Abstract
Objective
To investigate the suitable combination ratio of low-residue diet (LRD) and parenteral nutrition (PN) for nutritional support of surgical patients.
Summary Background Data
Bacterial translocation (BT) is a severe complication of total parenteral nutrition (TPN). However, it is sometimes impossible to supply sufficient amounts of nutrients to surgical patients by the enteral route. The authors reported previously that concomitant use of LRD with PN provided preferable nutritional support for patients undergoing surgery for colorectal cancer.
Methods
Ninety male Donryu rats were used for three experiments. In experiment 1, rats were divided into two groups to receive TPN or total enteral nutrition with LRD. In experiment 2, rats were divided into six groups, receiving variable amounts of LRD. In experiment 3, rats were divided into five groups to receive isocaloric nutritional support with variable proportions of PN and LRD. Intestinal permeability was assessed by monitoring urinary excretion of phenolsulfonphthalein. BT was assessed in tissue cultures of mesenteric lymph nodes and spleen.
Results
In experiment 1, increases in intestinal permeability and BT were observed in rats maintained on 7-day TPN, but not in those maintained on total enteral nutrition for up to 14 days. In experiment 2, the changes in body weight of rats were correlated with the dose of LRD. However, the intestinal permeability was increased only in rats receiving LRD at 15 kcal/kg per day. In experiment 3, additive LRD corresponding to 15% of total caloric intake prevented increases in intestinal permeability and BT.
Conclusion
Combined nutritional therapy consisting of PN and small amounts of LRD can provide better nutritional support than TPN for surgical patients.
Total parenteral nutrition (TPN) provides significant benefit in surgical patients who are not allowed to eat. However, TPN is complicated by bacterial translocation (BT). 1 Disuse of the alimentary tract in TPN causes not only a decrease in its own function, but also a decrease in the intestinal mucosal integrity. TPN has also been reported to increase sympathetic nervous activity. 2 Such conditions caused by derangement of intestinal defense mechanisms cause the development of BT or increases in intestinal permeability of macromolecules. 3,4 BT is considered one of the main causes of severe postoperative infections, including septicemia and pneumonia. Further, BT or increases in intestinal permeability may lead to the development of severe liver injury, especially in infants. 5,6 Therefore, It is important to maintain intestinal mucosal integrity during nutritional support in surgical patients. Elemental diet (ED) has also been reported to cause BT. 7–9 In contrast, low-residue diet (LRD) has not been reported to induce BT. However, it is sometimes impossible to supply sufficient amounts of nutrients to surgical patients with LRD.
We have reported previously that concomitant use of parenteral nutrition (PN) and LRD provided preferable nitrogen balance and protective effects on the liver in patients undergoing surgery for colorectal cancer. 10 Such combined nutritional therapy (CNT) preserved the normal pattern of enteroglucagon secretion (unpublished data). CNT consisting of a small amount of LRD and PN can prevent the decrease in intestinal mucosal integrity during nutritional support in surgical patients. In this study, we investigated whether total enteral nutrition (TEN) using LRD can preserve intestinal mucosal integrity; we also assessed the effects of varied amounts of LRD on intestinal permeability and tried to determine a suitable combination ratio of LRD and PN for nutritional support in surgical patients.
MATERIALS AND METHODS
Three sets of experiments were performed. Ninety male Donryu rats (Shizuoka Laboratory Animal Inc., Hamamatsu, Japan), weighing 200 to 250 g, were housed under barrier-sustained conditions in temperature-controlled rooms under a light/dark cycle. All rats received humane care in accordance with the National Research Council’s guide for the care and use of laboratory animals. Before experiments, they were fed rat chow and water ad libitum for 7 days. The LRD used throughout the study was Isocal (Mead Johnson Nutritionals, Evansville, IN). PN solution was prepared using Hicaliq NC-H (Terumo Corp., Tokyo, Japan) as a dextrose solution containing minerals, Amiparen (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) as a mixture of amino acids, and Intralipos (Green Cross Co., Ltd., Tokyo, Japan) as a lipid emulsion. The composition of PN solution was adjusted to that of Isocal (Table 1).
