Abstract
Objective
To determine molecular mechanisms involved in angiogenesis of hepatocellular carcinoma (HCC).
Summary Background Data
Tumor angiogenesis is believed to derive from the balance between angiogenic stimulators and inhibitors. It has been suggested that the switch to the angiogenic phenotype requires both upregulation of the first and downregulation of the second. However, its molecular basis in vivo remains obscure. In this study the authors analyze the participation of two factors in angiogenesis of HCC—human macrophage metalloelastase (HME), a matrix metalloproteinase responsible for the generation of angiostatin, a potent angiogenesis inhibitor, and vascular endothelial growth factor (VEGF), the most potent endogenous angiogenic factor.
Methods
Tumorous and contiguous nontumorous tissues from 25 patients with HCC who underwent curative partial hepatectomy were subjected to Northern blot analysis to detect HME and VEGF messenger RNA (mRNA) expression. Western blot analysis was used to detected angiostatin. Tumor vascularity was evaluated using hepatic angiography.
Results
Eleven of the 15 cases expressing the HME gene showed hypovascular tumors, whereas hypervascular tumors were seen in 9 of the 10 HME-negative cases. The median of HME mRNA expression (tumorous/nontumorous ratio) was 6.5 (range 0–264.5) in the hypovascular group and 0 (range 0—3.2) in the hypervascular group. A stepwise logistic analysis revealed that HME and VEGF mRNA expression were two independent variables significantly affecting the vascularity of HCC tumors.
Conclusion
HME gene expression is significantly associated with hypovascular tumors; moreover, angiogenesis in HCC is not determined by a single factor, but depends on the net balance between HME and VEGF gene expressions.
The growth of solid tumors requires new blood vessel formation to facilitate the delivery of nutrients and oxygen and the removal of catabolites, as has been experimentally demonstrated. 1,2 Thus, long-term suppression of angiogenesis may become a therapeutic option for malignancies. 3,4 However, the mechanisms that control it are not completely understood. Recently, it has been proposed that a balance between endogenous angiogenic stimulators, including members of the vascular endothelial growth factor (VEGF), fibroblast growth factor, and platelet derived-endothelial cell growth factor families, among many others, and endogenous angiogenic inhibitors, such as angiostatin, endostatin, and thrombospondin, is a critical condition for the promotion of the neovascularization of tumors. Indeed, several reports have stated that the switch to the angiogenic phenotype requires both upregulation of the first and downregulation of the second. 5,6 These molecular bases, in association with the lack of detailed studies on this aspect in human tumors, encouraged us to explore possible molecules regulating tumor angiogenesis in hepatocellular carcinoma (HCC), which is generally considered to be a hypervascular tumor with an unsatisfactory long-term survival rate after surgical resection and conventional adjuvant chemotherapy.
The matrix metalloproteinase (MMP) family includes an increasing list of enzymes characterized by their ability to degrade extracellular matrix as well as nonmatrix substrates. Although MMPs have long been associated with tumor invasion and metastasis, 7 their association with angiogenesis remains controversial. In our recent report, 8 we demonstrated, for the first time to our knowledge, that human macrophage metalloelastase (HME), identified as MMP-12, and formerly isolated from alveolar macrophages of cigarette smokers, 9,10 can be directly expressed by some human HCCs (62.5%), and that its expression is strongly associated with the generation in situ of human angiostatin, 11 a potent inhibitor of angiogenesis. Previously, only one basic experimental approach had been reported, in which a mouse counterpart of HME produced by tumor-infiltrating macrophages was responsible for the generation of angiostatin from plasminogen, inhibiting the growth and metastasis of Lewis lung carcinoma in mice. 12
Among angiogenic factors, VEGF is the most potent endogenous mediator of angiogenesis known. 13,14 VEGF is a diffusible endothelial cell-specific mitogen and angiogenic factor that also increases vascular permeability. These actions are mediated through two endothelial cell tyrosine kinase receptors, the fms-like tyrosine kinase and the kinase domain receptor. 15,16 Both VEGF and its receptors have been found to be expressed in a variety of tumors, 17–19 where overexpression of VEGF results in dramatically increased angiogenesis and subsequent tumor expansion, whereas the blockade of the VEGF receptors inhibits angiogenesis, leading to a reduction of new vessels, a higher degree of necrosis, and a reduction in size of certain tumors. In HCC, it has been demonstrated that 60% of these tumors exhibit high expression of VEGF, which significantly correlates with the hypervascularity of the tumors. 20
We designed the present study to clarify the in vivo molecular role of HME gene expression in angiogenesis of HCC, and to examine whether VEGF expression can modify the angiogenic response of HME gene expression. The results presented here indicate that HME messenger RNA (mRNA) expression is significantly associated with hypovascular tumors and, moreover, that the final angiogenic phenotype of HCC tumors does not depend on a single factor, but on the net balance between HME and VEGF.
