Abstract
Objective
To report the authors’ experience with living donor liver transplantation in adults using right lobe liver grafts, performed by a modified technique.
Summary Background Data
The initial results of seven living donor liver transplants in adults using extended right lobe grafts were satisfactory, but serious complications occurred in two donors, and six recipients required repeat laparotomy. Another 11 similar operations were performed. Further evaluation was made with the aim of improving the postoperative outcome.
Methods
From December 1996 to August 1998, 11 patients underwent living donor liver transplantation using right lobe grafts. The first four patients underwent surgery using methods previously designed and the next seven underwent a modification designed to minimize devitalized tissues on the liver transection surface, improve hepatic venous drainage, and reduce the number of hepatic duct orifices.
Results
There were no donor deaths. Donor complications included cholestasis (n = 1) and minor wound infection (n = 1). All the first four recipients required a repeat laparotomy for infected necrotic liver transection surface (n = 1), acute pancreatitis (n = 1), hepatic vein thrombosis (n = 1), and leakage from one of the two bilioenteric anastomoses (n = 1). The patient with hepatic vein thrombosis died. In the last seven recipients, all of whom survived the operation, one required a repeat laparotomy with the discovery of a methicillin-resistant Staphylococcus aureus culture of fibrinous exudate at the left subphrenic peritoneum, and another had right hepatic duct stump necrosis. The latter was likely related to hypovolemic shock secondary to bleeding from the right saphenous vein on removal of a hemofiltration catheter. Comparison of the incidence of repeat laparotomy between the first four and the remaining seven recipients showed a significant trend of improvement. Combining the result of the seven patients reported previously, the improvement in terms of relaparotomy rate is significant.
Conclusion
With modification of surgical technique, living donor liver transplantation in adults using right lobe liver grafts can become a relatively safe procedure.
An extended right lobe liver graft was used to overcome the problem of inadequate graft volume in adult-to-adult living donor liver transplantation. The initial result was satisfactory. 1 However, complications developed in both donors and recipients, some of which could have been related to deficiencies in technical design. We have modified the surgical procedure and in this article report the results of the modified procedure.
METHODS
From May 1996 to August 1998, 18 patients underwent living donor liver transplantation using extended right lobe grafts at Queen Mary Hospital, Hong Kong. The first seven patients underwent surgery between May 1996 and October 1996, and the results were previously reported. 1 The other 11 patients underwent surgery between December 1996 and February 1999. Of these 11 patients, 7 are men and 4 are women, with a median age of 42 (range 17–57). Indications for liver transplantation included reactivation of hepatitis B viral infection (n = 4), hepatitis B cirrhosis (n = 4), drug-induced fulminant hepatic failure (n = 1), fulminant hepatic failure of unknown etiology (n = 1), and fulminant Wilson disease (n = 1). The donors were the spouse (n = 5), daughter (n = 1), son (n = 1), aunt (n = 1), uncle (n = 1), nephew (n = 1) and brother-in-law (n = 1) of the recipients. The surgical procedure for the first four patients followed what had been reported previously. 1 The following is a detailed description of the modified procedure used in the last seven patients.
