Abstract
Objective
To clarify the effects of lung volume reduction surgery (LVRS) on cardiopulmonary circulation during exercise in comparison with pulmonary lobectomy for lung cancer.
Summary Background Data
LVRS improves pulmonary function and dyspnea symptoms acutely in selected patients with heterogeneous emphysema. However, there are few data concerning the effects of LVRS on the cardiopulmonary circulation, especially during exercise.
Methods
Pulmonary function tests and pulmonary hemodynamic study at rest and during exercise were performed before and 6 months after LVRS (seven patients) or pulmonary lobectomy (eight patients). In the workload test, an electrically braked bicycle ergometer (25 w) was used in the supine position for at least 2 minutes or until exhaustion or breathlessness developed.
Results
After lung lobectomy, the values of vital capacity, percentage of predicted vital capacity, forced expiratory volume in 1 second, percentage of predicted forced expiratory volume in 1 second, residual volume/total lung capacity, and maximal voluntary ventilation deteriorated significantly. Six months after LVRS, however, vital capacity, percentage vital capacity showed no significant change, and forced expiratory volume in 1 second, percentage of forced expiratory volume in 1 second, diffusing capacity for carbon monoxide, and maximal voluntary ventilation showed marked improvement. Cardiac index was changed neither at rest nor during exercise in either group by the operation. Although postoperative pulmonary arterial pressure in the lobectomy group was significantly increased by the exercise, LVRS did not affect postoperative pulmonary arterial pressure at rest or during exercise. Pulmonary capillary wedge pressure in the lobectomy group showed no significant change after the operation, whereas LVRS ameliorated the marked elevation of pulmonary capillary wedge pressure observed during exercise. After lobectomy, significant increases in the pulmonary vascular resistance index were observed at rest and during exercise. LVRS markedly increased the pulmonary vascular resistance index at rest but not during exercise. In the lobectomy group, the postoperative flow-pressure curve moved upward, and its gradient became steeper than the preoperative one. In the LVRS group, the curve moved upward in a parallel fashion. These results show that much more right-sided heart work is needed to achieve the same cardiac output against higher pulmonary arterial pressure, not only after lobectomy but also LVRS.
Conclusion
The current study demonstrated that the effects of LVRS on the cardiopulmonary circulation were not negligible, especially during exercise, and successful LVRS may depend on improved respiratory function and also preserved cardiac function that can tolerate the damage to the pulmonary vascular bed induced by this operation.
Lung volume reduction surgery (LVRS) has been introduced to treat severe emphysema 1 and is now an established procedure to treat patients with diffuse emphysema. 2,3 Recently, several reports 1,2,4–7 have demonstrated its beneficial effects on lung function and chest wall mechanisms. However, the range of improvement in lung functions, especially forced expiratory volume in 1 second (FEV1), has varied widely in each report. In the early reports, this was attributable to the use of different surgical techniques, but in more recent reports it has been mainly due to different patient selection criteria. 8 Pulmonary artery pressure (or other parameters demonstrating the condition of the pulmonary circulation) is one of the most important criteria for selection of LVRS at some centers, 1,9,10 where patients with a mean pulmonary artery pressure <30 or 35 mmHg are preferable for LVRS. However, there are no published data to support this criterion, and also no data to show the effect of LVRS on the cardiopulmonary circulation. In this study, we assessed the effects of LVRS on the cardiopulmonary circulation compared with the effects of pulmonary lobectomy for lung cancer. We also considered whether the criterion of pulmonary artery pressure <35 mmHg is appropriate in the preoperative assessment for LVRS.
PATIENTS AND METHODS
Patients
Eight patients with severe pulmonary emphysema and without giant bullae underwent bilateral LVRS at Shinshu University Hospital between March 1995 and April 1997. Because one of the eight patients died of asthma 5 months after surgery, the remaining seven patients participated in this study. They were all men, ranging in age from 56 to 75 years (mean 65 years). They were heavy smokers, with an average Brinkmann index (BI; cigarettes per day × years) of 1,367 ± 269. The patients, diagnosed by clinical history of exertional dyspnea and smoking and the clinical features of irreversible airway obstruction, lung hyperinflation, decrease in diffusing capacity for carbon monoxide (DLCO), and anatomic emphysema on computed tomography scanning of the chest, were selected in accordance with the criteria for surgery described by Cooper et al. 2
Eight male patients with bronchial carcinoma, considered to be candidates for lobectomy, participated in this study as a control group. The mean age was 66 years (range 55–75 years), and all were heavy smokers (BI 938 ± 361). They underwent pulmonary lobectomy in the same period as LVRS and had pre- and postoperative evaluation consisting of extensive pulmonary function testing and a graded exercise test with right-sided heart catheterization. In these patients, recurrence of the carcinoma was not observed during the 6-month follow-up period.