PATIENTS AND METHODS
Study Population
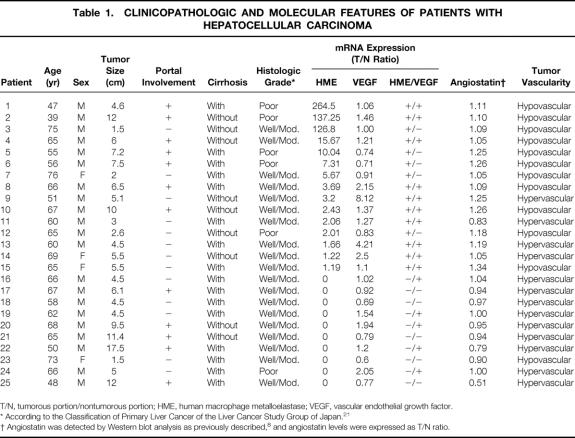
Twenty-five patients who had undergone curative partial hepatectomy for HCC at the Department of Surgery & Surgical Basic Science, Kyoto University Hospital, Kyoto, Japan, were included in this study. There were 21 men and 4 women with an average age of 61.56 ± 9.18 (mean ± SD; range 39–76). The central portion of the tumor without necrosis and contiguous nontumorous tissues were collected once we had informed consent. Specimens were macroscopically and histologically examined (Table 1) in accordance with the Classification of Primary Liver Cancer of the Liver Cancer Study Group of Japan 21 and stored at −70°C until used. All samples were subjected to Northern blot analysis, to detect HME and VEGF mRNA expression, and Western blot, to detect angiostatin.
Table 1. CLINICOPATHOLOGIC AND MOLECULAR FEATURES OF PATIENTS WITH HEPATOCELLULAR CARCINOMA
T/N, tumorous portion/nontumorous portion; HME, human macrophage metalloelastase; VEGF, vascular endothelial growth factor.
* According to the Classification of Primary Liver Cancer of the Liver Cancer Study Group of Japan. 21
† Angiostatin was detected by Western blot analysis as previously described, 8 and angiostatin levels were expressed as T/N ratio.
cDNA Probes and Northern Blot Hybridization
Total RNA was isolated from tumorous and nontumorous liver tissues by the acid guanidinium-thiocyanate/phenol/chloroform method; and poly (A)+ RNA was selected by Oligotex-dT30 (Takara, Japan). Northern blot hybridization was performed as previously described. 8 The probes used were 598 bp and 418 bp reverse transcription–polymerase chain reaction products corresponding to HME and human VEGF165 respectively, and S26 ribosomal protein cDNA as internal control. The probes were prepared as described elsewhere. 8,20 HME and human VEGF165 mRNA levels in the paired tumorous and nontumorous tissues were quantified by measuring the intensities of the appropriate bands in the autoradiograms (1.8 Kb band and 4.5 Kb band respectively) using an ATTO Densitograph (Tokyo, Japan) and were normalized to S26 ribosomal protein mRNA. Then, the tumorous/nontumorous (T/N) ratio of the former to the latter was determined (see Table 1).
