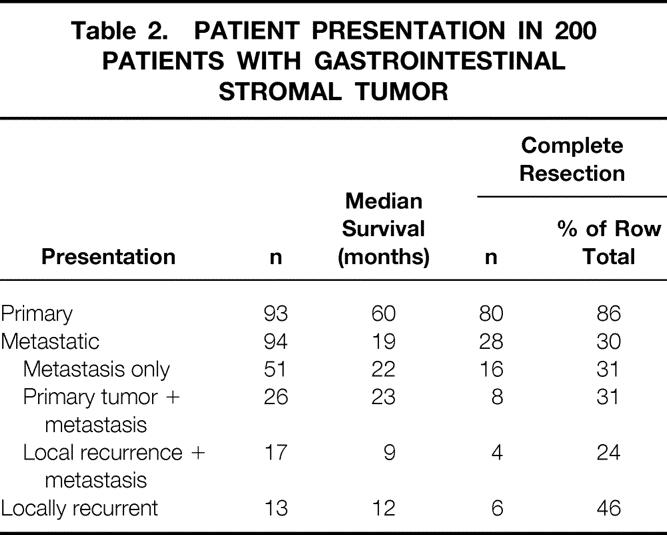
The cohort of 200 patients with GIST tumor represented 5.7% of the 3,505 patients with any type of sarcoma admitted for treatment to Memorial Hospital between July 1982 and February 1998. Retroperitoneal and extremity sarcomas represented 14.8% and 47.2% of all sarcomas, respectively. The clinicopathologic variables of the entire population with GIST are listed in Table 1. The group was made up of 56% men and 44% women. Age had a unimodal distribution, with a median of 58 years (range 16–94) (Fig. 1). The peak incidence in men occurred slightly before that in women. The distribution of race was 83% white, 8% black, 1% Hispanic, 4% other, and 4% unknown. A history of prior cancer was found in 10 (5%) patients—4 with breast cancer, 2 with prostate cancer, and 1 each with skin, uterine, lung, and renal cancer. Presentation at Memorial was primary in 93 (46%) patients, metastatic in 94 (47%), and locally recurrent in 13 (7%) (Table 2).
Table 1. CLINICAL AND PATHOLOGIC CHARACTERISTICS*
* In the entire population and in those with primary disease who underwent complete resection.
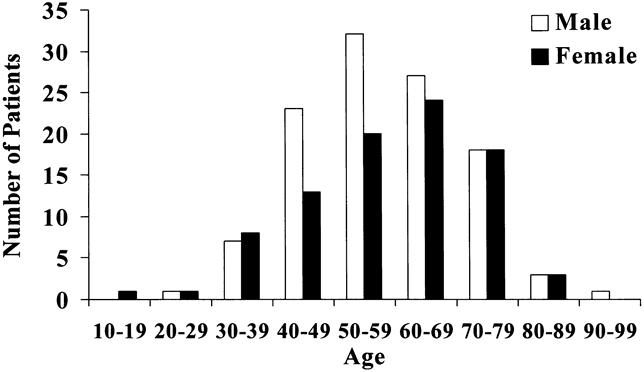
Figure 1. Age and sex distribution in all patients (n = 200).
Table 2. PATIENT PRESENTATION IN 200 PATIENTS WITH GASTROINTESTINAL STROMAL TUMOR
Tumor Characteristics
The most common anatomic sites of tumor origin were the stomach (39%) and the small intestine (32%); colorectal tumors accounted for 15% of the total (Table 3). In 18 (9%) patients, the site was either unclear or from another intraabdominal or retroperitoneal origin. More than two thirds of patients had tumors larger than 5 cm.