Donor Operation
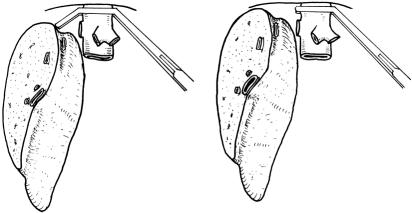
The donor was prepared on the operating table with care taken to prevent wound sepsis, pressure ulcers, and deep vein thrombosis. The abdomen was entered through a bilateral subcostal incision. Cholecystectomy and cannulation of the cystic duct were performed, followed by operative cholangiography using undiluted radiographic contrast and an image intensifier. The cholangiogram was obtained during injection of radiographic contrast. Intraoperative ultrasonography was then performed to determine the configuration of the junction of the middle hepatic vein with the left hepatic vein 2 and the presence of a right inferior hepatic vein, and to mark on the liver surface the position of the middle hepatic vein, which would be the liver transection plane. Because the middle hepatic vein could be seen by the ultrasound probe placed on the liver surface at various angles (Fig. 1A), a correct plane should be where the middle hepatic vein could be seen longitudinally, together with the inferior vena cava (see Fig. 1B). Hilar dissection was performed to free the right hepatic artery and right portal vein. Branches of the right portal vein supplying the caudate process were ligated and divided. Right hepatic duct dissection was not made until the liver was transected, because dissection for the right hepatic duct at this time could be difficult and might predispose to thinning and devascularization of the wall of the duct. 3 The right lobe of the liver was mobilized by dividing the triangular ligament and tiny hepatic veins draining the right caudate lobe into the inferior vena cava. 4 The right inferior hepatic vein, if sizable (>5 mm), was preserved. The inferior vena cava ligament or a layer of liver tissue covering the right side of the inferior vena cava was divided to encircle the right hepatic vein. The rotation of the right lobe of the liver was intermittent to prevent prolonged twisting of the inflow and outflow vascular pedicles of the liver. 3
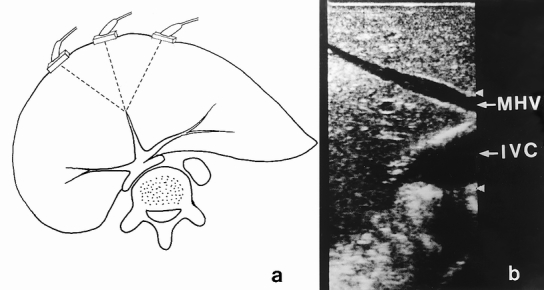
Figure 1. (A) The middle hepatic vein, which can be visualized by an ultrasound probe placed on the liver surface at various planes. (B) Ultrasound picture showing the middle hepatic vein (MHV) and the inferior vena cava (IVC) at one plane. This should be the correct transection plane.
Parenchymal transection was performed at the plane determined by intraoperative ultrasonography with an ultrasonic dissector without any inflow or outflow vascular occlusion. At the inferior surface of the liver, the transection plane approached the liver hilum, and dissection for the junction of the right and left hepatic ducts was made, but care was taken to avoid thinning the wall of the hepatic duct with the ultrasonic dissector. The liver tissue cranial to the right hepatic duct was cleared by the ultrasonic dissector down to the caudate process (Fig. 2). The right hepatic duct, together with the hilar plate, was encircled by passing a right-angled clamp at the level of the caudate process. The right hepatic duct was divided near the confluence of the bile ducts, and the hilar plate was divided by scissors without ligation of any tissue to expose the hepatic duct opening, which may represent an anomalous right hepatic duct or right caudate lobe branches missed by the operative cholangiogram. 4 The defect on the common hepatic duct was closed horizontally with 5-0 PDS monofilament absorbable sutures. Liver transection was then continued down to the posterior surface of the right caudate lobe and the junction of the middle hepatic vein and left hepatic vein or inferior vena cava. Bleeding points from the wall of the middle hepatic vein were carefully sutured with 6-0 prolene without narrowing the lumen of the middle hepatic vein. Harvest of the liver graft was initiated by clamping and cannulating the right portal vein. The cannula was connected to cold plasma solution. The harvesting procedure was completed by clamping and dividing the right hepatic artery, right portal vein, right hepatic vein(s), and middle hepatic vein. At the back table, the graft was immersed in ice sludge and flushed with 1 L University of Wisconsin (UW) solution through the right portal vein cannula. The hepatic artery stump was not flushed with UW solution to avoid injuring the intima of the artery. Dissection around the hepatic vein to increase its length was not made.