Angiostatin Detection
In all cases, Western blot analysis was performed to detect angiostatin by using murine monoclonal antibodies against kringle 1-3 elastase fragment of human plasminogen, subclass IgG1 (Product #P1517, Sigma Chemical Co., St. Louis, MO), as previously described. 8 The angiostatin levels in the paired tumorous and nontumorous tissues from the same patient were quantified by an ARGUS-50, 3.3 (Hamamatsu, Japan), and the ratio of the former to the latter was defined as the angiostatin T/N ratio (see Table 1).
Angiographic Evaluation
The correlation of HME and HME/VEGF mRNA expression with the angiographic findings was studied in all 25 patients who underwent selective hepatic angiography. The contrast dye was usually infused at 9 mL/sec for 3 seconds through the common hepatic artery. The vascularity of the tumor was evaluated by the degree of tumor-staining intensity into the parenchymal area of the liver at 3.667 seconds after the infusion of contrast dye; this time corresponded to the arterial phase of the angiography. The intensity of tumor staining was determined by detailed study of the angiogram by five surgeons masked to the results of HME and VEGF mRNA expression, and it was classified as hypervascular (strong staining) or hypovascular (moderate or weak staining). There was a consensus of opinion in 14 patients and a 4-to-1 vote in 11 patients.
Statistical Analysis
The Wilcoxon-Mann-Whitney test compared HME mRNA levels between the hypovascular and hypervascular groups. Fisher’s exact probability test was used to evaluate the association of vascularity with HME gene expression. To select an appropriate set of independent variables that significantly affect tumor vascularity in HCC, the clinicopathologic and molecular-biologic variables (see Table 1) were evaluated by stepwise logistic analysis using the likelihood ratio criterion. Statistical analyses were performed with JMP 3.0 (SAS Institute, Cary, NC) and with SPSS Professional Statistics 7.5 (SPSS Japan Inc.). P < .05 based on a two-tailed test was considered to indicate statistical significance.
RESULTS
Correlation of HME mRNA Expression Level and Tumor Vascularity
In 15 patients, the T/N ratio of HME mRNA expression increased by ≥1.2-fold in tumorous tissues compared with nontumorous tissues. In the other 10 patients, no expression of HME gene was detected in the tumorous portion by Northern blot analysis (see Table 1).
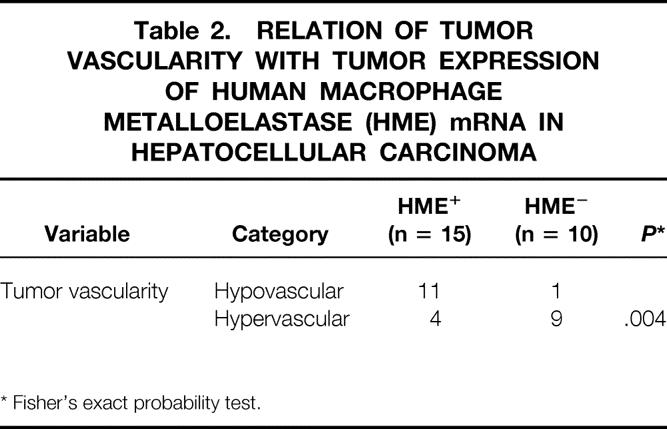
Eleven of the 15 patients expressing the HME gene (HME+) had hypovascular tumors. In contrast, hypervascular tumors were seen in 9 of the 10 HME-negative patients (Table 2).
Table 2. RELATION OF TUMOR VASCULARITY WITH TUMOR EXPRESSION OF HUMAN MACROPHAGE METALLOELASTASE (HME) mRNA IN HEPATOCELLULAR CARCINOMA
* Fisher’s exact probability test.