The patients gave written consent to participate in the surgery and the pulmonary hemodynamic study after they had been informed of the nature of all procedures and possible risks. This hemodynamic study was approved by the Research Committee of Shinshu University School of Medicine.
Surgical Approach
All patients underwent LVRS via a median sternotomy as described by Cooper et al. 1 Briefly, the most emphysematous areas that remained distended with air through lung deflation and that had been previously targeted by chest computed tomography scanning and V/Q scanning were excised, and linear staple lines were reinforced with bovine pericardium to minimize air leaks. The excised lung volume visually estimated was approximately 20% to 30% of the lung. After lung excision, apical pleural tents were created in the first five patients. Pulmonary lobectomy for patients with bronchial carcinoma was performed in the usual fashion via a posterolateral thoracotomy. No severe complications related to the LVRS or lobectomy were observed in this study.
Pulmonary Function Testing
Pulmonary function at rest was tested at the same time the hemodynamic studies were performed each time. Spirometry was performed with a water spirometer (Godart Expirograph; Godart-Statham, Bilthoven, Holland). The vital capacity (VC) and FEV1 were measured, and the percentage of predicted value of VC (%VC) and FEV1 (%FEV1) were calculated. Functional residual capacity was measured with a body plethysmography system (model 1085/D; MedGraphics Co., St. Paul MN), and total lung capacity (TLC) and residual volume (RV) were calculated from this lung volume. Maximal voluntary ventilation (MVV) was calculated from a tracing made while the patient breathed in and out of a spirometer as rapidly as possible for 15 seconds. The obtained value was then multiplied by four to give the volume in liters per minute. DLCO was measured by the single-breath method (Pulmocorder, model R1551S; Anima, Tokyo, Japan).
Hemodynamic Study and Blood Gas Analysis
A 7F Swan-Ganz thermodilution catheter was inserted through an internal jugular vein into the pulmonary artery for measurement of pulmonary arterial pressure (Ppa), pulmonary capillary wedge pressure (Ppcw), and right atrial pressure. Systemic arterial pressure was obtained using a radial artery cannula. All the pressures were continuously monitored using a disposable transducer system (SCK-580; Nihon Koden, Tokyo, Japan) and were recorded on an eight-channel recorder (WT-685G; Nihon Koden). Mean pressure was obtained by electronic averaging. Cardiac output was measured by the thermodilution method using an Edwards model 9520 cardiac output computer (Edwards Laboratories, Santa Ana, CA) and was presented as the mean of triplicate measurements. Heart rate was monitored by electrocardiography. Blood gas tensions was measured with a Radiometer model ABL2 blood gas analyzer (Radiometer, Copenhagen, Denmark).
From the directly measured parameters, the following indices were calculated: cardiac index (CI, L/min/m2) = cardiac output/body surface area; pulmonary vascular resistance index (PVRI, mmHg/L/min/m2) = (mean Ppa − mean Ppcw)/CI; driving pressure (mmHg) = mean Ppa − mean Ppcw.
Measurement of Exercise Performance
Exercise performance was assessed by the 6-minute walking test and an exercise test using a bicycle ergometer. The 6-minute walking distance was measured while the patient breathed supplemental oxygen through a nasal tube to avoid extreme hypoxemia, with SpO2 monitored with a pulse oxygen saturation monitor (Pulsox-8; Teijin Ltd., Osaka, Japan). The test measured the distance the patient could walk in 6 minutes in a corridor. Patients were allowed to rest when necessary but were encouraged to complete as many lengths of the corridor as possible.