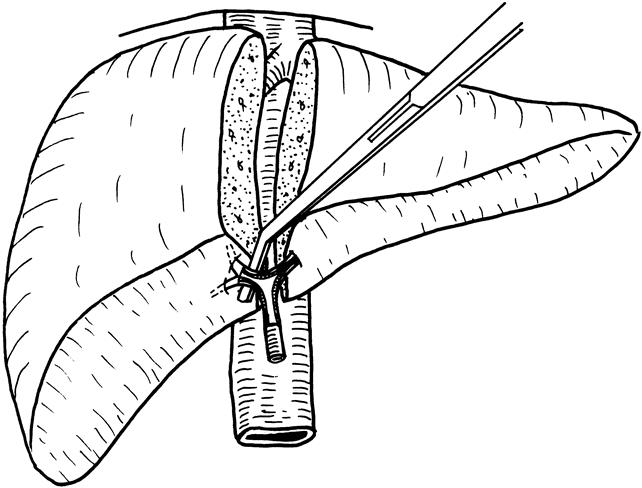
Figure 2. The line of transection (at the same site of encircling by a right-angled forcep) of the right hepatic duct at the time of liver transection. The right portal vein and right hepatic artery are not shown.
The stumps of the right hepatic artery, right hepatic vein, and right portal vein on the donor liver remnant were sutured. The falciform ligament was reconstructed to prevent the liver remnant from rotating into the right subphrenic cavity. 5 The raw area was covered with fibrin glue and then with the greater omentum. Before wound closure, care was taken to ensure that the small bowel had not migrated into the right subphrenic cavity. After surgery, parenteral nutrition support consisting of branched-chain amino acid-enriched solution, low-dose dextrose, phosphate, and medium-chain triglycerides was administered for 3 days to enhance regeneration of the liver remnant. 6
Recipient Operation
The recipient was prepared on the operating table in a similar manner as the donor. The abdomen was entered through a bilateral subcostal incision with midline extension. Hilar dissection was performed to isolate and divide branches of the hepatic artery and portal vein. Maximum length of the hepatic artery and portal vein branches was preserved. A microvascular clip was used to control the proximal end while the distal end of the hepatic artery was clamped, divided, and ligated. The microvascular clips were released intermittently throughout the procedure until anastomosis to prevent clogging of the lumen of the hepatic artery. Venovenous bypass was set up by cannulating the portal vein (by means of the left portal vein orifice), left saphenous vein, and left subclavian vein. The inferior vena cava was mobilized from the retroperitoneum. After the liver was freed from the inferior vena cava, a tape was used to control the infrahepatic inferior vena cava, and a vascular clamp without a “return” jaw was applied to the suprahepatic inferior vena cava (Fig. 3). The right hepatic vein was divided, and the liver was transected approximately 3 cm from the confluence of the middle and left hepatic veins. The liver tissues surrounding the middle and left hepatic veins were trimmed. The tiny holes on the wall of the middle and left hepatic veins were closed with 6-0 prolene sutures. Water-tightness of the inferior vena cava and the hepatic veins was checked by releasing the control tape at the infrahepatic inferior vena cava after the hepatic vein stumps were clamped at the edges. After that, the lumen of the inferior vena cava was thoroughly flushed with heparin-saline. Bleeding from the bare area of the diaphragm was controlled by suturing individual bleeding points. The bare area was not approximated and closed by continuous sutures as advocated for orthotopic full-size liver transplantation, 7 because by that the depth in the right subphrenic cavity could be substantially reduced and might be insufficient for accommodation of the right lobe graft.
Figure 3. Interference with approximation of recipient and donor right hepatic vein by a Satinsky vascular clamp (left). Use of a clamp without a “return” jaw can eliminate this problem (right).