The correlation between HME mRNA expression and the intensity of angiographic tumor staining is shown in Figure 1. A close relation was seen between HME mRNA expression and tumor vascularity in patients with HCC. The median of HME mRNA expression (T/N ratio) was 6.5 (range 0–264.5) in the hypovascular group and 0 (range 0–3.2) in the hypervascular group (P = .0004, Wilcoxon-Mann-Whitney test).
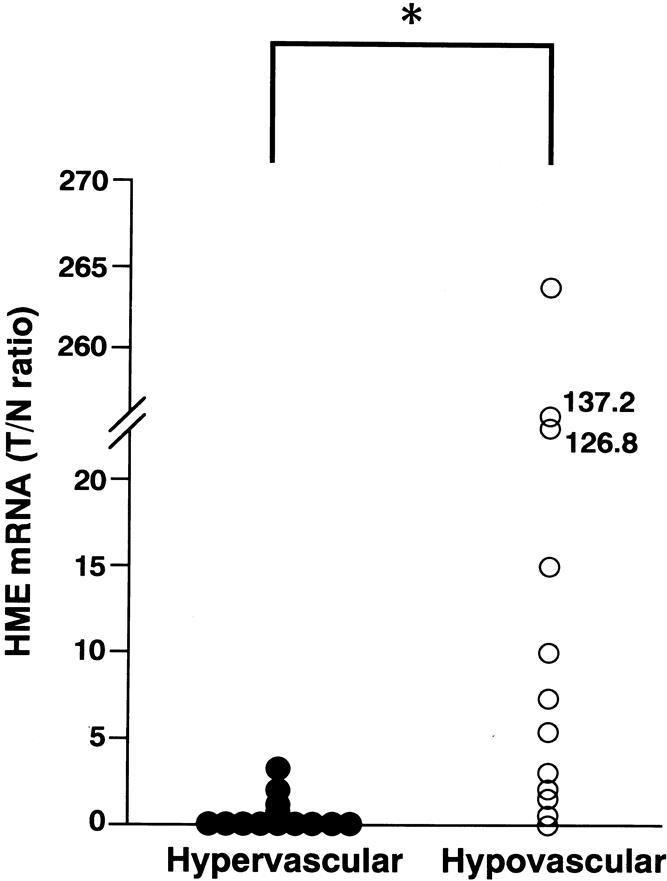
Figure 1. Scattergram showing the correlation between the intensity of angiographic tumor staining and human macrophage metalloelastase (HME) mRNA expression levels. The y axis represents HME mRNA in the tumorous portion/HME mRNA in the nontumorous portion (T/N ratio). The HME T/N ratio varied from 0 to 264.5 (median 6.5) in the patients with hypovascular tumors (n = 12) and from 0 to 3.2 (median 0) in the patients with hypervascular tumors (n = 13). (*P = .0004 by Wilcoxon-Mann-Whitney test).
Balance Between HME and VEGF mRNA Expression and its Correlation with Tumor Vascularity
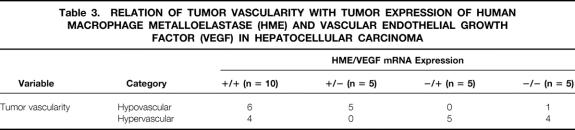
A total of 25 patients with HCC were divided, according to their HME and VEGF mRNA expressions (T/N ratios), into the following subgroups: HME ratio > 1.00 and VEGF ratio > 1.00; HME ratio > 1.00 and VEGF ratio ≤ 1.00; HME ratio ≤ 1.00 and VEGF ratio > 1.00; and HME ratio ≤ 1.00 and VEGF ratio ≤ 1.00. For the purpose of discrimination, the following terms—HME+/VEGF+, HME+/VEGF−, HME−/VEGF+, and HME−/VEGF−—were coined to describe the subgroups respectively (Table 3).
