Abstract
Objective
To compare prognostic results in patients with gastric stump cancer (GSC) versus those with primary gastric cancer (PGC).
Summary Background Data
Gastric stump carcinomas have often been described as having low resectability rates and a poor prognosis.
Methods
Results of surgical treatment of 50 patients with GSC were compared with that of 516 patients with PGC.
Results
The resectability rate was 94% for GSC patients and 96.5% for PGC patients, without significant differences in terms of postoperative complications, death rate, and median survival time (31.6 vs. 32.9 months). The multivariate analysis showed an independent prognostic effect for R0 resection, pT1 and pT2 category, and age older than 65 years.
Conclusion
The prognosis after resection and adequate lymphadenectomy does not differ between patients with GSC and PGC.
Cancer of the gastric remnant, defined as a carcinoma detected more than 5 years after primary surgery for a benign disease, was first described in 1922. 1 The incidence is reported to range from 2.4% to 5%. 2,3 Several studies have established an increased cancer risk in the gastric remnant. 4,5 Despite efforts to detect cancer early in patients who have undergone gastrectomy, the reported resectability rates at the time of diagnosis are approximately 40%, compared with 80% for patients with in primary gastric cancer. 5–8 Five-year survival rates remain poor (ranging from 7–25%), even in patients undergoing curative (R0) resection. 6–8 Recent reports of patients with early cancer of the gastric remnant or patients with advanced disease without evidence of nodal or distant metastases showed 5-yearsurvival rates of approximately 50% and 74%, respec-tively. 9–12
Because we were convinced that only patients with gastric stump cancer (GSC) could be compared with patients with primary gastric cancer (PGC) in the proximal third of the nonoperated stomach, we analyzed the survival and prognosis of these patients treated during the same period at our department.
METHODS
Between July 1982 and December 1998, 1,317 consecutive patients underwent surgery for histologically confirmed gastric cancer at the Department for Surgery, Technische Universität Munich. Carcinoma developed in the gastric remnant in 52 patients who had previously undergone distal gastrectomy for a gastric or duodenal ulcer, representing 3.9% of all our gastric cancer patients. We found 50 patients with GSC and 516 patients with PGC in the proximal third of the stomach, including type II and III adenocarcinoma of the gastroesophageal junction, to be eligible for further analysis. All patients with type I adenocarcinoma of the gastroesophageal junction or of the middle and distal third of the stomach (n = 762), 2 (3.8%) patients in the GSC group, and 37 (3.9%) patients in the PGC group were excluded from the study because they had received prior chemotherapy.
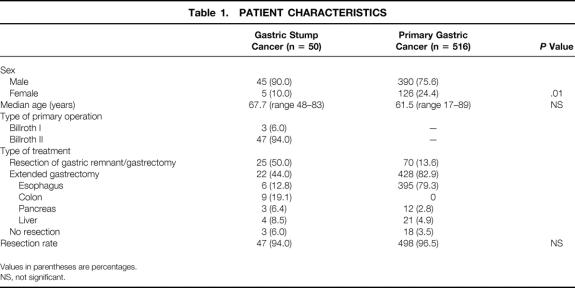
Three of the 50 patients with GSC had undergone a Billroth I procedure; the remaining 47 had undergone a Billroth II resection. Forty-five patients (90.0%) in this series were men. The mean age at the time of diagnosis was 67.7 years (range 48–83 years). The mean interval between the first operation and the development of cancer was 26.5 years (range 7–43 years). An en bloc resection of the gastric remnant and the anastomotic site of the jejunum and jejunal mesentery, together with the lymph nodes of compartment I (proximal part) and II, was recommended as the surgical procedure of choice. In 22 (44%) of the GSC patients, the resection was extended to the distal esophagus, pancreas, spleen, and colon as required by tumor spread or location. The demographic data, primary tumor site, and surgical therapy are shown in Table 1.