Table 3. SITE OF TUMOR ORIGIN IN 200 PATIENTS WITH GASTROINTESTINAL STROMAL TUMOR
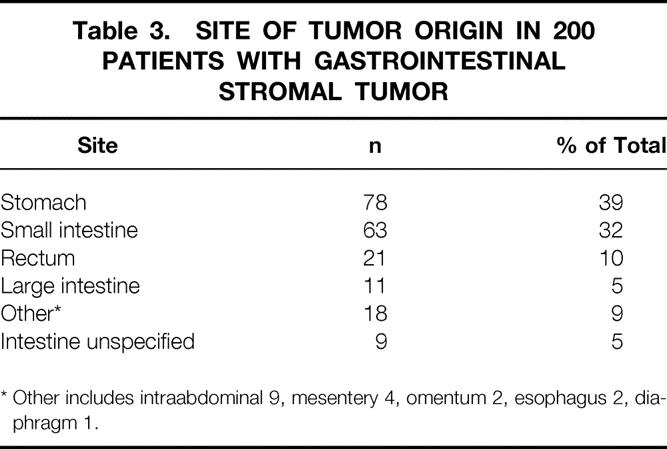
* Other includes intraabdominal 9, mesentery 4, omentum 2, esophagus 2, diaphragm 1.
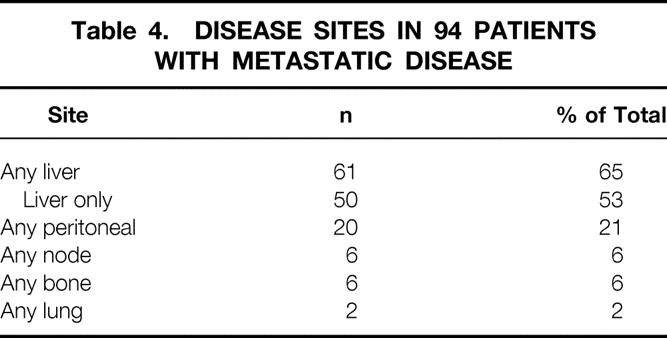
The sites of disease in the 94 patients with metastases are listed in Table 4. Nearly two thirds of patients with metastatic disease had hepatic involvement, and 53% had disease isolated to the liver. Extraabdominal metastasis occurred in eight (8%) patients. Lymph node involvement was uncommon and was documented in only six (6%) patients.
Table 4. DISEASE SITES IN 94 PATIENTS WITH METASTATIC DISEASE
Treatment
All patients were considered for surgical resection, given that there is no effective alternative form of therapy. Complete resection was accomplished in 80 (86%) patients with primary disease, 28 (30%) with metastasis, and 6 (46%) with local recurrence (see Table 2). Of the 114 patients who underwent complete resection, 82% had negative microscopic margins. There were 86 (43%) patients with residual gross disease after surgery or with tumors considered to be unresectable. Adjuvant therapy was used in a nonrandomized fashion. Radiation therapy was used in 11% of patients—6 patients before they sought treatment at Memorial and another 15 patients at our institution. Five of this latter group had brachytherapy primarily for rectal lesions. Chemotherapy was received by 33% of the study population—19 patients before they sought treatment at Memorial and 46 patients at our institution.
Survival
The survival of the entire population is shown in Figure 2. With a median follow-up of 14 (range 1–175) months, the disease-specific survival rate was 69% at 1 year, 44% at 3 years, and 35% at 5 years. Survival status was alive in 91 (46%), with 57 (29%) free of disease; dead of disease in 99 (50%); and dead of other causes in 10 (5%).
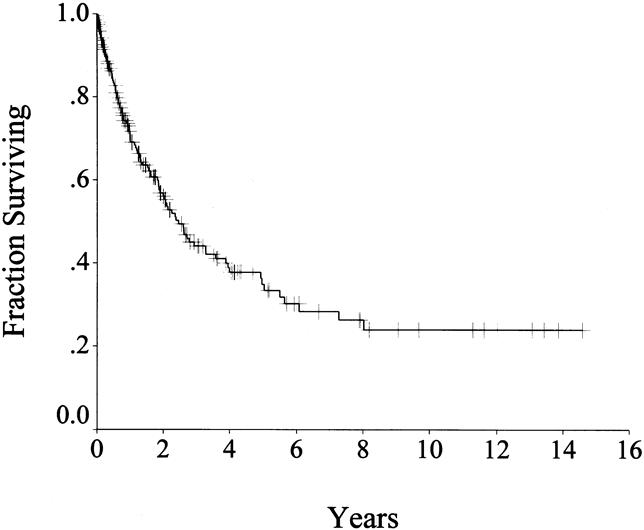
Figure 2. Disease-specific survival in all patients (n = 200).