Table 3. RELATION OF TUMOR VASCULARITY WITH TUMOR EXPRESSION OF HUMAN MACROPHAGE METALLOELASTASE (HME) AND VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) IN HEPATOCELLULAR CARCINOMA
All five patients (100%) from the group HME−/VEGF+ had hypervascular tumors, but in the group HME+/VEGF+ the hypervascular pattern of the tumors decreased to 40% (4/10 patients). In contrast, all patients (100%) in the group HME+/VEGF− exhibited a hypovascular pattern of staining in their tumors. In the group HME−/VEGF−, the vascular phenotype of the tumors again shifted to a hypervascular type (80%).
Four patients with high levels of HME mRNA expression (T/N ratio > 1.2) in their tumors also had hypervascular tumors. However, in three of them, the VEGF ratio increased by more than twofold compared with the HME ratio (patients 9, 13, and 14). In the fourth patient (patient 11), the level of angiostatin generation by the tumor was lower than in its corresponding nontumorous tissue (angiostatin T/N ratio = 0.83) (see Table 1).
Results of Stepwise Logistic Analysis
HME expression and VEGF mRNA expression were selected as two independent variables that significantly affect the vascularity of HCC (likelihood ratio chi-square = 15.2, P = .0001; likelihood ratio chi-square = 5.4, P = .02, respectively).
Angiography Findings
To illustrate the relation of HME and VEGF mRNA expression with hepatic angiographic findings, four representative cases were chosen. Figure 2 shows the Northern blot results for HME and VEGF mRNA expression;Figure 3 shows the angiograms. Patient 21, who had HME and VEGF T/N ratios of 0 and 0.79 respectively, with no generation of angiostatin (T/N ratio 0.79), showed strong angiographic staining (see Fig. 3A). In patient 22, the HME and VEGF T/N ratios were 0 and 1.2 respectively, also with no generation of angiostatin (T/N ratio 0.79). This patient showed stronger angiographic staining than patient 21 (see Fig. 3B). Conversely, patient 7, who showed HME mRNA expression (T/N ratio 5.67) with a very low expression of VEGF (T/N ratio 0.91) and angiostatin generation (T/N ratio 1.05), showed weak tumor staining in the angiogram (see Fig. 3C). Patient 8, who expressed a relatively high level of VEGF mRNA (T/N ratio 2.15), but with a higher expression of HME gene (T/N ratio 3.69) and angiostatin generation (T/N ratio 1.09), showed weak tumor staining as well (see Fig. 3D).
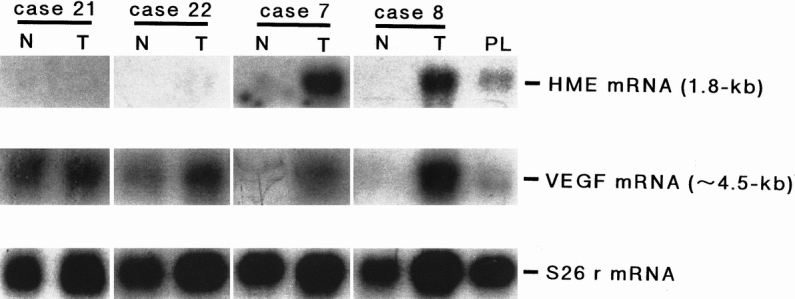
Figure 2. Northern blot analysis for human macrophage metalloelastase (HME) mRNA expression (upper panel) and vascular endothelial growth factor (VEGF) mRNA expression (middle panel) in tumorous and nontumorous liver tissues from four patients with hepatocellular carcinoma (patients 21, 22, 7, and 8). Five micrograms of polyA+ RNA from each human tissue were used. Hybridization was performed with HME and VEGF165 cDNA as described in text. The 1.8-kb band and ∼4.5-kb band, corresponding to the HME and VEGF transcripts respectively, were detected. S26 mRNA expression is shown as the corresponding internal control in the lower panel. N, nontumorous portion; T, tumorous portion; PL, human placenta.