The diameters of the right, middle, and left hepatic veins and the right portal vein were measured. Information about the diameter of the corresponding orifices of the liver graft was also obtained in preparation for anastomoses. The vascular clamp applied on the suprahepatic inferior vena cava was not elevated to 90°, as in the case with orthotopic full-size liver graft transplantation, because the orientation of the right hepatic vein might be distorted by such a maneuver. Implantation started with the right hepatic vein anastomosis. The inferior vena cava was incised longitudinally and caudally if the right hepatic vein was smaller than the liver graft. Application of corner stitches on the graft right hepatic vein was guided by the direction of the groove of the inferior vena cava on the posterior surface of the liver graft (Fig. 4). The corner stitches of the recipient right hepatic vein were applied near the inferior vena cava, so that the resultant length of the right hepatic vein anastomosis was as short as possible to prevent kinking of the right hepatic vein after reperfusion. In the presence of the right inferior hepatic vein, a slit was made in the recipient inferior vena cava for a short anastomosis. The middle hepatic vein anastomosis was made with the line of anastomosis obliquely on the recipient middle hepatic vein because the lumen of the distal part of middle hepatic vein was usually smaller than that near the orifice at the inferior vena cava (Fig. 5). When the diameter of the donor middle hepatic vein was grossly larger than that of the recipient middle hepatic vein, the left hepatic vein of the recipient was used instead for the anastomosis. On completion of the hepatic vein anastomosis, the sutures were not pulled tight and the knots were made loosely. During the middle hepatic vein anastomosis, cold plasma was used to flush the graft free of UW solution through the portal vein cannula, and the UW solution escaped through the left or middle hepatic vein orifice. End-to-end right portal vein anastomosis was made using 6-0 prolene; the venovenous bypass (by the left portal vein) was kept intact. A growth factor of 1 cm was allowed at the time of knotting of sutures. 8 The lumen of the right portal vein was filled with heparin-saline and a vascular clamp was applied to the junction of the right portal vein and the main trunk (Fig. 6). The bypass cannula was removed and the main portal vein was thoroughly flushed with heparin-saline before clamping of the left portal vein and left or middle hepatic vein and reperfusion of the liver graft. Hepatic artery anastomosis was performed using microvascular technique with 9-0 nylon. Bilioenteric anastomosis was performed using 5-0 PDS and a short internal stent.
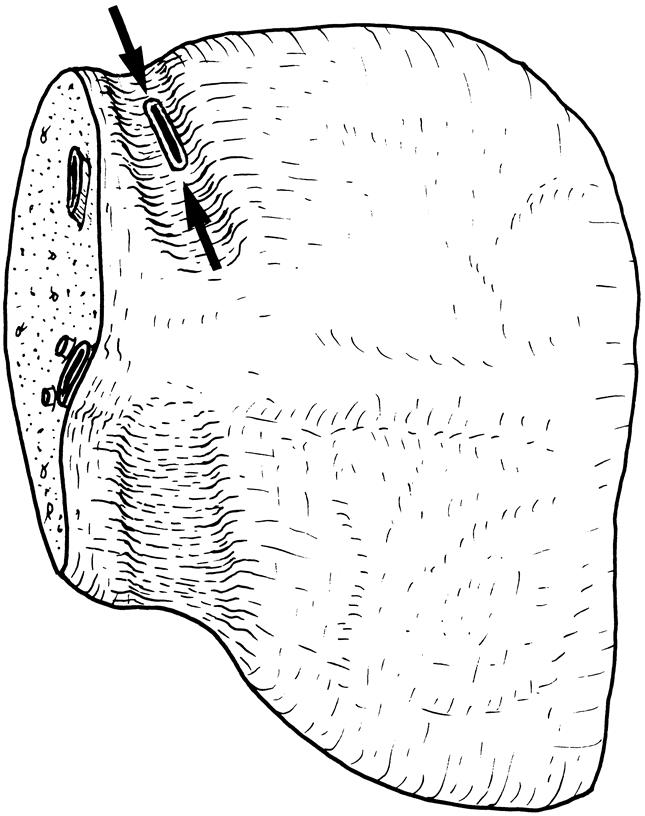
Figure 4. Correct locations (arrows) of placement of corner stitches at the time of implantation. The inferior vena cava groove serves as the landmark for correct orientation.