Table 1. FORMULAS OF NUTRITIONAL SOLUTIONS
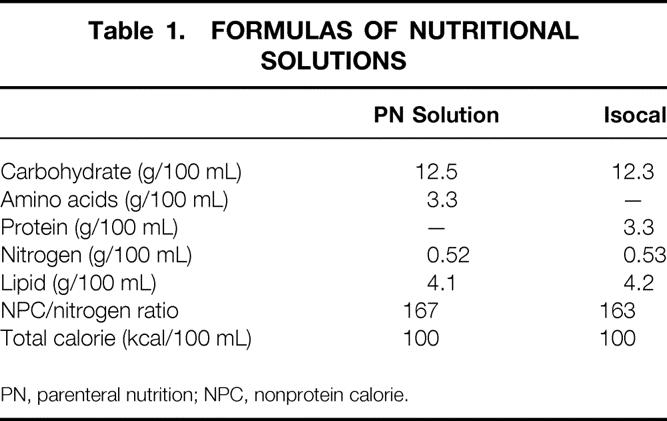
PN, parenteral nutrition; NPC, nonprotein calorie.
Experiment 1
This experiment was designed to observe the changes in intestinal permeability and the incidence of BT in TPN- or TEN-nourished rats. Twenty-four rats underwent placement of a central venous catheter from the jugular vein. The incision was closed in one layer. Then, the animals were randomized and received one of two feeding regimens: all of the nutrients with PN via the catheter (TPN groups) for 7 (n = 6) or 14 days (n = 6); or all of the nutrients with oral LRD (TEN groups) for 7 (n = 6) or 14 days (n = 6). The caloric intake in each group was carefully adjusted to 300 kcal/kg per day. The nitrogen intake and fat intake of each group were equalized. The changes in body weight were measured during the 7- or 14-day study period. Twenty-four hours before being killed, animals were gavaged with 10 mg phenolsulfonphthalein (PSP), and 24-hour urinary excretion of PSP was quantified. After the animals were killed, the mesenteric lymph nodes (MLNs) and spleen were removed, homogenized, and expanded to 5 mL with sterile 0.8% saline. Portions (1 μL) of the MLNs and splenic homogenate were cultured on blood agar for detection of gram-positive cocci, or on bromothymol blue agar for gram-negative enteric bacilli. The plates were examined after 24 or 48 hours of incubation at 37°C. Portions (1 μL) of the MLNs and splenic homogenate were also cultured anaerobically inside an anaerobic globe box on Brucella agar for 48 hours at 37°C.
Experiment 2
This experiment was designed to observe the effects of variable amounts of LRD on the changes in intestinal permeability and the incidence of BT. Thirty-six rats were randomized to receive one of six feeding regimens for 7 days: 15, 30, 60, 120, 180, or 300 kcal/kg per day of Isocal. Each group contained six rats. The caloric intake of each group was strictly regulated, but the rats were allowed free access to water. The changes in body weight were measured during the 7-day experimental period. Twenty-four hours before being killed, animals were gavaged with PSP, and 24-hour urinary excretion of PSP was quantified. After the animals were killed, the MLNs and spleen were removed. Culture of MLNs and spleen was performed in the same way as in experiment 1.