Table 1. PATIENT CHARACTERISTICS
Values in parentheses are percentages.
NS, not significant.
The lymph node dissection of compartment I (proximal part) and II (D2 lymphadenectomy) was performed according to the recommendations of the Japanese Research Society for Gastric Carcinoma. 13 All resected lymph nodes remained attached to the resected stomach (en bloc resection). For quality control, the extent of the lymph node dissection was evaluated by the pathologist by a careful count of the removed lymph nodes, and the ratio between the number of positive lymph nodes and the number of removed nodes was calculated. 14 Lymph nodes in the mesentery of the jejunum anastomosed to the stomach after a Billroth II reconstruction have been classified as a second tier of nodes (N2 category).
Laurén classification, macroscopic tumor type (Borrmann classification), depth of invasion (T category), lymph node involvement (N category), and distant metastasis (M category) were analyzed. All histopathologic data and the presence of metastases were determined according to the TNM system and the recommendations of the International Union Against Cancer. 15
Data of the GSC patients were compared with those of the 516 patients with PGC in the proximal third of the stomach, as described in the recommendations of the Japanese Research Society for Gastric Cancer. 13 All patients with type I carcinoma of the gastroesophageal junction, as defined previously, 16 were excluded from the study.
Statistics
Survival curves were calculated according to the Kaplan-Meier method. Survival data are given as observed overall survival and include postoperative deaths. The log-rank test was used to evaluate statistically significant differences between the subgroups;P < .05 was regarded as statistically significant. For survival curves, only patients with curative resection (R0 resection) were eligible. Independent prognostic factors were identified by stepwise regression analysis (Cox model). Significance was assessed by chi-square analysis. All analyses were performed using the BMDP software package (BDMP Statistical Software, Los Angeles, CA) and the CDC computer at the Leibniz Rechenzentrum München.
RESULTS
Clinical Findings
The resection rate was 94.0% (n = 47) in patients with GSC and 96.5% (n = 498) in patients with PGC. In 15 (31.9%) of the 47 GSC patients, the tumor was confined to the gastric remnant separated from the gastroenterostomy, whereas in 32 (68.1%) of these patients the primary tumor site was located at the gastrojejunostomy. Nine (19.1%) patients had tumor in the whole gastric stump. In 22 (44.0%) of the GSC patients and 428 (82.9%) of the PGC patients, the resection was extended to the esophagus (12.8%/79.3%), to the colon (19.1%/0%), to the pancreas (6.4%/2.8%), or to the liver (8.5%/4.9%) as required by tumor spread (see Table 1).
Postoperative complications occurred in 19 (40.4%) of the GSC patients and 177 (35.5%) of the PGC patients, whereas anastomotic leakage was found in 9 (1.8%) patients in the PGC group and in none of the GSC group. The difference in the incidence of postoperative complications was significant (40.4% vs. 35.5%). The hospital death rate was 2.1% in GSC patients and 2.2% in PGC patients (Table 2).
Table 2. SURGICAL RESULTS AFTER TUMOR RESECTION
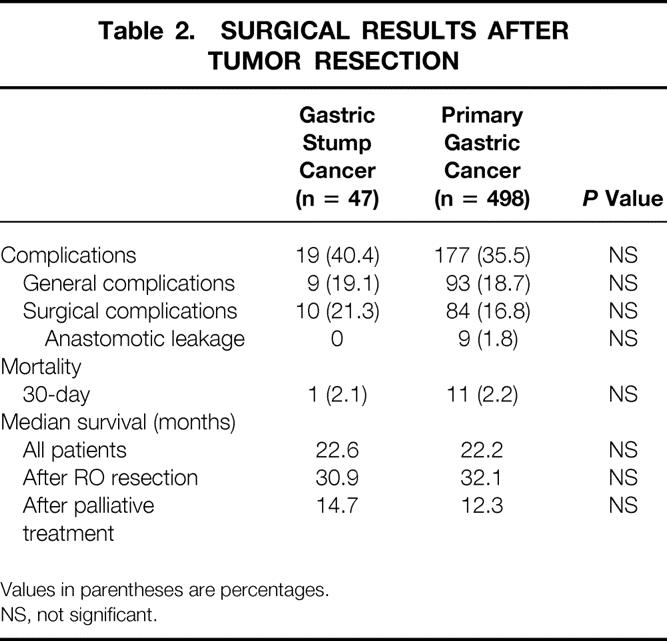
Values in parentheses are percentages.