In the test using an electrically braked bicycle ergometer (Reclining Ergometer Model WLP-300 ST; Lode Co., Netherlands), each patient was kept in the supine position for 30 minutes before examination. Before the start of the exercise, the patient rested for a few minutes with his feet lifted onto the bicycle ergometer (with the legs at a 0° to 30° angle to the horizontal) for baseline measurements. The workload was 25 W for ≥2 minutes or until exhaustion or breathlessness developed. The measurements at exercise were performed when it became impossible for the patient to continue exercising. Arterial blood samples were obtained at rest and during exercise. All of this exercise study was done under room air. No complications occurred during or after either study.
Experimental Protocol
Pulmonary function tests and pulmonary hemodynamic studies at rest and during exercise were performed before and 6 months after LVRS or pulmonary lobectomy in the same fashion. Only patients undergoing LVRS participated in the 6-minute walking test before and 6 months after surgery.
Statistical Analysis
The measurements are presented as mean ± standard error. Statistical analysis was performed using analysis of variance, P < .05 considered significant.
RESULTS
Effects on Pulmonary Function and Arterial Gas Analysis
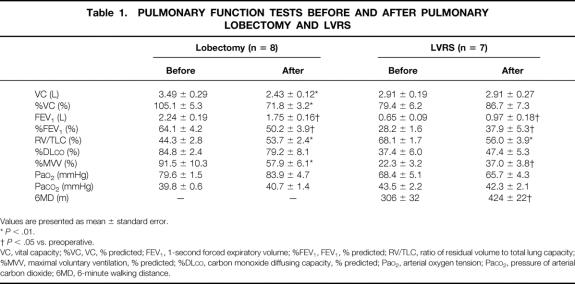
The results of the pulmonary function tests before and after the two operations are summarized in Table 1. After lung lobectomy, the values of VC, %VC, FEV1, %FEV1, RV/TLC, and MVV, all of which indicate the ventilation capacity, deteriorated significantly. %DLCO tended to be worse after surgery. In the arterial blood gas analysis, no statistical change was observed. Six months after LVRS, however, VC and %VC showed no significant change, and FEV1, %FEV1, %DLCO, and MVV showed marked improvement. RV/TLC decreased significantly after LVRS. Arterial gas concentration at rest was not affected by LVRS.
Table 1. PULMONARY FUNCTION TESTS BEFORE AND AFTER PULMONARY LOBECTOMY AND LVRS
Values are presented as mean ± standard error.
*P < .01.
†P < .05 vs. preoperative.
VC, vital capacity; %VC, VC, % predicted; FEV1, 1-second forced expiratory volume; %FEV1, FEV1, % predicted; RV/TLC, ratio of residual volume to total lung capacity; %MVV, maximal voluntary ventilation, % predicted; %DLCO, carbon monoxide diffusing capacity, % predicted; PaO2, arterial oxygen tension; PaCO2, pressure of arterial carbon dioxide; 6MD, 6-minute walking distance.
Effects on Exercise Performance
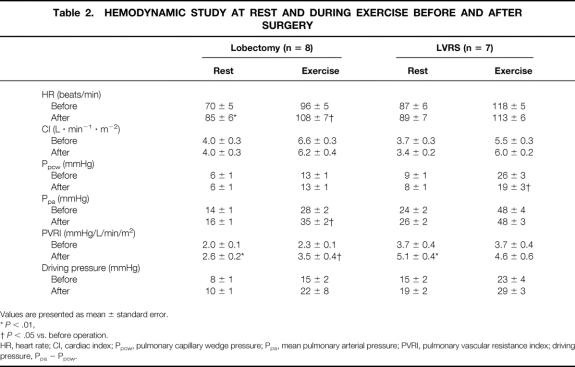
Effects of LVRS on the 6-minute walk are shown in Table 1. Six months after LVRS, marked improvement in the 6-minute walk, from 306 ± 32 m to 424 ± 22 m (P < .01), was observed. Table 2 shows the values of the hemodynamic studies on the patients before and after LVRS and pulmonary lobectomy. Before surgery (Fig. 1), CI in both the LVRS and lobectomy groups was significantly increased by the exercise loading in the same fashion. Ppa and Ppcw values at rest in the LVRS group were higher than in the lobectomy group, but there was no significant difference. After exercise loading, Ppa and Ppcw in both groups were markedly increased. However, these postexercise values in the LVRS group were significantly higher than those in the lobectomy group (P < .01).
Table 2. HEMODYNAMIC STUDY AT REST AND DURING EXERCISE BEFORE AND AFTER SURGERY
Values are presented as mean ± standard error.