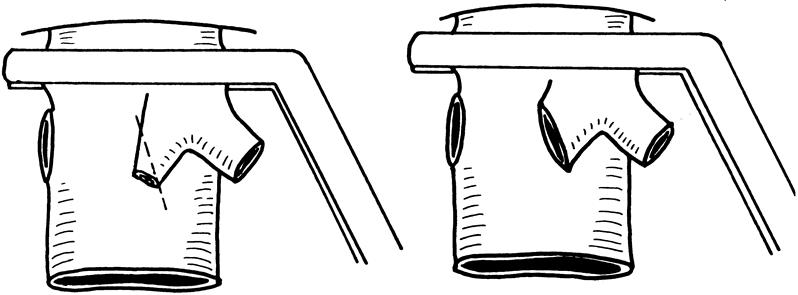
Figure 5. The diameter of the middle hepatic vein, which is narrower than that of the orifice at the inferior vena cava (left). To avoid narrowing of an end-to-end anastomosis, an oblique anastomosis must be made (right).
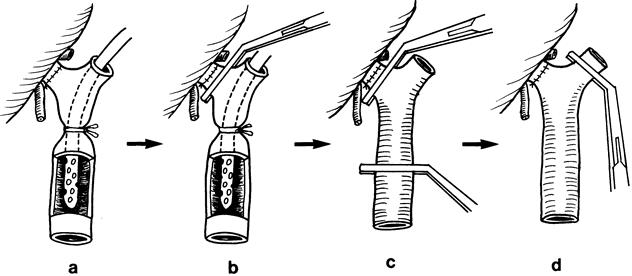
Figure 6. Sequence of clamping of the portal vein and its branches before reperfusion. (A) After completion of the right portal vein anastomosis. (B) A vascular clamp is applied to the junction of the right portal vein and the main trunk to prevent blood clot released from the portal vein from flushing into the liver graft; the control tape of the venovenous bypass and the cannula were then removed. (C) A vascular clamp is applied to the main trunk. Transient release of the vascular clamp applied to the main portal vein allows blood clot, if any, to come out. The lumen of the portal vein is flushed free of blood clot and fibrin. (D) A vascular clamp is then applied horizontally to the left hepatic vein while the lumen of the main portal vein is filled with heparin-saline. After the left hepatic vein is controlled and the suprahepatic inferior vena cava vascular clamp is released, the vascular clamps controlling the right and main portal veins are removed to allow reperfusion of the graft.
RESULTS
All donors survived the hepatectomy and did not require repeat laparotomy. Only two donors had postoperative complications. Donor 1 had prolonged hyperbilirubinemia, the cause of which thorough investigation could not identify. He was eventually free from jaundice. Donor 5 had minor wound infection. The average blood loss during the donor operation was 500 mL (range 200–1,200). The median surgical time was 12.2 hours (range 10.1–15.5).
The average duration for the three or four venous anastomoses of the recipient operation was 66 minutes. The duration decreased from 99 minutes in recipient 1 to 47 minutes in recipients 10 and 11. There were no problems during the anhepatic phase.
After reperfusion with portal blood, all liver grafts, except in recipient 3, functioned immediately and were soft in consistency. In recipient 3, the liver graft was turgid after reperfusion. Lowering of central venous pressure led to softening of the graft, but bile production was delayed.
Repeat laparotomy was required in recipients 1, 2, 3, 4, 6, and 9. In recipient 1, necrotic material was found covering the cut surface of liver, and Klebsiella pneumoniae and methicillin-resistant S. aureus were identified. He did well after débridement of necrotic material. In recipient 2, acute pancreatitis was found. She did well after conservative treatment. The postoperative course of recipient 3 was stormy. He continued to show clinical and hematologic evidence of portal hypertension. Doppler ultrasonography could not show the lumen of the right and middle hepatic vein anastomoses clearly. Bleeding esophageal varices developed that were not controlled by injection sclerotherapy. At laparotomy, splenic artery ligation was performed to reduce the portal pressure, but portal vein and hepatic vein thrombosis subsequently developed. He died of liver failure and fulminant sepsis. In retrospect, he had stenosis of both the right and middle hepatic vein anastomoses. Recipient 4 was found to have leakage from one of the two bilioenteric anastomoses. He required two more laparotomies before sepsis was controlled. Recipient 6 was found to have a small amount of fibrinous exudate under the diaphragm, and culture was positive for methicillin-resistant S. aureus. He made a rapid recovery after the laparotomy. Recipient 9 underwent repeat laparotomy on postoperative day 3 for evacuation of blood clot in the subphrenic area. Examination of the bilioenteric anastomosis showed that it was healthy and intact. On the next day, the catheter inserted into the right saphenous vein for hemofiltration was removed, but he had massive bleeding from the insertion site, leading to shock. Thereafter, he had clinical manifestations of sepsis. Repeat laparotomy on postoperative day 11 showed necrosis of the right hepatic duct stump. Intubation of the right hepatic duct branches was made. He recovered well and is waiting for reanastomosis. Recipients 5, 7, 8, 10, and 11 recovered from surgery without complications or repeat laparotomy.