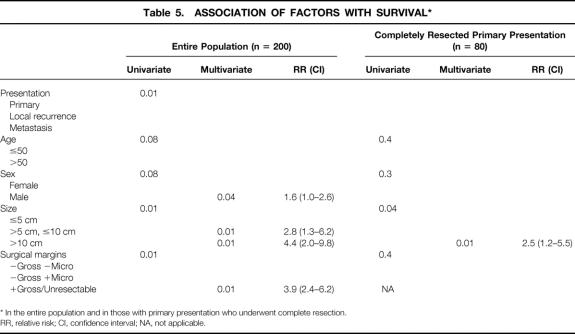
On univariate analysis (Table 5), presentation status, tumor size, and complete resection of gross disease were significant predictors of survival. Median disease-specific survival was 60 months for primary disease, 19 months for metastasis, and 12 months for local recurrence (see Table 2). Multivariate analysis showed that male sex, tumor size >5 cm, and incomplete resection or unresectable tumors were poor prognostic signs (see Table 5). Tumor size >10 cm was the most significant factor and carried a relative hazard of 4.4 (confidence interval 2.0–9.8). There was no effect of age on survival.
Table 5. ASSOCIATION OF FACTORS WITH SURVIVAL*
* In the entire population and in those with primary presentation who underwent complete resection.
RR, relative risk; CI, confidence interval; NA, not applicable.
Patients With Primary Disease Completely Resected
Clinicopathologic Variables
To analyze a homogeneous group of patients with GIST treated in a similar fashion, we performed all further analyses in the 80 patients who sought treatment at Memorial for primary disease and subsequently underwent complete resection of all gross disease and then were followed up prospectively. The clinicopathologic variables for this subgroup are listed in Table 1. There were 46 (57%) men and 34 (43%) women. Median age was 65 (range 16–94) years. The predominant sites of disease were the stomach (54%), rectum (16%), and small intestine (15%). Fewer than one third of the tumors were small (≤5 cm). Overall, the patient and tumor characteristics were similar to those of the entire cohort of 200 patients, except there was a greater preponderance of tumors originating in the stomach and fewer tumors arising from the small intestine. Of the 80 resections, 65 (81%) had negative microscopic margins.
Recurrence
With a median follow-up of 24 months (range 1–175), recurrence occurred in 32 (40%) patients, 29 of whom have died of disease. Complete data regarding the pattern of recurrence were available in 27 patients. There was local recurrence alone in 9 (33%) patients, metastasis alone in 13 (48%) patients, and both in 5 (19%) patients. The sites of recurrence are listed in Table 6. Recurrence was predominantly intraabdominal. It involved the liver in 17 (63%) patients and was isolated to the liver in 12 (44%) instances. Isolated local recurrence accounted for 33% of the patients with recurrent disease. Four patients had extraabdominal recurrence. There was a trend for tumors >10 cm to recur earlier, but no other factor, including age, sex, and surgical margins, predicted recurrence. The disease-free survival rate was 80% at 1 year, 67% at 2 years, and 45% at 5 years (Fig. 3).
Table 6. SITES OF FIRST RECURRENCE IN PATIENTS WITH PRIMARY DISEASE WHO UNDERWENT COMPLETE RESECTION

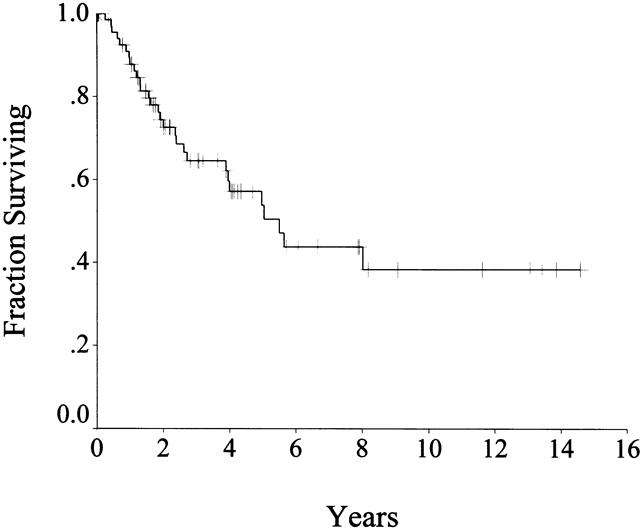
Figure 3. Disease-specific survival in patients with primary disease who underwent complete resection (n = 80).