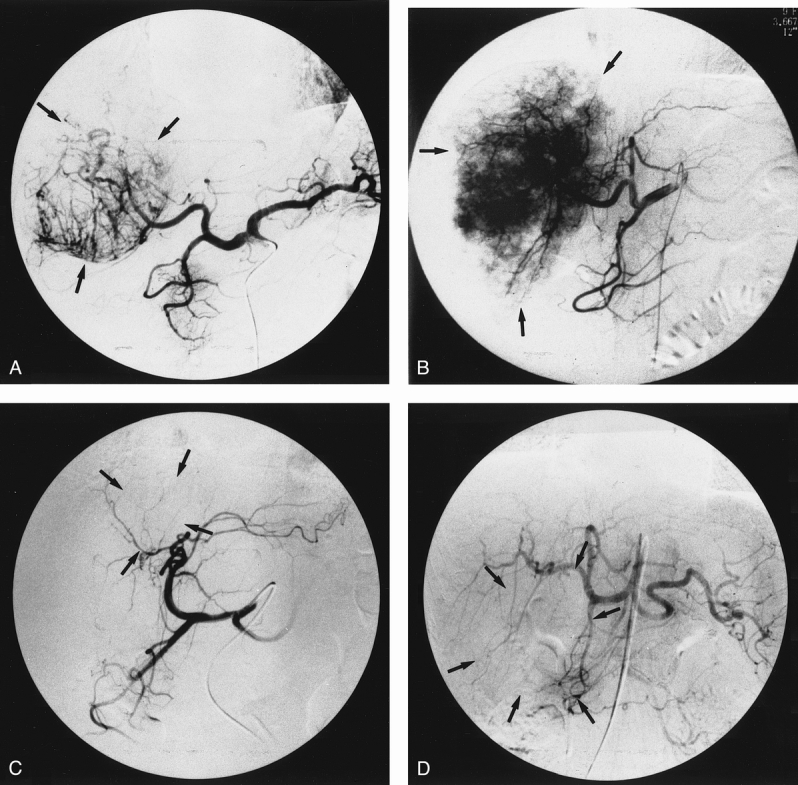
Figure 3. Hepatic angiograms through the celiac trunk with the catheter tip positioned in the common hepatic artery, showing the degree of tumor-staining intensity in patients 7, 8, 21, and 22. (A) Angiogram of patient 21, exhibiting strong tumor staining; human macrophage metalloelastase (HME) and vascular endothelial growth factor (VEGF) tumorous/nontumorous (T/N) ratios were 0 and 0.79, respectively. (B) Angiogram of patient 22, showing the strongest tumor staining; HME and VEGF T/N ratios were 0 and 1.2, respectively. (C) Angiogram of patient 7 and (D) angiogram of patient 8, both showing weak tumor staining; HME and VEGF T/N ratios were 5.67 and 0.91 respectively in patient 7 and 3.69 and 2.15 respectively in patient 8. Tumor location is denoted by the arrows.
Association Between HME or VEGF mRNA Expression and Histopathologic Findings
Table 1 shows the clinicopathologic features in each patient. No significant association of HME or VEGF mRNA expression levels with the tumor size, portal vein invasion, pathologic grade of anaplasia, or cirrhosis was found. Moreover, these clinicopathologic features did not show significant association with tumor vascularity (data not shown). Thus, the clinicopathologic backgrounds were uniform between the four HME/VEGF groups.
DISCUSSION
HCCs are commonly regarded as hypervascular tumors, although their vascularity ranges from a relatively hypovascular to a hypervascular pattern. 20,22 Previous reports have demonstrated that angiogenesis is a prerequisite for the development of HCC from precancerous foci. 23–26 Thus, more studies regarding the mechanism of angiogenesis in HCC are important because a better understanding of the molecular network involved in new vessel formation may contribute to the establishment of novel therapeutics (as well as biologic interest).