Experiment 3
This experiment was designed to observe the effects of CNT regimens consisting of variable ratios of LRD and PN on the changes in intestinal permeability and the incidence of BT. Thirty rats underwent placement of a central venous catheter from the jugular vein and tube gastrostomy with laparotomy. After tube placement, the intestines were displaced from the abdomen, exposed to the air for 30 minutes, then returned to the abdomen. This gut exposure is considered to provide additive stress, which better reflects the clinical conditions in surgical patients. 11 The cervical incision was closed in one layer, the abdominal incision in two layers. Then, the animals were randomized to receive one of five feeding regimens via the catheters for 7 days: 300 kcal/kg per day of TPN, 15 kcal/kg per day of Isocal + 285 kcal/kg per day of TPN, 30 kcal/kg per day of Isocal + 270 kcal/kg per day of TPN, 45 kcal/kg per day of Isocal + 255 kcal/kg per day of TPN, or 60 kcal/kg per day of Isocal + 240 kcal/kg per day of TPN. Each group contained six rats. The nitrogen intake and fat intake of the five groups were equalized. The changes in body weight were measured during the 7-day experimental period. Twenty-four hours before being killed, the animals were gavaged with PSP, and 24-hour urinary excretion of PSP was quantified. After being killed, the MLNs and spleen were removed. Culture of MLNs and spleen was performed in the same way as in experiment 1.
Statistical Analysis
The results are expressed as means ± SD. Changes in body weight, urinary excretion of PSP, wet liver weight ratio, and wet spleen weight ratio were analyzed using analysis of variance. Proportion comparisons for cultures of MLNs and spleen were analyzed using the chi-square test. Statistical significance was taken as P < .05.
RESULTS
Experiment 1
Rats underwent only placement of a central venous catheter and were used as nearly nonstressed model animals. Both groups studied showed a gain in whole body weight during the study period, although the rats in the TPN group gained more weight than those in the TEN group (Table 2, P < .05). Average weight changes of the two groups over 7 days were comparable with that of normal rats fed standard laboratory chow (data not shown). The urinary excretion rate of PSP in each group showed no change throughout the study period. However, it was significantly higher in the TPN group than in the TEN group on days 7 and 14 (P < .01).
Table 2. RESULTS OF EXPERIMENT 1
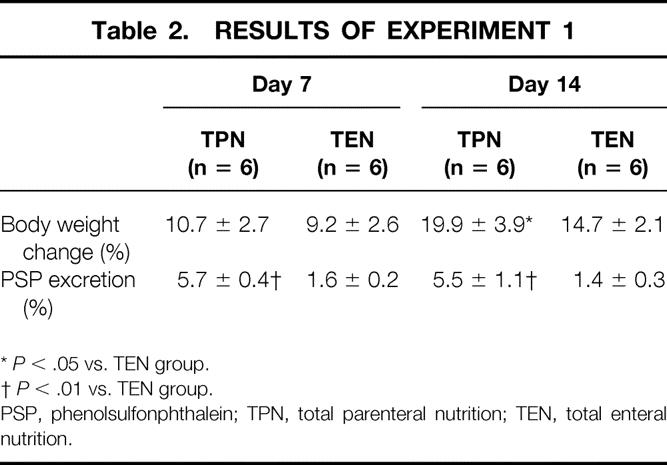
*P < .05 vs. TEN group.
†P < .01 vs. TEN group.
PSP, phenolsulfonphthalein; TPN, total parenteral nutrition; TEN, total enteral nutrition.
BT did not occur in the TEN group on day 7. However, one third of the MLNs and spleen were culture-positive in the TPN group on day 7. The culture-positive rate of the spleen in the TPN group was further increased on day 14. The culture-positive frequency of the spleen in the TPN group was significantly higher than that in the TEN group on day 14 (Table 3, P < .01).
Table 3. CULTURE-POSITIVE RATIOS IN EXPERIMENT 1
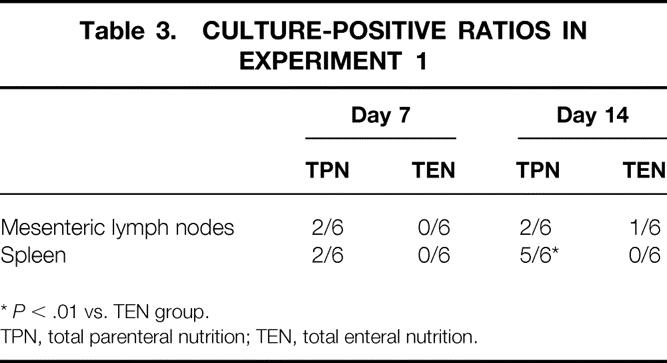
*P < .01 vs. TEN group.