NS, not significant.
Pathologic Findings
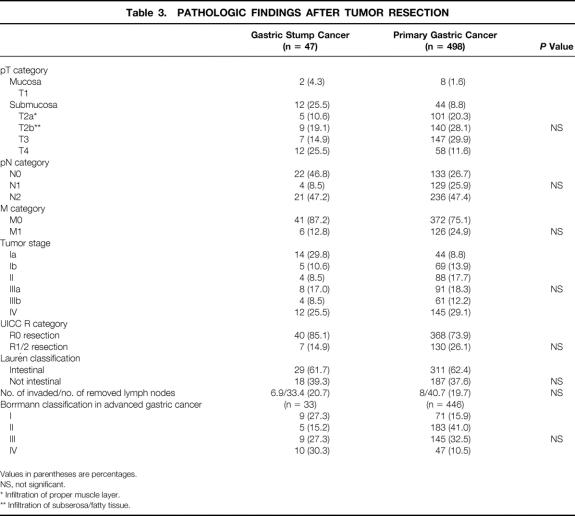
An R0 resection, according to the UICC, was achieved in 85.1% of the GSC patients and in 73.9% of the PGC patients. The distributions of TNM categories, tumor stage, and R category showed no significant differences between GSC and PGC patients (Table 3). We found a high incidence of early cancer (29.8%) in patients with GSC. No statistical differences were seen between the groups in terms of the ratio of positive lymph nodes, the number of removed nodes, and the Laurén classification. In terms of macroscopic tumor type (Borrmann classification), there was no significant difference between the two groups. Fifteen (46.8%) of 32 GSC patients with tumor infiltration around the gastrojejunostomy had lymph node metastases in the jejunal mesentery; in 17 (53.1%) patients the jejunum was infiltrated, and 13 (40.6%) of these patients had lymph node metastases, in contrast to only 2 (6.0%) patients without infiltration of the jejunum. From the nine patients in whom the whole stump was replaced by tumor, only one had tumor infiltration of the jejunum. Nine (19.1%) of the GSC patients and 140 (28.1%) of the PGC patients with pT2 category showed transmural tumor growth and infiltration of the perigastric fat tissue (T2b category).
Table 3. PATHOLOGIC FINDINGS AFTER TUMOR RESECTION
Values in parentheses are percentages.
NS, not significant.
* Infiltration of proper muscle layer.
** Infiltration of subserosa/fatty tissue.
Survival
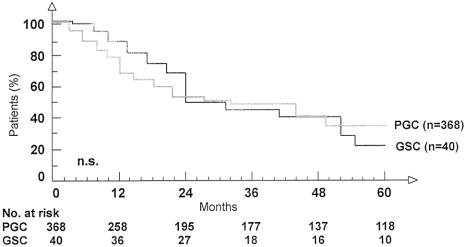
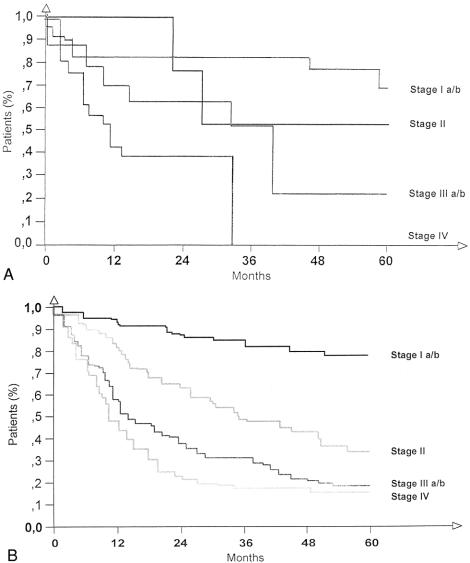
The median survival for the GSC patients undergoing R0 resection was 30.9 months; for the PGC patients it was 32.1 months (not significant; see Table 2). The overall survival rate of GSC patients (Fig. 1) was not significantly different from that of PGC patients. Based on tumor stage, there was no significant difference in survival between GSC and PGC patients (Fig. 2). In 15 GSC patients with lymph node metastases in the jejunal mesentery, the median survival was only 13.2 months.