*P < .01,
†P < .05 vs. before operation.
HR, heart rate; CI, cardiac index; Ppcw, pulmonary capillary wedge pressure; Ppa, mean pulmonary arterial pressure; PVRI, pulmonary vascular resistance index; driving pressure, Ppa − Ppcw.
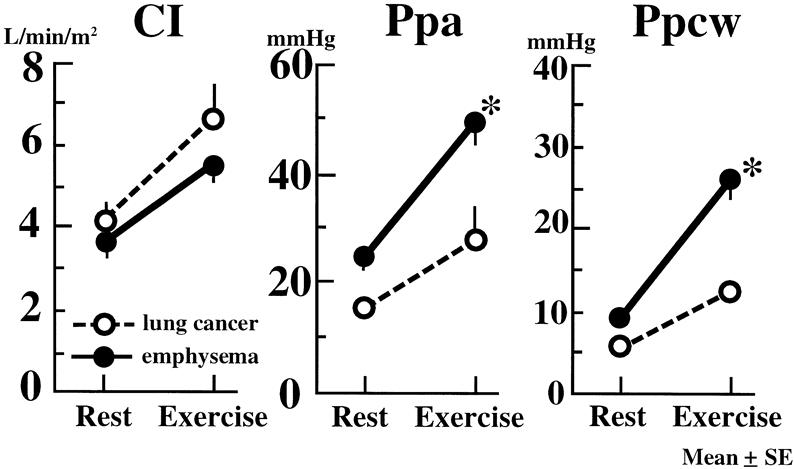
Figure 1. Effects of exercise on cardiac index (CI), pulmonary artery pressure (Ppa), and pulmonary capillary wedge pressure (Ppcw) in patients before each operation. Vertical bar shows mean ± SEM. *P < .01 vs. before operation.
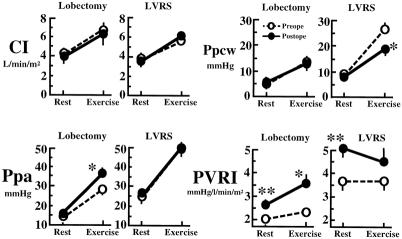
On the postoperative examination, only in the lobectomy group did HR significantly increase at rest and by exercise loading from preoperative values. CI was changed neither at rest nor during exercise in either group by surgery (Fig. 2). Although Ppa in the lobectomy group was markedly increased in the postoperative period by exercise loading, LVRS did not affect postoperative Ppa at rest or during exercise loading. Ppcw in the lobectomy group showed no significant change after surgery. However, LVRS ameliorated the marked elevation of Ppcw observed during exercise (P < .01). After lobectomy, significant increases in PVRI were observed at rest (P < .01) and during exercise (P < .05). LVRS markedly increased PVRI at rest (P < .05) but not during exercise.
Figure 2. Change in cardiac index (CI), pulmonary artery pressure (Ppa), pulmonary capillary wedge pressure (Ppcw), and pulmonary vascular resistance index (PVRI) before and after surgery. The open circles and the dotted lines show preoperative data; the closed circles and solid lines show postoperative data. Vertical bar shows mean ± SEM. *P < .05, **P < .01 vs. before operation.
Driving Pressure in Pulmonary Circulation
The relation between the driving pressure (Ppa − Ppcw) and CI is shown in Figure 3. In the lobectomy group, the postoperative flow-pressure curve moved upward, and its gradient became steeper than the preoperative one. This result shows that after lobectomy, more right-sided heart work is needed to achieve the same cardiac output. In the LVRS group, the curve moved upward in a parallel fashion. These results also show that after LVRS, much more right-sided heart work is needed to achieve the same cardiac output against higher Ppa, not only after exercise but also at rest.