TPN, total parenteral nutrition; TEN, total enteral nutrition.
Experiment 2
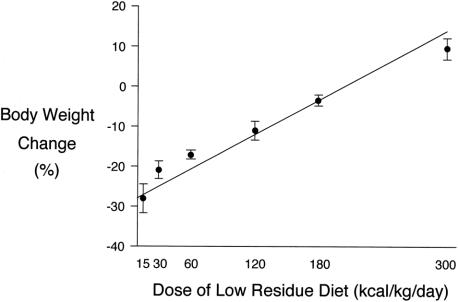
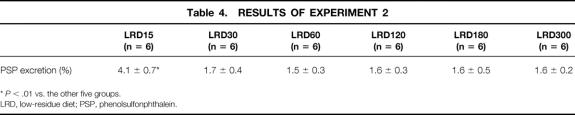
The rats in this experiment underwent no stress. The whole body weight gain ratio at the time of death was correlated with the calories provided in each group (Fig. 1, Y = 0.14X–27.88, r = 0.981, P < .01). The rats that received 15 kcal/kg Isocal showed a significantly higher urinary excretion rate of PSP than those in the other five groups (Table 4, P < .01). There were no significant differences in the urinary excretion rates of PSP between the other groups. BT occurred in none of the groups.
Figure 1. Body weight changes in experiment 2. The body weight changes of the rats were significantly correlated with the calories provided in each group.
Table 4. RESULTS OF EXPERIMENT 2
*P < .01 vs. the other five groups.
LRD, low-residue diet; PSP, phenolsulfonphthalein.
Experiment 3
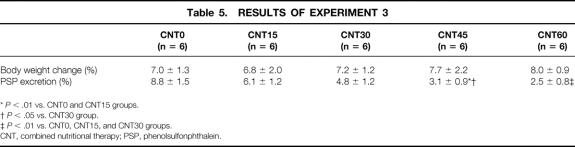
These animals were used as stressed models. All groups studied showed a gain in whole body weight during the study period. The changes in body weight were almost identical among the five groups (Table 5). The urinary excretion rate of PSP decreased with increases in the ratio of LRD. Significant decreases in the urinary excretion rate of PSP were observed in the groups that received 45 kcal/kg Isocal + 255 kcal/kg TPN and 60 kcal/kg Isocal + 240 kcal/kg TPN compared with the other three groups. Culture of MLNs and spleen were positive in half and one third of the rats that received 300 kcal/kg TPN, respectively (Table 6). The incidence of BT was reduced when the ratio of LRD was increased. In the groups that received 45 kcal/kg Isocal + 255 kcal/kg TPN and 60 kcal/kg Isocal + 240 kcal/kg TPN, cultures of MLNs and spleen were negative. The culture-positive frequency of MLNs in the group that received 300 kcal/kg TPN was significantly higher than those in the groups that received 45 kcal/kg Isocal + 255 kcal/kg TPN and 60 kcal/kg Isocal + 240 kcal/kg TPN (P < .05).
Table 5. RESULTS OF EXPERIMENT 3
*P < .01 vs. CNT0 and CNT15 groups.
†P < .05 vs. CNT30 group.
‡P < .01 vs. CNT0, CNT15, and CNT30 groups.
CNT, combined nutritional therapy; PSP, phenolsulfonphthalein.
Table 6. CULTURE-POSITIVE RATIOS IN EXPERIMENT 3
*P < .05 vs. CNT45 and CNT60 groups.
CNT, combined nutritional therapy.