Figure 1. Overall survival in patients with gastric stump cancer and primary gastric cancer undergoing R0 resection.
Figure 2. Overall survival in patients with gastric stump cancer (A) and primary gastric cancer (B) undergoing R0 resection according to tumor stage.
Multivariate Analysis
Results of the multivariate analysis showed an independent, positive prognostic effect in all patients for curative resection (R0 resection), pT1 and pT2 category, and age older than 65 years. In patients with R0 resection, the positive effect was seen only for the pT1 and pT2 category and age older than 65 years.
DISCUSSION
Gastric stump carcinomas have often been described as having low resectability rates and a poor prognosis, with 5-year survival rates of 3% to 10% in numerous series. 3,5,7,9 However, Sasako et al 18 found no significant difference in 5-year survival rates between patients with GSC and PGC: the resectability rate was 90% and the curative resection rate (R0 resection) was 69%.
A recent study by Newman et al 19 supported the opinion that the outcome after resection in GSC patients does not differ from that of patients with other primary proximal gastric cancers of the same stage. Other authors found a 5-year survival rate of 74% in patients with early cancer of the gastric remnant; even in patients without lymph node involvement but with advanced tumor stages, a 5-year survival rate of 47% could be achieved. 9,10 In the present analysis, the resectability rate and overall survival rate in patients who underwent R0 resection were high for both GSC and PGC patients; there were no significant differences between the groups.
Although a male preponderance of 3:1 is common in gastric cancer, in this study the male:female ratio—9:1—in GSC patients was unusually high. This may reflect the distribution of the surgical procedure or the type of peptic ulcer that is more common in men.
Most authors have reported a median age of 60 to 65 years for PGC patients, which is no different from that of the general cancer population. 6 We found a higher median age in GSC patients than in PGC patients. Univariate analysis showed that in GSC and PGC patients, age has prognostic value, 17 but on multivariate analysis only by GSC patients at age older than 65 years survival was significantly favorably influenced. Two studies with a small number of patients had reported this finding previously. 19,20 Further, the significantly better prognosis with increased age at surgery remained unaffected when adjusted for the duration of follow-up after surgery, sex, surgical procedure, and diagnosis at surgery. This may have resulted from some other risk factors (e.g., socioeconomic status), different responses to carcinogens in patients of different ages, or deaths of older patients from other causes before stomach cancer can develop.