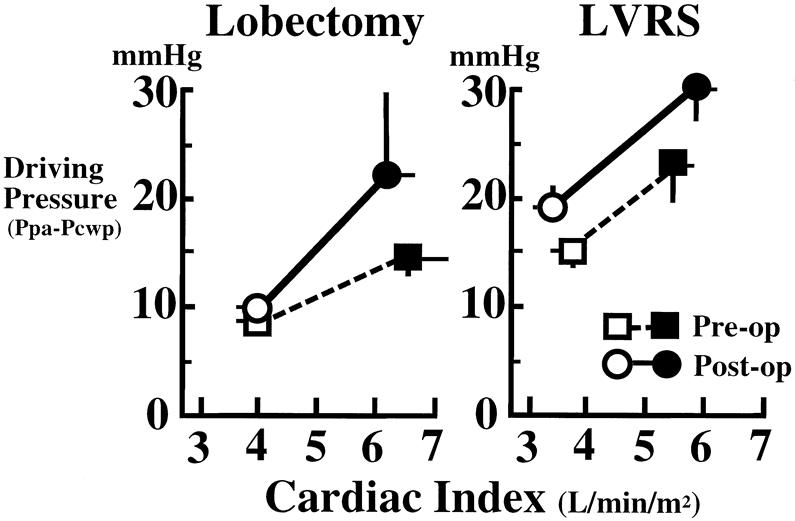
Figure 3. Relation between pulmonary driving pressure (pulmonary artery pressure minus pulmonary capillary wedge pressure) and cardiac index at rest (open circles and squares) and during exercise (closed circles and squares). Dotted lines and solid lines show pre- and postoperative data, respectively. Vertical and horizontal bars show mean±SEM.
DISCUSSION
This study demonstrated that pulmonary lobectomy decreased VC, FEV1, and MVV 6 months after surgery in patients with lung cancer. It also showed unfavorable effects of the lobectomy on the cardiopulmonary circulation—for instance, significant elevations of Ppa and PVRI. Six months after LVRS, in contrast, the pulmonary function test results and exercise performance were clearly improved. VC was not affected by this operation, and FEV1 increased from 0.65 L to 0.97 L, %MVV increased from 22.3% to 37.0%, and the 6-minute walking distance was prolonged from 306 ± 32 m to 424 ± 22 m. These results were similar to reports from other centers 2,4,10,11 and also demonstrated that our LVRS was successfully performed in this regard.
Preoperative Ppa and Ppcw in the patients with emphysema were elevated during exercise. Although the mechanisms underlying these elevations could not be sufficiently explained, at least two mechanisms are involved: hypoxic vasoconstriction during exercise and compression of alveolar vessels and/or the left atrium by the hyperinflated lung. Because LVRS is simply intended to decrease the lung volume, it will be expected to decrease these unfavorable effects of dynamic hyperinflation induced by exercise on the pulmonary circulation when LVRS has no or only a minimal effect on the pulmonary vascular bed. In contrast, pulmonary lobectomy, which is intended to decrease the pulmonary vascular bed, can be easily predicted to induce abnormalities of the postoperative cardiopulmonary circulation.
In terms of the effects on the cardiopulmonary circulation, no beneficial change was observed after the pulmonary lobectomy; postoperative Ppa and PVRI were significantly elevated during exercise. These effects were mainly derived from a decrease in the pulmonary vascular bed, because Ppcw and CI were minimally influenced by this operation. However, LVRS showed an excellent effect on postoperative Ppcw. After LVRS, the marked elevation in Ppcw during exercise that was observed before LVRS was significantly ameliorated. Although Ppcw measured by the Swan-Ganz catheter is influenced by many factors, including the pressure surrounding the cardiac chambers, 12 this improvement in Ppcw did not reflect a change in postoperative left-sided heart function, because there was no marked change in CI between the two stages. Further, a significant decrease in RV and a significant increase in FEV1 were observed after LVRS. These findings suggest that the elevation in Ppcw during exercise in patients with chronic obstructive pulmonary disease is associated with dynamic hyperinflation, whereas the decrease in lung volume induced by LVRS may help suppress the exercise-induced elevation in Ppcw.