DISCUSSION
The gastrointestinal tract has important immunologic and barrier functions, in addition to its roles in nutrient digestion and absorption. TPN or TEN using ED causes loss of intestinal barrier function, which allows the translocation of indigenous intestinal bacteria into the lymphatic system and the portal vein and systemic circulation. 9 TPN or TEN using ED also produces local and systemic immunologic impairment, including degenerative changes within the gut-associated lymphoid tissue and defective host pulmonary antimicrobial immune responses. 12,13 The immunologic impairments cause the infectious complications after BT to become more severe. Therefore, breakdown of the mucosal barrier due to TPN sometimes results in increased infection, which leads to death. Consequently, prevention of the decrease in intestinal mucosal integrity during nutritional support is needed in the fields of surgical metabolism and nutrition.
Early enteral feeding after a severe burn injury or major surgery prevents hypermetabolism, 14,15 maintains immunocompetence, 16–19 and improves wound healing. 20 Further, enteral feeding is considered to reduce septic complications, shorten the hospital stay, and reduce the risk of death. 21,22 However, it is often impossible to provide nutritional support in surgical patients solely by enteral feeding. We have reported previously that CNT provided excellent nutritional support and should overcome the drawbacks of TPN or TEN using ED. 10 CNT consisting of parenteral nutrition and small amounts of LRD seems to be better tolerated by surgical patients than TEN.
In this study, we used 24-hour urinary excretion of PSP as an index of intestinal permeability of macromolecules. PSP (molecular weight 354) is not metabolized in the body and is excreted in the urine from proximal renal tubules at a ratio of 90% when it is present in the blood. However, its absorption ratio is low when administered into the normal gastrointestinal tract because of its low affinity for the gastrointestinal mucosa as well as its poor lipid solubility. 23 Consequently, the increase in urinary excretion of PSP is considered to be the result of increased intestinal permeability. 24 Nakamura et al 25 reported that drug- or stress-induced gastric mucosal damage can be assessed by measurement of the urinary recovery rate of PSP after its oral administration—that is, the recovery rate of PSP correlated well with the extent of mucosal damage. Moreover, the measurement of PSP is simple and costs less than quantification of the other macromolecules used to assess intestinal permeability, including lactulose, polyethyleneglycol, and 51Cr-ethylenediaminetetraacetic acid. However, there is no evidence that the intestinal permeability assessed using PSP correlates with the passage of endotoxin by the intestinal mucosa.
The results of experiment 1 indicated that intestinal permeability was increased even in nearly nonstressed rats maintained on TPN. Further, BT developed in the TPN group on day 7. In contrast, BT did not develop in rats maintained on TEN using LRD. The absence of enteral stimulation should result in a decrease in intestinal integrity, even when the animals undergo almost no stress and gain sufficient body weight with TPN. Several components of food or enteral nutrition, including dietary fiber and protein, should have protective effects on the intestinal mucosa. Some studies have suggested that dietary fiber added to enteral nutrition prevents BT. 26,27 Deitch 28 reported that the type of fiber is important: bulk-forming but nonfermentable fiber was more effective in maintaining barrier function than easily fermentable types during systemic inflammation. However, Isocal contains no dietary fiber. The efficacy of dietary supplementation with glutamine remains controversial. 29–31 Isocal contains casein, a glutamine-containing protein, but no added glutamine. The type of nitrogen source is also considered to be important. Zaloga et al 32 compared the gut mass of rats maintained on three isocaloric and isonitrogenous liquid feeding formulas differing in their types of protein—an intact protein formula, a peptide formula, and an amino acid formula. Proximal and midgut mass were comparable in animals fed all three of these diets, but distal gut mass was significantly reduced in the group given the amino acid diet. Because amino acids are easily absorbed, they cannot reach the distal small intestine and therefore have no stimulatory effect. Casein probably played an important role in preventing the increase in intestinal permeability and BT observed in this study.