In the GSC patients, 29.8% with UICC stage Ia disease had a considerably better prognosis compared with patients with advanced tumors. The frequency of early gastric cancer in GSC patients has varied from 10% to 35% in different studies. 9,12,21 The incidence of early gastric cancer in PGC 2,17 is approximately 50% in Japan versus 17% in U.S. series and less than 10% in all gastric cancers in Europe. At our hospital, 52 (10.4%) PGC patients and 14 (29.8%) GSC patients were identified as having early cancers; the operated stomach seems to be a precancerous factor. Increased surveillance of patients who have undergone gastrectomy means that tumors are seen at an earlier stage, when they have a considerably better prognosis. Endoscopic diagnosis of early lesions offers the best hope for cure. Starting screening at the 15th year after previous gastric surgery led to an increased detection of malignant mucosal lesions at a curable stage. 3,6,22 However, the also high incidence of GSC (34.0%) with advanced disease (stage IIIb/IV) in our series worsens the prognosis in GSC patients. 6,22–24
In some series, 2–4,25–27 approximately 90% of the GSCs occurred around the gastrojejunostomy and were caused by mucosal changes; the gastrojejunal anastomosis favored the development of carcinoma. There is an abrupt transition between carcinoma located on the gastric side of the anastomosis and normal jejunal mucosa. Even in our nine patients in whom the entire stomach was replaced by tumor, deep infiltration of the jejunum was found in only one (2.2%). Unlike other reports that 25% to 40% of the primary tumors were located around the gastrojejunostomy, 9,21,27,28 we found in 68.1% a clear point of origin near the gastrojejunostomy; in only 31.9% was the tumor confined to the cardia and the gastric remnant alone. We agree with recent reports that in Western countries for PGC, that there is an increased number of tumors in the proximal part of the stomach in contrast to earlier reports. 17,28–32 GSCs are rare at the cardia of the stomach: in our series only six (13.3%) patients had tumor at this site. An explanation is lacking; increased exposure at the gastric mucosa to refluxed bile and duodenal secretions is supposed to be responsible for the high incidence of carcinomas at the gastrojejunostomy. 4,6,8,31 In GSC patients, there was no difference in prognosis between patients with a primary tumor site at the cardia compared with patients with a primary tumor site at the gastrojejunostomy. The predominant site of tumor location of GSC patients at the gastrojejunostomy and lesser curvature is confirmed by Yonemura et al 11 and Pointner et al. 9
Important prognostic factors in PGC patients are the TNM classification (depth of tumor invasion, nodal state, and distant metastasis) and R0 resection (curative resection). 17,29,30,33 The data of our multivariate analysis suggest that the prognosis in GSC patients may be improved by complete resection of the primary tumor, and that the prognosis depends on the depth of tumor infiltration (pT1/2 category) and the patient’s age. Thus, there are no differences in prognostic factors between PGC and GSC patients.
The pT2 category predominates in tumors of the proximal third of the stomach, and this part of the stomach is not entirely covered by serosa. Therefore, tumors of the proximal part often do not reach the serosal lining and are understaged as pT2 tumors. 33 We found that 64.3% of the GSC patients and 64% of the PGC patients with pT2 category showed transmural tumor growth and infiltration of the perigastric fat tissue. Correction for the real depth of tumor invasion results in a decrease in downstaging of patients with stage I and stage II cancer and might be responsible for the different prognosis between patients with stage III GSC and PCG (GSC, 34% vs. 14.9%; PGC, 56.6% vs. 30.1%).
Another important factor related to the prognosis of gastric cancer appears to be lymph node metastasis. Lymph node metastases in GSC patients are most common in perigastric node stations (compartment I), but nonperigastric node stations are also frequently involved, especially those around the left gastric artery or in the splenic hilum (compartment II). 14,17,29,34 In our series, the ratio of invaded versus examined lymph nodes was similar for GSC and PGC patients (20.7% vs. 19.7%). Four pathways of lymphatic drainage of the gastric stump were noted by Maruyama et al, 35 with a high incidence of metastases in the nodes along the mesenteric root. To improve the prognosis of GSC, some authors recommend wide resection of the jejunal mesentery from the origins of each involved jejunal artery in patients with jejunal tumor infiltration. 11,21 In our study, 15 (46.8%) of 32 GSC patients with tumor infiltration around the gastrojejunostomy had lymph node metastases in the jejunal mesentery, with poor survival (median survival 13.2 months). Therefore, resection of the jejunal mesentery is recommended when resecting a GSC.