Unlike Ppcw, LVRS significantly elevated PVRI, much like lobectomy did. CI and Ppa after LVRS showed no marked changes. These findings suggest that LVRS, in which we tried to resect the avascular area of the lung on the preoperative perfusion scan, had nonnegligible, harmful effects on the pulmonary vascular bed comparable to those of pulmonary lobectomy. The most interesting finding was a discrepancy in the postoperative changes in Ppcw, Ppa, and PVRI between the lobectomy and LVRS groups, especially during exercise. In the assessment at rest, there was no significant change in Ppa and Ppcw compared with the preoperative value, and PVRI was elevated significantly in both the lobectomy and LVRS groups. During exercise, however, postoperative elevations of Ppa and PVRI were not observed in the LVRS group. Although a quantitative study was not performed, improvement of Ppcw after LVRS might have modified and suppressed further elevation of Ppa and PVR during exercise. Another possibility is participation of hypoxic pulmonary vasoconstriction. Recently, Kubo et al 13 demonstrated that oxygen administration significantly decreased Ppa during exercise before and after LVRS. This finding suggests that the hypoxic pulmonary vasoconstriction is still an important factor after LVRS, and improvement of dynamic hyperinflation induced by LVRS may affect the supply of oxygen to the remaining lung, modifying the postoperative change in PVRI. Further investigation is necessary to clarify this issue.
The relation of Ppa to pulmonary blood flow (CI) is an important parameter demonstrating the condition of the pulmonary vascular bed. 14,15 Mahler et al 16 studied the relation of Ppa and CI during exercise in patients with chronic obstructive pulmonary disease and demonstrated that Ppa rose inordinately against CI during exercise. After major pulmonary resection, it was reported that the relation of Ppa to CI reflects the faculty of the pulmonary vasculature. 17 In the present study, we investigated the relation between driving pressure (Ppa − Ppcw) and ΔCI, which showed the condition of the pulmonary vascular bed more clearly. When the change in the driving pressure/ΔCI is steep, Ppa reaches its limit for increasing pulmonary blood flow with mild exercise, and the right ventricle fails to preserve cardiac output, easily leading to respiratory distress.
The current study showed that after lobectomy, the Ppa at rest and during exercise was higher than the preoperative values, and the driving pressure/flow curve shifted upward and became steeper than the preoperative one. Although the post-LVRS improvement in Ppcw was one of the causes of the increase in the driving pressure, the curve after LVRS shifted upward, reflecting the deteriorated condition of the pulmonary circulation in the remaining lung. These results demonstrate that LVRS had considerable effects on the condition of the pulmonary vascular bed, and the cardiac workload was increased at rest and during exercise. The important point is that successful LVRS depends on improved respiratory function and preserved cardiac function to tolerate the damage to the pulmonary vascular bed induced by this operation.
There is still no convincing evidence that the criterion of a mean pulmonary pressure <30 or 35 mmHg is appropriate for selection of patients for LVRS. Recently, Thurnheer et al 18 showed that in patients with severe emphysema who were candidates for LVRS, Ppa did not change significantly after the surgery; they stated that routine right-sided heart catheterization is not mandatory for preoperative evaluation. No significant change was observed in Ppa before and after LVRS in the current study. However, we also demonstrated that the effects of LVRS on the cardiopulmonary circulation were not negligible, especially during exercise, and the improved postoperative respiratory function of the patients with emphysema was still less than that of the patients who underwent lobectomy. These observations underscore the difficulty of selecting a reliable safety cutoff level for LVRS based on only the preoperative mean Ppa. We believe that preoperative right-sided heart catheterization is necessary to determine the condition of the pulmonary circulation, including the Ppa.
It has been suggested that preoperative measurements of VCO2max, 19,20 O2D, 21 or CI 22 during exercise are more precise than the preoperative Ppa to predict the postlobectomy condition. However, these data were obtained from patients undergoing lobectomy, whose postoperative respiratory function deteriorated after lobectomy. Because pulmonary function after LVRS usually improves and differs from lobectomy, no data are available as to whether it is appropriate to apply these criteria to LVRS. Careful study of patients undergoing LVRS will be necessary to clarify this issue.
Footnotes
Correspondence: Masayuki Haniuda, MD, Dept. of Surgery, Shinshu University School of Medicine, Matsumoto 390, Japan.
Accepted for publication May 21, 1999.