In experiment 2, intestinal permeability was significantly increased in the group that received 15 kcal/kg of Isocal compared with the other groups. A decrease in intestinal integrity, assessed by macromolecular permeability, developed in nonstressed rats under conditions of severe malnutrition. However, there were no significant differences in intestinal permeability between the other five groups, regardless of the variations in body weight changes. In the group that received 30 kcal/kg Isocal, the rats lost >20% of their body weight, but 24-hour urinary excretion ratio of PSP was similar to those in the groups receiving 60 kcal/kg per day of LRD or more. Small amounts of LRD, at a dose of only 30 kcal/kg per day, prevented the increase in intestinal permeability in this experimental model. BT occurred in none of the groups. These observations supported the conclusion that BT and macromolecular permeability are not strongly correlated. 33,34 Zaloga et al 11 studied gut weight and protein content after the postoperative provision of varying amounts of peptide diet in rats. Chow diet, which supplied only 30% of the recommended daily allowance, was almost as good for maintaining gut mass as a defined, fiber-free peptide diet given at a dose of 100% of the recommended daily allowance. Peptide diets given at lower rates induced greater body weight loss but did not decrease mucosal protein levels in various parts of the small intestine. However, intestinal permeability or BT was not assessed in their study. Gut mass and mucosal protein levels may be irrelevant to intestinal permeability under some conditions.
The results of experiment 3 suggested that small amounts of LRD, administered concomitantly with PN, prevent the increase in intestinal permeability and BT in stressed rats. Intestinal permeability was significantly decreased when LRD was added to >15% of the total caloric intake. The urinary excretion ratio of PSP was reduced to <40% in the group that received 45 kcal/kg Isocal + 255 kcal/kg TPN compared with the group that received 300 kcal/kg TPN. The urinary excretion ratios of the groups that received 300 kcal/kg TPN and the group that received 15 kcal/kg Isocal + 285 kcal/kg TPN were both >6%. No such high excretion ratios were observed in experiment 1 or 2. The rats in experiment 3 underwent far more stress than those in the other two experiments, which may have been responsible for the high urinary excretion ratios observed in experiment 3. BT did not occur when LRD was added to >15% of the total calorie intake. Sax et al 35 reported that partial enteral nutrition, with 25% of the total calories as enteral nutrition, improved nitrogen balance and reduced the incidence of BT. They administered a semisolid, nonscatterable 20% protein, casein-based paste orally as enteral nutrition. However, partial enteral nutrition did not alter intestinal permeability to macromolecules, as determined by monitoring urinary excretion of lactulose and mannitol. In our study, the increase in intestinal permeability assessed by urinary excretion of PSP was prevented by adding LRD accounting for 15% of the total caloric intake. The molecular weight of lactulose is approximately 342, similar to that of PSP. We started enteral nutrition using LRD via gastrostomy just after catheter placement. In contrast, Sax et al 35 began administering nutrients 24 hours after central venous catheter placement. The difference in the interval between surgery and commencement of enteral feeding in these two studies may have been responsible for the discrepancies regarding intestinal permeability. Immediate enteral feeding seems to be better for maintaining intestinal integrity than that started after a delay, even if the amount supplied is small.