In PGC of the proximal third of the stomach, to complete lymph node dissection in the retroperitoneum, a pancreas-preserving splenectomy and lymphadenectomy of the renal hilus in advanced cases is recommended and tends to improve survival. 33,34 The death rate associated with extended gastric surgery in Japan is less than 3%, comparable with the data for standard gastrectomy in Western countries. 11,12
In conclusion, we found no difference between GSC patients and PGC patients in terms of resectability rate, death rate, and survival. Therefore, the consequences of surgical therapy in these patients are identical. Patients at risk (e.g., those who have undergone previous gastric surgery) should be examined carefully, because GSC that is diagnosed early has a better prognosis than more advanced disease. Finally, our results add to the data suggesting that surgery can be performed safely in GSC patients and can achieve results as good as those in patients with PGC.
Footnotes
Correspondence: Stefan Thorban, MD, Dept. of Surgery, Technische Universität München, Ismaningerstr. 22, 81675 Munich, Germany.
Accepted for publication June 17, 1999.
References
- 1.Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg 1922; 76:405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer AB. Twenty-five years after Billroth II gastrectomy for duodenal ulcer. World J Surg 1984; 8:293–403. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls JC. Stump cancer following gastric surgery. World J Surg 1979; 3:731–736. [DOI] [PubMed] [Google Scholar]
- 4.Gross Fisher S, Davis F, Nelson R, et al. A cohort study of stomach cancer risk in men after gastric surgery for benign disease. J Natl Cancer Inst 1993; 85:1303–1310. [DOI] [PubMed] [Google Scholar]
- 5.Orlando R, Welch JP. Carcinoma of the stomach after gastric operation. Am J Surg 1981; 141:487–491. [DOI] [PubMed] [Google Scholar]
- 6.Domellof L. Gastric carcinoma promoted by alkaline reflux gastritis. Med Hypotheses 1979; 5:463–476. [DOI] [PubMed] [Google Scholar]
- 7.Viste A, Edie GE, Real C, et al. Cancer of the gastric stump: analyses of 819 patients and comparison with other stomach cancer patients. World J Surg 1986; 10:454–461. [DOI] [PubMed] [Google Scholar]
- 8.Dilin C, Sarfati E, Chevrel JP. Les cancers dits du moignon gastrique. Revue de la littérature à propos de quattre observations. J Chir 1985; 122:193–200. [PubMed] [Google Scholar]
- 9.Pointner R, Schwab G, Köningstrainer E, et al. Early cancer of the gastric remnant. Gut 1988, 29:298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianello P, Lerut J, Otte JB, et al. Cancer d l’ estomac restant après chirurgie pour maladie ulcéreuse bénigne. Acta Gastroent Belg 1983; 46:27–38. [PubMed] [Google Scholar]
- 11.Yonemura Y, Ninomiya I, Tsugawa K, et al. Lymph node metastases from carcinoma of the gastric stump. Hepato-Gastroenterol 1994; 41:248–252. [PubMed] [Google Scholar]
- 12.Yonemura Y, Sugiyama K, Fujimura T, et al. A new surgical technique (left upper abdominal evisceration) for advanced carcinoma of the gastric stump. Hepato-Gastroenterol 1994; 41:130–133. [PubMed] [Google Scholar]
- 13.Japanese Research Society for Gastric Cancer. The general rules for the gastric cancer study in surgery and pathology. Jpn J Surg 1981; 11:127–139. [DOI] [PubMed] [Google Scholar]
- 14.Siewert JR, Böttcher K, Roder JD, et al. Prognostic relevance of systematic lymph node dissection: results of the German Gastric Carcinoma Study 1992. Br J Surg 1993; 80:1015–1018. [DOI] [PubMed] [Google Scholar]
- 15.Wittekind C, Wagner G, editors. UICC TNM classification of malignant tumors, 5th ed. New York: Springer; 1997.