Survival
Survival status in patients with primary disease who underwent complete resection after a median follow-up of 24 months (range 1–175) was 44 (55%) patients alive, with 41 (51%) free of disease; 29 (36%) patients dead of disease; and 7 (9%) patients dead of other or unknown causes. The disease-specific survival rate was 88% at 1 year, 65% at 3 years, and 54% at 5 years (see Fig. 3). Patients with primary disease who underwent complete resection had a median survival of 66 months, compared with 22 months for those who had incomplete resection or whose tumor was unresectable. On univariate analysis of clinicopathologic factors, only tumor size predicted survival (see Table 5, Fig. 4). Age, sex, and tumor site were not important. Microscopic margins did not influence outcome (Fig. 5). On multivariate analysis, only tumor size >10 cm was significant: it carried a relative risk of 2.5 (confidence interval 1.2–5.5).
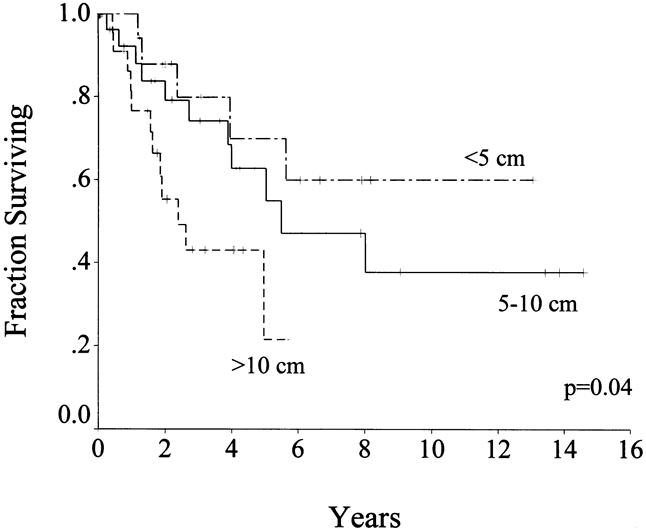
Figure 4. Disease-specific survival by tumor size in patients with primary disease who underwent complete resection (n = 80).
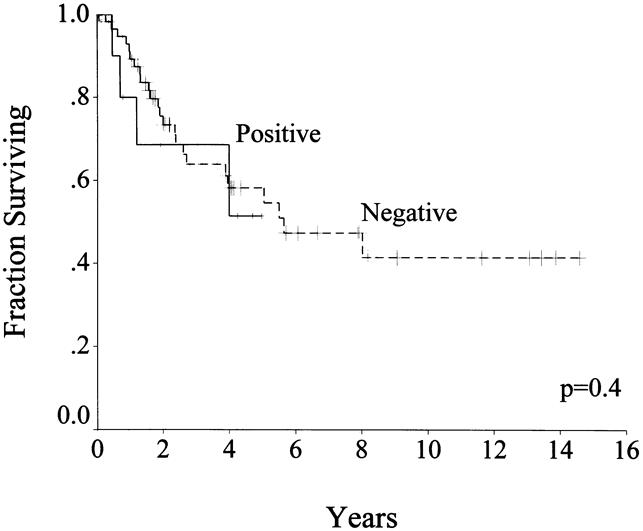
Figure 5. Disease-specific survival by margin status in patients with primary disease who underwent complete resection (n = 80).
Because of the lack of any effective alternative therapies, all patients with recurrent disease were considered for surgical re-resection (Table 7). Of the nine patients with isolated local recurrence, four could undergo complete resection. Four patients with metastatic recurrence underwent complete resection, and their median survival was 16 months from resection of the metastasis. Survival was poor in patients with incomplete resection, with local recurrence (median 5 months) or combined local and metastatic recurrence (median 5 months).