Comparison of the incidence of repeat laparotomy between the first four and the last seven recipients showed a significant trend of improvement (4/4 vs. 2/7, P = .06, Fisher’s test, two-tailed). Combining the results of the seven patients reported previously, 1 the improvement in terms of the incidence of repeat laparotomy is significant (10/11 vs. 2/7, P = .001, Fisher’s exact test, two-tailed).
DISCUSSION
From our past and present experience, we identified the reasons for unsatisfactory outcome: the presence of necrotic tissue on the liver transection surface, inadequate hepatic venous drainage, missed right posterior hepatic duct, and more than one right hepatic duct orifice on the graft, which made reconstruction difficult.
In our early design of the procedure, a 1-cm margin was deliberately left in the left medial segment to protect the middle hepatic vein. 1 The harvesting operation, thus designed, is easier to perform. However, the layer of liver tissue is not well vascularized and is prone to becoming necrotic and infected. Division of liver parenchyma is therefore preferred along the Cantlie line. Marking of the Cantlie line on the liver surface can be made by clamping the inflow vascular pedicle of either side, but in our experience the demarcation is not always clearly discernible. To define the Cantlie line, the middle hepatic vein can be used as a reference and can be seen on the intraoperative ultrasonogram. However, as shown in Figure 1, the middle hepatic vein can be seen at various angles from the liver surface. To obtain the correct plane, the inferior vena cava must be seen with the middle hepatic vein in the longitudinal plane. With this change in technique, we have renamed the procedure “living donor liver transplantation using right lobe graft.”
Transection of the liver parenchyma at the Cantlie line without inflow or outflow vascular occlusion is more difficult than liver transection on either side of Cantlie line. To avoid serious injury to the middle hepatic vein, and hence massive bleeding, repeated intraoperative ultrasonography to locate the main trunk of the middle hepatic vein in relation to the transection plane, cautious and slow application of the ultrasonic dissector, a decreased power setting on the ultrasonic dissector, 9 and immediate suturing of bleeding sites with fine prolene sutures are necessary. Wearing a magnifying loupe and headlamp helps in the identification and suturing of bleeding points and in the liver transection. Decreasing the central venous pressure also reduces the bleeding rate. 10
Adequate venous drainage must be provided for the reperfused graft. A major venous obstruction was responsible for the death of recipient 3, and a minor degree of venous obstruction was probably responsible for hyperamylasemia 11 in recipient 2. In our design, the right hepatic vein, the right inferior hepatic vein, if present, and the middle hepatic vein are harvested with the graft to meet this purpose, but attention to the resultant length, orientation, and diameter of the hepatic vein anastomosis must be made to achieve adequate drainage. On the recipient side, it is possible to preserve a long length of the right hepatic vein by cutting into the liver during hepatectomy. However, in normal anatomy, the right hepatic vein is usually short. Reconstruction of the right hepatic vein of any length is undesirable because reperfusion and expansion of the liver graft, which may be larger than the right subphrenic cavity, will lead to rolling over to the left side and folding or kinking of the anastomosis. Close approximation without twisting is therefore desirable. Approximation is facilitated if a vascular clamp with a “return” jaw (e.g., a Satinsky clamp) is avoided in this situation (see Fig. 3) and the inferior vena cava groove of the graft can fit into the recipient inferior vena cava (see Fig. 4).