References
- 1.Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995; 109:106–119. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JD, Patterson GA, Sundaresan RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996; 112:1319-1330. [DOI] [PubMed] [Google Scholar]
- 3.Gaissert HA, Trulock EP, Cooper JD, Sundaresan RS, Patterson GA. Comparison of early functional results after volume reduction or lung transplantation for chronic obstructive pulmonary disease [see comments]. J Thorac Cardiovasc Surg 1996; 111:296–307. [DOI] [PubMed] [Google Scholar]
- 4.McKenna R Jr, Brenner M, Fischel RJ, Gelb AF. Should lung volume reduction for emphysema be unilateral or bilateral? J Thorac Cardiovasc Surg 1996; 112:1331–1339. [DOI] [PubMed] [Google Scholar]
- 5.Gelb AF, Brenner M, McKenna R Jr, Zamel N, Fischel R, Epstein JD. Lung function 12 months following emphysema resection. Chest 1996; 110:1407–1415. [DOI] [PubMed] [Google Scholar]
- 6.Bingisser R, Zollinger A, Hauser M, Bloch KE, Russi EW, Weder W. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996; 112:875–882. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell DE, Webb KA, Bertley JC, Chau LK, Conlan AA. Mechanisms of relief of exertional breathlessness following unilateral bullectomy and lung volume reduction surgery in emphysema. Chest 1996; 110:18–27. [DOI] [PubMed] [Google Scholar]
- 8.McKenna R Jr, Brenner M, Fischel RJ, et al. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg 1997; 114:957–967. [DOI] [PubMed] [Google Scholar]
- 9.Miller J Jr, Lee RB, Mansour KA. Lung volume reduction surgery: lessons learned. Ann Thorac Surg 1996; 61:1464–1469. [DOI] [PubMed] [Google Scholar]
- 10.Date H, Goto K, Souda R, et al. Bilateral lung volume reduction surgery via median sternotomy for severe pulmonary emphysema. Ann Thorac Surg 1998; 65:939–942. [DOI] [PubMed] [Google Scholar]
- 11.Yusen RD, Trulock EP, Pohl MS, Biggar DG. Results of lung volume reduction surgery in patients with emphysema. The Washington University Emphysema Surgery Group. Semin Thorac Cardiovasc Surg 1996; 8:99–109. [PubMed] [Google Scholar]
- 12.Raper R, Sibbald WJ. Misled by the wedge? The Swan-Ganz catheter and left ventricular preload. Chest 1986; 89:427–434. [DOI] [PubMed] [Google Scholar]
- 13.Kubo K, Koizumi T, Fujimoto K, et al. Effects of lung volume reduction surgery on exercise pulmonary hemodynamics in severe emphysema. Chest 1998; 114:1575–1582. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Haniuda M, Morimoto M, Kubo K. Cardiopulmonary function after pulmonary lobectomy in patients with lung cancer. Ann Thorac Surg 1993; 55:1477–1484. [DOI] [PubMed] [Google Scholar]
- 15.Harrison RW, Adamus WE, Long ET, Burrows B, Reimann A. The clinical significance of cor pulmonale in the reduction of cardiopulmonary reserve following extensive lung resection. J Thorac Surg 1958; 36:352–368. [PubMed] [Google Scholar]
- 16.Mahler DA, Brent BN, Loke J, Zaret BL, Matthay RA. Right ventricular performance and central circulatory hemodynamics during upright exercise in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1984; 130:722–729. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Ota T, Okada M, Matsuda H, Okada K, Ishii N. Right ventricular dysfunction after major pulmonary resection. J Thorac Cardiovasc Surg 1994; 108:503–511. [PubMed] [Google Scholar]
- 18.Thurnheer R, Bingisser R, Stammberger U, et al. Effect of lung volume reduction surgery on pulmonary hemodynamics in severe pulmonary emphysema. Eur J Cardiothorac Surg 1998; 13:253–258. [DOI] [PubMed] [Google Scholar]
- 19.Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989; 139:902–910. [DOI] [PubMed] [Google Scholar]
- 20.Smith TP, Kinasewitz GT, Tucker WY, Spillers WP, George RB. Exercise capacity as a predictor of post-thoracotomy morbidity. Am Rev Respir Dis 1984; 129:730–734. [DOI] [PubMed] [Google Scholar]
- 21.Olsen GN, Weiman DS, Bolton JW, et al. Submaximal invasive exercise testing and quantitative lung scanning in the evaluation for tolerance of lung resection. Chest 1989; 95:267–273. [DOI] [PubMed] [Google Scholar]
- 22.Fee HJ, Holmes EC, Gewirtz HS, Ramming KP, Alexander JM. Role of pulmonary vascular resistance measurements in preoperative evaluation of candidates for pulmonary resection. J Thorac Cardiovasc Surg 1978; 75:519–524. [PubMed] [Google Scholar]