Table 7. OUTCOME AFTER FIRST RECURRENCE IN PATIENTS WITH PRIMARY DISEASE WHO UNDERWENT COMPLETE RESECTION
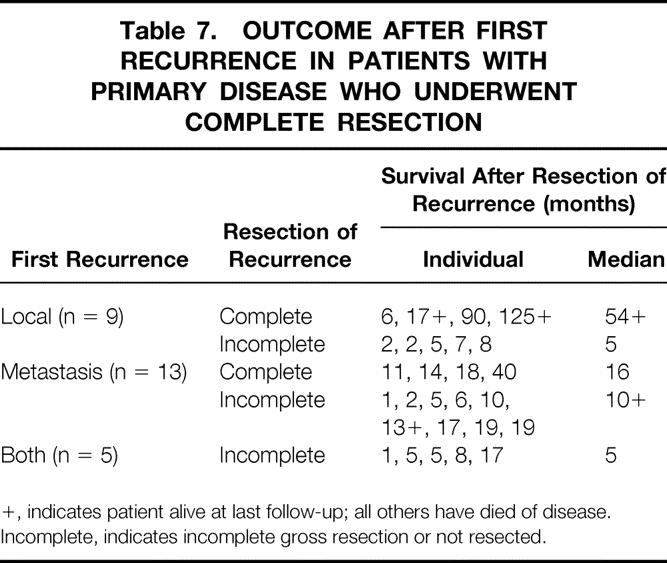
+, indicates patient alive at last follow-up; all others have died of disease.
Incomplete, indicates incomplete gross resection or not resected.
DISCUSSION
GISTs are encountered infrequently. In this report, we analyzed our results with 200 patients with GIST treated at Memorial Sloan-Kettering Cancer Center during the past 16 years.
The overall survival rate for all 200 patients in this study was 35% at 5 years, which is similar to the results of Ng et al, who reported 28% survival at 5 years in a group of 191 patients encountered during 30 years. 12 Outcome depended on the status and extent of disease at the time they sought treatment: median survival was 19 months in those with metastasis and 12 months in those with local recurrence. This difference may simply reflect bias from referrals to our institution. Patients who could not undergo complete resection (n = 86) had a median survival of only 12 months; others have reported a median survival of 9 to 12 months for such patients. 16,17 Complete resection in patients with a primary presentation of disease at our institution (who lacked metastases) was possible in 80 of 93 patients (86%), and these patients had a 54% 5-year survival rate. These results are similar to those published previously (Table 8) , with reported 5-year survival rates of 40% to 65%. 12,17–19
Table 8. PUBLISHED SERIES OF GASTROINTESTINAL STROMAL TUMORS

Although there is no accepted staging system for GISTs, several prognostic variables identified previously were examined in this series. In the entire population, sex, tumor size, and incomplete resection were significant predictors of survival on multivariate analysis. That size is important is in concordance with prior experience from our institution with stomach 18 and intestinal 14 leiomyosarcomas and the data of others. 10,12,17,20,21 The impact of resectability on outcome has also been demonstrated. 17,22
The patients who had their initial presentation at our institution, did not have metastasis, and underwent complete gross resection of disease were analyzed separately. This population is more homogeneous, and the patients underwent relatively uniform therapy. For this group of 80 patients, size was an important predictor of survival on both univariate and multivariate analyses. The status of the microscopic margin of resection did not affect survival. This may be explained by the fact that many of these tumors are exophytic and protrude into the abdominal cavity. Therefore, the microscopic margin of resection of the organ from which they arise may not be as important as whether the tumor sheds cells directly into the peritoneal cavity.
The stomach was the most common site of tumor origin in this series. The distribution of sites was similar to that published in 1965 by Skandalakis in a review of the literature. 23 In his report, the distribution of 725 malignant smooth muscle tumors of the gastrointestinal tract was stomach 47.3%, small intestine 35.4%, colon 4.6%, and rectum 7.4%. A small fraction of tumors in our experience originated from other intraabdominal sites, including the mesentery and omentum, as others have reported. 12 The site of origin did not predict survival (data not shown), as had been found previously. 24
Recurrence of GIST is usual, and one report found only 10% of patients to be free of disease after long-term follow-up. 22 Recurrence tends to involve the peritoneal surface or the liver. In the 80 patients with primary presentation who underwent complete resection, the liver was involved in 63% of patients who had recurrence; this was the only site of disease in 44% of recurrences. A similar pattern was seen in the 94 patients with metastasis at presentation: 65% had liver disease and 53% had isolated liver recurrence. Meanwhile, in the patients with primary disease who underwent complete resection, there was a component of local failure in 52% of patients with recurrence. Extraabdominal metastasis was much less common (15%). The status of the microscopic margins of resection did not predict recurrence, although the relatively small numbers prohibit a definitive conclusion. At 2 years, only 30% of the patients had recurrent disease; others have reported a median disease-free interval of 18 months. There may be benefit for resecting recurrent disease in highly selected patients with isolated local or metastatic disease when all gross tumor can be excised.