In our previous experience, division of the right hepatic duct was performed near the right side of the liver hilum because we were afraid of damaging the confluence of the hepatic ducts. As a result, the right anterior and right posterior ducts were divided separately, and occasionally the right posterior duct was ligated when the right anterior hepatic duct was thought to be the only right hepatic duct. Anastomosing two hepatic ducts to the small bowel is obviously more difficult than a single duct. To avoid this problem, we consider it mandatory to study the anatomy thoroughly by obtaining a good-quality cholangiogram, to perform deliberate but careful dissection at the confluence of the hepatic ducts to obtain, wherever possible, a single duct opening, and to divide the hilar plate without any ligation to identify any anomalous branch missed by the cholangiogram.
The blood supply to the right hepatic duct is dependent on a peribiliary plexus, which in turn is dependent on the gastroduodenal artery and the arterial supply to the caudate lobe. 12 Because in the harvesting of the right lobe graft, the arterial supply from the gastroduodenal artery is interrupted and the caudate lobe is divided at the plane between the right and left part, the arterial supply to the right hepatic duct becomes precarious: the only blood supply is from tiny arterial arcades from the right portion of the caudate lobe. 12 Hypotensive episodes will therefore render the right hepatic duct stump vulnerable to ischemia and necrosis. The experience gained from managing recipient 9 reinforced our belief that the surgeon should attend to every detail for such a complicated operation, the results of which can be influenced by many unpredictable factors.
With this modification of the technique, we have achieved improved results. We are now more confident that the operation can be offered to patients with terminal liver disease. Further evaluation of long-term results of a large-scale study will be performed to confirm the result.
Footnotes
Correspondence: S.T. Fan, Dept. of Surgery, Queen Mary Hospital, 102 Pokfulam Rd., Hong Kong.
Accepted for publication June 4, 1999.
References
- 1.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997; 226:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawasaki S, Makuuchi M, Miyagawa S, et al. Extended lateral segmentectomy using intraoperative ultrasound to obtain a partial liver graft. Am J Surg 1996; 171:286–288. [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Lai ECS, Lo CM, Ng IOL, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995; 130:198–203. [DOI] [PubMed] [Google Scholar]
- 4.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M. Hilar cholangiocarcinoma-surgical anatomy and curative resection. J Hep Bil Panc Surg 1995; 2:239–248. [Google Scholar]
- 5.Pitre J, Panis Y, Belghiti J. Left hepatic vein kinking after right hepatectomy: a rare cause of acute Budd-Chiari syndrome. Br J Surg 1992; 79:7948–7949. [DOI] [PubMed] [Google Scholar]
- 6.Fan ST, Lo CM, Lai ECS, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med 1994; 331:1547–1552. [DOI] [PubMed] [Google Scholar]
- 7.Casavilla A, Gordon RD, Starzl TE. Techniques of liver transplantation. In: Blumgart LH, ed. Surgery of the Liver and Biliary Tract, vol. 2. Edinburgh: Churchill Livingstone; 1994: 1863–1888.
- 8.Starzl TE, Iwatsuki S, Shaw BW Jr. A growth factor in fine vascular anastomoses. Surg Gynecol Obstet 1984; 159:164–165. [PMC free article] [PubMed] [Google Scholar]
- 9.Fan ST, Lai ECS, Lo CM, Chu KM, Liu CL, Wong J. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg 1996; 83:117–120. [DOI] [PubMed] [Google Scholar]
- 10.Johnson M, Mannar R, Wu AVO. Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg 1998; 85:188–190. [DOI] [PubMed] [Google Scholar]
- 11.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T, Hayashi K, Kasai H. Serum amylase elevation following hepatic resection in patients with chronic liver disease. Am J Surg 1996; 171:235–238. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton GN, Hickman R, Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg 1998; 85:202–207. [DOI] [PubMed] [Google Scholar]