- 16.Siewert JR, Stein HJ. Carcinoma of the cardia: carcinoma of the gastroesophageal junction—classification, pathology and extent of resection. Dis Esophagus 1995; 9:172–182. [Google Scholar]
- 17.Roder JD, Böttcher K, Siewert JR, and the German Gastric Carcinoma Study Group. Prognostic factors in gastric carcinoma: results of the German Gastric Carcinoma Study 1992. Cancer 1993; 72:2089–2097. [DOI] [PubMed] [Google Scholar]
- 18.Sasako M, Maruyama K, Kinoshita T, et al. Surgical treatment of carcinoma of the gastric stump. Br J Surg 1991; 78:822–824. [DOI] [PubMed] [Google Scholar]
- 19.Newman E, Brennan MF, Hochwald SN, et al. Gastric remnant carcinoma: just another proximal gastric cancer or a unique entity? Am J Surg 1997; 173:292–297. [DOI] [PubMed] [Google Scholar]
- 20.Erikson SBS. The operated stomach [PhD thesis]. Lund, Sweden: University of Lund; 1984.
- 21.Brollo A, Velsig M, Di Bonito L, et al. Elevato rischio relativo di carcinoma del moncone gastrico in pazienti gastrorescati in giovane età. Minerva Med 1981; 72:1921–1924. [PubMed] [Google Scholar]
- 22.Stael von Holstein C, Eriksson S, Huldt B, et al. Endoscopic screening during 17 years for gastric stump carcinoma: a prospective clinical trial. Scand J Gastroenterol 1991; 26:1020–1026. [DOI] [PubMed] [Google Scholar]
- 23.Caygill CP, Hill MJ, Kirkham JS, et al. Mortality from gastric cancer following gastric surgery for peptic ulcer. Lancet 1986; 26:929–931. [DOI] [PubMed] [Google Scholar]
- 24.Schnapka G, Hofstaedter F, Schwamberger K, et al. Gastric stump carcinoma following Billroth II resection for peptic ulcer disease: comparison with cancer in non-operated stomach. Endoscopy 1984; 16:171–174. [DOI] [PubMed] [Google Scholar]
- 25.Hioki K, Nakane Y, Yamamoto M. Surgical strategy for early gastric cancer. Br J Surg 1990; 77:1330–1334. [DOI] [PubMed] [Google Scholar]
- 26.Abe S, Ogawa Y, Nagasue N, et al. Early gastric cancer. Results in a general hospital in Japan. World J Surg 1984; 8:308–314. [DOI] [PubMed] [Google Scholar]
- 27.Morgenstern L, Vamakawa T, Seltzer D. Carcinoma of the gastric stump. Am J Surg 1973; 125:29. [DOI] [PubMed] [Google Scholar]
- 28.Debray VC, Bouvry M, Roches P. Stump carcinoma after gastric resection for ulcer. Schweiz Med Wocheschr 1958; 88:631. [PubMed] [Google Scholar]
- 29.Maruyama K. The most important prognostic factors for gastric cancer patients: a study using univariate and multivariate analyses. Scand J Gastroenterol 1987; 22:63–68. [Google Scholar]
- 30.Shiu MH, Moore E, Sanders M, et al. Influence of the extent of resection on survival after curative treatment of gastric carcinoma: a retrospective multivariate analysis. Arch Surg 1987; 122:1347–1352. [DOI] [PubMed] [Google Scholar]
- 31.Kalish RJ, Clancy PE, Oringer MB, et al. Clinical, epidemiologic and morphologic comparison between adenocarcinomas arising in Barett’s esophageal mucosa and in the gastric cardia. Gastroenterology 1984; 86:461–467. [PubMed] [Google Scholar]
- 32.Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach: a patient care study by the American College of Surgeons. Ann Surg 1993; 218:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siewert JR, Böttcher K, Stein HJ, et al. Problem of proximal third gastric carcinoma. World J Surg 1995; 19:523–531. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama K, Gunvén P, Okabayashi K, et al. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg 1989; 210:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987; 11:418–425. [DOI] [PubMed] [Google Scholar]