Although approximately half of the patients with primary disease who undergo complete resection will survive 5 years, surgery alone is inadequate for the treatment of GISTs. The high rate of local and distant recurrence underscores the need for adjuvant therapy. The use of radiation therapy is limited by the associated toxicity to adjacent structures; thus, radiation therapy is of uncertain value. 18 It may be of benefit in reducing local recurrence for rectal lesions. Systemic chemotherapy with the currently available agents is unproven, as is the case with retroperitoneal sarcomas. 24 In a collective review, the response rate of visceral sarcomas to mostly doxorubicin-based regimens was reported to be 12% to 43%. 25 In contrast, in a prospective randomized trial the response rate was only 7%. 26 Ifosamide has also been disappointing for leiomyosarcoma. 27 Despite the poor results, up to 76% of patients will receive chemotherapy without demonstrable effect. 22 At our institution, in an effort to reduce the recurrence rate after complete or near-complete resection of GISTs, we are initiating a phase I protocol of adjuvant intraperitoneal chemotherapy, which is already under investigation elsewhere (personal communication, F. Eilber, UCLA, March 1999).
Until more effective adjuvant therapy becomes available, the treatment of GISTs will remain primarily surgical. Several therapeutic principles deserve mention. Preoperative percutaneous biopsy carries the theoretical risk of peritoneal seeding or tumor rupture. It is indicated only for clearly unresectable disease or when treatment would be altered, as would be the case if the mass proved to be a lymphoma or germ cell tumor. The diagnosis of GIST may be suggested on a preoperative computed tomography scan or at laparotomy by the presence of a large mass without adenopathy that may or may not be associated with liver metastases. Intraoperative frozen section is usually not necessary; only highly experienced pathologists are likely to provide a diagnosis. The surgeon should make every effort to achieve complete resection of all gross disease, which may necessitate the removal of adjacent organs. In this series, 86% of the 93 patients with primary disease were able to undergo complete resection. Incomplete resection should be performed only for the palliation of symptoms due to bleeding, pain, or mass effect. Because the status of the microscopic margins does not appear to be important for survival, vital structures should not be sacrificed if gross tumor clearance has already been attained. For gastric sarcoma, limited resection is known to achieve results comparable to extended resection. 18 The low incidence of lymph node metastases in this cohort (and as shown by others 18,28–30) confirms that lymphadenectomy is not routinely warranted. The management of patients with asymptomatic recurrence detected by surveillance radiologic imaging is not clearly defined. These patients should be considered for investigational therapies.
Acknowledgments
The authors thank Nicole Maurice and Gina DiMartino for data management.
Footnotes
Correspondence: Murray F. Brennan, MD, Dept. of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
Presented in part at the Annual Meeting of the American College of Surgeons, October 25–30, 1998, Orlando, Florida.
Supported by USPHS Grant CA 47179 (MFB).
Accepted for publication July 26, 1999.