In our previous study, we reported that HME, a type of MMP, can be expressed in some HCCs (62.5%), and that its expression is strongly associated with the production of angiostatin, an endogenous inhibitor of angiogenesis. 8 The present study provides clinical evidence that the angiogenesis of HCC mainly depends on the expression of the HME gene, because 73% of the patients whose tumors expressed HME gene showed moderate or weak staining in hepatic angiograms, and 90% of the patients whose tumors did not express HME gene showed strong staining, indicating that the expression of HME effectively inhibits angiogenesis in HCC (P = .004; see Table 2). In fact, the Wilcoxon-Mann-Whitney test revealed a median HME T/N ratio of 6.5 (range 0–264.5) in the hypovascular group, compared with a median HME T/N ratio of 0 (range 0–3.2) in the hypervascular group (P = .0004).
The correlation of both HME and VEGF gene expression with tumor vascularity confirmed that both factors are significantly associated with the vascularity of HCC; however, it seems to be mainly determined by HME gene expression. In addition, the hypovascular phenotype shown by HME-expressing HCC shifted toward the hypervascular phenotype only in two situations: first, when the VEGF T/N ratio increased by more than twofold over HME mRNA expression levels (patients 9, 13, and 14; see Table 1), and second, when the tumors did not produce angiostatin despite HME gene expression (patient 11). Moreover, in the patients who did not express the HME gene and therefore had hypervascular tumors, a VEGF mRNA expression level of 1.2 or more was associated with stronger vascular staining in the angiograms (as shown in Fig. 3B, patient 22) than in patients whose tumors showed low levels (<1.00) of VEGF mRNA expression (see Fig. 3A, patient 21). Thus, although overproduction of angiogenic stimulators is necessary for neovascularization in HCC, they are not sufficient for the angiogenic switch.
In our series, four patients with HCC whose tumors did not express HME and in addition showed low levels of VEGF mRNA expression had hypervascular tumors (patients 17, 18, 21, and 25). The Northern blot and angiogram results of one of these patients (patient 21) are shown in Figures 2 and 3A, respectively. This might be explained, to a lesser extent, by the participation of other angiogenic factors, such as scatter factor (hepatocyte growth factor), basic fibroblast growth factor, platelet derived-endothelial cell growth factor, or others. 3
This study provides insights into the molecular basis of angiogenesis in HCC. We confirmed that HME-expressing HCCs will develop tumors with a relatively hypovascular phenotype and that it might be turned into a hypervascular phenotype, depending on the levels of VEGF mRNA expression. Thus, it is not difficult to speculate that the hypovascular phenotype shown by HME-expressing HCCs might be due to the presence and activity of angiostatin generated by these tumors through HME (see Table 1).
In conclusion, HME mRNA expression is significantly associated with hypovascular tumors. We demonstrated that the vascularity of HCC does not depend on a single factor, but on a net balance between HME and VEGF. Finally, considering that the development of HCC is strongly dependent on neovascularization, the molecular mechanism of angiogenesis demonstrated here provides bases for the establishment of a novel therapeutic and preventive strategy against HCC. Thus, further studies are needed focusing on the blockade of angiogenesis by enhancing HME gene expression by specific gene transfer or through the suppression of VEGF gene expression by using an antisense approach, such as VEGF gene targeting or antibodies. Then, combinations of conventional chemotherapy and antiangiogenic gene therapy may improve the survival rate after surgery.
Acknowledgments
The authors thank Dr. Shunzo Maetani and Dr. Yoshihiro Yamazoe for assistance with statistical analyses and critical reading of this manuscript and Beth Chamberlin and Didier Guillot for their excellent technical assistance.