TPN is used in patients unable to absorb nutrients from the gastrointestinal tract, including those with short bowel, pancreatitis, and ileus. However, it is sometimes possible to provide limited enteral nutrition by a nasojejunal tube or jejunostomy in such patients, with complementary parenteral nutrition. Additional LRD is considered to prevent decreases in intestinal mucosal integrity. Therefore, CNT consisting of PN and small amounts of LRD should be of benefit for surgical patients who cannot absorb their full nutritional need. CNT should provide better nutritional support for patients in the early stages of short-bowel syndrome. The hypermetabolic state after major surgery with a severe decrease in digestive function is also a good indication for CNT. Pancreatoduodenectomy is a typical surgical procedure that causes such a situation. It is also possible to introduce enteral nutrition to patients with severe burns or pancreatitis much earlier by CNT. Immediate postoperative CNT also provides better nutritional support to some patients undergoing surgery for diseases of the gastrointestinal tract. 10
In conclusion, an increase in intestinal permeability and BT occurred in nearly nonstressed rats receiving TPN for 7 days but not in those receiving TEN using LRD. An increase in intestinal permeability also develops in nonstressed rats receiving LRD at only 15 kcal/kg per day. However, it did not occur when the amount of LRD was increased to 30 kcal/kg per day, even if severe body weight loss occurred. Additive LRD with PN prevented the increase in intestinal permeability and BT in stressed rats. Further, additive LRD corresponding to 15% of the total caloric intake seemed to be sufficient to prevent such phenomena. CNT consisting of PN and small amounts of LRD can provide better nutritional support than TPN for surgical patients. Further studies are required to estimate the changes in intestinal permeability and the incidence of BT when CNT is given for >7 days. It is also necessary to determine the effects of small amounts of LRD on the other negative consequences of gut disuse, including immunologic impairment and hypermetabolism.
Footnotes
Correspondence: Kenji Omura, MD, Department of Surgery 1, Kanazawa University Faculty of Medicine School of Medicine, Takaramachi 13-1, Kanazawa, 920-8641 Japan.
Accepted for publication May 19, 1999.
References
- 1.Alverdy JC, Ayos E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988; 104:185–190. [PubMed] [Google Scholar]
- 2.Helton WS, Rockwell M, Garcia RM, et al. TPN-induced sympathetic activation is related to diet, bacterial translocation and an intravenous line. Arch Surg 1995; 130:209–214. [DOI] [PubMed] [Google Scholar]
- 3.Deitch EA, Winterton J, Li M, Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg 1987; 205:681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama M, Xu D, Specian RD, Deitch EA. Role of bacterial adherence and the mucus barrier on bacterial translocation: effect of protein malnutrition and endotoxin in rats. Ann Surg 1997; 225:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pallares R, Sitges-Serra A, Fuentes J, et al. Cholestasis associated with total parenteral nutrition. Lancet 1983; 8327:758–759. [PubMed] [Google Scholar]
- 6.Brown RS. Cholestasis in association with short-term parenteral alimentation. Crit Care Med 1976; 4:313–316. [DOI] [PubMed] [Google Scholar]
- 7.Haskel Y, Xu D, Lu Q, Deitch E. Elemental diet-induced bacterial translocation can be hormonally modulated. Ann Surg 1993; 217:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Lu Q, Deitch EA. Elemental diet-induced bacterial translocation associated with systemic and intestinal immune suppression. JPEN 1998; 22:37–41. [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA, Xu D, Naruhn MB, et al. Elemental diet and IV-TPN induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg 1995; 221:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omura K, Kanehira E, Ishida F, et al. Efficacy of combined nutritional therapy in nutritional support following colorectal surgery. Jpn J Surg Metabol Nutr 1993; 27:317–324. [Google Scholar]
- 11.Zaloga GP, Black KW, Prielipp R. Effect of rate of enteral nutrient supply on gut mass. JPEN 1992; 16:39–42. [DOI] [PubMed] [Google Scholar]