References
- 1.Suster S. Gastrointestinal stromal tumors. Sem Diag Pathol 1996; 13:297–313. [PubMed] [Google Scholar]
- 2.Erlandson RA, Klimstra DS, Woodruff JM. Subclassification of gastrointestinal stromal tumors based on evaluation by electron microscopy and immunohistochemistry. Ultrastruct Pathol 1996; 20:373–393. [DOI] [PubMed] [Google Scholar]
- 3.Starr GF, Dockerty MB. Leiomyomas and leiomyosarcomas of the small intestine. Cancer 1955; 8:101–111. [DOI] [PubMed] [Google Scholar]
- 4.Herrera GA, Demoraes HP, Grizzle WE, Han SG. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma): light and electron microscopic study confirming the origin of the neoplasm. Dig Dis Sci 1984; 29:275–284. [DOI] [PubMed] [Google Scholar]
- 5.Herrera GA, Cerezo L, Jones JE, et al. Gastrointestinal autonomic nerve tumors: “plexosarcomas.” Am J Surg Pathol 1989; 17:887–897. [PubMed] [Google Scholar]
- 6.Lauwers GY, Erlandson RA, Casper ES, Brennan MF, Woodruff JM. Gastrointestinal autonomic nerve tumors. A clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol 1993; 17:887–897. [DOI] [PubMed] [Google Scholar]
- 7.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998; 152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 8.Miettinen M, Virolainen M, Maarit SR. Gastrointestinal stromal tumors—value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol 1995; 19:207–216. [DOI] [PubMed] [Google Scholar]
- 9.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279:577–580. [DOI] [PubMed] [Google Scholar]
- 10.Sanders L, Silverman M, Rossi R, Braasch J, Munson L. Gastric smooth muscle tumors: diagnostic dilemmas and factors affecting outcome. World J Surg 1996; 20:992–995. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DJ, Traverso LW. Gut stromal tumors and their clinical behavior. Am J Surg 1997; 173:390–394. [DOI] [PubMed] [Google Scholar]
- 12.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992; 215:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans HL. Smooth muscle tumors of the gastrointestinal tract. A study of 56 cases followed for a minimum of 10 years. Cancer 1985; 56:2242–2250. [DOI] [PubMed] [Google Scholar]
- 14.Shiu MH, Farr GH, Egeli RA, Quan SH, Hajdu SI. Myosarcomas of the small and large intestine: a clinicopathologic study. J Surg Oncol 1983; 24:67–72. [DOI] [PubMed] [Google Scholar]
- 15.Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol 1995; 2:26–31. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty MJ, Compton C, Talbert M, Wood WC. Sarcomas of the gastrointestinal tract. Separation into favorable and unfavorable prognostic groups by mitotic count. Ann Surg 1991; 214:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath PC, Neifeld JP, Lawrence WJ, Kay S, Horsley JS, Parker GA. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg 1987; 206:706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiu MH, Farr GH, Papachristou DN, Hajdu SI. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 1982; 49:177–187. [DOI] [PubMed] [Google Scholar]
- 19.Akwari OE, Dozois RR, Weiland LH, Beahrs OH. Leiomyosarcoma of the small and large bowel. Cancer 1978; 42:1375–1384. [DOI] [PubMed] [Google Scholar]
- 20.Chiotasso PJ, Fazio VW. Prognostic factors of 28 leiomyosarcomas of the small intestine. Surg Gynecol Obstet 1982; 155:197–202. [PubMed] [Google Scholar]
- 21.Kimura H, Yonemura Y, Kadoya N, et al. Prognostic factors in primary gastrointestinal leiomyosarcoma: a retrospective study. World J Surg 1991; 15:771–776. [DOI] [PubMed] [Google Scholar]
- 22.Ng EH, Pollock RE, Romsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leiomyosarcomas. Cancer 1992; 69:1334–1341. [DOI] [PubMed] [Google Scholar]
- 23.Skandalakis JE, Gray SW. Smooth muscle tumors of the alimentary tract. In: Ariel IM, ed. Progress in Clinical Cancer. New York: Grune & Stratton; 1965: 692–708. [PubMed]
- 24.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998; 228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licht JD, Weissmann LB, Antman K. Gastrointestinal sarcomas. Sem Oncol 1988; 15:181–188. [PubMed] [Google Scholar]
- 26.Zalupski M, Metch B, Balcerzak S, et al. Phase III comparison of doxorubicin and dacarbazine given by bolus versus infusion in patients with soft-tissue sarcomas: a Southwest Oncology Group study. JNCI 1991; 83:926–932. [DOI] [PubMed] [Google Scholar]
- 27.Elias A, Ryan L, Sulkes A, Collins J, Aisner J, Antman KH. Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol 1989; 7:1208–1216. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay PC, Ordonez N, Raaf JH. Gastric leiomyosarcoma: clinical and pathological review of fifty patients. J Surg Oncol 1981; 18:399–421. [DOI] [PubMed] [Google Scholar]
- 29.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 1993; 217:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YT. Leiomyosarcoma of the gastro-intestinal tract: general pattern of metastasis and recurrence. Cancer Treat Rev 1983; 10:91–101. [DOI] [PubMed] [Google Scholar]