Footnotes
Correspondence: Manuel J. Gorrin-Rivas, MD, Dept. of Surgery & Surgical Basic Science, Graduate School of Medicine, Kyoto University, 54 Shogoin, Kawara-cho, Sakyo-ku, Kyoto 606-8507, Japan.
Supported in part by grant 07457271 from the Ministry of Education, Science, Sports and Culture of Japan.
Accepted for publication July 22, 1999.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285:1182–1186. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. What is the evidence that tumors are angiogenesis dependent? JNCI 1990; 82:4–6. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 1995; 333:1757–1763. [DOI] [PubMed] [Google Scholar]
- 4.Uhr JW, Scheuermann RH, Street NE, Vitetta ES. Cancer dormancy: opportunities for new therapeutic approaches. Nat Med 1997; 3:505–509. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1:27–31. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86:353–364. [DOI] [PubMed] [Google Scholar]
- 7.Powell WC, Matrisian LM. Complex roles of matrix metalloproteinases in tumor progression. Curr Top Microbiol Immunol 1996; 213:1–21. [DOI] [PubMed] [Google Scholar]
- 8.Gorrin-Rivas MJ, Arii S, Furutani M, et al. Expression of human macrophage metalloelastase gene in hepatocellular carcinoma: correlation with angiostatin generation and its clinical significance. Hepatology 1998; 28:986–993. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produce by human alveolar macrophages. J Biol Chem 1993; 268:23824–23829. [PubMed] [Google Scholar]
- 10.Belaaouaj A, Shipley JM, Kobayashi DK, et al. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem 1995; 270:14568–14575. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79:315–328. [DOI] [PubMed] [Google Scholar]
- 12.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 1997; 88:801–810. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Houck KA, Jakeman LB, et al. The vascular endothelial growth factor family of polypeptides. J Cell Biochem 1991; 47:211–218. [DOI] [PubMed] [Google Scholar]
- 14.Connolly DT. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem 1991; 47:219–223. [DOI] [PubMed] [Google Scholar]
- 15.De Vries C, Escobedo JA, Ueno H, et al. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992; 255:989–991. [DOI] [PubMed] [Google Scholar]
- 16.Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992; 187:1579–1586. [DOI] [PubMed] [Google Scholar]
- 17.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995; 26:86–91. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Kitadai Y, Bucana CD, et al. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55:3964–3968. [PubMed] [Google Scholar]
- 19.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993; 53:4727–4735. [PubMed] [Google Scholar]
- 20.Mise M, Arii S, Higashitsuji H, et al. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology 1996; 23:455–464. [DOI] [PubMed] [Google Scholar]
- 21.Liver Cancer Study Group of Japan. Classification of Primary Liver Cancer, 1st English ed. Tokyo: Kanehara; 1997: 8–38.
- 22.Okuda K, Kondo Y. Primary carcinoma of the liver. In: Haubrich W, Schaffner F, Berk JE, eds. Gastroenterology, Vol. 3, 5th Ed. Philadelphia: Saunders; 1995: 2444–2487.
- 23.Sakamoto M, Ino Y, Fujii T, Hirohashi S. Phenotype changes in tumor vessels associated with the progression of hepatocellular carcinoma. Jpn J Clin Oncol 1993; 23:98–104. [PubMed] [Google Scholar]
- 24.Terada T, Nakanuma Y. Arterial elements and perisinusoidal cells in borderline hepatocellular nodules and small hepatocellular carcinomas. Histopathology 1995; 27:333–339. [DOI] [PubMed] [Google Scholar]
- 25.Mazzanti R, Messerini L, Monsacchi L, et al. Chronic viral hepatitis induced by hepatitis C but not hepatitis B virus infection correlates with increased liver angiogenesis. Hepatology 1997; 25:229–234. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H, Nakajima T, Kagawa K, et al. Angiogenesis in hepatocellular carcinoma as evaluated by CD34 immunohistochemistry. Liver 1998; 18:14–19. [DOI] [PubMed] [Google Scholar]