- 12.Shou J, Lappin J, Daly JM. Impairment of pulmonary macrophage function with total parenteral nutrition. Ann Surg 1994; 219:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janu P, Li J, Reneger KB, Kudsk KA. Recovery of gut-associated lymphoid tissue and upper respiratory tract immunity after parenteral nutrition. Ann Surg 1997; 225:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mochizuki H, Trocki O, Dominioni L, et al. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg 1984; 200:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bower RH. Nutritional and metabolic support of critically ill patients. JPEN 1990; 14(Suppl):257–259. [DOI] [PubMed] [Google Scholar]
- 16.Alverdy J, Chi HS, Sheldon GF. The effect of parenteral nutrition on gastrointestinal immunity: the importance of enteral stimulation. Ann Surg 1985; 202:681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer J, Yurt RW, Duhaney R, et al. Differential neutrophil activation before and after endotoxin infusion in enterally versus parenterally fed volunteers. Surg Gynecol Obstet 1988; 167:501–509. [PubMed] [Google Scholar]
- 18.Gianotti L, Alexander JW, Nelson JL. Role of early enteral feeding and acute starvation on postburn bacterial translocation and host defense: prospective, randomized trials. Crit Care Med 1994; 22:265–272. [DOI] [PubMed] [Google Scholar]
- 19.Shirabe K, Matsumata T, Shimada M, et al. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection—the results of a randomized prospective study. Hepato-Gastroenterology 1997; 44:205–209. [PubMed] [Google Scholar]
- 20.Schroeder D, Gillanders L, Mahr K, et al. Effects of immediate postoperative enteral nutrition on body composition muscle function, and wound healing. JPEN 1991; 15:376–383. [DOI] [PubMed] [Google Scholar]
- 21.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma: reduced septic morbidity. J Trauma 1989; 29:916–923. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JW, Gottschlich MM. Nutritional immunomodulation in burn patients. Crit Care Med 1990; 18(Suppl):149–153. [PubMed] [Google Scholar]
- 23.Nakamura J, Yoshizaki Y, Yasuhara M, et al. Mechanism of the absorption of water-soluble dyes from the rat small intestine. Chem Pharm Bull 1976; 24:683–690. [DOI] [PubMed] [Google Scholar]
- 24.Toh Y, Korenaga D, Maekawa S, et al. Assessing the permeability of the gastrointestinal mucosa after oral administration of phenolsulfonphthalein. Hepato-Gastroenterology 1997; 44:1147–1151. [PubMed] [Google Scholar]
- 25.Nakamura J, Yoshizaki Y, Yasuhara M, et al. Role of membrane components, glycocalyx and lipid in absorption of water-soluble dyes from the rat small intestine. Chem Pharm Bull 1976; 24:691–697. [DOI] [PubMed] [Google Scholar]
- 26.Frankel W, Zhang W, Singh A, et al. Fiber: effect on bacterial translocation and intestinal mucin content. World J Surg 1995; 19:144–148. [DOI] [PubMed] [Google Scholar]
- 27.Zapata Sirvent RL, Hansbrough JF, Ohara MM, et al. Bacterial translocation in burned mice after administration of various diets including fiber- and glutamine-enriched enteral formulas. Crit Care Med 1994; 22:690–696. [DOI] [PubMed] [Google Scholar]
- 28.Deitch EA. Bacterial translocation: the influence of dietary variables. Gut 1994; 35S:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaeth G, Gottwald T, Haas W, Holmer M. Glutamine peptide does not improve gut barrier function and mucosal immunity in total parenteral nutrition. JPEN 1993; 17:317–323. [DOI] [PubMed] [Google Scholar]
- 30.Gianotti L, Alexander JW, Gennari R, et al. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. JPEN 1995; 19:69–74. [DOI] [PubMed] [Google Scholar]
- 31.Morlion BJ, Stehle P, Wachtler P, et al. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg 1998; 227:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaloga GP, Ward KA, Prielipp RC. Effect of enteral diets on whole body and gut growth in unstressed rats. JPEN 1991; 15:42–47. [DOI] [PubMed] [Google Scholar]
- 33.O’Dwyer ST, Michie HR, Ziegler TR, et al. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg 1988; 123:1459–1464. [DOI] [PubMed] [Google Scholar]
- 34.Illig KA, Ryan CK, Hardy DJ, et al. Total parenteral nutrition-induced changes in gut mucosal function: atrophy alone is not the issue. Surgery 1992; 112:631–637. [PubMed] [Google Scholar]
- 35.Sax HC, Illig KA, Ryan CK, et al. Low-dose enteral feeding is beneficial during total parenteral nutrition. Am J Surg 1996; 171:587–590. [DOI] [PubMed] [Google Scholar]